Abstract
During the industrial manufacturing of pomegranate juice, large amounts of pomace are produced. The aim of this work was to find the effective method to dry pomegranate pomace to open new commercial applications for this co-product. The effects of three drying methods: (i) convective drying (CD) at 50, 60, and 70 °C; (ii) vacuum microwave drying (VMD) at 240, 360, and 480 W, and (iii) a combined method (CPD–VMFD); convective pre-drying (60 °C) followed by vacuum microwave finish drying (360 W), on drying kinetics and quality of PomP (pomegranate pomace obtained after preparing pomegranate juice by squeezing only arils) were evaluated. The shortest treatments were VMD at 240 and 360 W (52 and 33 min, respectively); besides, these treatments led to interesting values of the green–red coordinate, a*, (12.2 and 4.1, respectively), total phenolic content (4.0 and 4.1 mg eq gallic acid g−1 dry weight, respectively), and antioxidant activity (30.8 and 29.0 µmol g−1 dry weight, respectively). On the other hand, this study demonstrated that this co-product is a rich source of punicic acid (average value = 66.4%), being a good opportunity for the pharmaceutical and nutraceutical industries. Moreover, no significant changes in the fatty acid profile was observed as affected by the drying treatments, and no off-flavors were generated by any of the drying methods.
Keywords: Convective drying, Vacuum-microwave drying, Color, Descriptive sensory analysis, Antioxidant activity, Polyphenols
Introduction
Industrial processing of vegetables and fruits usually generates large quantities of co-products which are rich in bioactive compounds, for example polyphenols (Lario et al. 2004); thus, they can be converted into useful food ingredients (Bhol et al. 2015). As an example, during the industrial production of pomegranate juice, important amounts of pomace (composed mainly of non-edible rind, and residual arils pulp, and carpelar membranes) are produced, and represent as much as 50% on average in fresh weight. In the last years, attention has been paid to pomegranate non-edible parts (Hasnaoui et al. 2014), mainly because of their high antioxidant capacity. In this way, the re-use of pomegranate pomace (PomP), obtained from the juice industry to take advantage of the huge quantity of beneficial compounds, can be of interest in products requiring hydration, viscosity development, antibacterial agents and freshness preservation, such as baked foods or cooked meat products (Viuda-Martos et al. 2012; Hasnaoui et al. 2014; Gullon et al. 2016; Akhtar et al. 2015). Moreover, a recent study about PomP reported that an extract from this material can be used as a washing agent due to its antimicrobial properties against Listeria monocytogenes (Kang and Song 2017).
Drying is known as the best method to preserve fruits and vegetables. The use of this technique on PomP may reduce its volume and weight, making its transport easier and cheaper. Water removal prevents microorganism evolution (Mathlouthi 2001) and harmful chemical reactions, and leads to longer storage time (Calín-Sánchez et al. 2013); besides, an extra-value is added to this low-cost industrial waste, which main use at the current time is its inclusion in animal feed. Despite of its disadvantages, the most common method to produce industrial dried fruits and vegetables, due to its low cost, is convective drying (CD), using hot air. The normal conditions of this method (high temperature and/or long time) can lead to significant degradation of functional compounds and color pigments, significantly decrease of antioxidant capacity, and development of non-desirable off-flavors (Singh and Kingsly 2008). A more modern drying method is vacuum-microwave drying (VMD), which leads to better quality products, with a proper preservation of nutritional and sensory components. However, one of its major limitations is the non-uniformity of the microwave radiation which can induce over-heating in the borders and corners of the food (Clark 1996; Nijhuis et al. 1998). In order to overcome these problems, the combination of convective pre-drying with hot air (CPD) followed by vacuum-microwave finish drying (VMFD) has been successful in leading to high quality dried products (Figiel 2010; Nowicka et al. 2014; Cano-Lamadrid et al. 2017). The working hypothesis of this study was that this could be the case of PomP, because in previously studies on other pomegranate products, CPD–VMFD resulted in high quality dried arils and rind (Calín-Sánchez et al. 2013).
Therefore, this study was focused on evaluating the effect of three drying methods [(i) convective drying (CD) at different temperatures, (ii) vacuum microwave drying (VMD) at different powers, and (iii) a combination of convective pre-drying and vacuum-microwave drying (CPD–VMFD)] on drying kinetics and quality of PomP; the quality was studied by evaluating instrumental color, phenolic content, antioxidant capacity, and fatty acid profile. The aim of this work was to find the most effective drying method for PomP to open new commercial applications for this abundant co-product.
Materials and methods
Materials
Pomegranate pomace, PomP (Punica granatum L, cultivar Mollar de Elche) was obtained after extraction of juice in a Spanish company manufacturing pomegranate juice by squeezing only pomegranate arils. The PomP was express-posted in a refrigerated package to the Institute of Agricultural Engineering (Wrocław, Poland). After drying the material using different methods, the samples were posted back to the Universidad Miguel Hernández de Elche (Orihuela, Alicante, Spain) for the descriptive sensory analysis.
Convective drying (CD)
The process of CD was conducted using the drier shown in Fig. 1. Pomegranate samples (~ 60 g) were spread on a round 100-mm tray, and the CD was operated at 3 temperatures: 50, 60, and 70 °C, with an air velocity of 0.8 m s−1.
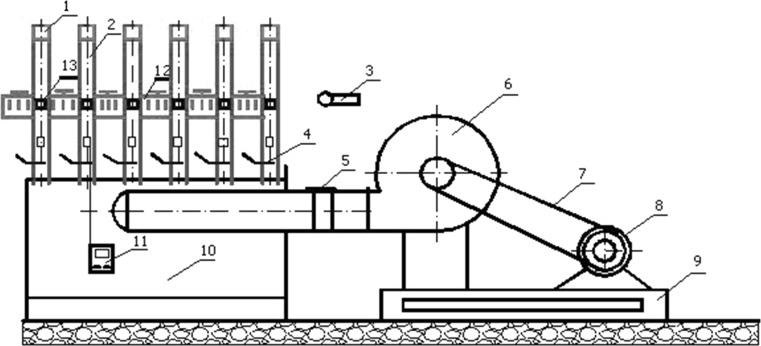
Fig. 1.
Diagram of the convective dryer. 1—tray, 2—air pipe, 3—anemometer, 4—air stream strangler, 5—elastic connector, 6—fan, 7—transmission belt, 8—electric motor, 9—base, 10—surge chamber, 11—thermometer, 12—autotransformer, 13—heating element
Vacuum-microwave drying (VMD)
The process of VMD was conducted using a SM 200 dryer (Plazmatronika, Wrocław, Poland) connected to a vacuum system, consisting of a vacuum pump BL 30 P (“Tepro,” Koszalin, Poland), a vacuum gauge MP 211 (“Elvac,” Bobolice, Poland), and a compensation reservoir of 0.15 m3. The dryer was operated at 3 power levels: 240, 360, and 480 W. Samples were placed in a cylindrical container of organic glass (6.8 L), with a pressure ranging from 4 to 6 kPa. To avoid local overheating of the material, the container rotated at a speed of 6 rpm, and additionally, an electric fan was installed at the bottom of the dryer producing an air stream of 22 °C at a velocity of 1 m s−1.
Combined drying (CPD–VMFD)
The process of CPD–VMD consisted of CPD at 60 °C for 2 h, followed by VMFD at 360 W.
Mathematical modeling and temperature measurement
Table Curve 2D (Systat Software, San Jose, California, USA) was used to calculate the moisture ratio (MR) by fitting the mathematical model to experimental points with the highest possible values of determination coefficient (R2) and the lowest values of root mean square error (RMSE).
On the other hand, this manuscript presents a new model (Lech et al. 2015). This fitted model includes the effect of two independent variables (i) temperature of air (T) and (ii) drying time (τ) during CD drying with MR = f(τ, T), and microwave power (P) and drying time (τ) versus MR values during VM drying with MR = f(τ, P).
The temperature of PomP was measured using an infrared camera i50 (Flir Systems AB, Stockholm, Sweden).
Color measurement
Instrumental color of fresh and dried PomP was evaluated using a Minolta Chroma Meter CR-200. The results, using Illuminant D65 and 10° observer angle, were expressed as L* (lightness), a* (green–red) and b* (blue–yellow) coordinates.
Antioxidant capacity and total polyphenol content (TPC)
The radical scavenging activity was evaluated using the DPPH• radical method (Brand-Williams et al. 1995), with the only modification of using a reaction time of 15 min. The decrease in absorbance was measured at 515 nm using a UV–Visible Spectrophotometer (Helios Gamma model, UVG 1002E, Mercers Row, Cambridge, UK).
The TPC was quantified using the Folin-Ciocalteu reagent (Singleton et al. 1999). Absorbance was measured at 760 nm using a spectrophotometer (Helios, Therm Spectronic, Cambridge, UK). Results were expressed as milligrams of gallic acid per gram of dry weight.
Fatty acids profile
Fatty acids were methylated in situ (Trigueros and Sendra 2015). FAMEs (fatty acid methyl esthers) were identified and semi-quantified using GC–MS with a SupraWax-280 column, 100% polyethylene glycol (Teknokroma S. Co. Ltd., 165 Barcelona, Spain; 30 m × 0.25 mm × 0.25 µm film thickness). Analyses were carried out using helium as carrier gas at a flow rate of 1.1 mL min−1 in a split ratio of 1:10, and a program: (i) initial temperature 80 °C, hold for 2 min, (ii) rate of 8.0 °C min−1 to 160 °C; (iii) rate of 4 °C min−1 from 160 to 220 °C and hold for 13 min, and (iv) rate of 10 °C min−1 from 220 to 260 °C and hold for 6 min. Injector and detector temperatures were held at 230 and 260 °C, respectively; 0.5 µL of the extract was injected.
Descriptive sensory evaluation
Eight trained panelists (aged 25–50 years; 4 females and 4 males) from the Department of Agro-Food Technology (UMH) participated in this study. Panelists received two orientation sessions of 60 min, on sensory evaluation of dried pomegranate arils and pomace (appearance, flavor, and texture). Dehydrated samples of PomP (~ 4 g) were served monadically in a randomized order into odor-free, disposable plastic plates (coded using 3 digit numbers), at room temperature (21 °C). Unsalted crackers and low mineralization still water were provided to panelists to clean their palates between samples.
The panel evaluated only the following attributes: appearance: color; basic tastes: sourness and bitterness; flavor: pomegranate ID, fruity, caramel, woody, and off-flavors; and, texture: crispiness, adhesiveness, solubility in saliva, and seed hardness. The panel used a numerical scale for quantifying the intensity of the pomegranate products attributes, where 0 represents no intensity and 10 extremely strong intensity, and with increments of 0.5 units.
Statistical analysis
The program Table Curve 2D Windows v2.03 enabled mathematical modeling with the best parameters of fitting (R2 and RMSE). All analyses were run in triplicate. The results obtained were evaluated by statistical analysis with the use of the Statgraphics Plus 5.0 software (Manugistics, Inc., Rockville, MD, USA). In order to find out if the differences in the mean values estimated were statistically significant, the one-way analysis of variance was applied (ANOVA). Later, homogeneous groups were determined with the Duncan’s multiple range test (at significance level α = 0.05).
Results and discussion
Drying kinetics
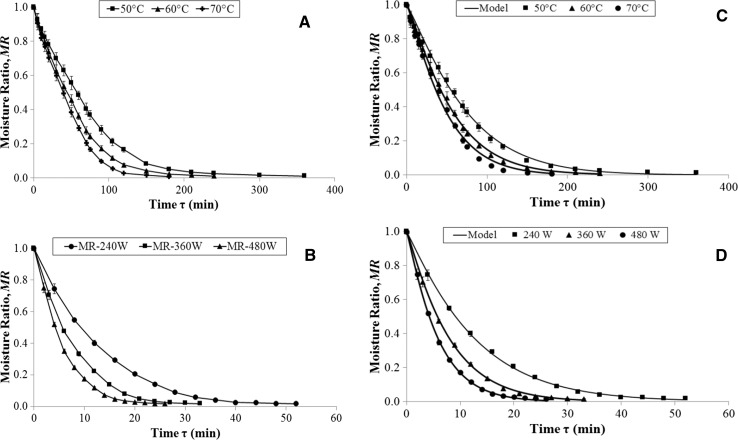
Drying kinetics curves [moisture ratio (MR) versus drying time (τ)] for CD (50, 60 and 70 °C), VMD (240, 360 and 480 W), and the combined method (CPD at 60 °C followed by VMFD at 360 W) are shown in Figs. 2a, b and 3. The selected drying model constants, their coefficients of determinations (R2), and their root mean square error (RMSE) are presented in Table 1. The R2 and RMSE values ranged between 0.9974 and 0.9998, and between 0.0004 and 0.0197, respectively; these values clearly showed a good agreement among the experimental data and the thin-layer modelling equations (the R2 values should be close to 1, and the RMSE values should be close to 0 for a good fitting). The drying constant k, ranged from 0.0023 to 0.0350 and from 0.0956 to 0.2274 during CD and VMD, respectively; the values of this constant were higher as the air temperature or microwave power were higher, and thus the drying process was shorter (Calín-Sánchez et al. 2015; Lech et al. 2015). In case of the combined drying method (CPD–VMFD), the k value, 0.2284, was the highest one; this value of the CPD–VMFD meant that the process was fast in both parts (the values of τ were 120 and 18 min for CPD and VMFD periods, respectively).
Fig. 2.
Drying kinetics for pomegranate pomace (PomP) dehydrated using CD (a) and VMD (b); Adjustment of drying kinetics for pomegranate pomace (PomP) dehydrated using CD (c) and VMD (d) using Lech’s model (Lech et al. 2015)
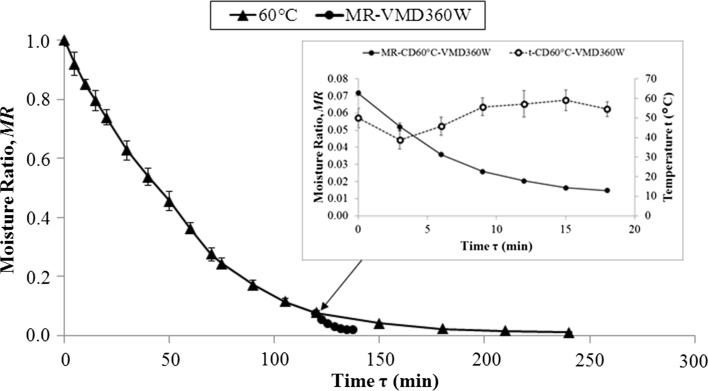
Fig. 3.
Drying kinetics for pomegranate pomace (PomP) dehydrated using the combined method [CPD (60 °C)–VMFD (360 W)]
Table 1.
Effect of the drying conditions on parameters k, a, b, and c of the functions used to describe the drying kinetics, including Lech’s model, of dehydrated pomegranate pomace (PomP)
| Treatment | |||||||
|---|---|---|---|---|---|---|---|
| [min] | k | A | b | c | R 2 | RMSE | |
| CD 50 °C | 360 | 0.0023e† | 0.0111c | 1.409c | 29.16a | 0.9995 | 0.0075 |
| CD 60 °C | 240 | 0.0275de | 0.0043c | 1.721c | 9.90b | 0.9992 | 0.0107 |
| CD 70 °C | 180 | 0.0350d | 0.0006c | 1.480c | 19.01a | 0.9974 | 0.0197 |
| VMD 240 W | 52 | 0.0956c | 0.0001a | 3.276a | − 8.68e | 0.9996 | 0.0072 |
| VMD 360 W | 33 | 0.1526b | 0.0a | 3.451a | − 5.89d | 0.9995 | 0.0087 |
| VMD 480 W | 26 | 0.2274a | 0.0114b | 2.689b | − 2.36c | 0.9996 | 0.0069 |
| CPD 60 °C–VMFD 360 W | 138 | 0.2284b | 0.0131d | 0.124d | − 0.46e | 0.9998 | 0.0004 |
| CD | – | – | – | – | – | 0.9971 | 0.0185 |
| VMD | – | – | – | – | – | 0.9994 | 0.0073 |
†Values (mean of 3 replications) followed by the same letter, within the same column, were not significantly different (p < 0.05), according to Tukey’s least significant difference test
Based on the experimental results obtained (Figs. 2a, b, 3), the decrease in moisture ratio MR of PomP during drying by CD, VMD, and CPD–VMFD can be described by a sigmoid equation (Eq. 1):
| 1 |
This model is more complicated than others [e.g. Page, successfully used in chokeberry drying (Calín-Sánchez et al. 2015)] but shows the drying kinetics within the time interval regardless of the temperature (CD) or microwave power (VMD) (Figiel 2010). The parameter “a” in this model belongs to an asymptotic value of water content not crossing the x-axis. The parameter “b” is the theoretical interval of MR values. Parameter “c” corresponds to the coordinates of the x-axis in the inflexion point of the drying curve. During VMD of PomP at 240, 360, and 480 W, and CPD–VMFD, the inflexion point did not exist (c < 0) because the drying continued with decreasing rate.
One of the most important parameters in the drying kinetics is the drying time, τ, which decreased as the temperature increased (ranging from 360 to 180 min for 50 and 70 °C) and the microwave power also increased (ranging from 52 and 26 min for 240 and 480 W). The combined method led to a drying time of 138 min, which was significantly lower than that reached when only CD at 60 °C was applied (240 min); the time of the VMFD (360 W) was only 18 min.
Recently, a new drying model was developed (Lech et al. 2015) to describe the decrease of MR during CD and VMD considering two parameters: drying time (τ) and air temperature (T) or microwave power (P), respectively. The coefficients of determinations (R2) and root mean square error (RMSE) of this new model (CD and VMD) are also listed in Table 1. The decrease of MR during CD (Fig. 2c) and during VM (Fig. 2d) can be described by Eqs. 2 and 3, respectively:
| 2 |
| 3 |
Examples of CD and VM drying kinetics measured at different air temperatures and microwave powers for PomP are shown in Fig. 2c, d, respectively. This study revealed that both high air temperature and high microwave power increased the drying rate, and resulted in shorter drying time (Fig. 2c, d). This positive effect of reducing the drying time agreed with previous studies (Lech et al. 2015).
The drying of the PomP significantly decreased the water activity, aw, of the samples, with mean values reaching of 0.18, 0.27, and 0.28 for CD-, VMD-, and CPD-VMFD-dried samples, as compared to the value of 0.86 found in the fresh material. This reduction of aw is very important to extend the shelf life of the dried product, because the mean value of aw in all dried samples, 0.23, drastically reduces the risk of microbial proliferation.
Temperature profile
A primordial factor influencing the bioactivity of the finished product is the temperature reached during drying (Zubair et al. 2011), which basically depends on the microwave wattage/power applied (Figiel 2010). It is, therefore, of high importance to properly control the temperature during the drying process. During VMD dying, an increase in the microwave power from 240 to 480 W resulted in the maximum temperatures increasing from 48.7 to 58.6 °C. In case CPD–VMFD, the temperature never exceeded 59.5 °C during the 120 min of drying. Thus, the temperature reached was not as high as previously cited in pomegranate products by other authors (Calín-Sánchez et al. 2013); those previous results were from pomegranate arils, which probably had higher internal pressure created by water inside the arils while microwaving.
CIEL*a*b* coordinates
Considering as the control values (L*, a* and b*) those of the fresh PomP, it can be stated that the drying method significantly (p < 0.05) influenced the color of the dried products (Table 2). In the case of the parameters L* (lightness) and b* (blue–yellow coordinate), there were not significant differences (p > 0.05) among the dried samples, with mean values reaching values of 39.83 and 20.25, respectively. On the other hand, the green–red coordinate, a*, was significantly higher in the combined method, 13.45, as compared to the rest of the dried samples, 11.21. Samples dried using CPD–VMFD had the highest values of the color coordinates, L*, a* and b*, although differences were only significant for the a* coordinate.
Table 2.
Effect of drying conditions on instrumental color coordinates, total polyphenol content (TPC), and antioxidant activity (DPPH•) of dehydrated pomegranate pomace (PomP) as affected by the drying method
| Treatment | aw | CIEL*a*b* coordinates | TPC (mg eq gallic acid g−1 dw) | DPPH• (µmol g−1 dw) | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| Fresh | 0.86 ± 0.02a† | 35.08 ± 0.31b | 15.78 ± 0.20a | 17.69 ± 0.31b | 4.96 ± 0.27a† | 35.2 ± 0.6a |
| CD 50 °C | 0.18 ± 0.01c | 38.38 ± 0.78a | 10.41 ± 0.27c | 19.58 ± 0.65a | 3.81 ± 0.24b | 26.3 ± 0.2b |
| CD 60 °C | 0.18 ± 0.01c | 39.70 ± 0.56a | 11.49 ± 0.23c | 20.13 ± 0.54a | 4.12 ± 0.39b | 28.7 ± 0.4b |
| CD 70 °C | 0.16 ± 0.01c | 39.03 ± 0.41a | 10.59 ± 0.16c | 19.72 ± 0.35a | 2.63 ± 0.22c | 19.2 ± 0.4c |
| VMD 240 W | 0.27 ± 0.01b | 40.41 ± 0.01a | 12.15 ± 0.01c | 20.60 ± 0.01a | 4.04 ± 0.16b | 30.8 ± 0.4b |
| VMD 360 W | 0.27 ± 0.01b | 40.64 ± 0.73a | 12.20 ± 0.28c | 20.69 ± 0.71a | 4.05 ± 0.33b | 29.8 ± 0.2b |
| VMD 480 W | 0.26 ± 0.01b | 39.08 ± 0.67a | 10.43 ± 0.19c | 19.87 ± 0.56a | 3.60 ± 0.27b | 27.4 ± 0.7b |
| CPD 60 °C–VMFD 360 W | 0.28 ± 0.01b | 41.55 ± 0.77a | 13.45 ± 0.25b | 21.16 ± 0.71a | 2.72 ± 0.16c | 23.1 ± 0.1b |
†Values (mean of 3 replications) followed by the same letter, within the same column, were not significantly different (p < 0.05), according to Tukey’s least significant difference test
The changes of color in the dried fruits or vegetal materials are mainly due to Maillard reactions, which involve reducing sugars. In the current study, the pomace was generated after squeezing the juice; thus, the sugars contents were not too high because they were extracted with juice. As compared to the fresh product, drying increased the values of the lightness, L*, and b*, while a* reduced its value. These changes led to a lighter, greener, and more yellow dried products as compared to the fresh material. This experimental situation was completely different to those reported in other fruits with higher sugar contents, such as pomegranate juice, pumpkin, and green pepper (Guiné and Barroca 2012).
Antioxidant activity
PomP drying led to a significant reduction of both TPC and DPPH• activity (Table 2) as compared to the values found in the fresh material.
The mean TPC values for fresh, CD-, VMD-, and CPD–VMFD-dried samples were 4.96, 3.52, 3.90, and 2.72 mg eq gallic acid g−1, respectively; these values represented decrease of 29.0, 21.4, and 45.2%, respectively, as compared to the value in the fresh material. The mean value of TPC of all dried PomP samples was 3.57 mg eq gallic acid g−1, which was higher than that previously reported in dried pomegranate arils, 2.03 mg eq gallic acid g−1 (Calín-Sánchez et al. 2013).
A similar trend was observed with the antioxidant activity, with the highest DPPH• value being found in the VMD-dried samples (mean of 29.34 µmol g−1 = 7.34 mg Trolox g−1), followed by CD-dried samples (mean of 24.71 µmol g−1 = 6.18 mg Trolox g−1), and CPD–VMFD (mean of 23.1 µmol g−1 = 5.78 mg Trolox g−1). The mean value of the DPPH• of all dried samples was 26.47 µmol g−1 (6.62 mg Trolox g−1), which was considerably higher than the one previously reported in dried arils, 0.66 mg eq Trolox g−1 (Calín-Sánchez et al. 2013).
Fatty acids profile
The fatty acid contents were expressed as percentage of the total fatty acids area. The order observed in PomP (mean of all treatments) was: C18:3 (78.1%) >> C18:2 (7.9%) ≈ C18:1 (7.0%) > C16:0 (4.3%) > C18:0 (2.7%) ≈ C20:1 (Table 3). The percentage of the punicic acid (C18:3) in the dried PomP ranged between 77.2 and 78.9%; similar results have been previously reported in pomegranate seed oil (~ 80%) (Aruna et al. 2016). Similarly, Alcaraz-Mármol et al. (2015) reported the fatty acid profile of 20 Spanish pomegranate cultivars and found the following mean content (%) for the following fatty acids in sweet cultivars, such as the one under study here (Mollar de Elche): palmitic 3.79%, linoleic 4.65%, oleic 4.76%, stearic 0.60%, punicic 66.7%, CLNA1 10.4%, CLNA2 2.77%, CLNA3 5.54%, and arachidic 0.61%. The importance of this experimental finding was that PomP is considered a waste, but it is a rich source of punicic acid, which is a compound of high interest for the pharmaceutical and nutraceutical industries.
Table 3.
Fatty acids profile (% of total area) of dehydrated pomegranate pomace (PomP) as affected by the drying method
| Parametera | ANOVA† | CD | VMD | CPD–VMFD | ||||
|---|---|---|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 240 W | 360 W | 480 W | 60 °C–360 W | ||
| C16:0 | NS | 4.3b | 4.4 | 4.4 | 4.4 | 4.3 | 4.3 | 4.2 |
| C18:3 (punicic) | NS | 66.6 | 65.7 | 67.0 | 65.9 | 66.1 | 66.1 | 67.5 |
| CLNAc 1 (C18:3) | NS | 6.6 | 6.7 | 6.6 | 7.0 | 7.1 | 6.8 | 6.7 |
| CLNA 2 (C18:3) | NS | 4.5 | 4.9 | 4.7 | 4.9 | 5.1 | 5.3 | 4.7 |
| C18:2 | NS | 8.2 | 8.1 | 8.1 | 7.7 | 7.9 | 7.9 | 7.6 |
| C18:1 | NS | 7.2 | 7.6 | 6.4 | 7.4 | 6.9 | 7.1 | 6.6 |
| C18:0 | NS | 2.7 | 2.8 | 2.8 | 2.8 | 2.7 | 2.57 | 2.6 |
| SFAc | NS | 7.0 | 7.2 | 7.2 | 7.1 | 7.0 | 6.9 | 6.8 |
| MUFAc | NS | 7.2 | 7.6 | 6.4 | 7.4 | 6.9 | 7.1 | 6.6 |
| PUFAc | NS | 85.8 | 85.3 | 86.4 | 85.5 | 86.1 | 86.0 | 86.6 |
†NS, not significant at p < 0.05
aThe results are expressed in % in the total fatty acid profile
bValues are the mean of 3 replicates
cCLNA, conjugated linolenic acid; SFA, saturated fatty acids (C16:0, C18:0, and C20:0); MUFA, monounsaturated fatty acids (C16:1, C18:1, and C20:1); PUFA, polyunsaturated fatty acids (C18:2, and C18:3)
Although the resistance of polyunsaturated fatty acids to oxidation depends on factors such as temperature and contact with air (Stewart et al. 2003), the most important result was that the fatty acids were not sensitive to the drying conditions and were not affected neither by air temperature nor the microwave power.
Descriptive sensory analysis
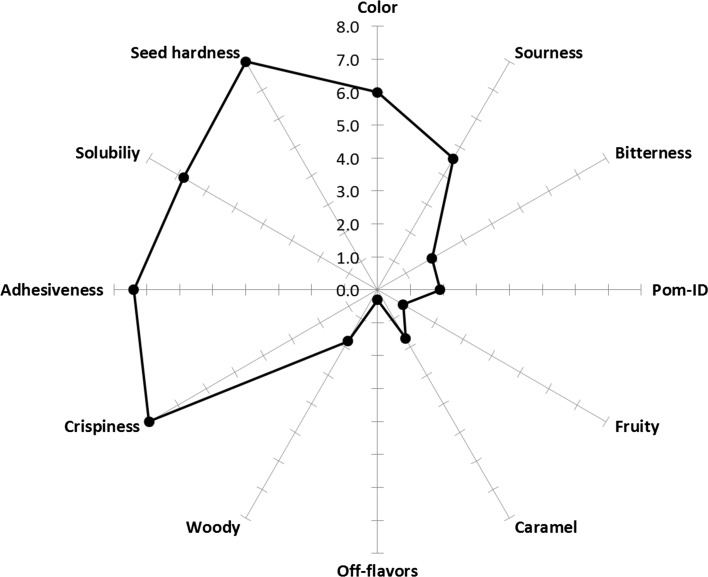
Because no significant differences were found among the sensory profiles of the pomaces dried using different methods; thus, Fig. 4 shows the “mean” sensory profile of all dried PomP samples. This profile was characterized by high values in attributes such as seed-hardness (8.0), solubility (6.8), crispiness (8.0), and sourness (4.6). Besides low notes of pomegranate ID (1.9), bitterness (1.9), caramel (1.7), and woody (1.8) were also found. It was necessary to evaluate the presence of off-flavors and burnt notes because in previous studies, it was concluded that drying of pomegranate and chokeberry led to significant increases on the intensity of these two negative attributes (Calín-Sánchez et al. 2013). However, one of the most relevant finding in the current experiment was that no off-flavors were generated during none of the studied drying methods.
Fig. 4.
Mean sensory profile of dried pomegranate pomace (PomP)
Conclusion
The vacuum-microwave drying (at 240 or 360 W) was found to be the most appropriate method to dry pomegranate pomace. This treatment led to short drying times (52 and 33 min, respectively), and acceptable total phenolic content (4.04 and 4.05 mg eq gallic acid g−1 dw, respectively) and antioxidant activity (30.8 and 29.0 µmol g−1 dw, respectively). The traditional drying (CD) with 50 and 60 °C also gave good results, but require long drying time (360 and 240 min, respectively) and energy. The dried pomegranate pomace was rich source of punicic acid (66.4% of total fatty acid profile) without any measurable off-flavors.
Acknowledgements
The authors are grateful to the project AGL2013-45922-C2-2-R (Ministerio de Economía y Competitividad, MINECO, Spain). Author Marina Cano-Lamadrid was funded by a FPU grant (FPU15/02158) from the Spanish Ministry of Education..
Abbreviations
- a, b
Function parameters
- AA
Antioxidant activity
- ANOVA
Analysis of variance
- CD
Convective drying
- CLNA
Conjugated linolenic acid
- CPD
Convective pre-drying
- CPD–VMFD
Combined drying (convective predrying–vacuum-microwave finish drying)
- HPLC
High-performance liquid chromatography
- K
Drying constants (min−1)
- MR
Moisture ratio
- MUFA
Monounsaturated fatty acids
- P
Microwave power (W)
- PomP
Pomegranate pomace
- PUFA
Polyunsaturated fatty acids
- R2
Coefficient of determination
- RMSE
Root mean square error
- SFA
Saturated fatty acids
- T
Air temperature (°C)
- τ
Time (min)
- TPC
Total polyphenols content
- VMD
Vacuum-microwave drying
- VMFD
Vacuum-microwave finish drying
References
- Akhtar S, Ismail T, Fraternale D, Sestili P. Pomegranate peel and peel extracts: chemistry and food features. Food Chem. 2015;174:417–425. doi: 10.1016/j.foodchem.2014.11.035. [DOI] [PubMed] [Google Scholar]
- Alcaraz-Mármol F, Nuncio-Jáuregui N, Calín-Sánchez Á, Carbonell-Barrachina ÁA, Martínez JJ, Hernández F. Determination of fatty acid composition in arils of 20 pomegranates cultivars grown in Spain. Sci Hortic. 2015;197:712–718. doi: 10.1016/j.scienta.2015.11.004. [DOI] [Google Scholar]
- Aruna P, Venkataramanamma D, Singh AK, Singh RP. Health benefits of punicic acid: a review. Compr Rev Food Sci Food Saf. 2016;15(1):16–27. doi: 10.1111/1541-4337.12171. [DOI] [PubMed] [Google Scholar]
- Bhol S, Lanka D, Bosco SJD. Quality characteristics and antioxidant properties of breads incorporated with pomegranate whole fruit bagasse. J Food Sci Technol. 2015;53(3):1717–1721. doi: 10.1007/s13197-015-2085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Calín-Sánchez Á, Figiel A, Hernández F, Melgarejo P, Lech K, Carbonell-Barrachina ÁA. Chemical composition, antioxidant capacity, and sensory quality of pomegranate (Punica granatum L.) arils and rind as affected by drying method. Food Bioprocess Technol. 2013;6:1644–1654. doi: 10.1007/s11947-012-0790-0. [DOI] [Google Scholar]
- Calín-Sánchez Á, Figiel A, Lech K, Szumny A, Martínez-Tomé J, Carbonell-Barrachina ÁA. Dying methods affect the aroma of Origanum majorana L. analyzed by GC–MS and descriptive sensory analysis. Ind Crops Prod. 2015;74:218–227. doi: 10.1016/j.indcrop.2015.04.067. [DOI] [Google Scholar]
- Cano-Lamadrid M, Lech K, Michalska A, Wasilewska M, Figiel A, Wojdyło A, Carbonell-Barrachina AA. Influence of osmotic dehydration pre-treatment and combined drying method on physico-chemical and sensory properties of pomegranate arils, cultivar Mollar de Elche. Food Chem. 2017;232(1):306–315. doi: 10.1016/j.foodchem.2017.04.033. [DOI] [PubMed] [Google Scholar]
- Clark D. Microwave processing of materials. Annu Rev Mater Sci. 1996;26:299–331. doi: 10.1146/annurev.ms.26.080196.001503. [DOI] [Google Scholar]
- Figiel A. Drying kinetics and quality of beetroots dehydrated by combination of convective and vacuum-microwave methods. J Food Eng. 2010;98(4):461–470. doi: 10.1016/j.jfoodeng.2010.01.029. [DOI] [Google Scholar]
- Guiné RPF, Barroca MJ. Effect of drying treatments on texture and color of vegetables (pumpkin and green pepper) Food Bioprocess Technol. 2012;90(1):58–63. [Google Scholar]
- Gullon B, Pinatado ME, Perez-Alvarez JA, Viuda-Martos M. Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum L.) flour obtained from co-product of juice extraction. Food Control. 2016;59:94–98. doi: 10.1016/j.foodcont.2015.05.025. [DOI] [Google Scholar]
- Hasnaoui N, Wathelet B, Jiménez-Araujo A. Valorization of pomegranate peel from 12 cultivars: dietary fibre composition, antioxidant capacity and functional properties. Food Chem. 2014;160:196–203. doi: 10.1016/j.foodchem.2014.03.089. [DOI] [PubMed] [Google Scholar]
- Kang JH, Song KB. Effect of pomegranate (Punica granatum L.) pomace extract as a washing agent on the inactivation of Listeria monocytogenes inoculated on fresh produce. Int J Food Sci Technol. 2017;52(10):2295–2302. doi: 10.1111/ijfs.13511. [DOI] [Google Scholar]
- Lario Y, Sendra E, García-Pérez J, Fuentes C, Sayas-Barberá E, Fernández-López J, Pérez-Alvarez JA. Preparation of high dietary fiber powder from lemon juice by-products. Innov Food Sci Emerg. 2004;5(1):113–117. doi: 10.1016/j.ifset.2003.08.001. [DOI] [Google Scholar]
- Lech K, Figiel A, Wojdyło A, Korzeniowska M, Serowik M, Szarycz M. Drying kinetics and bioactivity of beetroot slices pretreated in concentrated chokeberry juice and dried with vacuum microwaves. Dry Technol. 2015;33(13):1644–1653. doi: 10.1080/07373937.2015.1075209. [DOI] [Google Scholar]
- Mathlouthi M. Water content, water activity, water structure and the stability of foodstuffs. Food Control. 2001;12:409–417. doi: 10.1016/S0956-7135(01)00032-9. [DOI] [Google Scholar]
- Nijhuis HH, Torringa HM, Muresan S, Yuksel D, Leguijt C, Kloek W. Approaches to improving the quality of dried fruit and vegetables. Trends Food Sci Technol. 1998;9(1):13–20. doi: 10.1016/S0924-2244(97)00007-1. [DOI] [Google Scholar]
- Nowicka P, Wojdyło A, Lech K, Figiel A. Influence of osmodehydration pretreatment and combined drying method on the bioactive potential of sour cherry fruits. Food Bioprocess Technol. 2014;8(4):824–836. doi: 10.1007/s11947-014-1447-y. [DOI] [Google Scholar]
- Singh DB, Kingsly ARP. Effect of convective drying on quality of anardana. Indian J Hortic. 2008;65(4):413–416. [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Stewart OJ, Raghavan GSV, Orsat V, Golden KD. The effect of drying on unsaturated fatty acids and trypsin inhibitor activity in soybean. Process Biochem. 2003;39(4):483–489. doi: 10.1016/S0032-9592(03)00130-4. [DOI] [Google Scholar]
- Trigueros L, Sendra E. Fatty acid and conjugated linoleic acid (CLA) content in fermented milks as assessed by direct methylation. LWT Food Sci Technol. 2015;60(1):315–319. doi: 10.1016/j.lwt.2014.09.053. [DOI] [Google Scholar]
- Viuda-Martos M, Ruiz-Navajas Y, Martin-Sánchez A, Sánchez-Zapata E, Fernández-López J, Sendra E, Sayas-Barberá E, Navarro C, Pérez-Álvarez JA. Chemical, physico-chemical and functional properties of pomegranate (Punica granatum L.) bagasses powder co-product. J Food Eng. 2012;110(2):220–224. doi: 10.1016/j.jfoodeng.2011.05.029. [DOI] [Google Scholar]
- Zubair M, Nybom H, Lindholm C, Rumpunen K. Major polyphenols in aerial organs of greater plantain (Plantago major L.), and effects of drying temperature on polyphenol contents in the leaves. Sci Hortic. 2011;128(4):523–529. doi: 10.1016/j.scienta.2011.03.001. [DOI] [Google Scholar]