Abstract
Thermodynamic compatibility and probable interactions between Speckled Sugar been protein (SSBP) and xanthan gum for production of multilayer O/W emulsion (30% oil) were investigated. Different interactions were observed between SSBP and xanthan at different pH (3–7) including electrostatic interactions and hydrogen bonding. These interactions were predominant at pH 3. When low xanthan gum concentration (0.1%) was used, phase separation and complex coacervation observed at this pH (negative effect of interactions). However, at pH 5, only 0.1% xanthan was enough to drastically reduce non-dissolved protein and its precipitation which normally occurs at this pH. In addition, incompatibility or segregative phase behavior which normally occurs when protein and polysaccharide have same charges was not observed (positive effects of interactions). Protein-gum interactions influenced emulsion properties (zeta potential, particle size, PDI, rheology, emulsion capacity, heat stability and creaming rate). Interactions had considerable influence on emulsion shelf life and produced completely stable emulsions at all pH values. Results confirmed that SSBP-xanthan gum mixture has a high potential for production of multilayer emulsions.
Keywords: Speckled Sugar been protein, Polysaccharide-protein interaction, Xanthan gum, Compatibility, Emulsion
Introduction
Multilayer emulsions consist of small oil droplets dispersed in an aqueous medium, surrounded by a multilayered interfacial membrane generally composed of various interfacial layers of proteins (emulsifier) and a charged biopolymer (polysaccharides) (Bortnowska 2015).
Mixtures of protein-polysaccharide have already been used and their physico-chemical and functional properties have been investigated (Azarikia and Abbasi 2015; Chang et al. 2016; Qiu et al. 2015; Khouryieh et al. 2015; Martínez-Padilla et al. 2015). Different types of interactions such as hydrogen bond, electrostatic attractive and electrostatic repelling forces have been reported in the mixtures between two biopolymers. Each interaction could be an indicative of compatibility or incompatibility between two polymers.
Several studies have shown that vegetable proteins can be used to prepare multilayer emulsions. Proteins possess both hydrophilic and hydrophobic characters; therefore, they are surface active biopolymers and are widely used for their appropriate emulsifying activity (Lam and Nickerson 2013). The use of vegetable proteins as emulsifier reflects the present “green” trend in the pharmaceutical, cosmetics and food industries. In food applications, vegetable proteins are known to be less allergenic compared to animal derived proteins (Nesterenko et al. 2013). Common bean (Phaseolus vulgaris) with 15–30% protein is the second legume seed after soy, cultivated throughout the world (Xu and Chang 2008). The main fractions of this protein are 7S (mainly phaseolin) and 11S (mainly legumin). Phaseolin as the most important subunit in 7S fraction comprises about 50% of the common bean seed storage protein (Siddiq and Uebersax 2013; Makri and Doxastakis 2006).
Despite their functional properties and benefits, emulsions stabilized by proteins are highly sensitive to environmental stresses such as pH, ionic strength and temperature (Yin et al. 2012). As a way to overcome this disadvantage, the strategy is the incorporation of additional polysaccharide coating layers that stabilize the O/W emulsions by means of electrostatic interaction with the protein layer. The interactions between protein and polysaccharide could also increase the interfacial adsorption of proteins onto the oil droplets (Khouryieh et al. 2015; Long et al. 2013).
Xanthan is an anionic gum and has been widely used because of its unique properties such as high resistance to acidic and alkaline conditions and its considerable ability to increase viscosity (Long et al. 2013). These characteristics have converted xanthan into one of the most applicable gums in food industries. From the structure of xanthan gum, we can infer that the polysaccharide could bind with protein via electrostatic interaction.
To create stable multilayer emulsions, it is essential to choose a suitable combination of biopolymers. Therefore, the ionic behavior (electrical charge) between protein and polysaccharides used in the system should be evaluated as a function of pH (Kaushik et al. 2015). Thus, the main aims of this research were to investigate the probable interactions between Speckled Sugar bean (Phaseolus vulgaris) protein (as a new source of protein) and xanthan gum and to assess the potential of their mixture in stabilizing oil in water multilayer emulsion. The other purposes were to understand how the interactions between these two macromolecules may affect emulsion properties and demonstrate how some common environmental conditions such as various pH values and thermal treatments can change emulsion specifications.
Materials and methods
Materials
Speckled Sugar bean (cultivar red mottled Pache baghala) was purchased from Arak researches center for agriculture (Arak, Iran). Other materials were used in this research were xanthan gum (Sigma Aldrich Co.) and commercial sunflower oil (Nina brand, Iran), both provided from market. Chemical materials including NaCl, NaOH, HCl were all of analytical grade (Merck and Mojallali Co.).
Protein extraction
Alkaline extraction was used for protein extraction (Rahmati et al. 2017a). Alkaline extraction (pH 9, 1:10 bean flour to water for 45 min, room temperature) was followed by centrifugation at 15,000 g for 15 min to complete precipitation of remained flour. After that, the extracted protein was isolated at pH 4.5. The protein precipitate was then recovered by centrifugation (7000 g for 15 min), washed twice and neutralized to pH 7. Afterwards, protein was freeze dried, packaged and stored at 4 °C.
Phase diagram
Stock solutions (water as solvent) of protein and xanthan gum were prepared and mixed together to have protein-xanthan mixture solutions (pH 3–7). The final concentration for protein and xanthan were 0.5 and 0–0.3%, respectively. The mixtures were then left for 12 h and after that the state behavior of mixtures was rated as cloudy solution, cloudy solution + solid sediment and clear solution + solid sediment (Moschakis et al. 2010).
FTIR spectroscopy
FTIR spectroscopy (400–4000 cm−1) was performed to investigate the probable interactions between SSBP and xanthan gum. The protein-xanthan mixtures were prepared as explained above. These mixtures were freeze-dried and evaluated by FTIR spectrometer.
Emulsion preparation
Emulsions were made similar to the method described by Ghorbanian et al. (2015). Protein (0.5% w/v) and xanthan solutions (0–0.3% w/v) were prepared separately and kept overnight at 4 °C for complete hydration. All emulsions were prepared through a three-stage process. The initial emulsion was prepared by mixing sunflower oil (30%) with protein solution using a magnetic stirrer followed by pre-homogenization with a homogenizer (Ultra Turrax, T-25, IKA Instruments, Germany) at a speed of 20,000 rpm for 2 min at room temperature. Xanthan gum solution was then added to the initial emulsion, the pH was adjusted to 3–7 and the emulsion was homogenized for 3 min at the speed of 20,000 rpm. Then, for final homogenization emulsion was sonified at room temperature and 70% power output for 3 min.
Zeta potential and particle size measurement
The surface charge (zeta potential) on oil droplets of freshly prepared emulsions was evaluated by a zetasizer (Zetasizer, Malvern, USA). Zeta potential is the effective surface potential of a droplet suspended in a medium. For measurement of particle size, emulsions were diluted by SDS solution (0.1%) and then oil droplet size (z-average) and polydispersity index (PDI, degree of non uniformity of a distribution) of freshly prepared emulsions were measured using a dynamic light scattering particle sizer (Vasco3 particle size analyzer, Cordouan, French) (Rahmati et al. 2017b).
where, Si is the scattered intensity from particle i and Di is the diameter of particle i. The measurements were done with three readings made on each sample.
Rheological measurement
Rheological behavior of emulsions was investigated using a rotational viscometer (DV III ULTRA, Brookfield Engineering Laboratories, USA) in the shear rate range of 0–300/s. Rheological data were fitted to Power law model:
where τ is the shear stress (Pa), k is the consistency coefficients (Pa sn), is the shear rate (s−1) and n is the flow behavior index (dimensionless).
Emulsifying properties
Emulsions were centrifuged at 1000 g for 2 min. The emulsifying capacity (EC) was calculated as:
where eh is the final emulsion height and th is initial total height.
Emulsion stability (ES) against high temperatures, were determined in the emulsions that were heated at 80 °C for 30 min, and centrifuged at 1000 g for 2 min. ES was calculated as:
where feh is the final emulsion height and ieh is initial emulsion height.
Creaming rate
Immediately after preparation, 10 ml of freshly prepared emulsion was transferred into a test tube, capped, and stored at 4 °C. Creaming of emulsions was monitored after 28 days storage. During storage, some of the samples separated into an opaque cream layer at the top and a transparent serum layer at the bottom. The height of the cream after 28 days (H) and the initial emulsion height transferred into the tube (H0) were measured. The creaming rate (CR) was expressed as:
Statistical analysis
The experiments were organized as a randomized full factorial design with xanthan concentration (0, 0.1, 0.2 and 0.3%) and pH (3, 5, 7) as the main effects. The whole design was replicated three times. The results were analyzed by one-way analysis of variance (ANOVA), means were compared by SPSS software (version 19.0.0, IBM SPSS Statistics, New York) at the significance level α = 0.05.
Results and discussions
Phase diagram and thermodynamic compatibility
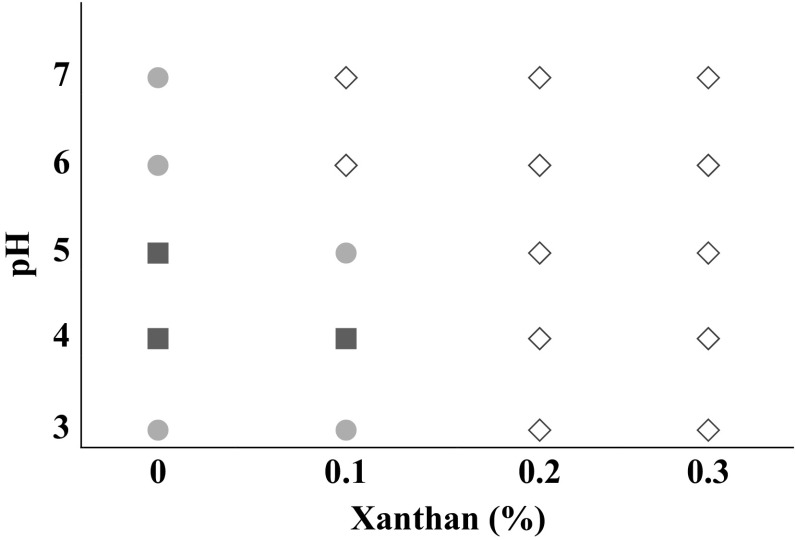
Phase diagram qualitatively shows protein-polysaccharide compatibility or incompatibility (Rodríguez Patino and Pilosof 2011). Protein solutions (samples without any gum) showed two different states (Fig. 1). At pHs 3, 6 and 7, a non-clear solution with little precipitate was observed. This appearance confirms suitable solubility of SSBP at these pH values. Our previous research on SSBP showed that this protein has 66 and 83% solubility at pHs 3 and 7, respectively (Rahmai et al. 2017a). However, at pHs 4 and 5, the phase on the top was clear and more amount of precipitate was seen at the bottom of the tube. At pH near protein iso-electric point (IpH for SSBP is 4.5, Rahmai et al. 2017b), the repulsive forces between protein molecules are not sufficient enough to inhibit the formation of protein aggregates. These high molecular weight aggregates do not have high solubility. Similar to our results Azarikia and Abbasi (2015) also reported higher protein solubility for whey protein and casein at pHs 3, 6 and 7, while high precipitation was observed at pHs 4 and 5.
Fig. 1.
Phase diagram of Speckled Sugar bean protein-xanthan gum mixtures at different pH values. Hollow diamond: cloudy solution (no solid sediment), filled circle: cloudy solution + solid sediment, filled square: clear solution + solid sediment
Generally, at pHs lower than protein iso-electric point (3 and 4), more phase separation occurred when 0.1% xanthan gum was added into protein solution. Complex coacervation which mainly occurs between proteins and gums with counter electrical charges is responsible for such phenomenon (Rodríguez Patino and Pilosof 2011). When this happens, two phases of coacervate (precipitate) and solvent will be formed. In fact, the electrical charges on protein will be neutralized by opposite charges of polysaccharide which consequently produces two phases, an aqueous solution rich in solvent and a precipitate-rich phase (Rodríguez Patino and Pilosof 2011). Same results were observed by other researchers who also reported precipitation of protein-gum coacervate at acidic pHs (Azarikia and Abbasi 2015).
At higher gum concentrations (0.2 and 0.3%) phase separation of mixtures decreased and conversion of insoluble complexes into soluble ones increased. According to the results reported by the other researchers, when higher levels of anionic gum is used, negative charges of gum predominate (Azarikia and Abbasi 2015; Qiu et al. 2015). It seems that this negative charge might have reduced the precipitation of protein-gum mixture.
When xanthan was added to the solution at pHs higher than protein iso-electric point (5, 6, and 7), protein-gum mixtures showed better phase behavior than solutions without gum. Addition of xanthan reduced protein normal precipitation at these pHs.
At pH 5, only 0.1% xanthan was enough to drastically reduce non-dissolved protein and high precipitation which normally occurs at this pH. However, at higher gum concentrations (0.2 and 0.3%) this effect was surprisingly higher (Fig. 1). Thus, SSBP could be used around its iso-electric pH in combination with an anionic gum to decrease its precipitation and conversion of insoluble protein into a soluble complex.
Incompatibility or segregative phase behavior (formation of two phases, each containing different biopolymer) which normally occurs when protein and polysaccharide have same charge (Rodríguez Patino and Pilosof 2011; Doublier et al. 2000) was not observed for none of protein-gum mixtures.
Probable interactions between SSBP and xanthan gum
Below protein iso-electric point
Generally, at pH lower than protein iso-electric point two main types of non-covalent interactions namely hydrogen bonding and attractive electrostatic interactions occurred between SSBP and xanthan gum (Table 1). At pH below iso-electric point, protein is positively charged and is able to attract an anionic negatively charged gum like xanthan.
Table 1.
Probable interactions between Speckled Sugar bean protein (0.5%) and xanthan gum at different pH values
| pH | Xanthan (%) | Main interactions | Thermodynamic protein-gum compatibility |
|---|---|---|---|
| 3 | 0.1 | Electrostatic interaction (attractive) | Compatible/insoluble complex (coacervation) |
| 0.2 | Electrostatic interaction (attractive), Hydrogen bond | Compatible/soluble complex | |
| 0.3 | Like 0.2% | Compatible/soluble complex | |
| 5 | 0.1 | Electrostatic interactions (mainly repulsive and in lower amount attractive), Hydrogen bond | Compatible/soluble complex |
| 0.2 | Electrostatic interaction (mainly repulsive and in lower amount attractive) | Compatible/soluble complex | |
| 0.3 | Like 0.2% | Compatible/soluble complex | |
| 7 | 0.1 | Electrostatic interaction (mainly repulsive and in lower amount attractive), Hydrogen bond | Compatible/soluble complex |
| 0.2 | Like 0.1% | Compatible/soluble complex | |
| 0.3 | Like 0.2% | Compatible/soluble complex |
In order to detect the hydrogen bonding between protein and gum at pH 3, FTIR bonds at 400–4000 cm−1 were evaluated. Results of this experiment indicated that there were some obvious differences in FTIR peaks of protein and protein-gum mixtures at this pH. At pH 3 (Table 2), the bands for sample containing 0.1% xanthan was similar to control. This shows that the only probable interaction between positively charged protein and negatively charged gum at this concentration was attractive electrostatic interaction.
Table 2.
Probable hydrogen bonds between Speckled Sugar bean protein (0.5%) and xanthan gum at different pH values based on FTIR spectra
| pH | Xanthan (%) | |||
|---|---|---|---|---|
| 0 | 0.1 | 0.2 | 0.3 | |
| 3 | 3294 cm−1 (NH stretching) |
3299 | 3300 | 3397 (xanthan OH) |
| 1654 cm−1 (C = O stretching) |
1653 | 1654 | 1654 | |
| 1541 cm−1 (CN streching, NH bending) |
1561 | 1540 | 1560 | |
| 1240 cm−1 (CN streching, NH bending) |
1233 | 1240 | 1241 | |
| 670 cm−1 (out of plane NH bending) |
671 | 602 (xanthan COO−) | 603 (xanthan COO−) | |
| Probable hydrogen bond | No hydrogen bond | Protein NH–xanthan COO− | Protein NH–xanthan COO− protein NH–xanthan OH |
|
| 5 | 3301 cm−1 (NH stretching) |
3411 | 3316 | 3316 |
| 1655 cm−1 (C=O stretching) |
1654 | 1653 | 1654 | |
| 1546 cm−1 (CN streching, NH bending) |
1546 | 1556 | 1551 | |
| 1245 cm−1 (CN streching, NH bending) |
1245 | 1243 | 1242 | |
| 662 cm−1 (out of plane NH bending) |
603 (xanthan COO−) | 665 | 671 | |
| Probable hydrogen bond | Protein NH-xanthan OH protein NH-xanthan COO− |
No hydrogen bond | No hydrogen bond | |
| 7 | 3300 cm−1 (NH stretching) |
3302 | 3300 | 3428 (xanthan OH) |
| 1654 cm−1 (C=O stretching) |
1654 | 1653 | 1653 | |
| 1544 cm−1 (CN streching, NH bending) |
1544 | 1546 | 1561 | |
| 1240 cm−1 (CN streching, NH bending) |
1245 | 1242 | 1241 | |
| 674 cm−1 (out of plane NH bending) |
606 (xanthan COO−) | 602 (xanthan COO−) | 672 | |
| Probable hydrogen bond | Protein NH-xanthan COO− | Protein NH-xanthan COO− | Protein NH-xanthan OH | |
However, there were differences between the FTIR bands for samples having 0.2 and 0.3% xanthan gum with those of protein. In the spectra of these samples the intensity of peak at 670 cm−1 (NH bending) was very low (almost disappeared). Instead, a new peak was recognized at about 600 cm−1, for both of these two samples. This peak is attributed to COO− group in xanthan structure (Moosavi-Nasab et al. 2010). This observation reflects the formation of a hydrogen bond between NH of SSBP and COO− of xanthan gum. Hydrogen bond occurs between an electron acceptor (like NH3+ or NH2) and an electron donor groups (such as COO− or OH). NH3+, as an electron acceptor, and COO−, as an electron donor, are two favorable groups for hydrogen bonding (Petsko and Ringe 2004).
In addition, for the mixture of 0.3% xanthan with protein, the NH stretching band at 3300 cm−1 was not pronounced, instead a new peak was observed at about 3400 cm−1 (OH group in xanthan) which can be a sign for formation of another H-bond (Table 2).
Above protein iso-electric point
At pH higher than protein iso-electric point, same kinds of non-covalent interactions occurred between protein and gum (including H-bond and electrostatic interactions) (Table 2). In this case, electrostatic interaction was mainly repulsive. Above iso-electric pH, protein has overall negative electrical net charge on its surface due to the presence of negatively charged carboxyl groups.
However, there are still positively charged groups on the protein surface because all amino acids do not have the same iso-electric point. Therefore, some attractive electrostatic interactions may also occur but in lower amounts. Attractive electrical interactions are originated from oppositely charged groups of protein and gum, either in pHs above or lower than protein iso-electric (Table 1). Other researchers have also mentioned that even at pHs where protein overall net charge is negative, as stated above, there are still some local positive patches on the surface of protein which could cause electrostatic attractive force between protein and an anionic gum (Long et al. 2013; Azarikia and Abbasi 2015; Qiu et al. 2015).
After evaluation of FTIR spectroscopy, results demonstrated that at pH 5, for sample containing 0.1% gum, the peaks at 3300 and 670 cm−1 (NH stretching and NH bending vibrations) were displaced by two new peaks; one at about 3400 cm−1 and the other at about 600 cm−1 (Table 2). The former is related to OH and the latter is attributed to the COO− group of xanthan. This result once again shows that there are hydrogen bonds between SSBP and xanthan gum which converted the mixture into a complex.
There were no hydrogen bonds between protein and gum in the mixtures containing 0.2 or 0.3% gum at pH 5. However, there were electrostatic interactions between Specked Sugar bean protein and xanthan (Table 1) since normal protein precipitation at pH 5 was not detected when xanthan was added to the protein solution. As shown in Fig. 1, no phase separation occurred in these samples which indicate both biopolymers coexist in the same phase.
Likewise, at pH 7, the changes in FTIR bands confirmed existence of hydrogen bonds (Table 2). In agreement with our results, Zhao et al. (2014) explained that the adsorption of negatively charged polysaccharide onto the surface of a negatively charged protein is possible through H-bonding that overcomes the repulsive electrostatic interactions. Interactions between protein and polysaccharide are controlled by thermodynamically favorable enthalpy and entropy changes (Rodríguez Patino and Pilosof 2011). Therefore, based on these results it appears that SSBP and xanthan gum were thermodynamically compatible since repulsive forces between these two biopolymers could not prevent the formation of interactions. In addition, segragative phase separation which is a sign of incompatibility was not observed in the concentration ranges studied.
Evaluation of phase diagram showed that the highest phase separation and therefore interactions were occurred at pH 3, where protein is positively charged. It should be reminded that the amount of NH3+ group at pH 3 on the protein surface is much higher than the other evaluated pHs. As a result, at this pH attractive electrostatic forces would be more powerful. On the other hand, at pHs 5 or 7 some of the NH3+ groups are converted into NH2. NH3+ is more active than NH2 in hydrogen bonding because it has been shown that the strongest hydrogen bonds are formed between charged groups (Petsko and Ringe 2004).
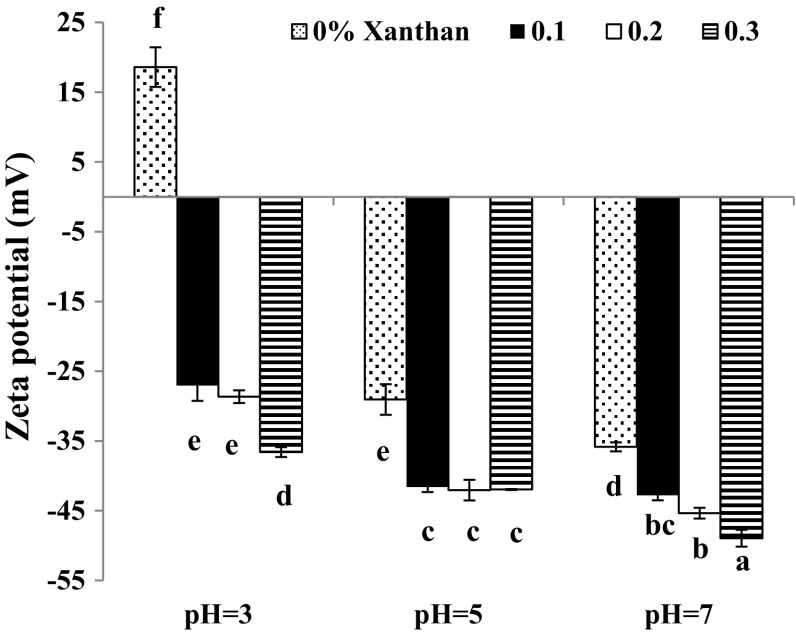
Zeta potential
For emulsion stabilized by only protein, the zeta potential was positive at pH 3; while, at pH 5 and 7 the surface charge on oil droplets was negative (Fig. 2). Generally, changing the pH of the aqueous phase surrounding the protein affects the ionization of amino acids and distribution of charged groups on the protein molecule (Zayas 1997). Other researchers also reported that the surface charge of protein gradually changes from positive to negative when pH changes from values lower than protein isoelectric point to the values higher than protein isoelectric pH (Chang et al. 2016). Protonation of carboxyl groups (acidic pH) and deprotonation of amino groups (alkaline pH) existing throughout the protein structure are responsible for these charge changes in different pH regions.
Fig. 2.
Effect of xanthan gum on zeta potential of oil/water emulsions at different pHs (0.5% protein)
At pH 3, zeta potential varied from positive to negative values, when gum concentration increased from 0 to 0.3% indicating the adsorption of xanthan molecules on the surface of protein coated oil droplets (Fig. 2). As discussed before, protein below iso-electric pH had positive charge and xanthan was attracted to the protein through attractive electrostatic forces (Table 1). These interactions consequently increased negative zeta potential on the protein-coated oil droplets and the electrical charges on the protein may be neutralized by the opposite charges of polysaccharide (Azarikia and Abbasi 2015). Similar data were reported for various mixtures such as whey protein-gum arabic, milk protein-tragacanth and milk protein-Persian gum (Azarikia and Abbasi 2015).
In addition to attractive electrostatic interactions, hydrogen bonding between SSBP and xanthan gum at pH 3 (Tables 1, 2) may have led to higher adsorption of xanthan on the protein-coated oil droplets surface.
At pHs 5 and 7 negative zeta potential was higher when xanthan gum increased to higher contents (Fig. 2). Similar to pH 3, the interactions between protein and gum, caused higher negative charge on the surface of protein-coated oil droplets. Likewise other researchers reported gradual increase in negative zeta potential when concentration of an anionic gum increased in the emulsion stabilized with a negatively charged protein. Zhao et al. (2014) stated that when the concentration of negatively charged fenugreek gum increased, the negative zeta potential on the surface of protein coated oil particles increased. They explained that this result is due to the interactions such as H-bonding between protein and gum which produce more negative zeta potential.
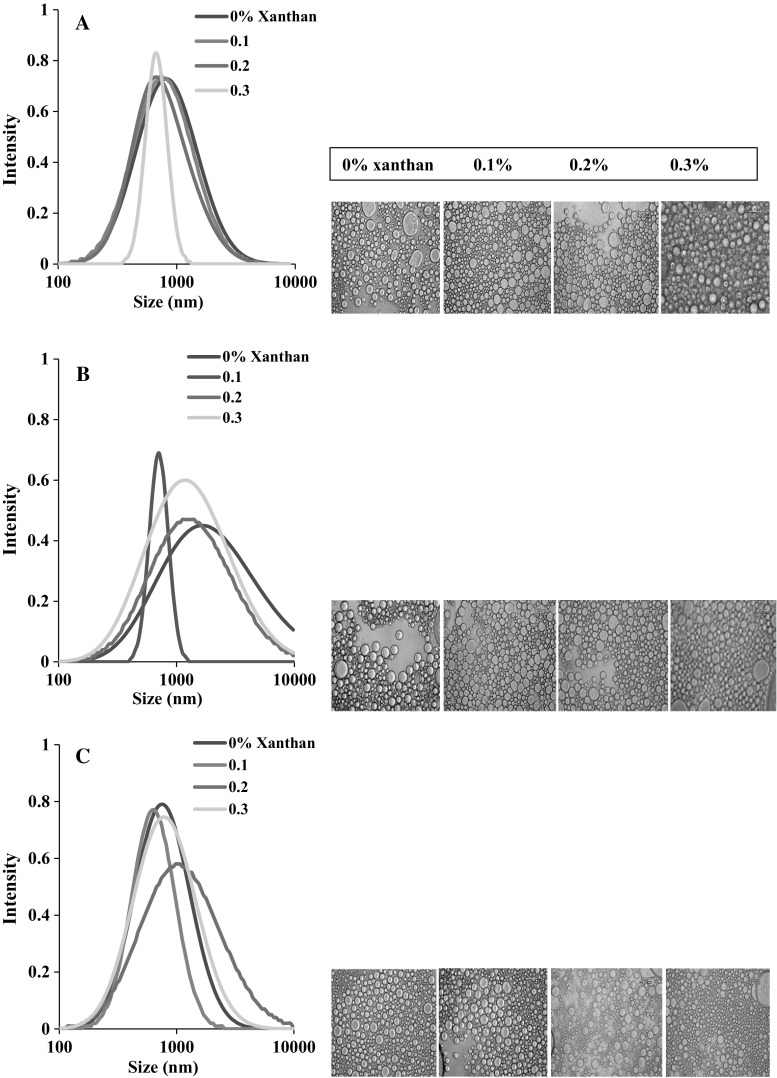
Particle size and Poly dispersity index (PDI)
Emulsion droplet size and microstructure at different pHs are shown in Table 3 and Fig. 3, respectively. Results showed that when no gum was used, the largest oil droplets were
Table 3.
Effect of xanthan gum concentration on O/W emulsion properties containing Speckled Sugar bean protein (0.5%)
| Xanthan (%) | pH | Particle size (nm) | PDI | Emulsifying capacity (%) | Heat stability (%) | Creaming (%) |
|---|---|---|---|---|---|---|
| 0 | 3 | 794.83 ± 3.06cd | 0.72 ± 0.16c | 58.86 ± 2.77ef | 52.27 ± 2.28f | 49.28 ± 1.94e |
| 5 | 1072.00 ± 171.38g | 1.37 ± 0.10b | 53.58 ± 2.87f | 50.5 ± 1.42gh | 41.38 ± 4.05d | |
| 7 | 729.73 ± 24.19b | 0.46 ± 0.10e | 67.86 ± 0.94bc | 63.3 ± 0.34de | 35.04 ± 1.79c | |
| 0.1 | 3 | 740.98 ± 22.75bc | 0.47 ± 0.16b | 58.79 ± 2.27ef | 56.75 ± 0.39fg | 37.88 ± 2.33c |
| 5 | 707.23 ± 11.21b | 0.42 ± 0.05b | 60.91 ± 2.24d | 59.82 ± 2.93ef | 25.92 ± 2.43b | |
| 7 | 620.24 ± 5.73a | 0.13 ± 0.01a | 79.76 ± 1.32b | 70.76 ± 0.94bc | 0 ± 0.00a | |
| 0.2 | 3 | 700.14 ± 42.87b | 0.35 ± 0.04b | 64.59 ± 3.1 cd | 65.07 ± 2.50de | 27.2 ± 3.20c |
| 5 | 987.56 ± 19.80f | 1.29 ± 0.09d | 70.25 ± 2.19bc | 64.89 ± 1.26de | 0 ± 0.00a | |
| 7 | 850.88 ± 26.31d | 0.90 ± 0.04c | 100 ± 0.00a | 75.23 ± 2.41b | 0 ± 0.00a | |
| 0.3 | 3 | 683.29 ± 21.05b | 0.32 ± 0.08a | 69.14 ± 5.21bc | 67.5 ± 2.30de | 0 ± 0.00a |
| 5 | 926.88 ± 29.83e | 0.92 ± 0.06c | 100 ± 0.00a | 100 ± 0.00a | 0 ± 0.00a | |
| 7 | 737.36 ± 9.65b | 0.47 ± 0.13b | 100 ± 0.00a | 100 ± 0.00a | 0 ± 0.00a |
Reported values correspond to the mean ± standard deviation. Different letters in the same column indicate significant differences (P < 0.05)
PDI poly dispersity index
Fig. 3.
Effect of xanthan gum on emulsion particle size distribution and its microstructure at pHs a 3 b 5 and c 7 (0.5% protein)
produced at pH 5 where protein molecule charge has its minimum value. Therefore, poor repulsive between oil particles could result in higher coalescence and larger particle size. Other authors also reported that due to the lack of enough repelling force between protein-coated oil droplets, particle size would be larger at pHs near to the protein iso-electric point (Cheng e al. 2009; Wang et al. 2010). In contrast, the smallest droplets were seen at pH 7 where the oil droplets had higher zeta potential (Fig. 2). This high zeta potential produces higher repulsive force and prevents oil droplets from flocculation and coalescence.
Addition of xanthan gum at pH 3 slightly decreased emulsion oil droplet size while this decrease was not significant (Table 3). The main reason for such trend could be related to the formation of network in the aqueous phase. This network increases the aqueous phase viscosity and is able to prevent movements and collisions of emulsion oil droplets (Qiu et al. 2015). Likewise, in agreement with our results it is stated that emulsion stabilizers such as gums, stabilize emulsions by limiting oil droplets motions through absorbing the water exist in the continuous phase (Long et al. 2013; Rodríguez Patino and Pilosof 2011).
At pHs 5 and 7, when 0.1% gum was added to the emulsion, more viscous continuous phase inhibited oil droplets flocculation and produced smaller particle size. However, further increase in gum concentration, increased droplets particle size (Table 3). Apparently, the interactions occurred between protein and gum were the reasons for production of larger oil particle sizes. Compared to pH 3, at these pHs the interactions were lower both in strength and amount. Therefore, gum adsorbed at protein-coated droplets had more freedom in the continuous phase which might have resulted in larger particle size.
Similar trends were also observed for PDI (Table 3). At all pHs, poly dispersity index was higher when particles size was larger and was lower in the emulsions with smaller size of oil droplets. In fact, for samples with smaller particle size, oil droplets were more uniform in size.
Figure 3 shows droplet size distribution curves for different emulsion samples stabilized by SSBP and xanthan gum. Mono-modal (single-peak) distribution curves were observed for all emulsions irrespective of xanthan concentration and pH value. The curves shifted to the right (smaller size) or to the left (larger size) according to the explanations given before for mean droplet size. In addition, it is inferred from this figures that emulsions with smaller particle size and lower PDI had narrower particle size distribution curves and vise versa. It means that emulsions with smaller particle size had oil droplets more similar in size because the amount of flocculation and coalescence of oil droplets in these emulsions are lower.
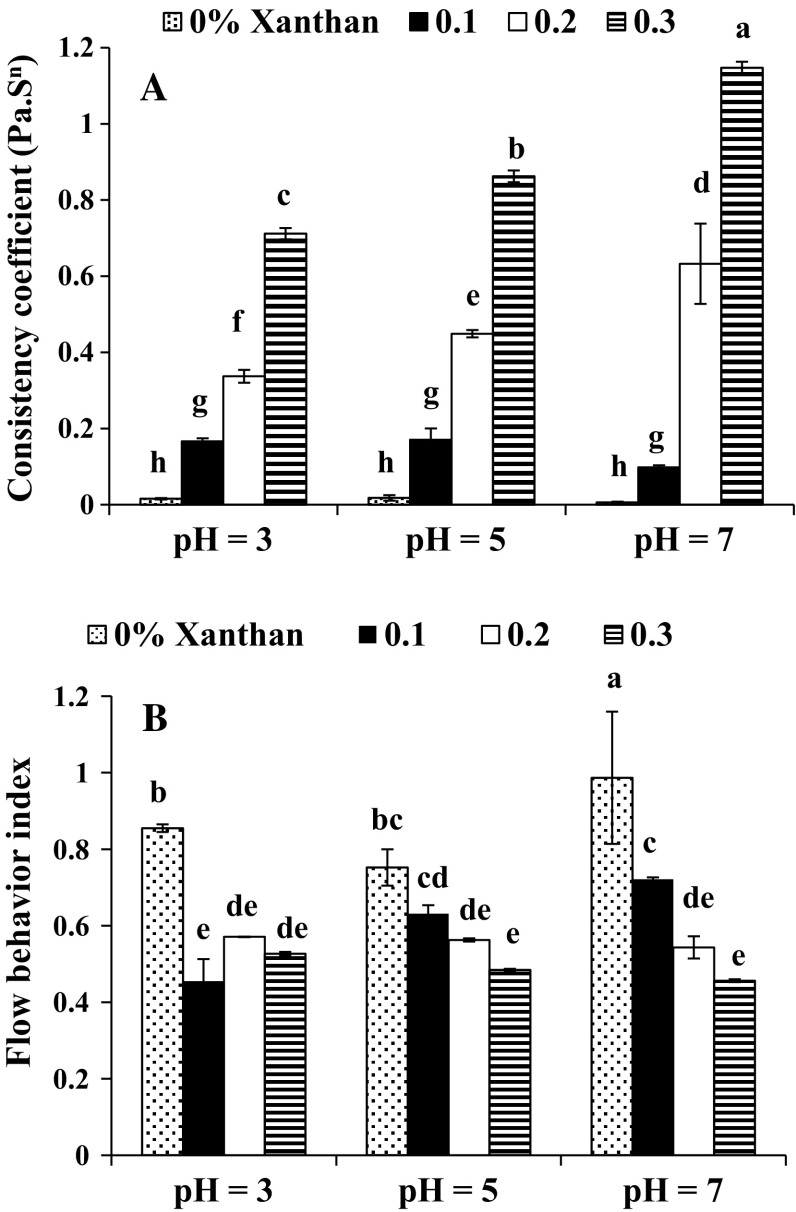
Rheological behavior
Results showed that consistency coefficient and flow behavior index of emulsion were influenced by pH and xanthan concentration (Fig. 4). The maximum consistency coefficient was observed at pH 5 (Fig. 4a). Similar to our result, Demetriades et al. (1997) also reported that the most consistency index was obtained at pH 5 for whey protein stabilized emulsions. According to this research, flocculated oil droplets and larger oil particles in this pH are the reasons for this result. They stated that the water trapped within flocculated oil droplets increases the effective volume fraction of oil particles and this phenomenon may be responsible for higher consistency in samples prepared at pH 5.
Fig. 4.
Effect of xanthan gum on emulsion consistency coefficient (a) and flow behavior index (b) at different pHs (0.5% protein)
Increasing xanthan concentration from 0 to 0.3% produced higher consistency coefficient at all pHs. Other researchers also reported the same result when higher concentration of gums such as locust bean, xanthan and flaxseed gum was used for preparation of O/W emulsion (Farshchi and Ettelaie 2013; Long et al. 2013).
As discussed above, at low xanthan concentration (0 and 0.1%) the consistency coefficient was higher at pH 5. However, when higher xanthan concentrations were used, results were different. At 0.2 and 0.3% xanthan concentrations, the consistency coefficient was higher at pH 7 followed by 5 and 3. It seems that the interactions between protein and gum were the reasons of this result. As discussed before (Table 2), COO– group of xanthan is less involved in hydrogen bonding between protein and gum at pHs 7 and 5. It is well known that this group is responsible for hydrogen bonding of xanthan with water and also other xanthan molecules. These hydrogen bonds are the reasons for high thickness of xanthan O/W emulsions.
Pseudoplastic behavior was observed for all emulsions prepared by only SSBP (Fig. 4b). Other researchers also reporetd shear thinning behavior for protein-stabilized emulsions (Wang et al. 2010; Guo and Mu 2011). According to Damodaran (1997) during shearing, protein molecules orient toward the direction of shearing force. After stopping the shear, protein molecules tend to re-orient to their initial state; thus, this redirecting permits the protein solutions to show pseudoplasticity (Damodaran 1997).
For samples without gum the most shear thinning behavior was observed at pH 5 followed by pHs 3 and 7. This result was completely similar to the observations (Demetriades et al. 1997; Farshchi and Ettelaie 2013). Emulsions with flocculated and larger oil droplets demonstrate more shear thinning behavior because these flocs and particles are deformed and disrupt when shear rate is increased. This phenomenon may be the origin of higher shear thinning behavior of emulsion (Demetriades et al. 1997). Farshchi et al. (2013) also concluded that when shear rate is used, disruption of oil droplets takes place and this disruption is more considerable at pHs where emulsions have aggregated oil droplets and could cause lower flow behavior index.
It has been well known that xanthan gum has noticeable shear thinning behavior (Martínez-Padilla et al. 2015). This property of xanthan originates from its ability to form a gel like network that in this network xanthan molecules are attached to each other by hydrogen bonding. Therefore, all samples containing xanthan exhibited pseudo plasticity and their flow behavior index was lower than those stabilized by only protein (Fig. 4b).
Emulsifying capacity and heat stability
Among protein stabilized emulsions, the lowest emulsion capacity was measured at pH 5 (Table 3). As mentioned before, at this pH repulsive forces between oil droplets are not sufficient, thus lower emulsion capacity would be expected because protein-stabilized emulsions are pH-sensitive. At pH 7, higher zeta potential on the oil droplets (Fig. 2) restricted the flocculation of oil droplets and made the emulsions more stable.
Emulsifying capacity is defined as the ability of emulsion to retain its system after subjected to centrifugal force (Noorlaila et al. 2015). As it was expected, addition of gum increased emulsion capacity. At pH 3, interactions between xanthan (-) and protein (+) (Table 2) made a bi-layer interfacial film. This bi-layer can retain adsorbed protein and improve emulsion capacity.
Xanthan is able to increase solution viscosity and develop a network which limits motion of oil droplets. Addition of xanthan markedly increased emulsion capacity in such a way that this parameter reached to 100% (at pH 7, at xanthan concentration of 0.2% and at pH 5 at gum concentration of 0.3%).
At pH 7 and specially 5, the interactions between protein and xanthan made the protein more soluble (Fig. 1). As mentioned before, the normal precipitation of protein at pH 5 was not observed. This high amount of protein available in the aqueous phase could be able to increase emulsion capacity. In addition, the interactions between SSBP and gum xanthan produced oil particles with higher zeta potentials on the surface (Fig. 2). Higher zeta potential is also another positive parameter affecting emulsion capacity. It is also possible that as a result of repulsive forces between protein and gum xanthan, adsorbed protein increases (Rodríguez Patino and Pilosof 2011).
Generally emulsion heat stability values were lower compared to emulsifying capacity (Table 3). Heat treatment can cause more movements of oil droplets, their flocculation and unraveling of protein structure which leads to the formation of interactions between protein molecules (hydrophobic or di-sulphyd bonds) (McClements 2004). These phenomena reduce emulsion heat stability. Therefore, when heat treatment is necessary for food processing, higher amount of gum must be used.
Creaming rate
Generally, as discussed for emulsifying capacity, addition of xanthan provided higher stability against creaming (Table 3) due to the protein-gum interactions which results in multilayer formation at pH 3 and higher protein surface absorption at pH 5 and 7. Effect of xanthan on droplet zeta potential and aqueous phase viscosity is another reason for such trend. At pH 5, 0.2% and at pH 7 only 0.1% xanthan made the emulsions completely stable against creaming after 4 weeks of storage.
It was observed that the most unstable emulsions after 4 weeks of storage were the ones prepared at pH 3, followed by pHs 5 and 7. This result was exactly the same as the data achieved before for emulsion creaming of emulsion stabilized with Specked Sugar bean protein (Rahmati et al. 2017a). Due to the smaller hydrodynamic size of SSBP at pH 3, which reflects the compact structure of protein, rearrangement of protein molecule at oil droplet surface does not occur as easy as other pHs. Thus, protein absorption might have been temporary.
Creaming was lower at pH 5 compared to those of pH 3. There are reports which show some proteins such as gelatin, bovine serum albumin and soluble muscle protein have appropriate absorption at around their iso-electric point. High protein absorption can form a viscous emulsifier film with good cohesiveness which produces more stable emulsion (Zayas 1997). Consequently, at acidic pH values there should be more amount of xanthan gum in the aqueous phase to have stable emulsion. Data showed that there was no creaming at 0.3% of xanthan at pH 3.
Conclusion
Interactions between SSBP and xanthan gum influenced their physico-chemical properties and performance at oil–water interface. At pHs lower than protein iso-electric point, more phase separation occurred when the content of xanthan gum was low. However, at high gum concentrations insoluble complexes converted into soluble ones. Furthermore, at pH values higher than protein iso-electric point adding xanthan gum reduced protein precipitation. Incompatibility or segregative phase behavior was not observed for protein-gum mixtures. Consequently, the interactions between SSBP and xanthan gum were highly effective on emulsion stability. However, it is suggested to use this negatively-charged gum at acidic pHs to produce more stable emulsion.
References
- Azarikia F, Abbasi S. Mechanism of soluble complex formation of milk proteins with native gums (tragacanth and Persian gum) Food Hydrocolloid. 2015;59:35–44. doi: 10.1016/j.foodhyd.2015.10.018. [DOI] [Google Scholar]
- Bortnowska G. Multilayer oil in water emulsions: formation, characteristics and application as the carries for lipophilic bioactive food components- a review. Pol J Food Nutr Sci. 2015;65:157–166. [Google Scholar]
- Chang Y, Hu Y, McClements DJ. Competitive adsorption and displacement of anionic polysaccharides (fucoidan and gum arabic) on the surface of protein-coated lipid droplets. Food Hydrocolloid. 2016;52:820–826. doi: 10.1016/j.foodhyd.2015.08.023. [DOI] [Google Scholar]
- Cheng J, Zhou S, Wu D, Chen J, Liu D, Ye X. Bayberry (Myrica rubra Sieb. et Zucc.) kernel: a new protein source. Food Chem. 2009;112:469–473. doi: 10.1016/j.foodchem.2008.05.106. [DOI] [Google Scholar]
- Damodaran S. An overview. In: Damodaran S, Paraf A, editors. Food proteins and their applications. New York: Marcel Dekker; 1997. pp. 1–2. [Google Scholar]
- Demetriades K, Coupland JN, McClements DJ. Physical properties of whey protein stabilized emulsions as related to pH and NaCl. J Food Sci. 1997;62:342–347. doi: 10.1111/j.1365-2621.1997.tb03997.x. [DOI] [Google Scholar]
- Doublier JL, Garnier C, Renarda D, Sanchez C. Protein-polysaccharide interactions. Curr Opin Colloid Interface Sci. 2000;5:202–214. doi: 10.1016/S1359-0294(00)00054-6. [DOI] [Google Scholar]
- Farshchi A, Ettelaie R. Holmes M, Influence of pH value and locust bean gum concentration on the stability of sodium caseinate-stabilized emulsions. Food Hydrocolloid. 2013;32:402–411. doi: 10.1016/j.foodhyd.2013.01.010. [DOI] [Google Scholar]
- Ghorbanian F, Kochaki A, Milani E, Razavi SMA (2015) Effect of xanthan gum on physicochemical properties of grass pea (Lathyrus sativus) isolate stabilized oil in water emulsion (Text in Persian). Food Sci Tech, 115–123
- Guo Q, Mu TH. Emulsifying properties of sweet potato protein: effect of protein concentration and oil volume fraction. Food Hydrocolloid. 2011;25:98–106. doi: 10.1016/j.foodhyd.2010.05.011. [DOI] [Google Scholar]
- Kaushik PD, Dowling K, Barrow CJ, Adhikari B. Complex coacervation between flaxseed protein isolate and flaxseed gum. Food Res Int. 2015;72:91–97. doi: 10.1016/j.foodres.2015.03.046. [DOI] [Google Scholar]
- Khouryieh H, Puli G, Williams K, Aramouni F. Effects of xanthan–locust bean gum mixtures on the physicochemical properties and oxidative stability of whey protein stabilised oil-in-water emulsions. Food Chem. 2015;167:340–348. doi: 10.1016/j.foodchem.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Lam RSH, Nickerson MT. Food proteins: a review on their emulsifying properties using a structure–function approach. Food Chem. 2013;141:975–984. doi: 10.1016/j.foodchem.2013.04.038. [DOI] [PubMed] [Google Scholar]
- Long Z, Zhao Q, Liu T, Kuang W, Xu J, Zhao M. Influence of xanthan gum on physical characteristics of sodium caseinate solutions and emulsions. Food Hydrocolloid. 2013;32:123–129. doi: 10.1016/j.foodhyd.2012.12.017. [DOI] [Google Scholar]
- Makri EA, Doxastakis GI. Study of emulsions stabilized with Phaseolus vulgaris or Phaseolus coccineus with the addition of Arabic gum, locust bean gum and xanthan gum. Food Hydrocolloid. 2006;20:1141–1152. doi: 10.1016/j.foodhyd.2005.12.008. [DOI] [Google Scholar]
- Martínez-Padilla LM, García-Rivera JL, Romero-Arreola V, Casas-Alencáster NB. Effects of xanthan gum rheology on the foaming properties of whey protein concentrate. J Food Eng. 2015;156:22–30. doi: 10.1016/j.jfoodeng.2015.01.018. [DOI] [Google Scholar]
- McClements DJ. Protein-stabilized emulsions. Curr Opin Colloid Interface Sci. 2004;9:305–313. doi: 10.1016/j.cocis.2004.09.003. [DOI] [Google Scholar]
- Moosavi- Nasab M, Pashangeh S, Rafsanjani M. Effect of fermentation time on xanthan gum production from sugar beet molasses. Int Scholarl Sci Res Innov. 2010;4:8–29. [Google Scholar]
- Moschakis T, Murray BS, Biliaderis CG. Modifications in stability and structure of whey protein-coated o/w emulsions by interacting chitosan and gum arabic mixed dispersions. Food Hydrocolloid. 2010;24:8–17. doi: 10.1016/j.foodhyd.2009.07.001. [DOI] [Google Scholar]
- Nesterenko A, Alric I, Oise Silvestre F, Durrieu V. Vegetable proteins in microencapsulation: a review of recent interventions and their effectiveness. Ind Crop Product. 2013;42:469–479. doi: 10.1016/j.indcrop.2012.06.035. [DOI] [Google Scholar]
- Noorlaila A, Siti Azah A, Asmeda R, Norizzah AR. Emulsifying properties of extracted okra (Abelmoschus esculentus L.) mucilage of different maturity index and its application in coconut milk emulsion. Int Food Res J. 2015;22:782–787. [Google Scholar]
- Petsko GA, Ringe D. Protein structure and function. London: New science Press; 2004. [Google Scholar]
- Qiu C, Zhao M, McClements DJ. Improving the stability of wheat protein-stabilized emulsions: effect of pectin and xanthan gum addition. Food Hydrocolloid. 2015;43:377–387. doi: 10.1016/j.foodhyd.2014.06.013. [DOI] [Google Scholar]
- Rahmati NF, Koocheki A, Varidi M, Kadkhodaee R. Adsorption of Speckled Sugar bean protein isolate at oil-water interface: effect of ionic strength and pH. Int J Biol Macromol. 2017;95:1179–1189. doi: 10.1016/j.ijbiomac.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Rahmati NF, Koocheki A, Varidi M, Kadkhodaee R. Structural and functional properties of three genotypes of common bean proteins (Phaseolus vulgaris) Iran Food Sci Tech Res J. 2017;13:79–91. [Google Scholar]
- Rodríguez Patino JM, Pilosof AMR. Protein-polysaccharide interactions at fluid interfaces. Food Hydrocolloid. 2011;25:1925–1937. doi: 10.1016/j.foodhyd.2011.02.023. [DOI] [Google Scholar]
- Siddiq M, Uebersax MA. Dry beans and pulses production and consumption-an overview. In: Siddiq M, Uebersax MA, editors. Dry beans and pulses production, processing and nutrition. 1. Iowa: Wiley and Blackwell; 2013. pp. 3–22. [Google Scholar]
- Wang B, Li D, Wang LJ, Adhikari B, Shi J. Ability of flaxseed and soybean protein concentrates to stabilize oil-in-water emulsions. J Food Eng. 2010;100:417–426. doi: 10.1016/j.jfoodeng.2010.04.026. [DOI] [Google Scholar]
- Xu BJ, Chang SK. Total phenolic content and antioxidant properties of eclipse black beans (Phaseolus vulgaris L.) as affected by processing methods. J Food Sci. 2008;73:19–27. doi: 10.1111/j.1750-3841.2007.00625.x. [DOI] [PubMed] [Google Scholar]
- Yin B, Deng W, Xu K, Huang L, Yao P. Stable nano-sized emulsions produced from soy protein and soy polysaccharide complexes. J Colloid Interface Sci. 2012;380:51–59. doi: 10.1016/j.jcis.2012.04.075. [DOI] [PubMed] [Google Scholar]
- Zayas J. Functionality of proteins in food. New York: Springer; 1997. [Google Scholar]
- Zhao Q, Long Z, Kong J, Liu T, Sun-Waterhouse D, Zhao M. Sodium caseinate/flaxseed gum interactions at oil-water interface: effect on protein adsorption and functions in oil-in-water emulsion. Food Hydrocolloid. 2014;43:137–145. doi: 10.1016/j.foodhyd.2014.05.009. [DOI] [Google Scholar]