Abstract
The present study was performed to evaluate the phytochemicals profiles of some cereal milling by-products such as wheat (bran, germ and shorts), rice (bran, germ and husk) and corn (bran, germ and germ meal) to assess their potentiality as bioactive compounds sources. Distilled water, ethanol, methanol, and acetone separately were used as solvents for the extraction of phytochemicals compounds. The antioxidant activity (AOA), total phenolics content (TPC), and total flavonoids content (TFC) of the extracts were investigated using various in vitro assays. The results showed that tannins content was ranged from 113.4 to 389.5 (mg/100 g sample).The study revealed that TPC and TFC of cereal by-products extracts were significantly different for various solvents. TPC content varied from 366.1 to 1924.9 mg/100 g and TFC content varied from 139.3 to 681.6 mg/100 g. High carotenoids content was observed for corn germ meal and minimum for wheat bran. Distilled water, ethanol and methanol extracts showed significantly different antioxidant activity. Significant variations were observed with regard to AOA of different cereal by-products by using various solvents. The ethanol and methanol were observed to be the best solvents to extract phenolic compounds and antioxidant activity, while acetone extract showed less efficiency. Also, the cereal milling by-products were rich in bioactive compounds and could be used as a value added products.
Keywords: Cereal milling by-products, Phytochemicals, Antioxidant activity, Carotenoids, TPC
Introduction
Cereals constitute a major part of human nutrition, being the important source of proteins and energy, particularly in developing countries. Cereals and their products are rich in antioxidant phytochemicals that make them ideal for developing functional foods and ingredients (Serafini et al. 2002). Cereals and its by-products like bran have antioxidative, antimutagenic, and anticarcinogenic activities (Nam et al. 2005).
Cereals such as wheat and rice represent more than half of the world’s grain production, accounts for a chief source of wastes in several countries (Zhang et al. 2011).These wastes are also a good source of dietary fibers (Crittenden et al. 2002) and bioactive compounds like phenolics, pigments, flavonoids, tannins and vitamins. Hence, the production of value added products such as food additives and supplements from food processing wastes have gained worldwide attention (Wang and Chen 2010). The valorizations of wastes are not only economical but also environmentally benign (Djilas et al. 2009).
The phenolics compound in cereals could be grouped into benzoic acid or cinnamic acid derivatives, where ferulic acid is the major phenolic acid in cereals (Abdel-Aal et al. 2001). The phytochemicals are concentrated in the outer layers compared to the internal layers of the cereals. Thus, the cereals by-products have a high content of bioactive, particularly antioxidant compounds such as phenolics compound, tannins, and carotenoids (Heinio et al. 2008). The antioxidant potential of these compounds could be attributed to several mechanisms including free radical scavenging, minimizing the formation of peroxides, metal ion chelation and enzyme activation of defense system (Zhou and Yu 2004a).
The different solvent can yield extracts with variable antioxidant activity, consequently, the capabilities of extracts might be variable to prevent lipid oxidation. In the last decade there has been a great demand from consumers for whole grains because of their high nutritional value, antioxidant compounds and their health benefits against various diseases such as cardiovascular diseases, cancer (breast and prostate cancer), reduce the risk of diabetes (Broekaert et al. 2011).
Considering the nutritional value and associated health benefits of consuming cereal by-products the present study was planned to evaluate the phytochemicals compounds in different cereal by-products. In addition, four different solvents, distilled water, ethanol, methanol and acetone separately were used for the extraction of the bioactive compounds and its antioxidant activity. The concentrations of total phenolic compounds (TPC), total flavonoids content (TFC) and the capabilities of antioxidant activity; scavenging free radicals and ferric reducing power of cereal by-products were studied and compared with different solvent extracts separately.
Materials and methods
Raw materials
Cereals by-products such as wheat milling by-products (bran, shorts and germ) obtained from South Cairo & Giza Flour Mills, Al Haram, Giza, Egypt, Corn wet milling by-products (bran, germ and germ meal) obtained from National Company for Maize Products, El Asher men Ramadan city, Sharqiyah, Egypt., Rice milling by-products (bran, husk and germ) obtained from Rice mills company, Sharqiyah, Egypt were used.
Chemicals and solvents
2,2-Diphenyl-1-picrylhydrazyl (DPPH), sodium carbonate, gallic acid, catechin, β-carotene, 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), 2,2′-azinobis(3-ethylbenothiazoline-6-sulfonic acid) diammonium salt (ABTS+), Folin–Ciocalteu’s reagent, aluminium chloride, FeSO4, FeCL3·6H2O, sodium nitrite were purchased from Sigma–Aldrich Chemical Co., Denmark. All other chemicals and solvents used were of analytical grade.
Samples preparation
Cereals by-products were ground using a coffee grinder then sieved through a 50-mesh sieve; to remove broken pieces of cereal by-products and impurities. Finally, mixed homogenously, stored in tight polyethylene bags and kept at − 20 °C until analysis.
Determination of phytochemicals profiles
Extraction and determination of total phenolics content (TPC) and total flavonoids content (TFC)
Methods of extraction
Distilled water, ethanol, methanol and acetone separately were used to extract bioactive phytochemicals from cereal by-products according to Dent et al. (2013) with some modification. Recovered extracts were evaluated in terms of total phenolic compounds, total flavonoids content, and antioxidant properties. In brief, an amount of 5 g of cereal by-products (in triplicate) was extracted with 100 ml of distilled water, ethanol, methanol and acetone, respectively, in separate Erlenmeyer flasks and kept for extraction in a water bath at 50 °C. After 60 min, the supernatants were separated by filtration using Whatman filter paper no. 1.
Total phenolic content (TPC)
Total phenolic compounds of various solvent extracts were determined colorimetrically using Folin–Ciocalteau reagent according to the method described by Adom et al. (2005); using Spectramax i3x multi-mode microplate reader system (Molecular Devices, Wokingham, UK) spectrophotometer at 760 nm against the blank. Gallic acid was used as a standard for quantification; expressed as milligram (mg) gallic acid equivalents (GAE) per 100 g of cereal milling by-products.
Total flavonoids content (TFC)
The determination of total flavonoid content of various extracts was carried out using Liu et al. (2002) method. Total flavonoid content was calculated as milligram (mg) of catechin equivalent (CE) per 100 g of sample against a standard curve of catechin.
Determination of carotenoids
The carotenoids were assessed on cereal by-products using AACC-approved method 14–50 (AACC 2000). Briefly, A saturated mixture of n-butanol and distilled water (8:2 ratio) was used for carotenoids extraction. Ten mL of water-saturated butyl alcohol was added to 1 g of different cereal milling by-products, shaken and extracted for 16 h. Extracts were then filtered through Whatman No. 1 filter paper, and absorbance measured at 440 nm using a Spectramax i3x (Molecular Devices, Wokingham, UK) spectrophotometer. A calibration curve was made from pure β-carotene. The carotenoids content was expressed as µg β-carotene/g samples.
Determination of tannins content
Quantitative determination of tannins were carried out as described by Price et al. (1978) followed with minor modification. The formed color was measured at 500 nm by using spectrophotometer (UV-1280 spectrophotometer, Japan). Catechin was used to prepare the standard curve. Tannins were calculated as mg/100 g on a dry weight basis.
Determination of antioxidant activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay
The antioxidant activity was determined using the method described by (Yu et al. 2002).The absorbance of the various extracts and control was measured at 515 nm. The lower absorbance of the reaction mixture indicated higher free radical scavenging activity.
Antioxidant activity was calculated as a percentage of radical scavenging activity (% RSA) using the following equation:
ABTS•+ scavenging assay
The scavenging activity using 2,2-asino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS•+) was measured by the method described by Li et al. (2015). The radical scavenging activity (% RSA) of the samples was calculated as follows:-
Ferric-reducing antioxidant power (FRAP) assay
FRAP assay was carried out according to the literature (Benzie and strain 1996). The absorbance of the reaction mixture was detected at 593 nm. The standard curve was constructed using FeSO4 solution, and the results were expressed as mmol L−1 FeSO4 g−1 dry weight of cereal milling by-products.
Statistical analysis
All the analyses were carried out in triplicates and represented as mean ± SD. The statistical analysis was performed using SPSS software (version 24.0, USA). One way ANOVA was applied following Tukey’s post hoc test to compare the mean and differences were considered as significant when P < 0.05.
Results and discussion
Total phenolics content (TPC)
Total phenolics content (TPC) of cereal milling by-products extracted with various solvents are shown in Table 1.
Table 1.
Total phenolics content (TPC) of cereal milling by-products (dw)
| Cereal milling by-products | TPC (mg GAE/100 g dw) | |||
|---|---|---|---|---|
| Distilled water | Ethanol | Methanol | Acetone | |
| Wheat milling by-products | ||||
| Bran | 844.7 ± 5.7a | 566.6 ± 8.5c | 630.0 ± 15.1b | 430.6 ± 4.2d |
| Germ | 1861.9 ± 22.9a | 1351.2 ± 42.5c | 1704.5 ± 12.2b | 987.7 ± 15.4d |
| Shorts | 698.2 ± 11.5a | 423.1 ± 10.4c | 500.5 ± 16.3b | 366.1 ± 29.3d |
| Rice milling by-products | ||||
| Bran | 1084.8 ± 12.2c | 1335.9 ± 36.5a | 1176.9 ± 57.8b | 953.6 ± 7.4d |
| Germ | 652.7 ± 23.81c | 1012.2 ± 59.4a | 865.4 ± 15.8b | 555.5 ± 18.6d |
| Husk | 845.7 ± 20.0c | 1060.0 ± 26.5a | 933.4 ± 25.6b | 776.9 ± 22.8d |
| Corn milling by-products | ||||
| Bran | 1925.0 ± 16.3a | 1779.5 ± 17.43b | 1814.0 ± 7.8b | 1538.0 ± 82.8c |
| Germ | 691.1 ± 6.2c | 1235.1 ± 21.5a | 1182.7 ± 3.9b | 676.6 ± 14.6d |
| Germ meal | 932.8 ± 11.2c | 1356.4 ± 11.9a | 1158.0 ± 12.5b | 638.1 ± 7.1d |
TPC total phenolics content, dw dry weight, GAE gallic acid equivalent
Means in the same row with different letters are significantly different (P < 0.05)
Results of the assays for phenolics indicated a wide variation in TPC in the different extracts of cereal milling by-products. The results in Table 1 showed that the highest amount of TPC was obtained by using distillate water for wheat milling by-products (germ, bran and shorts 1861.9, 844.7 and 698.2 mg GAE/100 g, respectively) and corn bran (1925 mg GAE/100 g) followed by methanol, ethanol and acetone. These results were similar to those obtained by Liu et al. (2017) who stated that total phenolics content of defatted wheat germ was 10.55 mg GAE/g. On the other hand, the lowest amount of TPC was achieved by using acetone. In this respect, Zhao et al. (2017) reported that total phenolics content of wheat bran as a gallic acid was 4206.16 µg/g. Ciccoritti et al. (2017) reported different wheat bran fractions had total phenolics ranged from 5698.8 to 6820.3 mg Ferulic acid/kg dw. Also, Thakur et al. (2017) reported that corn grit containing a high amount of bound phenolics such as gallic acid, ferulic acid, and sinapic acid would be beneficial for human health.
The results revealed that TPC of rice milling by-products (husk, germ and bran) and corn milling by-products with ethanol were significantly high compared to methanol, distillate water and acetone, respectively. The polyphenols extraction from plants have received great interest due their chemical diversity and interaction with other food molecules (Kosar et al. 2005). Therefore, studies have been conducted to evaluate the efficacy of various solvents such as methanol and acetone in the extraction of polyphenols (Złotek et al. 2016). Sun et al. (2006) mentioned that methanol was the best solvent for extracting the phenolic compound from oat bran. They have also reported that the amount of phenols extracted by methanol was higher than those extracted with acetone and hexane (three and four folds, respectively). In this regards Do et al. (2014) reported that ethanol more efficiency in extraction of TPC may be caused by some phenolic compounds (have higher molecular weights) are soluble in ethanol than the phenolics in the water. Indeed, the choice of solvents depends mainly on the polarity of target compounds, safety and cost (Yu et al. 2002).
Total flavonoids content (TFC)
Total flavonoids content (TFC) of different cereal milling by-products were extracted with various solvents and the results are presented in Table 2.
Table 2.
Total flavonoids content (TFC) of cereal milling by-products (dw)
| Cereal milling by-products | TFC (mg CE/100 g dw) | |||
|---|---|---|---|---|
| Distilled water | Ethanol | Methanol | Acetone | |
| Wheat milling by-products | ||||
| Bran | 163.6 ± 8.6c | 276.7 ± 12.4b | 160.6 ± 7.5c | 330.7 ± 12.7a |
| Germ | 316.8 ± 7.9d | 500.5 ± 3.6b | 338.6 ± 12.4c | 681.6 ± 6.6a |
| Shorts | 139.3 ± 1.2c | 257.3 ± 9.5a | 154.4 ± 8.4b | 262.4 ± 2.6a |
| Rice milling by-products | ||||
| Bran | 272.4 ± 17.6c | 530.1 ± 12.4b | 310.4 ± 13.9c | 673.5 ± 13.4a |
| Germ | 180.3 ± 16.6d | 386.4 ± 8.0b | 250.8 ± 13.4c | 673.5 ± 13.4a |
| Husk | 143.3 ± 1.5d | 241.0 ± 11.6b | 217.2 ± 1.5c | 307.3 ± 15.5a |
| Corn milling by-products | ||||
| Bran | 228.0 ± 5.8d | 539.0 ± 18.2b | 451. 0 ± 17.4c | 624.1 ± 14.2a |
| Germ | 257.6 ± 3.8c | 444.9 ± 20.4b | 437.3 ± 22.5b | 546.4 ± 9.0a |
| Germ meal | 237.9 ± 12.3d | 466.9 ± 9.6b | 378.3 ± 10.1c | 549.8 ± 6.1a |
TFC total flavonoids content, dw dry weight, CE catechin equivalent
Means in the same row with different letters are significantly different (P < 0.05)
The data in Table 2 revealed that the TFC of cereal milling by-products extracted by using different solvents (P < 0.05).The results also showed that TFC of cereal milling by-products (wheat milling by-products, rice milling by-products and corn milling by-products) extracted by acetone had the highest values followed by ethanol and methanol. On the other hand, distilled water had the less efficiency for TFC extraction. The results also showed that TFC of different cereal milling by-products extracted by distillate water was ranged from 139.3 to 316.8 mg/100 g, while the TFC of acetone extracts was varied from 262.4 to 681.6 mg/100 g.
Results showed in Table 2 were similar to those reported by Iloki-Assanga et al. (2015) who suggested that acetone was more efficient in extracting of TFC than methanol and ethanol from Bucid abuceras. In this respect, Abozed et al. (2014) noticed that TFC of Gemiza-9 bran had no significant difference between 70% methanol and 50% acetone extracts. Nevertheless, the other extract (Beni-suef-3bran) showed that 50% acetone containing a higher level of total flavonoid content than methanol.
Carotenoids
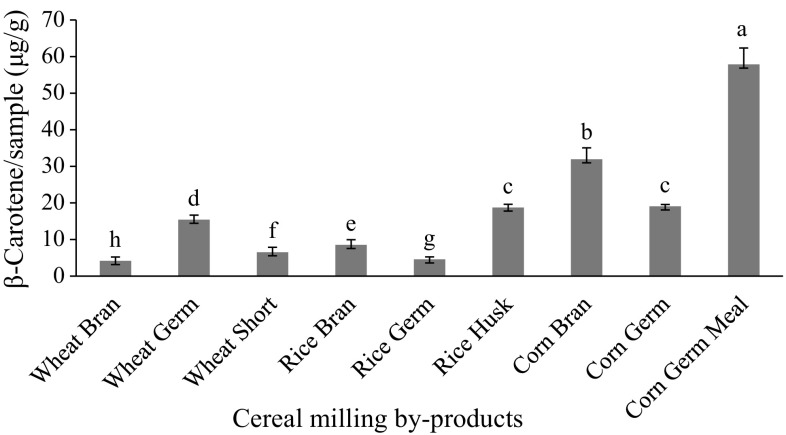
The total Carotenoids of cereal milling by-products was extracted by n-butanol and expressed as β-carotene and the results are presented in Fig. 1.
Fig. 1.
Carotenoids content as β-Carotene of cereal milling by-products (dw). The vertical bars represent the SD (n = 3), and values marked by the same letter are not significantly different (P < 0.05); dw dry weight
The present study shows that there was statistically significant different (P < 0.05) between cereal milling by-products in carotenoids. The results also revealed that corn germ meal showed the highest content of carotenoids (57.9 µg/g) followed by corn bran (32.0 µg/g). On the other hand, wheat bran showed the lowest value of carotenoids (4.2 µg/g). Hentschel et al. (2002) stated that pigments (yellow color compounds) of wheat varieties were concentrated in the outer layers than in the inner layers. In this respect, Ndolo and Beta (2013) reported that the total carotenoids content of wheat germ (9.11 µg/g) significantly higher than endosperm (1.92 µg/g) and bran (0.74 µg/g).
The obtained results are also was higher than those obtained by Al-Okbi et al. (2014) who found that the β-carotene of whole rice bran extracted by hexane was 101.0 μg/100 g. Zhou et al. (2004) suggested that bran samples might significantly differ in their carotenoid profiles due to the wheat variety and growing condition. Panfili et al. (2004) reported that wheat germ contained total carotenoids about 5.5 μg/g.
Tannins content
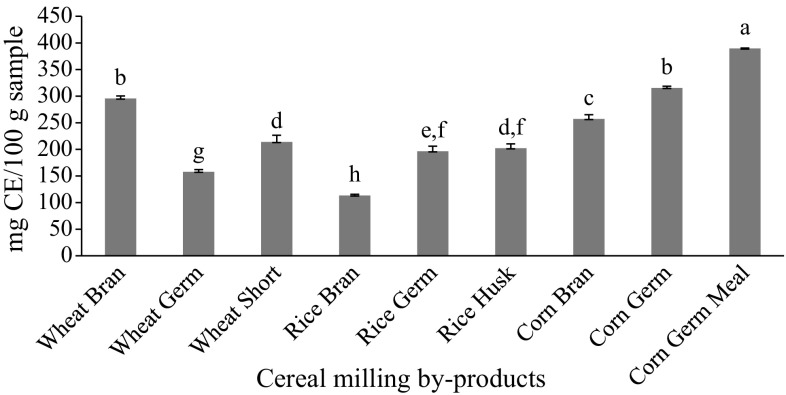
Tannins contents of different cereals milling by-products are presented in Fig. 2.
Fig. 2.
Tannins content of cereal milling by-products (dw). The vertical bars represent the SD (n = 3), and values marked by the same letter are not significantly different (P < 0.05). dw dry weight, CE catechin equivalent
Dykes and Rooney (2006) reported that tannins have an antioxidant effect as well as other therapeutic properties such as lowering cholesterol, anticarcinogenic and cardiovascular.
The results in Fig. 2 revealed that corn germ meal contained the highest value (389.5 mg CE/100 g) followed by corn germ and wheat bran (315.3 and 295.5 mg CE/100 g, respectively) when compared with than those of the rest of the tested cereal by-products. On the other hand, rice bran contained the lowest values (113.4 mg CE/100 g).
Antioxidant activity (AOA)
DPPH, ABTS+ radical scavenging activity and ferric reducing power of the various cereal milling extracts are illustrated in Table 3. The extracts of each cereal milling by-products were evaluated for their free radical scavenging properties, which are indicative of antioxidant capacities.
Table 3.
Antioxidant activity (AOA) of cereal milling by-products
| Cereal by-products | Antioxidant activity | Distilled water | Ethanol | Methanol | Acetone |
|---|---|---|---|---|---|
| Wheat by-products | |||||
| Bran | AOA DPPH (% RSA) | 39.3 ± 0.1b | 49.0 ± 0.5a | 50.9 ± 1.1a | 37.5 ± 1.7b |
| AOA ABTS (% RSA) | 32.4 ± 1.5c | 39.3 ± 0.7a | 40.6 ± 0.7a | 23.3 ± 0.7d | |
| FRAP (mmol L−1 ferrous sulfate/g sample) | 21.5 ± 0.5d | 31.3 ± 0.3b | 36.1 ± 0.4a | 25.9 ± 0.1c | |
| Germ | AOA DPPH (% RSA) | 43.9 ± 0.8c | 55.4 ± 0.6b | 69.2 ± 3.4a | 40.6 ± 1.2c |
| AOA ABTS (% RSA) | 88.3 ± 0.3a | 53.1 ± 1.6b | 88.5 ± 2.1a | 43.6 ± 1.1c | |
| FRAP (mmol L−1 ferrous sulfate/g sample) | 28.8 ± 0.5d | 39.2 ± 0.1a | 37.8 ± 0.1b | 29.7 ± 0.7c | |
| Shorts | AOA DPPH (% RSA) | 20.5 ± 0.7c | 23.7 ± 0.6b | 27.5 ± 1.7a | 17.0 ± 0.6d |
| AOA ABTS (% RSA) | 34.0 ± 1.3a | 24.8 ± 1.4c | 30.0 ± 1.9b | 18.8 ± 0.5d | |
| FRAP (mmol L−1 ferrous sulfate/g sample) | 13.7 ± 0.1b | 24.2 ± 0.4b | 28.4 ± 0.3a | 21.8 ± 0.3c | |
| Rice by-products | |||||
| Bran | AOA DPPH (% RSA) | 63.7 ± 0.9a | 59.0 ± 2.0b | 65.1 ± 2.2a | 47.3 ± 1.9c |
| AOA ABTS (% RSA) | 70.6 ± 2.0a | 43.6 ± 3.6b | 74.3 ± 3.1a | 35.4 ± 1.5c | |
| FRAP (mmol L−1 ferrous sulfate/g sample) | 20.6 ± 0.5d | 38.6 ± 0.2b | 40.3 ± 0.4a | 35.0 ± 0.1c | |
| Germ | AOA DPPH (% RSA) | 43.9 ± 1.02c | 47.5 ± 0.5b | 48.7 ± 0.2a | 33.6 ± 0.2d |
| AOA ABTS (% RSA) | 69.0 ± 0.7a | 35.2 ± 1.2c | 55.7 ± 2.1b | 32.6 ± 0.7d | |
| FRAP (mmol L−1 ferrous sulfate/g sample) | 19.1 ± 0.2d | 38.8 ± 0.3a | 36.1 ± 0.2b | 34.0 ± 0.8c | |
| Husk | AOA DPPH (% RSA) | 50.2 ± 0.3b | 50.4 ± 0.8b | 54.5 ± 0.2a | 34.3 ± 1.4c |
| AOA ABTS (% RSA) | 55.4 ± 0.2b | 43.5 ± 1.1c | 62.6 ± 0.7a | 29.7 ± 1.4d | |
| FRAP (mmol L−1 ferrous sulfate/g sample) | 23.5 ± 0.3c | 37.8 ± 0.1b | 39.0 ± 1.1a | 33.7 ± 0.5d | |
| Corn milling by-products | |||||
| Bran | AOA DPPH (% RSA) | 60.5 ± 2.2b | 70.0 ± 0.9a | 71.3 ± .7a | 40.3 ± 1.4c |
| AOA ABTS (% RSA) | 48.1 ± 2.1a | 32.8 ± 1.8b | 51.7 ± 2.7a | 26.1 ± 0.7c | |
| FRAP (mmol L−1 ferrous sulfate/g sample) | 12.6 ± 0.3c | 34.7 ± 0.2a | 33.7 ± 0.3b | 29.3 ± 0.2d | |
| Germ | AOA DPPH (% RSA) | 37.4 ± 1.0c | 49.8 ± 0.8b | 52.1 ± 0.4a | 29.8 ± 0.8d |
| AOA ABTS (% RSA) | 30.1 ± 0.8b,c | 32.7 ± 2.0b | 42.8 ± 1.8a | 27.6 ± 0.6c | |
| FRAP (mmol L−1 ferrous sulfate/g sample) | 16.3 ± 0.1c | 37.3 ± 0.1a | 37.1 ± 0.2a | 31.9 ± 2.0b | |
| Germ Meal | AOA DPPH (% RSA) | 41.2 ± 1.3c | 53.2 ± 0.5b | 59.1 ± 0.6a | 31.8 ± 0.8d |
| AOA ABTS (% RSA) | 28.9 ± 1.3c | 45.4 ± 1.8b | 59.5 ± 2.0a | 25.7 ± 0.8d | |
| FRAP (mmol L−1 ferrous sulfate/g sample) | 15.2 ± 0.1d | 37.1 ± 0.3b | 38.2 ± 0.2a | 32.6 ± 1.2c | |
AOA antioxidant activity, DPPH 2,2-Diphenyl-1-picrylhydrazyl, ABTS 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulphonic acid), FRAP ferric reducing antioxidant power, RSA radical scavenging activity
Means in the same row with different letters are significantly different (P < 0.05)
The results indicated that cereal milling by-products contained the high amounts of some phytochemicals (bioactive compounds). These bioactive compounds would have antioxidant activity as indicated in Table 3.
The data in Table 3, showed that antioxidant activity of different cereal milling by-products which determined by different Antioxidant Activity (AOA) methods such as DPPH, ABTS+ and FRAP were significantly different according to different solvent extracts (P < 0.05).
Wheat by-products results showed that germ had higher AOA compared to bran and shorts. It was also found that AOA of wheat germ ranged from 40.6 to 69.2% RSA as DPPH assay, 43.6 to 88.5% RSA as ABTS assay and 28.8 to 39.2 mmol L−1 sulfate/g samples as FRAP assay. These results are in the line of Li et al. (2015) who mentioned that the FRAP assay results of some wheat flour varieties were ranged from 31.7 to 42.3 mmol L−1 FeSO4 g−1. Liyana-Pathirana and Shahidi (2007) reported that the biologically active substances and antioxidant activity compounds were concentrated in the outer layers of grains, such as bran and shorts, compared to the inner layers (endosperm).
The results in Table 3 showed that AOA of wheat bran was ranged from 37.5 to 50.9% RSA as DPPH assay, 23.3 to 40.6% RSA as ABTS assay and 21.5 to 36.1 mmol L−1 sulfate/g samples as FRAP assay. These results are closed to those obtained by Abozed et al. (2014) who reported that both bran varieties of wheat Gemiza-9 and Beni-suef-3 showed strong DPPH free radical scavenging activities (28.1–31.8 and 33.8–36.8% RSA, respectively). Previous studies have used solvents of different polarity and mixture of water such as acetone 50% (Zhou and Yu 2004b); methanol 80% (Kim et al. 2006) and concluded that polar solvents are more effective in extracting of hydrophilic antioxidants compounds.
The results revealed that rice bran had the highest value of TPC and TFC and had antioxidant activity ranged from 47.3 to 65.1% RSA as DPPH assay, 35.4 to 74.3% RSA as ABTS+ assay and 20.6 to 40.3 mmol L−1 ferrous sulfate/g as FRAP assay followed by rice husk which had AOA 34.3 to 54.5% RSA, 29.7 to 62.6% RSA and 23.5 to 39.0 mmol L−1 ferrous sulfate/g as DPPH, ABTS+ and FRAP, respectively. In a food product, antioxidant plays an important role in extending the shelf life and improving the nutritional quality. This means the significant influence of the polarity solvent on antioxidant activity observed for different rice milling by-products.
Concerning corn wet milling by-products; i.e. bran, germ and germ meal, the results revealed that AOA extracts by various solvents were ranged from 37.4 to 60.5, 49.8 to 70.0, 52.1 to 71.3 and 29.8 to 40.3% RSA as DPPH assay for distillate water, ethanol, methanol and acetone, respectively. On the other hand, FRAP assay for each extract was ranged from 12.6 to 16.3, 34.7 to 37.3, 33.7 to 38.2 and 29.3 to 32.6 mmol L−1 ferrous sulfate/g for distillate water, ethanol, methanol and acetone, respectively. This indicated that ethanol and methanol were more efficient in the extraction of bioactive compounds of high antioxidant activity. The phytochemical profile and antioxidant activity of wheat and wheat milling by-products were different owing to the different type of solvent used. The low molecular weight phenolic compounds from cereal milling by-products were rapidly extracted by methanol and ethanol (Sun et al. 2006).
In general, distilled water, ethanol and methanol extracts were significantly different in antioxidant activity. The DPPH radical scavenging activity for the different cereal by-products extracts was varied from 17.0 to 71.3% RSA, while cereal by-products extracts showed ABTS+ scavenging activities varied from 18.8 to 88.5% inhibition. These data suggested that ethanol followed by methanol and distillate water were the best solvents to extract phenolic compounds and antioxidant activity. Therefore, ethanol and methanol might be a better choice for extracting antioxidants compounds from cereal milling by-products samples. Due to the polyphenol’s chemical diversity and its interaction with other matrix constituents, extraction from different plant sources are complex (Masisi et al. 2016). Since antioxidant capacity is mainly linked to the concentration of these compounds, it could also be affected as a property of the final extract mixture (Stefanello et al. 2018).
These outcomes demonstrated that high polarity solvents (ethanol and methanol) were more effective for extraction of antioxidants with efficient free radical scavenging properties versus an intermediate-polarity solvent (acetone). In the same line, Osman et al. (2009) reported that DPPH and ABTS activities varied with high levels of total phenols and total flavonoids and exhibited high free radical scavenging activity. In this respect, Saharan et al. (2017) revealed that a positive correlation was obtained between total phenol and flavonoid content with antioxidant activity. In this study, significant differences in free radical scavenging ability of extracts of different cereal milling by-products were observed depending on the kind of extraction solvent used.
Conclusion
In this study, antioxidant activities, total phenolics, flavonoids, tannins and carotenoids contents of wheat, rice and corn milling by-products were quantified, aiming to evaluate the different fractions. The research was tried to establish the relationship between the amount of bioactive compounds and the different solvents used for the extraction. In addition, the results indicate that TPC and antioxidant activities of wheat, rice and corn milling by-products achieved by ethanol were significantly high compared to methanol then distillate water. Also, the lowest amount of TPC was achieved by using acetone. The study, showing that corn milling by-products to be a rich source of carotenoids compared with wheat and rice milling by-products. These results could provide useful information for further studies and applications of cereal milling by-products for functional food or related products.
Acknowledgements
This work was supported by Danish Agency for Higher Education and Egyptian Cultural Affairs and Missions (Ministry of Higher Education). Sayed thanks to the Design and Consumer Behaviour section, Faculty of Science, University of Copenhagen, Denmark for the Guest Ph.D. student position.
Abbreviations
- AOA
Antioxidant activity
- TPC
Total phenolic content
- TFC
Total flavonoids content
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Sayed Saad Smuda, Phone: 00201150013307, Email: sayedsaad1910@agr.cu.edu.eg.
Sobhy Mohamed Mohsen, Email: sobmohsen1@hotmail.com.
Karsten Olsen, Phone: +45 3052 1918, Email: ko@food.ku.dk.
Mohamed Hassan Aly, Email: m_hassan1953@yahoo.com.
References
- AACC (2000) Approved methods of the AACC-method, 10th ed, USA
- Abdel-Aal ESM, Hucl P, Sosulski FW, et al. Screening spring wheat for midge resistance in relation to ferulic acid content. J Agric Food Chem. 2001;49:3559–3566. doi: 10.1021/jf010027h. [DOI] [PubMed] [Google Scholar]
- Abozed SS, El-kalyoubi M, Abdelrashid A, Salama MF. Total phenolic contents and antioxidant activities of various solvent extracts from whole wheat and bran. Ann Agric Sci. 2014;59:63–67. [Google Scholar]
- Adom KK, Sorrells ME, Rui HL. Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J Agric Food Chem. 2005;53:2297–2306. doi: 10.1021/jf048456d. [DOI] [PubMed] [Google Scholar]
- Al-Okbi SY, Hussein AMS, Hamed IM, et al. Chemical, rheological, sensorial and functional properties of gelatinized corn–rice bran flour composite corn flakes and tortilla chips. J Food Process Preserv. 2014;38:83–89. doi: 10.1111/j.1745-4549.2012.00747.x. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Broekaert WF, Courtin CM, Verbeke K, et al. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit Rev Food Sci Nutr. 2011;51:178–194. doi: 10.1080/10408390903044768. [DOI] [PubMed] [Google Scholar]
- Ciccoritti R, Taddei F, Nicoletti I, et al. Use of bran fractions and debranned kernels for the development of pasta with high nutritional and healthy potential. Food Chem. 2017;225:77–86. doi: 10.1016/j.foodchem.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Crittenden R, Karppinen S, Ojanen S, et al. In vitro fermentation of cereal dietary fibre carbohydrates by probiotic and intestinal bacteria. J Sci Food Agric. 2002;82:781–789. doi: 10.1002/jsfa.1095. [DOI] [Google Scholar]
- Dent M, Dragovi V, Peni M, Brn M. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol Biotechnol. 2013;51:84–91. [Google Scholar]
- Djilas S, Canadanovic-Brunet J, Cetkovic G. By-products of fruits processing as a source of phytochemicals. Chem Ind Chem Eng Q. 2009;15:191–202. doi: 10.2298/CICEQ0904191D. [DOI] [Google Scholar]
- Do QD, Angkawijaya AE, Tran-Nguyen PL, et al. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes L, Rooney LW. Sorghum and millet phenols and antioxidants. J Cereal Sci. 2006;44:236–251. doi: 10.1016/j.jcs.2006.06.007. [DOI] [Google Scholar]
- Heinio RL, Liukkonen KH, Myllymäki O, et al. Quantities of phenolic compounds and their impacts on the perceived flavour attributes of rye grain. J Cereal Sci. 2008;47:566–575. doi: 10.1016/j.jcs.2007.06.018. [DOI] [Google Scholar]
- Hentschel V, Kranl K, Hollmann J, et al. Spectrophotometric determination of yellow pigment content and evaluation of carotenoids by high-performance liquid chromatography in durum wheat grain. J Agric Food Chem. 2002;50:6663–6668. doi: 10.1021/jf025701p. [DOI] [PubMed] [Google Scholar]
- Iloki-Assanga SB, Lewis-Luján LM, Lara-Espinoza CL, et al. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res Notes. 2015;8:396. doi: 10.1186/s13104-015-1388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Tsao R, Yang R, Cui SW. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006;95:466–473. doi: 10.1016/j.foodchem.2005.01.032. [DOI] [Google Scholar]
- Kosar M, Dorman HJD, Hiltunen R. Effect of an acid treatment on the phytochemical and antioxidant characteristics of extracts from selected Lamiaceae species. Food Chem. 2005;91:525–533. doi: 10.1016/j.foodchem.2004.06.029. [DOI] [Google Scholar]
- Li Y, Ma D, Sun D, et al. Total phenolic, flavonoid content, and antioxidant activity of flour, noodles, and steamed bread made from different colored wheat grains by three milling methods. Crop J. 2015;3:328–334. doi: 10.1016/j.cj.2015.04.004. [DOI] [Google Scholar]
- Liu M, Li XQ, Weber C, Lee CY, Brown J, Liu RH. Antioxidant and antiproliferative activities of raspberries. J Agric Food Chem. 2002;50:2926–2930. doi: 10.1021/jf0111209. [DOI] [PubMed] [Google Scholar]
- Liu F, Chen Z, Shao J, et al. Effect of fermentation on the peptide content, phenolics and antioxidant activity of defatted wheat germ. Food Biosci. 2017;20:141–148. doi: 10.1016/j.fbio.2017.10.002. [DOI] [Google Scholar]
- Liyana-Pathirana CM, Shahidi F. Antioxidant and free radical scavenging activities of whole wheat and milling fractions. Food Chem. 2007;101:1151–1157. doi: 10.1016/j.foodchem.2006.03.016. [DOI] [Google Scholar]
- Masisi K, Beta T, Moghadasian MH. Antioxidant properties of diverse cereal grains: a review on in vitro and in vivo studies. Food Chem. 2016;196:90–97. doi: 10.1016/j.foodchem.2015.09.021. [DOI] [PubMed] [Google Scholar]
- Nam SH, Choi SP, Kang MY, et al. Antioxidative, antimutagenic, and anticarcinogenic activities of rice bran extracts in chemical and cell assays. J Agric Food Chem. 2005;53:816–822. doi: 10.1021/jf0490293. [DOI] [PubMed] [Google Scholar]
- Ndolo VU, Beta T. Distribution of carotenoids in endosperm, germ, and aleurone fractions of cereal grain kernels. Food Chem. 2013;139:663–671. doi: 10.1016/j.foodchem.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Osman H, Rahim AA, Isa NM, Bakhir NM. Antioxidant activity and phenolic content of Paederia foetida and Syzygium aqueum. Molecules. 2009;14:970–978. doi: 10.3390/molecules14030970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panfili G, Fratianni A, Irano M. Improved normal-phase high-performance liquid chromatography procedure for the determination of carotenoids in cereals. J Agric Food Chem. 2004;52:6373–6377. doi: 10.1021/jf0402025. [DOI] [PubMed] [Google Scholar]
- Price ML, Van Scoyoc S, Butler LG. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J Agric Food Chem. 1978;26:1214–1218. doi: 10.1021/jf60219a031. [DOI] [Google Scholar]
- Saharan P, Sadh PK, Singh Duhan J. Comparative assessment of effect of fermentation on phenolics, flavanoids and free radical scavenging activity of commonly used cereals. Biocatal Agric Biotechnol. 2017;12:236–240. [Google Scholar]
- Serafini M, Bellocco R, Wolk A, Ekström AM. Total antioxidant potential of fruit and vegetables and risk of gastric cancer. Gastroenterology. 2002;123:985–991. doi: 10.1053/gast.2002.35957. [DOI] [PubMed] [Google Scholar]
- Stefanello FS, dos Santos CO, Bochi VC, et al. Analysis of polyphenols in brewer’s spent grain and its comparison with corn silage and cereal brans commonly used for animal nutrition. Food Chem. 2018;239:385–401. doi: 10.1016/j.foodchem.2017.06.130. [DOI] [PubMed] [Google Scholar]
- Sun T, Xu Z, Godber JS, Prinyawiwatkul W. Capabilities of oat extracts in inhibiting cholesterol and long chain fatty acid oxidation during heating. Cereal Chem. 2006;83:451–454. doi: 10.1094/CC-83-0451. [DOI] [Google Scholar]
- Thakur S, Singh N, Kaur A, Singh B. Effect of extrusion on physicochemical properties, digestibility, and phenolic profiles of grit fractions obtained from dry milling of normal and waxy corn. J Food Sci. 2017;82:1101–1109. doi: 10.1111/1750-3841.13692. [DOI] [PubMed] [Google Scholar]
- Wang SY, Chen CT. Effect of allyl isothiocyanate on antioxidant enzyme activities, flavonoids and post-harvest fruit quality of blueberries (Vaccinium corymbosum L., cv. Duke) Food Chem. 2010;122:1153–1158. doi: 10.1016/j.foodchem.2010.03.106. [DOI] [Google Scholar]
- Yu L, Haley S, Perret J, et al. Free radical scavenging properties of wheat extracts. J Agric Food Chem. 2002;50:1619–1624. doi: 10.1021/jf010964p. [DOI] [PubMed] [Google Scholar]
- Zhang G, He L, Hu M. Optimized ultrasonic-assisted extraction of flavonoids from Prunella vulgaris L. and evaluation of antioxidant activities in vitro. Innov Food Sci Emerg Technol. 2011;12:18–25. doi: 10.1016/j.ifset.2010.12.003. [DOI] [Google Scholar]
- Zhao HM, Guo XN, Zhu KX. Impact of solid state fermentation on nutritional, physical and flavor properties of wheat bran. Food Chem. 2017;217:28–36. doi: 10.1016/j.foodchem.2016.08.062. [DOI] [PubMed] [Google Scholar]
- Zhou K, Yu L. Antioxidant properties of bran extracts from trego wheat grown at different locations. J Agric Food Chem. 2004;52:1112–1117. doi: 10.1021/jf030621m. [DOI] [PubMed] [Google Scholar]
- Zhou K, Yu L. Effects of extraction solvent on wheat bran antioxidant activity estimation. LWT Food Sci Technol. 2004;37:717–721. doi: 10.1016/j.lwt.2004.02.008. [DOI] [Google Scholar]
- Zhou K, Su L, Yu LL. Phytochemicals and antioxidant properties in wheat bran. J Agric Food Chem. 2004;52:6108–6114. doi: 10.1021/jf049214g. [DOI] [PubMed] [Google Scholar]
- Złotek U, Mikulska S, Nagajek M, Świeca M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J Biol Sci. 2016;23:628–633. doi: 10.1016/j.sjbs.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]