Abstract
The current study aimed to fabricate nisin-loaded chitosan (N-CS) nanoparticles through ionic interactions between positive amino groups of chitosan and negatively charged tripolyphosphate ions in the presence of nisin and to evaluate their efficacy against foodborne pathogens in orange juice. The synthesized nanoparticles were sphere-shaped and homogenous with an average size of 64.34 ± 2.1 and 147.93 ± 2.9 for chitosan and N-CS nanoparticles, respectively. The encapsulation efficiency of nisin into nanoparticles was 67.32 ± 0.63%. Both chitosan and N-CS nanoparticles showed greater stability, as indicated by a higher zeta potential value of + 49.3 and + 33.4 mV, respectively. The in vitro antibacterial activities of chitosan and N-CS nanoparticles were investigated against the Gram-positive bacteria Staphylococcus aureus and Listeria monocytogenes and the Gram-negative bacteria Escherichia coli O157:H7 and Salmonella Typhimurium. N-CS nanoparticles showed higher activity compared with chitosan nanoparticles. The highest reduction of microorganisms was recorded for S. aureus of 3.82 log CFU/ml and L. monocytogenes of 3.61 log CFU/ml. The antimicrobial activity of N-CS nanoparticles in orange juice for 48 h revealed higher activity compared with the control against all the tested strains. The highest microbial reduction was recorded for N-CS nanoparticles against S. aureus with a 3.84 log CFU/ml reduction. L. monocytogenes and E. coli 0157:H7 were reduced by 3.54 and 3.44 log CFU/ml, respectively. The results showed high potential for the N-CS nanoparticles to be used as potent antibacterial agents in food and other related areas.
Keywords: Antimicrobial activity, Chitosan, Foodborne pathogens, Nanoparticles, Nisin
Introduction
Each year, approximately 48 million people get sick, 128,000 are hospitalized, and 3000 die of foodborne diseases in the United States alone (CDC 2017). In the European Union in 2012, a total of 55, 453 human illnesses, 5118 hospitalizations and 41 deaths were reported (Perez Pulido et al. 2016). In developing and underdeveloped countries major food-borne pathogens are widely predominant and pose a major threat to human health (Akhtar et al. 2014). Food products frequently associated with foodborne outbreaks include beef and poultry, eggs and egg products, mixed foods and fish and fish products (Khan et al. 2015; Perez Pulido et al. 2016; Tango et al. 2016). In addition, outbreaks of illness associated with the consumption of fruit juice have been a growing public health problem since the early 1990s. According to the CDC, 39 juice-associated outbreaks were reported from 1991 through 2010; 15 implicated apple juice or cider, 10 were linked to orange juice, 3 were linked to mixed fruit, and 11 involved other types of fruit juice (Danyluk et al. 2012).
A significant number of studies have been carried out on the application of pulsed electric fields, essential oils, ozone and bacteriocins from lactic acid bacteria in the preservation of fruit juices. Among these, the use of bacteriocins is a promising alternative (de Oliveira Junior et al. 2015). Bacteriocins previously tested in fruit juices include nisin (Komitopoulou et al. 1999), enterocin AS48 (Grande et al. 2005), bovicin HC5 (De Carvalho et al. 2008), and bificin C6564 (Pei et al. 2014). Among these, only nisin has been approved as a food additive (de Oliveira Junior et al. 2015). However, the effectiveness of nisin in food products is influenced by many factors such as the interaction of nisin with the food matrix and its reactions with glutathione, sodium metabisulfite, proteases, and titanium oxide (Khan and Oh 2016; Quintavalla and Vicini 2002). The controlled release and delivery of nisin can be widely improved through the use of nanoparticulate systems (Khan and Oh 2016; Quintavalla and Vicini 2002). To overcome the issues linked with the use of nisin as a food preservative, the use of nanotechnology for the synthesis of nisin-loaded/coated nanoparticles has been introduced (da Silva Malheiros et al. 2010).
Chitosan is naturally obtained from chitin through N-deacetylation and is the second most abundant biopolymer after cellulose (Khan et al. 2015). Chitosan is considered to be the most widely distributed biopolymer having nontoxicity, biodegradability and biocompatibility properties. Chitosan has been extensively applied in the food industry as an antimicrobial and antioxidant agent (Khan et al. 2016). Moreover, chitosan has been extensively studied as a delivery agent in various fields, including medicine and the food industry (Alishahi 2014; Khan et al. 2015).
The aim of the current study was to enhance the antimicrobial activity of nisin in orange juice by encapsulation into chitosan nanoparticles. The synthesized nisin-loaded chitosan (N-CS) nanoparticles were characterized for morphology, zeta potential and encapsulation efficiency.
Materials and methods
Synthesis of nanoparticles
Chitosan (0.5%; LMW: 52 kDa; Sigma, USA) was dissolved in a 1% acetic acid (Sigma, USA) solution and stirred for 12 h to produce a homogeneous chitosan solution. Sodium tripolyphosphate (TPP; 0.5%; Sigma, USA) and nisin (990 IU/ml; Sigma, USA) were mixed in deionized water. A nisin/TPP solution at a 1:10 ratio of chitosan was added drop by drop to the chitosan solution to form ionically cross-linked nanoparticles. D-trehalose (3% w/v) was added to the reaction mixture to avoid nanoparticle aggregation and continuously stirred for 1 h. After centrifugation for 10 min at 20,000 rpm, the nanoparticles were collected and kept at − 80 °C for 24 h before freeze-drying. The nanoparticles were freeze-dried (Labtech Freeze Dryer, Daihan, Korea) at − 50 °C and 0.15 mbar pressure. The powdered nanoparticles were stored at 4 °C until further analysis.
Encapsulation efficiency
Nisin encapsulation efficiency was measured according to the method described by Zohri et al. (2010) with slight modifications. Briefly, the chitosan and N-CS nanoparticles dispersion was subjected to centrifugation at 13,800 rpm for 10 min at 4 °C by using 15 ml of a 100 kDa molecular weight cut off ultrafilter (Amicon Ultra-15 Ultracel-100 K). The amount of nisin in the upper tube was examined by Bradford protein assay. A calibration curve for nisin at various concentrations was produced before the experiment. The nisin encapsulation efficiency was determined using the following formula:
Zeta potential and size
The zeta potential and the size of the chitosan and N-CS nanoparticles were evaluated by a particle analyzer SZ-100 (Horibo, Japan). The diluted aqueous dispersion of nanoparticles was measured with the apparatus.
Morphology
The morphology of the chitosan and N-CS nanoparticles was analyzed by scanning electron microscopy (SEM). Prior to observation, an aliquot (10 µl) of the nanoparticles was mounted on SEM plastic coverslips (Rinzl Plastic Coverslips, Electron Microscopy Science, USA). The operation condition of the SEM was at an accelerating voltage of 5 kV. SEM images were observed at 50,000× magnification.
In vitro antimicrobial activity
Preparation of bacterial strains
Four foodborne pathogens, two Gram-positive bacteria Staphylococcus aureus ATCC 12600 and Listeria monocytogenes ATCC 19115 and two Gram-negative bacteria Escherichia coli 0157:H7 and Salmonella Typhimurium ATCC 14028 were used in the study. The stock culture of each strain was transferred to tryptic soy broth (TSB, 10 ml) and cultured at 37 °C for 18 h and then harvested. All the strains were washed two times with 0.1% buffer peptone water (BPW) and centrifuged for 10 min at 4000 rpm. The final culture was resuspended in BPW to obtain a final concentration of approximately 8 log CFU/ml. The cell counts of each strain were recorded by plating an aliquot on TSA plates.
Susceptibility testing
The antimicrobial effect of chitosan and N-CS nanoparticles was determined by standard cell counting methods with minor modifications (Khan et al. 2016). Briefly, nanoparticles at concentrations of 1.25 and 2.5 mg/ml were added into a 96-well plate. Approximately 0.1 ml of each strain (~ 8 log CFU/ml) was poured into each well of the 96-well plate and the total volume was adjusted to 250 using BPW. The microtiter plates were incubated at 37 °C for 24 h. After incubation, 0.1 ml of a suitable dilution was spread on TSA and incubated at 37 °C for the next 24 h. The experiment was repeated twice and expressed as the mean ± standard deviation.
Evaluation of antimicrobial activity in orange juice
Preparation of orange juice
Fresh navel oranges were purchased from a local market in Chuncheon, South Korea and transferred immediately into the laboratory. The oranges were washed with tap water two times and halved, then squeezed using an ordinary orange squeezer. Milder action was taken as the maximum point of penetration was reached so that the oil and juice from the orange rind were not released into the juice. The juice was then filtered using a stainless filter with a net square hole of 1 mm2. The juice was kept in a sterile reagent bottle at 4 °C until further use.
Microorganisms and growth conditions
S. aureus, L. monocytogenes, E. coli 0157:H7 and S. Typhimurium were grown in TSB according to the methods described by Tango et al. (2016).
Antimicrobial activity
In vivo antimicrobial activity of the test samples was evaluated against that of S. aureus, L. monocytogenes, E. coli 0157:H7 and S. Typhimurium. Test samples, i.e., chitosan (2.5 mg/ml), nisin (1 mg/ml), chitosan nanoparticles (2.5 mg/ml) and N-CS nanoparticles (2.5 mg/ml) were mixed with 25 ml of orange juice. Approximately 6 log CFU/mL (18 h culture) of the microorganisms was inoculated into the fruit juices that were supplemented with different test samples. The fruit juices were incubated at the optimal microorganism growth temperature, i.e., 25 °C for up to 48 h. After 0, 8, 24 and 48 h of incubation, samples were withdrawn and diluted (in tenfold increments), and viable cell numbers were determined by plate counting. Pure orange juice without any additive was used as a negative control.
Statistical analysis
All data are expressed as the mean ± standard deviation. The data were analyzed by one-way ANOVA using SPSS software (IBM SPSS version 21.0; IBM Corp, Chicago, USA). Tukey’s multiple range tests were used to determine the significant differences at P < 0.05 between treatments.
Results and discussion
Nanoparticle synthesis and characterization
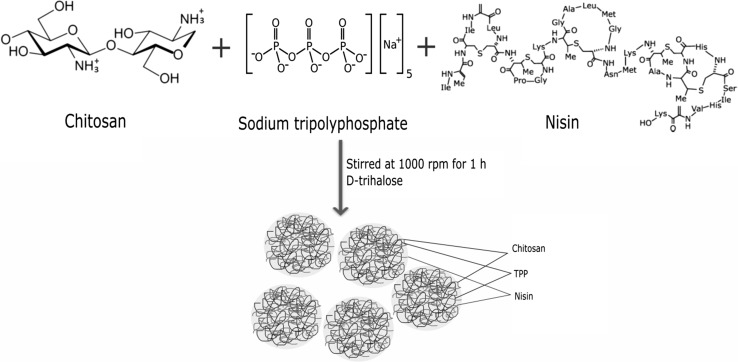
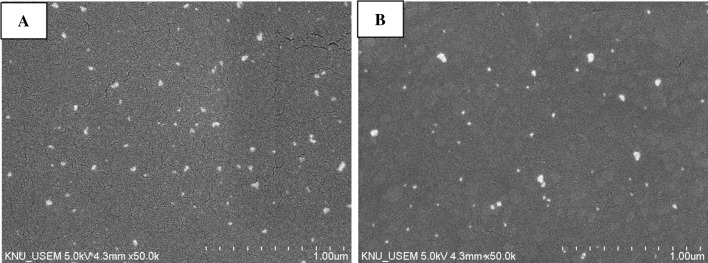
Chitosan and N-CS nanoparticles were successfully synthesized according to the scheme shown in Fig. 1. Chitosan/TPP interacted with nisin through hydrogen and ionic bonding (Alishahi 2014). Figure 2 shows the morphology of the chitosan and N-CS nanoparticles observed by SEM. The nanoparticles have spherical shapes and smooth surfaces (Mohammadpour Dounighi et al. 2012). Chitosan and N-CS nanoparticles have similar morphology. The mean size of the chitosan and N-CS nanoparticles was 64.34 ± 2.12 and 147.93 ± 2.9 nm, respectively. The size of chitosan nanoparticles is highly influenced by the solution pH, TPP and chitosan concentration (Zhao et al. 2011). Various studies have reported different sizes and shapes of chitosan nanoparticles that ranged from 42 to 350 nm (Khan et al. 2015; Liu and Gao 2009; Muhammed et al. 2010; Zhao et al. 2011; Zohri et al. 2010). In the present study, the N-CS nanoparticles were larger than the chitosan nanoparticles, possibly due to the molecular weight and size of the nisin peptide.
Fig. 1.
Schematic representation of N-CS nanoparticles
Fig. 2.
Scanning electron microscopy of chitosan nanoparticles (a) and N-CS nanoparticles (b)
The zeta potential of N-CS nanoparticles can greatly influence their stability in suspension by means of electrostatic repulsion between the particles (Mohammadpour Dounighi et al. 2012). Our results demonstrated the zeta potential of chitosan and N-CS nanoparticles was + 49.3 and + 33.4 mV, respectively. Similar results were obtained by Khan et al. (2017), who reported a zeta potential of + 48.34 and + 39.4 mV for chitosan and N-CS nanoparticles, respectively. The zeta potential of the nanoparticle was positive, indicating the presence of amino groups of chitosan on the surface (Alishahi 2014; Dorkoosh et al. 2002). However, these results showed that the nisin loading into the nanoparticles led to reduction of the particle’s zeta potential. It was supposed that the nisin encapsulation in long chain chitosan molecules was not uniform. This can be explained by the fact that chitosan can possibly adopt a diffuse conformation in an aqueous solution due to the electrostatic repulsion force present between amine groups through the long chitosan chain. Furthermore, the carboxyl groups of nisin may form hydrogen bonds with amine groups at certain locations along the chitosan chain but still maintain a compact structure without diffusing in the relatively acidic solution to keep an inner hydrophobic core. Therefore, nisin attachment did not sufficiently neutralize the positive surface charge of chitosan (Mohammadpour Dounighi et al. 2012).
The encapsulation efficiency of nisin into nanoparticles was 67.32 ± 0.63%. The encapsulation efficiency of nisin is consistent with previously reported work (Alishahi 2014; Gan et al. 2005). It is well established that increasing the chitosan to nisin concentration results in an increase of encapsulation efficiency. When the concentration of chitosan was 2.0%, the encapsulation efficiency of nisin was 78.8% (Alishahi 2014).
In vitro antimicrobial activity
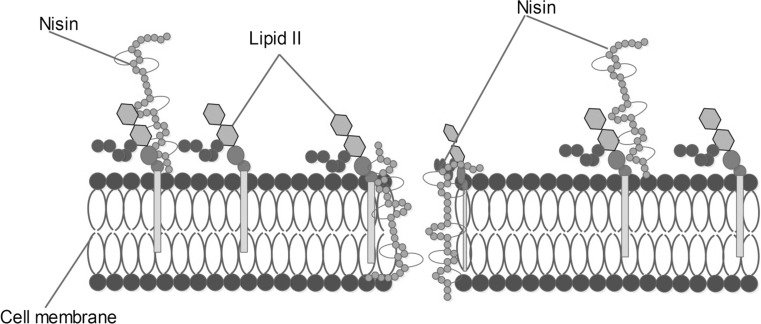
The in vitro antimicrobial activity of chitosan and chitosan nanoparticles against four foodborne pathogens is depicted in Table 1. The highest reduction was observed for S. aureus with a 3.82 log CFU/ml reduction followed by L. monocytogenes with a 3.61 ± 0.05 log CFU/ml reduction for N-CS nanoparticles compared to chitosan nanoparticles. However, Gram-negative bacteria have shown slight resistance to both chitosan and N-CS nanoparticles. Although various mechanisms of interaction between chitosan and bacteria have been reported, the most likely mechanism of interaction is facilitated by the electrostatic forces between the protonated-NH3+ groups of chitosan and the electronegative charges on the cell surface of microbes, causing the leakage of intracellular materials (Hosseini et al. 2016). Nisin has been shown to be effective against a wide range of Gram-positive bacteria. However, it shows little or no activity against Gram-negative bacteria (Deegan et al. 2006; Karam et al. 2013). This can be explained by the fact that Gram-positive bacteria have comparatively higher amounts of anionic lipid in the cytoplasmic membrane, facilitating nisin insertion, compared to Gram-negative bacteria (Breukink and de Kruijff 1999). Moreover, nisin disrupts the integrity of the microbial cell membrane by forming pores that lead to the efflux of small metabolites such as amino acids, nucleotides, ions and other cytoplasmic solutes, resulting in cell death as depicted in Fig. 3 (Reiners et al. 2017).
Table 1.
Antimicrobial activity of chitosan nanoparticles and N-CS nanoparticles against some foodborne pathogens
| Foodborne pathogens | Chitosan (2.5 mg/ml) | Nisin (1 mg/ml) | Chitosan nanoparticles (2.5 mg/ml) | N-CS nanoparticles (1.5 mg/ml) |
|---|---|---|---|---|
| Log reduction (CFU/ml) | ||||
| S. aureus | 1.75 ± 0.02d | 2.73 ± 0.01d | 2.21 ± 0.02d | 3.82 ± 0.03d |
| L. monocytogenes | 1.58 ± 0.01c | 2.34 ± 0.05c | 2.15 ± 0.04c | 3.61 ± 0.05c |
| E. coli O157:H7 | 1.45 ± 0.06b | 1.72 ± 0.03b | 2.03 ± 0.03b | 3.49 ± 0.01b |
| S. Typhimurium | 1.32 ± 0.03a | 1.56 ± 0.03a | 1.96 ± 0.01a | 2.88 ± 0.03a |
Initial count of the bacterial strains S. aureus, L. monocytogenes, E. coli O157:H7, S. Typhimurium was 8.35, 8.12, 7.98, and 8.02 log CFU/ml, respectively. Letters (a–d) are assigned according to increasing mean values. Values sharing the same letters (column wise) are not significantly different at P < 0.05
Fig. 3.
Mechanism of action of nisin by inhibiting cell wall synthesis and pore formation. In both cases, nisin interacts with lipid II molecules, a main transporter for peptidoglycan subunits, leading to the death of targeted cells
Interestingly, the effect of N-CS nanoparticles on E. coli O157:H7 was higher with 3.49 ± 0.04 log CFU/ml compared to chitosan nanoparticles with 2.03 ± 0.03 log CFU/ml reduction. The lowest log CFU/ml reduction was observed for S. Typhimurium with 2.88 ± 0.03 and 1.96 ± 0.01 for chitosan and N-CS nanoparticles, respectively. S. Typhimurium showed resistance against all the tested samples compared with the other tested foodborne pathogens.
The results have revealed that a synergistic antibacterial effect between chitosan nanoparticles and nisin has been observed. Our results coincide with those obtained by Alishahi (2014), where N-CS nanoparticles showed a synergistic effect compared with the control.
Inactivation of foodborne pathogens in orange juice
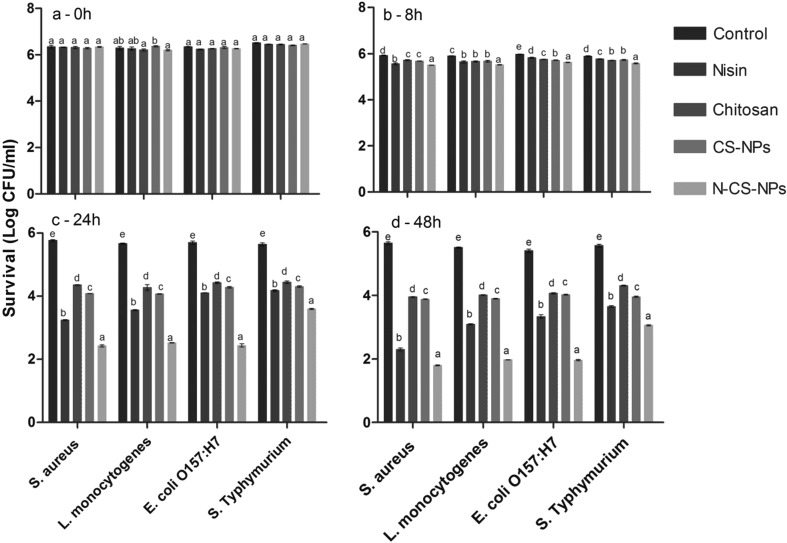
The inactivation effect of nisin, chitosan, CS nanoparticles and N-CS nanoparticles against S. aureus, L. monocytogenes, E. coli 0157:H7, and S. Typhimurium in orange juice (Fig. 4). The bacterial count in the control treatment was slightly reduced after 48 h of incubation (Fig. 4). The slight decline in the microbial count of the control samples can be attributed to the antimicrobial effect of orange juice itself (Oikeh et al. 2016). Tomotake et al. (2006) reported the antimicrobial effects of citrus juices against seven Vibrio spp. The results indicated that all juices were effective in inhibiting the growth of the Vibrio strains.
Fig. 4.
The effect of test samples on the inactivation of four foodborne pathogens in orange juice at different time intervals. The data are given as log CFU/ml survival. The letters a–e represent statistically significant differences among the same microorganism using different test samples. Bars showing the same letter are not significantly different at P < 0.05. CS-NPs: chitosan nanoparticles, N-CS-NPs: nisin loaded chitosan nanoparticles
The effect of N-CS nanoparticles was prominent among all the treatments against the microorganisms tested. At 8 h of incubation with test samples, the N-CS nanoparticles showed the highest (P < 0.05) antimicrobial activity against all the strains compared with other test samples (Fig. 4b). The antimicrobial effect of the test samples was significantly (P < 0.05) enhanced after 24 h of incubation against all the microorganisms with approximately 1.47–3.33 log CFU/ml reduction (Fig. 4c). The highest (P < 0.05) microbial reduction was observed for N-CS nanoparticles against S. aureus (3.33 log CFU/ml) followed by E. coli 0157:H7 (3.25 log CFU/ml). At 48 h of incubation with test samples, the highest (P < 0.05) microbial reduction was recorded for N-CS nanoparticles against S. aureus with 3.84 log CFU/ml, followed by L. monocytogenes with 3.54 log CFU/ml reduction in total count. The E. coli 0157:H7 count was reduced by 3.44 after 48 h of incubation with N-CS nanoparticles. However, S. Typhimurium was the most resistant strain with 2.51 log CFU/ml reduction after 48 h of incubation (Fig. 4d).
The antimicrobial effect of chitosan and nisin was lower (P < 0.05) compared with that of N-CS nanoparticles. The antimicrobial activity of nisin and chitosan was dose-dependent, and therefore, the activity varies from study to study (Bhatia and Bharti 2015; Goy et al. 2016). In addition, the antimicrobial effect of chitosan and nisin varies both from strain to strain and species to species (Bergholz et al. 2013; Goy et al. 2009). The enhanced antimicrobial effect of N-CS nanoparticles could be attributed to the stable structure of nanoparticles and controlled release of nisin into the medium (Alishahi 2014). The enhanced antimicrobial effect of N-CS nanoparticles against E. coli 0157:H7 and S. aureus could be explained by the fact that treatment with the nanoparticle causes a release of intracellular components from the cell membrane, which is increased through destroying the integrity of the bacterial cell membranes (Alishahi 2014).
Conclusion
Chitosan has the ability to form stable and homogenous nanoparticles for the successful encapsulation of nisin. N-CS nanoparticles were successfully prepared through ionic interactions. N-CS nanoparticles showed higher antimicrobial activity compared to free nisin and chitosan alone. Higher antimicrobial activity was observed against S. aureus and L. monocytogenes. N-CS nanoparticles showed a higher reduction of pathogenic microbes in orange juice compared with control samples. Higher encapsulation efficiency and stability make them a good candidate for the preservation of foods against pathogenic bacteria.
Acknowledgement
The current work is supported by BK21 Plus Program and partly supported by Kangwon National University in 2015.
Footnotes
Eun Hee Lee and Imran Khan shared first co-authorship.
References
- Akhtar S, Sarker MR, Hossain A. Microbiological food safety: a dilemma of developing societies. Crit Rev Microbiol. 2014;40:348–359. doi: 10.3109/1040841X.2012.742036. [DOI] [PubMed] [Google Scholar]
- Alishahi A. Antibacterial effect of chitosan nanoparticle loaded with nisin for the prolonged effect. J Food Saf. 2014;34:111–118. doi: 10.1111/jfs.12103. [DOI] [Google Scholar]
- Bergholz TM, Tang S, Wiedmann M, Boor KJ. Nisin resistance of Listeria monocytogenes is increased by exposure to salt stress and is mediated via LiaR. Appl Environ Microbiol. 2013;79:5682–5688. doi: 10.1128/AEM.01797-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia S, Bharti A. Evaluating the antimicrobial activity of Nisin, Lysozyme and Ethylenediaminetetraacetate incorporated in starch based active food packaging film. J Food Sci Technol. 2015;52:3504–3512. doi: 10.1007/s13197-014-1414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breukink E, de Kruijff B. The lantibiotic nisin, a special case or not? BBA-Biomembranes. 1999;1462:223–234. doi: 10.1016/S0005-2736(99)00208-4. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) (2017) Burden of foodborne illness: overview. https://www.cdc.gov/foodborneburden/estimates-overview.html. Accessed 8 Mar 2017
- Danyluk M, Goodrich-Schneider R, Schneider K, Harris L, Worobo R (2012) Outbreaks of foodborne disease associated with fruit and vegetable juices, 1922–2010. EDIS Publication FSHN12-04. http://edis.ifas.ufl.edu/pdffiles/FS/FS18800.pdf. Accessed 10 Mar 2017
- da Silva Malheiros P, Daroit DJ, Brandelli A. Food applications of liposome-encapsulated antimicrobial peptides. Trends Food Sci Technol. 2010;21:284–292. doi: 10.1016/j.tifs.2010.03.003. [DOI] [Google Scholar]
- De Carvalho A, Vanetti M, Mantovani H. Bovicin HC5 reduces thermal resistance of Alicyclobacillus acidoterrestris in acidic mango pulp. J Appl Microbiol. 2008;104:1685–1691. doi: 10.1111/j.1365-2672.2007.03710.x. [DOI] [PubMed] [Google Scholar]
- de Oliveira Junior AA, de Araújo Couto HGS, Barbosa AAT, Carnelossi MAG, de Moura TR. Stability, antimicrobial activity, and effect of nisin on the physico-chemical properties of fruit juices. Int J Food Microbiol. 2015;211:38–43. doi: 10.1016/j.ijfoodmicro.2015.06.029. [DOI] [PubMed] [Google Scholar]
- Deegan LH, Cotter PD, Hill C, Ross P. Bacteriocins: biological tools for bio-preservation and shelf-life extension. Int Dairy J. 2006;16:1058–1071. doi: 10.1016/j.idairyj.2005.10.026. [DOI] [Google Scholar]
- Dorkoosh F, Verhoef J, Rafiee-Tehrani M, Borchard G, Junginger H. Peroral drug delivery systems for peptides and proteins. STP Pharma Sci. 2002;12:213–221. [Google Scholar]
- Gan Q, Wang T, Cochrane C, McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf B. 2005;44:65–73. doi: 10.1016/j.colsurfb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Goy RC, Britto Dd, Assis OB. A review of the antimicrobial activity of chitosan. Polímeros. 2009;19:241–247. doi: 10.1590/S0104-14282009000300013. [DOI] [Google Scholar]
- Goy RC, Morais STB, Assis OBG. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev Bras Farmacogn. 2016;26:122–127. doi: 10.1016/j.bjp.2015.09.010. [DOI] [Google Scholar]
- Grande MJ, et al. Control of Alicyclobacillus acidoterrestris in fruit juices by enterocin AS-48. Int J Food Microbiol. 2005;104:289–297. doi: 10.1016/j.ijfoodmicro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Hosseini SF, Rezaei M, Zandi M, Farahmandghavi F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem. 2016;194:1266–1274. doi: 10.1016/j.foodchem.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Karam L, Jama C, Dhulster P, Chihib N-E. Study of surface interactions between peptides, materials and bacteria for setting up antimicrobial surfaces and active food packaging. J Mater Environ Sci. 2013;4:798–821. [Google Scholar]
- Khan I, Oh D-H. Integration of nisin into nanoparticles for application in foods. Innov Food Sci Emerg Technol. 2016;34:376–384. doi: 10.1016/j.ifset.2015.12.013. [DOI] [Google Scholar]
- Khan I, Khan M, Umar MN, Oh D-H. Nanobiotechnology and its applications in drug delivery system: a review. IET Nanobiotechnol. 2015;9:396–400. doi: 10.1049/iet-nbt.2014.0062. [DOI] [PubMed] [Google Scholar]
- Khan I, Ullah S, Oh D-H. Chitosan grafted monomethyl fumaric acid as a potential food preservative. Carbohydr Polym. 2016;152:87–96. doi: 10.1016/j.carbpol.2016.06.073. [DOI] [PubMed] [Google Scholar]
- Khan I, Tango CN, Miskeen S, Oh D-H. Evaluation of nisin-loaded chitosan-monomethyl fumaric acid nanoparticles as a direct food additive. Carbohydr Polym. 2017 doi: 10.1016/j.carbpol.2017.11.034. [DOI] [PubMed] [Google Scholar]
- Komitopoulou E, Boziaris IS, Davies EA, Delves-Broughton J, Adams MR. Alicyclobacillus acidoterrestris in fruit juices and its control by nisin. Int J Food Sci Technol. 1999;34:81–85. doi: 10.1046/j.1365-2621.1999.00243.x. [DOI] [Google Scholar]
- Liu H, Gao C. Preparation and properties of ionically cross-linked chitosan nanoparticles. Polym Adv Technol. 2009;20:613–619. doi: 10.1002/pat.1306. [DOI] [Google Scholar]
- Mohammadpour Dounighi N, Eskandari R, Avadi M, Zolfagharian H, Mir Mohammad Sadeghi A, Rezayat M. Preparation and in vitro characterization of chitosan nanoparticles containing Mesobuthus eupeus scorpion venom as an antigen delivery system. J Venom Anim Toxins Incl Trop Dis. 2012;18:44–52. [Google Scholar]
- Muhammed R, Junise V, Saraswathi P, Krishnan P, Dilip C. Development and characterization of chitosan nanoparticles loaded with isoniazid for the treatment of tuberculosis. Res J Pharm Biol Chem Sci. 2010;1:383–390. [Google Scholar]
- Oikeh EI, Omoregie ES, Oviasogie FE, Oriakhi K. Phytochemical, antimicrobial, and antioxidant activities of different citrus juice concentrates. Food Sci Nut. 2016;4:103–109. doi: 10.1002/fsn3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J, Yue T, Yuan Y. Control of Alicyclobacillus acidoterrestris in fruit juices by a newly discovered bacteriocin. World J Microbiol Biotechnol. 2014;30:855–863. doi: 10.1007/s11274-013-1491-1. [DOI] [PubMed] [Google Scholar]
- Perez Pulido R, Grande Burgos MJ, Galvez A, Lucas Lopez R. Application of bacteriophages in post-harvest control of human pathogenic and food spoiling bacteria. Crit Rev Biotechnol. 2016;36:851–861. doi: 10.3109/07388551.2015.1049935. [DOI] [PubMed] [Google Scholar]
- Quintavalla S, Vicini L. Antimicrobial food packaging in meat industry. Meat Sci. 2002;62:373–380. doi: 10.1016/S0309-1740(02)00121-3. [DOI] [PubMed] [Google Scholar]
- Reiners J, Lagedroste M, Ehlen K, Leusch S, Zaschke-Kriesche J, Smits SH. The N-terminal region of nisin is important for the BceAB-Type ABC Transporter NsrFP from Streptococcus agalactiae COH1. Front Microbiol. 2017;8:1643. doi: 10.3389/fmicb.2017.01643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tango CN, Khan I, Park YS, Oh DH. Growth of Staphylococcus aureus in cooked ready-to-eat ground fish as affected by inoculum size and potassium sorbate as food preservative. LWT-Food Sci Technol. 2016;71:400–408. doi: 10.1016/j.lwt.2016.03.048. [DOI] [Google Scholar]
- Tomotake H, Koga T, Yamato M, Kassu A, Ota F. Antibacterial activity of citrus fruit juices against Vibrio species. J Nutr Sci Vitaminol. 2006;52:157–160. doi: 10.3177/jnsv.52.157. [DOI] [PubMed] [Google Scholar]
- Zhao L-M, Shi L-E, Zhang Z-L, Chen J-M, Shi D-D, Yang J, Tang Z-X. Preparation and application of chitosan nanoparticles and nanofibers. Braz J Chem Eng. 2011;28:353–362. doi: 10.1590/S0104-66322011000300001. [DOI] [Google Scholar]
- Zohri M, Alavidjeh MS, Haririan I, Ardestani MS, Ebrahimi SES, Sani HT, Sadjadi SK. A comparative study between the antibacterial effect of nisin and nisin-loaded chitosan/alginate nanoparticles on the growth of Staphylococcus aureus in raw and pasteurized milk samples. Probiotics Antimicrob Proteins. 2010;2:258–266. doi: 10.1007/s12602-010-9047-2. [DOI] [PubMed] [Google Scholar]