Abstract
The purpose of current study was to purify and partially depolymerize guar gum by β-mannanase, HCl, Ba(OH)2 actions and subjected to inspect compositional, thermogravimetric analysis (TGA) and haemolytic activity. Chemical composition revealed mannose and galactose ratio remained un-altered even after process of purification and hydrolysis. TGA thermograms affirmed initial and final decomposition temperature in various zones. Major decomposition stages apparently revealed partially hydrolyzed guar gum (PHGG) exhibited better heat stable properties having more zones of degradation than crude one. Furthermore, all guar fractions (2.5–250 mg/mL) were subjected to haemolysis to evaluate toxic effects during process of hydrolysis. The crude and hydrolyzed guar galactomannans exhibited minor haemolytic activity (1.9 ± 0.03–7.24 ± 0.02%) when compared to 0.1% Triton-X 100 (100% haemolysis) showing no toxic effects to human RBC’s. Conclusively, hydrolyzed guar-galactomannans are safe and can be used in food products with improved heat stability.
Keywords: Hydrolyzed guar gum, Haemolysis, In vitro, Thermal stability (TGA), Compositional analysis
Introduction
Guar gum is a galactomannan obtained from ground endosperm of Cyamopsis tetragonoloba (L) and considered as an excellent source for dietary fiber (Greenberg and Sellman 1998). Chemically, guar gum is a polysaccharide comprised of mannose and galactose sugars units in ratio 2:1. The backbone is a linear chain of β 1,4-linked mannose residues to which galactose residues are 1,6-linked at every second mannose, forming short side-branches (Sabahelkheir Murwan et al. 2012). Guar gum is white to yellowish white, nearly odorless powder, disperses in hot or cold water and swells immediately to form a highly viscous solution. It is principally utilized as a natural fiber, stabilizer, emulsifier, thickener, flocculants, bonding and gelling agent (Rowe et al. 2006). Although guar gum has beneficial physiological effects but its incorporation in enteral solutions and food products is difficult because of its higher viscosity. Therefore, moderate hydrolysis techniques have been developed to reduce the molecular weight resulting in altered flow attributes in solution, without disturbing chemical nature of the gum (Cheng et al. 2002; Hussain et al. 2015).
Partially hydrolyzed guar gum (PHGG) has been revealed to be very comparable to crude guar gum with respect to structure and composition of polysaccharide. PHGG is acceptable to consumer with no toxic effect, except for the presence of salts in neutralization step involved in the process of manufacturing (Slavin and Greenberg 2003). PHGG even when ingested through parenteral route, can’t destabilize the hematological parameters and particularly no effects on histopathologic, hepatic and renal factors (Cho and Samuel 2009).
PHGG has higher solubility and minute physical impact on foods and can be utilized as excellent source of fermentable dietary fiber (Finley et al. 2013). PHGG is more heat stable as compared to the crude one (Yoon et al. 2008). Thermal stability of polymer is of significant characteristic that could make materials suitable for applications in food where material is thermally processed in unit operations (baking, pasteurization and sterilization etc.) Thermo-gravimetric analysis (TGA) is precise method to analyze thermal stability and decomposition configuration of polymers (Mudgil et al. 2012). Hence, the current research article deals to thermally characterize crude and purified guar galactomannans and its hydrolyzed forms (acid, base and enzyme modifications) using thermo-gravimetric analyzer and furthermore, explores to examine toxic residues formed during the process of depolymerization to haemolytic activities in a dose dependent manner as these guar gums would have to be utilized for quality enhancement of various food products.
Materials and methods
Purification of guar gum
Fine powder of crude guar gum (100 g) was dissolved in 200 mL of distilled water and allowed to stand for 24 h with intermittent stirring. The gum was strained with calico to remove any insoluble debris or impurities and precipitated with 350 mL of 96% ethanol. The precipitated gum was re-filtered, washed with diethyl ether and freeze dried (CHRIST, Alpha 1-4 LD Plus, version 1.26, Germany) at − 55 °C. The dried purified gum was milled to fine powder (Ofori-Kwakye et al. 2010).
Hydrolysis of guar gum
Hydrolysis of guar gum with acid, base and enzyme was performed according to method described by Hussain et al. (2015).
Acidic hydrolysis
Guar gum (10 g) was taken in 80% aqueous methanol (200 mL) containing 5% w/v HCl. The reaction mixture was heated for 2.5 h at 65 °C. The depolymerized guar gum was neutralized with 1.0 N NaOH solution and filtered under suction, washed with ethanol, freeze dried and milled to fine powder (Chauhan et al. 2009).
Basic hydrolysis
Guar gum (5 g) was basically hydrolyzed with a saturated barium hydroxide [Ba(OH)2] solution (200 mL) at 100 °C for 8 h. The hydrolyzed gum was neutralized with 1 M H2SO4, filtered, freeze-dried and milled to fine powder (Beltrán et al. 2008).
Enzymatic hydrolysis
Guar gum powder was hydrolyzed with the enzyme mannanase (Mannan endo-1,4-beta mannosidase, Mannaway 4.0T, Novozyme, DK) by the adopted method with some modifications (Cheng and Prud’homme 2000). Guar powder 1.5 g was sprinkled slowly onto 150 mL of deionized water. The mixture was stirred through magnetic stirrer during the reaction. Another 49 mL of deionized water was added to wash all the residual powder on the beaker walls into the solution. A 0.04 mg (0.04 units/200 mL) of mannanase enzyme was diluted in 2 mL of 0.1 M sodium acetate/acetic acid buffer solution with pH adjusted to 6 and mixed thoroughly for 60 min. The solution pH is adjusted to 7.0 using HCl (37%, sp. gravity, 1.19 g/mL). Finally, the polymer solution is transferred to a container and placed for approximately 20–24 h at 25 °C to complete hydration. The mixture was magnetically stirred during the reaction. Guar and enzyme mixture were immediately heated to 100 °C for 20 min to denature the enzyme and stop the reaction. The mixture was vacuum filtered (whatman filter paper 42) and collected residues were freeze dried at − 55 °C (CHRIST, Alpha 1-4 LD Plus, version 1.26, Germany) and ground to fine powder.
Chemical composition
Chemical composition of guar gum and its hydrolytic derivatives were estimated according to standard operating method (AACC 2000). The moisture content (method no. 44-15A) was analyzed gravimetrically by drying the samples in an air forced draft oven (MEMMERT Mod. 1430) at a temperature of 105 ± 5 °C till a constant weight of the dried material is attained. Protein estimation (no. 46-10) was done by Kjeldahl’s method and fat content (no. 30-10) was estimated by using soxhlet extraction with hexane (b.p. 65–70 °C). The ash content (no. 08-01) was determined by heating the sample in a muffle furnace at 550 °C for 5 h followed by cooling and weighing. The fiber content (no. 32-10) was calculated by placing the digested samples in a muffle furnace maintained for 3–5 h at temperature of 550–650 °C till grey or white ash was obtained. Galactose and mannose content of guar gum fractions were estimated with some modifications (Jahanbin et al. 2012). 10 mg pure freeze-dried gum was hydrolyzed by heating at 120 °C (Memmert 100 universal bench, Germany) for 3 h with 1 mL of 2 M trifluoroacetic acid (TFA, CF3COOH) in a sealed tube. Excess acid was removed by flash evaporation on a water bath at a temperature of 40 °C and co-distilled with three times of water. The hydrolyzed products were reduced with NaBH4 (50 mg) and filtered through a 0.45 µm filter, and 20 µL of the sample was injected into the HPLC column. A Perkin Elmer Shimadzu HPLC unit and Rezex RCM-Monosaccharide Ca2+, Phenomenex column was used to carry out the analysis. HPLC grade water was used as mobile phase (isocratic) at a flow rate of 0.6 mL/min. A refractive-index detector (Gradient LC) was used and the column oven temperature was 80 °C. Monosaccharides were identified by comparing their retention times with the standard sugars. They were quantified according to their percentage area, obtained by integration of the peaks.
Thermal analysis
Thermo-gravimetric (TGA) analysis was carried out with the help of a TGA instrument. Thermo-gravimetric and differential thermal analyzer (TG/DTA) (Perkin Elmer, USA) was used to evaluate the effect of rise in temperature with respect to time in crude, purified and hydrolyzed samples of guar gum. Analysis was carried out in a temperature range from 30 to 1200 °C with a uniform heating rate of 10 °C/min in nitrogen atmosphere (Sen et al. 2010).
Haemolytic activity assay
Haemolytic activity of various guar gums was assessed with the method followed by Riaz et al. (2012). Five milliliters freshly human blood was gently mixed, poured into a sterile 15 mL polystyrene screw-cap tube and centrifuged at 850×g for 5 min. The supernatant was poured off and the viscous pellet (erythrocyte) washed three times with 5 mL of chilled (4 °C) sterile isotonic phosphate-buffered saline (PBS) solution (amounts g/L: NaCl, 8; KH2PO4, 0.2; Na2HPO4, 1.2; KCl, 0.2, Adjusted to pH 7.4, mixed for 60 min to stabilize pH). The washed erythrocytes were suspended in a final volume of 20 mL chilled, sterile PBS and the cells counted on a haemacytometer (Marienfeld, Neubauer Improved, Germany). The erythrocyte suspension was maintained on wet ice and diluted with sterile PBS to 7.068 × 108 cells/mL for each assay. Aliquots of 50 µL of crude, purified and hydrolyzed guar galactomannans were aseptically placed into 2.0 mL microfuge tubes. For each assay, 0.1% Triton X-100 (BDH, UK) was the positive, 100% lytic control and PBS (Oxoid, UK) was the negative, background (0% lysis) control. Aliquots of 180 µmL diluted erythrocyte suspension were aseptically placed into each 2-mL tube and gently mixed. The guar galactomannans concentrations tested were 2.5, 5.0, 25, 50, 75, 100, 150, 200 and 250 mg/mL. Tubes were incubated for 35 min at 37 °C with agitation (80 rev/min). Immediately following incubation, the tubes were placed on ice for 5 min then centrifuged for 5 min at 1310×g. Aliquots of 100 mL of supernatant were carefully collected, placed into a sterile 1.5 mL microfuge tube, and diluted with 900 mL chilled, sterile PBS. Optical absorbance at 576 nm was measured on a Micro Quant (BioTek, USA) using a 96-well plates and % RBC lysis was calculated.
Statistical analysis
Each guar fraction was analyzed in triplicate and results are shown as arithmetic mean values ± standard deviation. In each concentration parameter, the differences between guar samples were analyzed using one-way analysis of variance (ANOVA) followed by LSD test (P ≤ 0.05). This treatment was carried out using Statistix v. 8.1 program (Analytical Software., Tallahassee FL 32317, USA).
Results and discussion
Purification and hydrolysis of guar gum
In current study, the guar gum was precipitated from the aqueous medium by adding ethanol (95%) slowly while stirring and then dried. The simplest change is achieved by removing the impurities fractions in purification procedures. The purified gum possesses good flow and compressional characteristics forming good compacts at low compaction force (Cunha et al. 2007; Lubambo et al. 2013). The hydrolysis process (acid, enzyme, base) expectedly reduce the chain length and molecular weight of the polymer and finally the lower viscosity makes it an innovative soluble fiber that bear a resemblance to the basic chemical structure with crude guar gum and possess a variety of applications in clinical nutrition (Cunha et al. 2007; Hussain et al. 2015; Mudgil et al. 2012; Slavin and Greenberg 2003).
Chemical composition
Chemical composition (protein, moisture, fat, fibre and ash content) of crude, purified and partially hydrolyzed guar gum precised in Table 1. Moisture contents decreased from 6.54% (CGG) to 5.09% (BHGG) and fat 1.18% (CGG) to 0.92% (AHGG) accordingly. The increase in ash was highly significant but lowest value was in PGG followed by second lowest EHGG. Whereas, BHGG exhibited the highest value for ash content (1.88%) followed by the second lowest in AHGG (1.49%), when compared to 0.42% ash in case of CGG.
Table 1.
Means values for chemical composition (%) for the crude and hydrolyzed guar gum
| Treatment | Moisture | Ash | Fat | Protein | Fiber |
|---|---|---|---|---|---|
| CGG | 6.54 ± 0.57 | 0.42 ± 0.16d | 1.18 ± 0.03 | 9.48 ± 0.77bc | 1.87 ± 0.04b |
| PGG | 5.37 ± 0.55 | 0.22 ± 0.70c | 1.05 ± 0.07 | 11.47 ± 0.77b | 1.88 ± 0.01b |
| AHGG | 6.03 ± 0.04 | 1.49 ± 0.06b | 0.92 ± 0.03 | 9.48 ± 0.76bc | 2.02 ± 0.02a |
| BHGG | 5.09 ± 0.63 | 1.88 ± 0.01a | 0.96 ± 0.08 | 7.98 ± 0.11c | 1.91 ± 0.03b |
| EHGG | 6.00 ± 0.54 | 0.25 ± 0.02c | 0.95 ± 0.07 | 14.96 ± 1.51a | 2.00 ± 0.02a |
The values are mean ± SD (n = 5)
Means with different letters differ significantly at (P ≤ 0.05). Comparisons are made within the column for each guar fractions
LSD value: Moisture = 1.3207, Ash = 0.2078, Fat = 0.1595, Protein = 2.1453, Fiber = 0.0845
CGG crude guar gum, PGG purified guar gum, AHGG acid hydrolyzed guar gum, BHGG base hydrolyzed guar gum, EHGG enzyme hydrolyzed guar gum
The results regarding protein contents showed variability as CGG (9.48%) was at par with AHGG whereas, significantly increased in EHGG (14.96%) and in PGG (11.47%). Fiber contents of the CGG was increased (highly significant) from 1.87 to 2.02% in AHGG and then to 2.00% in EHGG whereas, PGG and BHGG were at par. The results obtained in this study were comparable to Mudgil et al. (2012) who examined the decrease in moisture from 10.82 to 8.02% (difference of 2.80%) when compared the crude guar gum with hydrolyzed guar gum respectively. Their results showed increase in ash contents which were in one way or the other supportive to studies conducted in this manuscript particularly for ash increase AHGG and BHGG whereas, in our case ash contents PGG and EHGG were decreased.
Increase in fiber contents in our case was also reinforced by the studies (Mudgil et al. 2012) where they obtained considerable quantity of total fiber and can be regarded as an excellent source of total dietary fiber (TDF). The values for various chemical compositions in current study when compared with other study (López-Franco et al. 2013) showed moisture contents (6.54 vs. 5.9%), protein (9.48 vs. 5.1%), ash (0.66 vs. 0.42%) and fat (1.18 vs. 0.005%) accordingly showing higher values than the data reported by them for crude guar gum.
The varietal difference may exist in guar gum from different sources showing variation in chemical composition. The higher moisture (11.70%) and ash (0.72%) but lower protein (0.05%) content was observed in guar gum when compared to current results (Bourbon et al. 2010).
The overall results showed that guar gum analyzed from different sources and variety exhibited different values for moisture, ash, protein, fat and fiber. Chemical composition varied with the purification and hydrolysis (acid/base and enzymes), moisture (9–15%), protein (4.43%) and fiber (1.28%) contents for the crude guar gum was determined (Gupta et al. 2009).
The crude (CGG), purified (PGG) and hydrolysed (BHGG, AHGG and EHGG) guar gums were subjected to measurement of mannose and galactose ratio through HPLC (Shimadzu 10 AL, Japan). The standards were consisted of 1% solutions of galactose and mannose. The results are presented in Table 2. It had been deduced from the results that hydrolysis through acid, base and enzyme did not change the ratio of galactose and mannose. However, the percentage of mannose and galactose was significantly changed as depicted (Table 2). CGG showed 73:35 which was the highest value for mannose and galactose followed by PGG (71:33) and BHGG (67:33). The lowest values were obtained in EHGG (67:29) and AHGG (66:30). The current findings were supported by Cunha et al. (2007) who made purified guar gum by using various solvents and found that mannose and galactose ratio remained unchanged even after process of purification. Kurakake et al. (2006) also reinforced the current results while studying the production of galacto-manno-oligosaccharides from guar gum through mannanase enzyme. Tapie et al. (2008) conducted study on natural galactomannans and reported galactose and mannose ratio as 1:2 which supported results in this study.
Table 2.
Mean values for galactose-mannose (%) of crude and hydrolyzed guar gum
| Treatment | Mannose | Galactose | M/G ratio |
|---|---|---|---|
| CGG | 73 a | 35 a | 2:1 |
| PGG | 71 b | 33 b | 2:1 |
| AHGG | 66 d | 30 c | 2:1 |
| BHGG | 67 c | 33 b | 2:1 |
| EHGG | 67 c | 29 d | 2:1 |
Means with different letters are differ significantly at (P ≤ 0.05). Comparisons are made within the column for each guar fractions to evaluate the sugar composition
CGG crude guar gum, PGG purified guar gum, AHGG acidic hydrolyzed guar gum, BHGG basic hydrolyzed guar gum, EHGG enzymatic hydrolyzed guar gum
Thermo-gravimetric analysis (TGA)
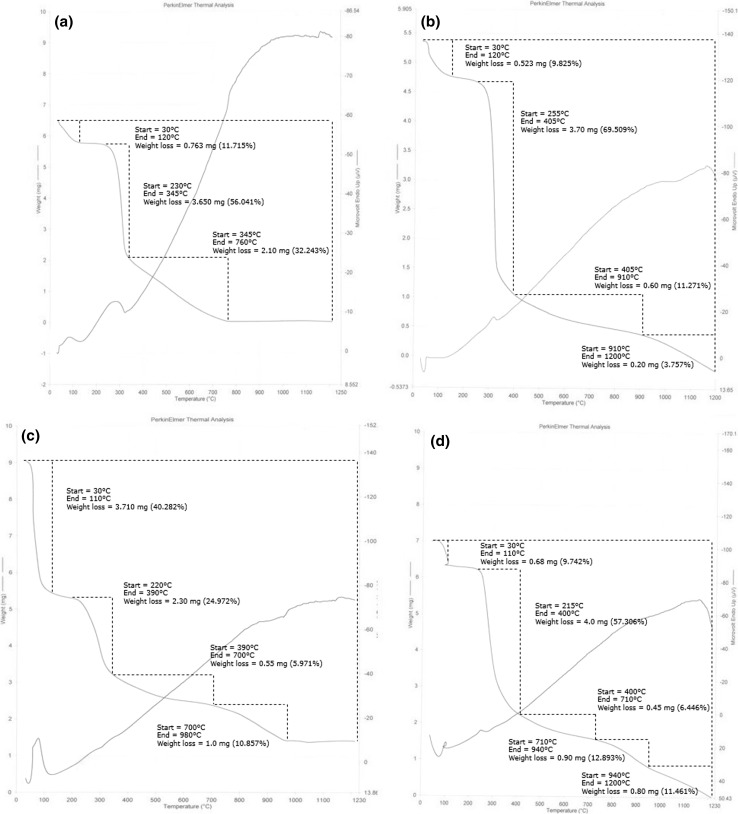
TGA curves of CGG as shown in Fig. 1a essentially indicate involvement of three distinctive zones of weight reduction. The initial weight loss was 11.715% at 30–120 °C which was due to the presence of moisture contents traces. The second zone of weight loss ranged from 230 to 345 °C showing the loss of 56.041% that could be attributed to degradation of secondary alcohol -CHOH whereas, the weight loss in third zone occurred at 345–760 °C resulting in mass reduction of 32.243% which could be due to degradation of backbone of polymer (primary alcohol –CH2OH). The results on the thermal stability declared that the mass loss of CGG occurs with the rise in temperature in three distinct zones which is in coordination to results for CGG in our studies (Sen et al. 2010).
Fig. 1.
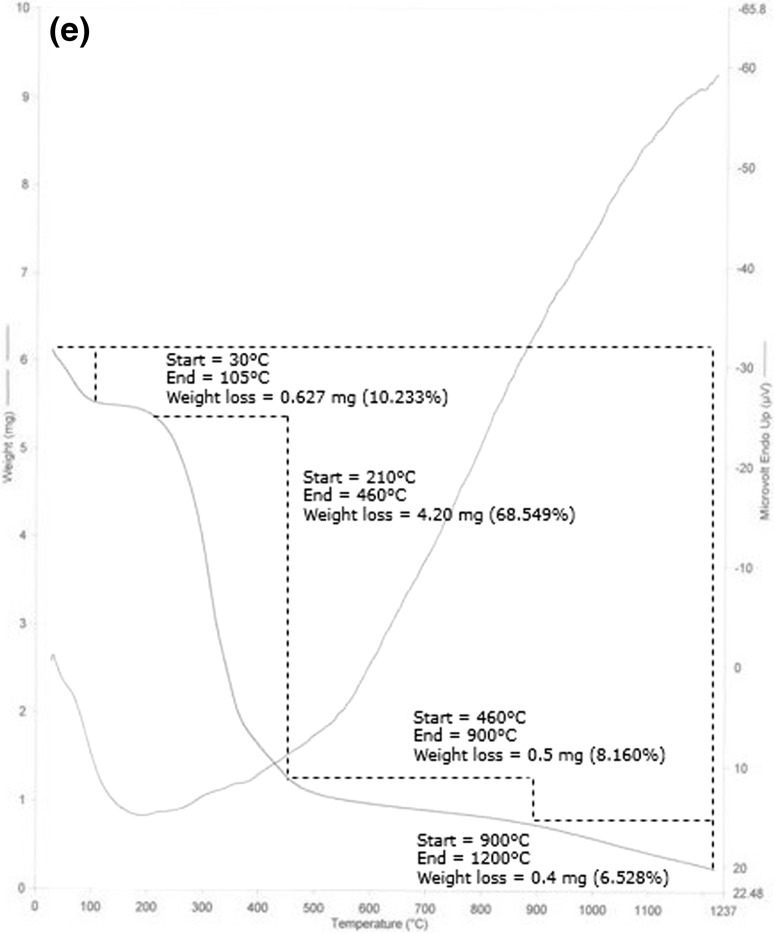
Thermo gravimetric analysis (TGA) curve of a crude guar gum CGG; b purified guar gum PGG; c base hydrolyzed guar gum (BHGG); d acid hydrolyzed guar gum (AHGG); e enzyme hydrolyzed guar gum (EHGG)
In case of PGG, weight loss occurred in four zones of temperature ranges as shown in Fig. 1b. The first, second and third zones were in the range of 30–120, 255–405 and 405–910 °C exhibiting the weight loss of 9.825, 69.509 and 11.271% accordingly. In addition to previous weight reduction zones, PGG had an extra zone which was in the range of 910–1200 °C displaying the weight reduction of 3.757%. The fourth extra zone of weight loss as compared to CGG, might be due to the amide group (–CONH2) of the synthesized polymer. Therefore, the existence of this additional zone is a clear sign that some functional groups have been attached onto the backbone of guar gum.
TGA curve for BHGG presented in Fig. 1c, declared weight loss in four distinct zones. Initial weight loss started at zone 30–110 °C. This zone indicated the major loss about 40.282%. The second zone of weight loss involves the range of 220–390 °C showing the weight loss of 24.972%. Weight reduction of about 5.971% occurred in the third zone of mass loss at 390–700 °C whereas, fourth zone (700–980 °C) contributing the weight loss of polymer as about 10.857%. In Fig. 1d, TGA profile of AHGG shows the weight loss in five zones. The first zone occurs at 30–110 °C contributing to loss of moisture about 9.742%. The second zone ranges in 215–400 °C and resulted in distinct mass loss of polymer (57.306%). The third zone (400–700 °C) gave rise to relatively less weight loss of about 6.446%. The fourth (710–940 °C) and fifth (940–1200 °C) zone contributed towards mass loss of 12.893 and 11.461% respectively.
In EHGG, TGA thermo-gram as presented in Fig. 1e, thermal degradation comprises of four major zones. Initial thermal degradation starts in the range of 30–105 °C giving rise to 10.233% of moisture loss. The second zone (210–460 °C) contributed distinct mass loss of 68.549%. The third (460–900 °C) and fourth (900–1200 °C) zones showed weight loss of 8.160 and 6.528% respectively.
Thermal decomposition of CGG, completed in two phases, 180–365 and 390–504 °C resulting in weight loss of about 60 and 18% respectively. While thermal degradation of modified polymer was completed in three steps, at 150–300, 320–410 and 420–500 °C with the weight loss of about 30, 20 and 27% in the first, second and third steps of degradation accordingly. An extra zone of weight reduction in modified guar gums declared the increased heat stability as compared to CGG (Prasad et al. 2012). In another study, thermo-gravimetric investigation exhibited reduction of weight in two phases. In the first phase, minor weight loss in the samples may be attributed to the loss of adsorbed and structural water of biopolymers or due to desorption of moisture as hydrogen bounded water to the saccharide structure. The second weight loss event may be attributed to the decomposition of polysaccharide (Bothara and Singh 2012).
An initial loss in weight (8–12%) in temperature range 80–120 °C was experienced (Iqbal et al. 2011). First major phase of degradation was characterized by initial decomposition temperature (IDT) in the range 220–270 °C and final decomposition temperature (FDT) in the range 310–375 °C. The initial stage caused a weight loss of about 39–56% while in the second main decomposition stage, the IDT and FDT range was 415–450 and 490–550 °C respectively. The loss in weight was about 20% that is credited to complete decomposition of hydrogels.
TGA profiles of residual mass verified increased stability of PHGG at higher range of temperature than crude guar gum. TGA outcomes also exposed that no principal variation was observed in chemical structure of PHGG (Mudgil et al. 2012). The integral procedural decomposition temperature (IPDT) values calculated based on the TGA thermo-grams showed that modified gums were documented to be more thermally stable than any other polysaccharides (Zohuriaan and Shokrolahi 2004).
Haemolytic effects
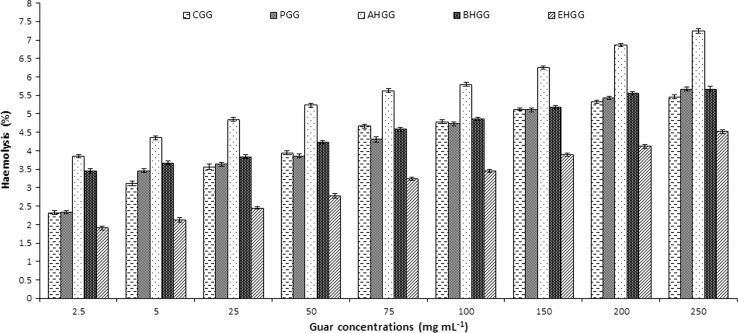
Haemolytic bioassays (Table 3) of crude, purified and hydrolyzed guar galactomannans were executed on human RBCs. Standard references used were PBS negative control (0% haemolysis) and Triton X-100 positive control (100% haemolysis). Absorbance at 576 nm wave length was in the range of 0.036 ± 0.022–0.136 ± 0.032 (Table 3) for all guar fractions and showed very low percentage of haemolysis when compared to positive and negative control. Maximum haemolysis was 7.24 ± 0.02% observed in acidically hydrolyzed guar fractions (250 mg/mL) while minimum was 1.9 ± 0.03% in enzymatically hydrolyzed guar gum (2.5 mg/mL) (Fig. 2).
Table 3.
Haemolytic activity of controls (Triton-X 100, PBS solution) and five guar samples at various concentrations (mg/mL)
| Conc. mg/mL | Optical absorbance at 576 nm | ||||||
|---|---|---|---|---|---|---|---|
| Positive control | Negative control | CGG | PGG | AHGG | BHGG | EHGG | |
| 2.5 | 1.876 ± 0.012a | 0.005 ± 0.007f | 0.044 ± 0.022d | 0.044 ± 0.022d | 0.072 ± 0.012b | 0.065 ± 0.031c | 0.036 ± 0.022e |
| 5.0 | 1.876 ± 0.012a | 0.005 ± 0.007g | 0.059 ± 0.032e | 0.065 ± 0.021d | 0.082 ± 0.021b | 0.069 ± 0.021c | 0.039 ± 0.028f |
| 25 | 1.876 ± 0.012a | 0.005 ± 0.007f | 0.067 ± 0.042d | 0.068 ± 0.012d | 0.091 ± 0.018b | 0.072 ± 0.022c | 0.046 ± 0.022e |
| 50 | 1.876 ± 0.012a | 0.005 ± 0.007f | 0.074 ± 0.021d | 0.073 ± 0.012d | 0.098 ± 0.022b | 0.079 ± 0.012c | 0.052 ± 0.034e |
| 75 | 1.876 ± 0.012a | 0.005 ± 0.007f | 0.088 ± 0.024c | 0.081 ± 0.021d | 0.106 ± 0.018b | 0.086 ± 0.021c | 0.061 ± 0.022e |
| 100 | 1.876 ± 0.012a | 0.005 ± 0.007f | 0.089 ± 0.021d | 0.089 ± 0.028d | 0.109 ± 0.023b | 0.091 ± 0.011cd | 0.065 ± 0.031e |
| 150 | 1.876 ± 0.012a | 0.005 ± 0.007e | 0.096 ± 0.023c | 0.096 ± 0.012c | 0.117 ± 0.021b | 0.097 ± 0.023c | 0.073 ± 0.012d |
| 200 | 1.876 ± 0.012a | 0.005 ± 0.007f | 0.100 ± 0.022d | 0.102 ± 0.013cd | 0.129 ± 0.012b | 0.104 ± 0.012c | 0.078 ± 0.021e |
| 250 | 1.876 ± 0.012a | 0.005 ± 0.007f | 0.103 ± 0.059d | 0.106 ± 0.022c | 0.136 ± 0.032b | 0.106 ± 0.032c | 0.085 ± 0.018e |
The values are mean ± SD (n = 3)
Means with different superscripts are significantly different (P ≤ 0.05). Comparisons are made within the row for each concentration of guar fractions to evaluate the effect on haemolysis activity
0.1% Triton-X 100, as positive control, PBS, phosphate buffer saline solution, as negative control
CGG, crude guar gum, PGG, purified guar gum, AHGG, acidic hydrolyzed guar gum, BHGG, basic hydrolyzed guar gum, EHGG, enzymatic hydrolyzed guar gum
Fig. 2.
Haemolytic activity, as a percentage of haemolysis caused by 0.1% triton X-100, for 2.5, 5.0, 25, 50, 75, 100, 150, 200, 250 mg/mL of guar gum fractions and controls. CGG crude guar gum, PGG purified guar gum, AHGG acidically hydrolyzed guar gum, BHGG basically hydrolyzed guar gum, EHGG enzymatically hydrolyzed guar gum. 0.1% Triton-X 100, as positive control (100% haemolysis), phosphate buffer saline, as negative control (0% haemolysis)
The European Food Safety Authority (EFSA) has documented the safety of PHGG as a stabilizer, emulsifier and thickener in food (EFSA 2007). But classical haemolysis of human RBCs includes saponins, which are glycosides of steroids, triterpenoids and sugars (Hassan et al. 2010). In human clinical study non-insulin dependent diabetic patients were examined to evaluate the effects of feeding a daily dose of 30 g of guar gum for a period of 16 weeks. No changes were observed in haematologic functions (McIvor et al. 1985).
In an acute and sub-chronic oral toxicity study, PHGG at a dose of 6000 mg/kg for 13 weeks (Koujitani et al. 1997) and 0, 500, and 2500 mg/kg/day for 28 days (Takahashi et al. 1994) proved to have no toxic and mutagenic potential. Healthy female students administered 12.5 g/day of PHGG in their meal, no adverse reactions to the treatment reported (Sakata and Shimbo 2006).
Conclusion
The compositional examination of guar gum and its hydrolyzed derivatives indicated that partial hydrolysis of guar gum reduced significantly moisture and fat content, although CGG, PGG, BHGG were at par for the later, when compared with native guar gum. However, there was marked increase in protein (except BHGG) and ash (except PGG and EHGG) content in comparison to native guar gum. Mannose and galactose ratio remained unchanged even after process of purification and hydrolysis by various ways. PHGG achieved by enzymatic, acidic and basic method possessed better heat stable property and showed more zones of degradation as compared to CGG. In addition, guar gum and its hydrolytic forms exhibited reduced haemolysis on human RBC’s with reference to standard and proved to be non-toxic for human consumption. There is no safety distress for partially depolymerized guar gum prepared either by alkaline, acid or enzymatic hydrolysis at estimated levels of ingestion and are generally recognized as safe (GRAS) in food processing industries with better heat stability.
Acknowledgements
Authors are cordially grateful to Higher Education Commission, Pakistan (Indigenous 5000 PhD Fellowship Program, Grant No. 106-1942-Av6-017) for financial support to accomplish this research project.
References
- AACC Approved methods of the American association of cereal chemists. Methods. 2000;54:21. [Google Scholar]
- Beltrán O, de Pinto GL, Rincón F, Picton L, Cozic C, Le Cerf D, Muller G. Acacia macracantha gum as a possible source of arabinogalactan–protein. Carbohydr Polym. 2008;72:88–94. doi: 10.1016/j.carbpol.2007.07.027. [DOI] [Google Scholar]
- Bothara SB, Singh S. Thermal studies on natural polysaccharide. Asian Pac J Trop Biomed. 2012;2:S1031–S1035. doi: 10.1016/S2221-1691(12)60356-6. [DOI] [Google Scholar]
- Bourbon A, Pinheiro A, Ribeiro C, Miranda C, Maia J, Teixeira J, Vicente A. Characterization of galactomannans extracted from seeds of Gleditsia triacanthos and Sophora japonica through shear and extensional rheology: comparison with guar gum and locust bean gum. Food Hydrocoll. 2010;24:184–192. doi: 10.1016/j.foodhyd.2009.09.004. [DOI] [Google Scholar]
- Chauhan K, Chauhan GS, Ahn J-H. Synthesis and characterization of novel guar gum hydrogels and their use as Cu2+ sorbents. Bioresour Technol. 2009;100:3599–3603. doi: 10.1016/j.biortech.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prud’homme RK. Enzymatic degradation of guar and substituted guar galactomannans. Biomacromolecules. 2000;1:782–788. doi: 10.1021/bm005616v. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Brown KM, Prud’homme RK. Preparation and characterization of molecular weight fractions of guar galactomannans using acid and enzymatic hydrolysis. Int J Biol Macromol. 2002;31:29–35. doi: 10.1016/S0141-8130(02)00046-6. [DOI] [PubMed] [Google Scholar]
- Cho SS, Samuel P. Fiber ingredients: food applications and health benefits. Boca Raton: CRC Press; 2009. [Google Scholar]
- Cunha PL, Paula RC, Feitosa JP. Purification of guar gum for biological applications. Int J Biol Macromol. 2007;41:324–331. doi: 10.1016/j.ijbiomac.2007.04.003. [DOI] [PubMed] [Google Scholar]
- EFSA Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food on a request from the commission related to an application on the use of partially depolymerized guar gum as a food additive Question No EFSA-Q-2006-122. EFSA J. 2007;514:1–17. [Google Scholar]
- Finley JW, Soto-Vaca A, Heimbach J, Rao T, Juneja LR, Slavin J, Fahey GC. Safety assessment and caloric value of partially hydrolyzed guar gum. J Agric Food Chem. 2013;61:1756–1771. doi: 10.1021/jf304910k. [DOI] [PubMed] [Google Scholar]
- Greenberg N, Sellman D. Partially hydrolyzed guar gum as a source of fiber. Cereal Foods World. 1998;43:703–707. [Google Scholar]
- Gupta S, Hooda K, Mathur N, Gupta S. Tailoring of guar gum for desert sand stabilization. Indian J Chem Technol. 2009;16:507–512. [Google Scholar]
- Hassan S, Haq A, Byrd J, Berhow M, Cartwright A, Bailey C. Haemolytic and antimicrobial activities of saponin-rich extracts from guar meal. Food Chem. 2010;119:600–605. doi: 10.1016/j.foodchem.2009.06.066. [DOI] [Google Scholar]
- Hussain M, Bakalis S, Gouseti O, Zahoor T, Anjum FM, Shahid M. Dynamic and shear stress rheological properties of guar galactomannans and its hydrolyzed derivatives. Int J Biol Macromol. 2015;72:687–691. doi: 10.1016/j.ijbiomac.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Iqbal MS, Akbar J, Saghir S, Karim A, Koschella A, Heinze T, Sher M. Thermal studies of plant carbohydrate polymer hydrogels. Carbohydr Polym. 2011;86:1775–1783. doi: 10.1016/j.carbpol.2011.07.020. [DOI] [Google Scholar]
- Jahanbin K, Moini S, Gohari AR, Emam-Djomeh Z, Masi P. Isolation, purification and characterization of a new gum from Acanthophyllum bracteatum roots. Food Hydrocoll. 2012;27:14–21. doi: 10.1016/j.foodhyd.2011.09.007. [DOI] [Google Scholar]
- Koujitani T, Oishi H, Kubo Y, Maeda T, Sekiya K, Yasuba M, Matsuoka N, Nishimura K. Absence of detectable toxicity in rats fed partially hydrolyzed guar gum (K-13) for 13 weeks. Int J Toxicol. 1997;16:611–623. doi: 10.1080/109158197226928. [DOI] [Google Scholar]
- Kurakake M, Sumida T, Masuda D, Oonishi S, Komaki T. Production of galacto-manno-oligosaccharides from guar gum by β-mannanase from Penicillium oxalicum SO. J Agric Food Chem. 2006;54:7885–7889. doi: 10.1021/jf061502k. [DOI] [PubMed] [Google Scholar]
- López-Franco Y, Cervantes-Montaño C, Martínez-Robinson K, Lizardi-Mendoza J, Robles-Ozuna L. Physicochemical characterization and functional properties of galactomannans from mesquite seeds (Prosopis spp.) Food Hydrocoll. 2013;30:656–660. doi: 10.1016/j.foodhyd.2012.08.012. [DOI] [Google Scholar]
- Lubambo AF, de Freitas RA, Sierakowski MR, Lucyszyn N, Sassaki GL, Serafim BM, Saul CK. Electrospinning of commercial guar-gum: effects of purification and filtration. Carbohydr Polym. 2013;93:484–491. doi: 10.1016/j.carbpol.2013.01.031. [DOI] [PubMed] [Google Scholar]
- McIvor M, Cummings C, Mendeloff AI. Long-term ingestion of guar gum is not toxic in patients with noninsulin-dependent diabetes mellitus. Am J Clin Nutr. 1985;41:891–894. doi: 10.1093/ajcn/41.5.891. [DOI] [PubMed] [Google Scholar]
- Mudgil D, Barak S, Khatkar B. X-ray diffraction, IR spectroscopy and thermal characterization of partially hydrolyzed guar gum. Int J Biol Macromol. 2012;50:1035–1039. doi: 10.1016/j.ijbiomac.2012.02.031. [DOI] [PubMed] [Google Scholar]
- Ofori-Kwakye K, Asantewaa Y, Kipo SL. Physicochemical and binding properties of cashew tree gum in metronidazole tablet formulations. Int J Pharm Pharm Sci. 2010;2:105–109. [Google Scholar]
- Prasad SS, Rao KM, Reddy PRS, Reddy NS, Rao KK, Subha M. Synthesis and characterisation of guar gum-g-poly (acrylamidoglycolic acid) by redox initiator. Indian J Adv Chem Sci. 2012;1:28–32. [Google Scholar]
- Riaz M, Rasool N, Bukhari IH, Shahid M, Zubair M, Rizwan K, Rashid U. In vitro antimicrobial, antioxidant, cytotoxicity and GC-MS analysis of Mazus goodenifolius. Molecules. 2012;17:14275–14287. doi: 10.3390/molecules171214275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe RC, Sheskey PJ, Weller PJ. Handbook of pharmaceutical excipients. London: Pharmaceutical Press; 2006. [Google Scholar]
- Sabahelkheir Murwan K, Abdalla Abdelwahab H, Nouri Sulafa H. Quality assessment of guar gum (endosperm) of guar (Cyamopsis tetragonoloba) ISCA J Biol Sci. 2012;1:67–70. [Google Scholar]
- Sakata Y, Shimbo S. How much does partially hydrolyzed guar gum affect the weight, moisture and hardness of feces? Jpn J Public Health. 2006;53:257–264. [PubMed] [Google Scholar]
- Sen G, Mishra S, Jha U, Pal S. Microwave initiated synthesis of polyacrylamide grafted guar gum (GG-g-PAM)—characterizations and application as matrix for controlled release of 5-amino salicylic acid. Int J Biol Macromol. 2010;47:164–170. doi: 10.1016/j.ijbiomac.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Slavin JL, Greenberg NA. Partially hydrolyzed guar gum: clinical nutrition uses. Nutrition. 2003;19:549–552. doi: 10.1016/S0899-9007(02)01032-8. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yang SI, Fujiki M, Kim M, Yamamoto T, Greenberg NA. Toxicity studies of partially hydrolyzed guar gum. Int J Toxicol. 1994;13:273–278. [Google Scholar]
- Tapie N, Malhiac C, Hucher N, Grisel M. Determination of galactose and mannose residues in natural galactomannans using a fast and efficient high-performance liquid chromatography/UV detection. J Chromatogr A. 2008;1181:45–50. doi: 10.1016/j.chroma.2007.12.027. [DOI] [PubMed] [Google Scholar]
- Yoon S-J, Chu D-C, Raj Juneja L. Chemical and physical properties, safety and application of partially hydrolized guar gum as dietary fiber. J Clin Biochem Nutr. 2008;42:1–7. doi: 10.3164/jcbn.2008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohuriaan M, Shokrolahi F. Thermal studies on natural and modified gums. Polym Test. 2004;23:575–579. doi: 10.1016/j.polymertesting.2003.11.001. [DOI] [Google Scholar]