Abstract
Lepidium sativum is widely used as a culinary and medicinal herb and is claimed to cure many diseases. In this study, an attempt was made to investigate the biochemical composition and functional properties of L. sativum ethanolic extract. The extract contained a total phenolic content of 11.03 ± 0.75 (mg GAE/g dw plant material) and a flavonoid content of 4.79 ± 0.24 (mg QE/100 g dw plant material). Further, the extract was characterized by LC–ESI-Q-TOF–MS/MS profiling and the results showed that the ethanolic fraction contains many important phenolics such as Kaempferol, Coumaroylquinic acid, p-Coumaroyl glycolic acid, Caffeic acid. The identified compounds are known for their biological properties and therefore, the functional properties of the extract as a whole were also studied. The extract showed significant antioxidant activity (IC50 values) 162.4 ± 2.3, 35.29 ± 1.02, 187.12 ± 3.4 and 119.32 ± 1.5 μg/ml in terms of DPPH, ABTS, Superoxide scavenging activity and metal chelating property respectively. Further, the extract showed IC50 values, 73.72 ± 1.23 and 121.78 ± 1.03 μg/ml in HRBC membrane stabilization ability and protein denaturation inhibition capacity respectively, which in turn is a measure of its anti-inflammatory activity. The results of the study are promising and serve basis for further investigation into the plant and possible consideration for use in nutraceuticals and functional foods.
Keywords: L. sativum, Phenolic compounds, LC–ESI-Q-TOF–MS/MS, Antioxidant capacity, Anti-inflammatory activity
Introduction
In the past few decades, extensive research have been carried out in the interest of identifying plant derived phytonutrients with antioxidant property to limit the damage caused by free radicals. Phenolics and flavonoids are widely studied phytonutrients and they appear to function as biological response modifiers, which modify immune responses during the cell and tissue injury by functioning as a free radical scavenger and metal chelators (Ravi et al. 2017). These class of phytonutrients exhibit stress relief compound, anti-aging, anti-inflammatory, as well as anti-carcinogenic activity (Lidija et al. 2007; Coulibaly et al. 2014; Doshi et al. 2015; Belwal et al. 2016).
In addition to this, many plant-derived compounds are well-known antioxidants and they help in reducing the oxidative damage occurring in the body, by stabilizing free radicals. Antioxidants play a significant role in maintaining optimum health and well-being. It also limits the occurence of numerous cognitive impairment, immune dysfunction as well as degenerative diseases including cardiovascular, cancer and diabetes (Prathapan et al. 2011; Shirzad et al. 2017). Oxygen molecule plays a vital role in a biological metabolic pathway. The paradox of oxygen, react rapidly with free radicals to become radical themselves called reactive oxygen species (ROS). ROS include the hydroxyl radical (·OH), hydrogen peroxide (H2O2), the superoxide anion radical (O2−), hypochlorite radical (–OCl), singlet oxygen (O2), nitric oxide radical (·NO) and various lipid peroxides which are capable of attacking the normal cells of the body causing several structural and functional damages (Doshi et al. 2015). Reports indicate that plant extracts, in the form of pure compounds and as standardized extracts, have unmatched chemical diversity and therefore, provide limitless prospects for the development of new drugs. Further, more than 80% of people around the globe rely on traditional medicines, especially on herbal medicines, for their primary healthcare needs. This number is increased further due to the high efficiency of plant medicines to treat chronic as well as infectious diseases and the development of adverse effects and microbial resistance of the chemically synthesized drugs (Sonmezdag et al. 2016). Hence, it is essential to study the phytochemical composition of various medicinally important plant species.
Lepidium sativum which belongs to the genus Lepidium under Cruciferae family, is one of such plant species with huge potential in the treatment of various diseases. It is locally called as pepper grass, garden grass, water cress and pepper wart. Its usage is reported in the treatment of skin disease, asthma, leprosy, hepatopathy, scurvy and seminal weakness (Ayedemir and Becerik 2011; Diwakar et al. 2017). The bitter seed is aphrodisiac, depurative, diuretic, tonic, aphrodisiac, thermogenic antiscorbutic, antihistaminic and ophthalmic (Diwakar et al. 2017).
Further, many studies showed that edible oil seed cakes contain many biologically active phytonutrients including natural antioxidants, phenolic acids, and flavonoids with tremendous health benefits. Therefore, oilseed cakes and their extracts can be used in nutraceuticals, dietary supplements, and pharmaceutical products (Ayala-zavala et al. 2011; Teh et al. 2014). Therefore, in this study, efforts were made to analyze the bioactive profile and antioxidant and anti-inflammatory potential of LSS-C ethanol fractions.
Material and method
Chemicals and reagents
2,2′-Diphenyl-1-picrylhydrazyl radical (DPPH), 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), gallic acid, Folin–Ciocalteu’s phenol reagent, and quercetin were purchased from Sigma-Aldrich Chemical Company (St. Louis, USA). Ethanol (EtOH) and other chemicals and organic solvents used were of analytical grade from Merck Company.
Plant material
L. sativum seeds were procured from a local market, in Mumbai, India. The seeds were manually cleaned and grounded. Oil from the seeds was extracted using Soxhlet apparatus with n-hexane as the solvent for 5 h. The defatted seed powder was then dried at 28 °C. Seed flour was further grounded, passed through a 60-mesh sieve and stored in a desiccator at 29 °C, protected from light.
Ethanolic extraction of LSS-C
LSS-Cs were extracted according to the method reported by Kadam and Lele (2017) with some modification. About 1 g samples of LSS-C were mixed with the 17 ml of 53% of ethanol and stirred with a magnetic stirrer at 500 rpm for 30 min at 28 °C. The samples were then centrifuged at 5000 rpm for 15 min, and the supernatants were recovered. The solvent parts were evaporated under reduced pressure and extracts were then stored at − 20 °C until the further use.
Phytochemical analysis
Total Polyphenol (TP) and Total flavonoid (TF) content were evaluated according to the method described by Belwal et al. (2016) and Kadam and Lele (2017). The anti-inflammatory activity of ethanolic fraction was determined by HRBC membrane stabilization and the inhibition of protein denaturation assay according to the method described by Kadam and Lele (2014).
Bioactive profiling of extract by LC-Q-TOF-MS/MS
Phenolic contents of LSS-C extracts were characterized by LC-Q-TOF-MS/MS according to the method described by Kadam and Lele (2017). An Agilent 6200 series Liquid Chromatography system utilizing a Zorbax Eclipse C18 (5 μm, 150 mm × 2.1 mm) column was used. The solvent programme comprised water 95% (Solvent A): acetonitrile 5% (Solvent B) applying the following gradient: 0.02–20 min (A: 95%, B: 5%), 20–26 min (A: 5%, B: 95%), 26–30 min (A: 95%, B: 5%) system with a flow rate 0.2 ml/min and a column temperature of 25 °C. The injection volume was set at 5 μl with a total run time of 30 min.
An Agilent G6550A ultra high definition Accurate-Mass Quadrupole Time of Flight Mass Spectrophotometer using Agilent Mass Hunter Software version B.05.01 (B5125) was utilized. Agilent personal Compound Database Library (PCDL) version B.05.01 build 92 was employed to create the custom database. The mass spectrophotometer was operated at 2 GHz and the full scan mass covered the range of m/z from 100 to 1000. MS2 analyses were carried out in the automatic mode.
Sample ionization was achieved using an Electrospray Ionisation (ESI) interface in both positive and negative ion mode. In negative ion mode, the gas and vaporizer temperatures were set to 250 °C, with a drying gas flow rate of 13 L/min. The nebulizer was set to 35 psig with a corona current of 20 mA, fragmentor 175 V, skimmer 65 V, Octopole RF 750 V and a capillary voltage of 5500 V.
Antioxidant activity and metal chelation property
Antioxidant activities of the LSS-C extracts were determined in terms of DPPH radical scavenging activity (Sonawane and Arya 2017), ABTS radical scavenging assay (Perez-Ramirez et al. 2015) and superoxide scavenging activity assay (Prathapan et al. 2011). Fe2+ chelation activities were also estimated (Decker and Barbara 1990).
Statistical analysis
All data obtained were represented as the mean value ± standard deviation (SD) of the three replicates. Analyses were performed using the SPSS Version 16.0 (SPSS Inc. Chicago, IL. USA) and the variance (p < 0.05) of the data was analyzed Duncan’s new multiple ranges.
Result and discussion
Total phenolic and flavonoid content of LSS-C extract
Phenolic and flavonoid compounds are viewed as important phytonutrients and are widely present in the many plants. In addition, these class of phytonutrients also recognized as antioxidants because of their free radicals scavenging activity active as well as oxygen species including singlet, superoxide free radicals and hydroxyl radicals quenching capacity (Vieira and Fatibello-filho 1997).
Table 1 shows the total phenolic and total flavonoid content of LSS-C extract. TPC and TFC values were expressed as gallic acid (mg GAE/g dw plant material) and quercetin equivalent (mg QE/g dw plant material) respectively.
Table 1.
Quantification of total phenolic and flavonoid content of LSS-C extract
| LSS-C extract | TPC (mg GAE/g dw plant material) | FAC (mg QE/100 g dw plant material) |
|---|---|---|
| 11.03 ± 0.75 | 4.79 ± 0.24 |
Each value is the mean ± SD of three independent measurements
TPC total phenolic content; TFC total flavonoid content; GA gallic acid equivalent; QE quercetin equivalent
The extract of LSS-C contained 11.03 ± 0.75 and 5.79 ± 0.24 mg/g dw of total phenolic and flavonoid content respectively. Indumathy and Ajithadas (2013) found similar results for total phenolic content in methanolic extract of L. sativum, 8.651 ± 0.321 mg gallic acid equivalent/gdw. In addition, a higher value of total phenolic content was found in acidified methanol extract produced by maceration at room temperature and were reported as 120.26 ± 1.52 mg Caffeic acid equivalent/g dw by Zia-Ul-Haq et al. (2012). Ayedemir and Becerik (2011) reported a phenolic content of 51.0 ± 1.95 mg gallic acid equivalent/100 g dw in ethanolic extract of L. sativum. Regarding the flavonoid content of L. sativum, Indumathy and Ajithadas (2013) reported a similar trend of 4.023 ± 0.081 mg/g de catechol equivalent in the methanolic extract.
Bioactive profiling of extract by LC-Q-TOF-MS/MS
Liquid chromatography coupled with a high-resolution quadrupole time-of-flight mass spectrometer (Q-TOF-MS) in dual ESI+ and ESI− ionization mode was performed in this study. This enabled the identification of different isobaric compounds and then to carry out a qualitative analysis of phenolic constituents occurring in 60% EtOH fractions in LSS-C extract. The mass spectrometer ionizes the compound to generate charged molecule and molecule fragment, measuring their mass-to-charge ratio. This analytical technique is highly sensitive and selective as compared with conventional LC methods due to its rapid metabolite analysis with proper peak separation.
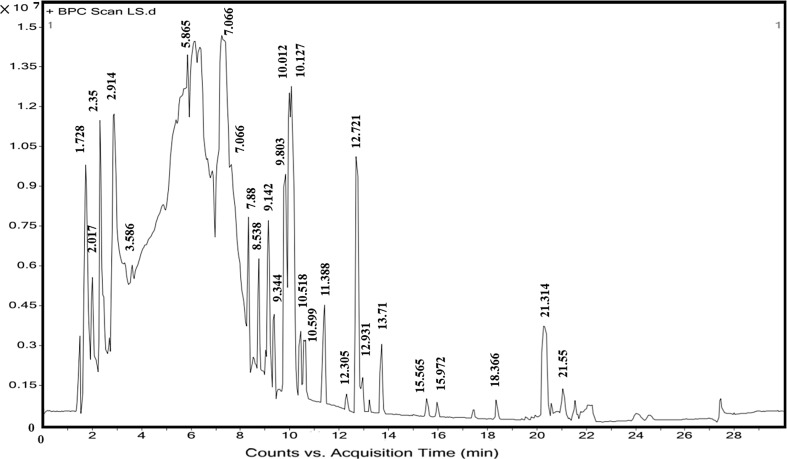
The LC-ESI-Q-TOF-MS–MS chromatograms of the LSS-C extract was relatively complex, containing peaks of phenolic acid, flavonoid and other methanolic compounds (Fig. 1). Thirteen phenolic compounds, twenty flavonoids, alkaloids, lignan and two stilbenes were identified using fragmentation patterns observed in tandem mass spectra (Table 2). The MS/MS spectra of the individual compounds were analyzed by referring it to retention times, by comparison with published libraries and fragmentation profile. Peaks were also authenticated from masses and chemical formulas (Table 2). Annotated compounds represent different classes include alkaloid, phenolic acid derivative (hydroxycinnamic acid) and flavonols.
Fig. 1.
Chromatogram of chemical constituent from LSS-C extract
Table 2.
List of the detected compounds from LSS-C extracts observed from negative ion LC–MS/MS with their retention times, UV spectra and mass spectral data
| Peak no. | Class | Phytochemical compound | RT (min) | Molecular formula | [M + H]+ or [M + Na]+, m/z | MS/MS fragment ions, m/z | Biological activity |
|---|---|---|---|---|---|---|---|
| Hydroxycinnamic acids (phenolic acid) | Coumaroylquinic acid | 1.728 | C16H18O8 | 337 | 173, 155, 129, 111, 85 | Antioxidant and anti-inflammatory | |
| Alkaloid | Sinapine | 2.017 | C16H24NO5 | 310 | 251.0909; 207.0647; 175.0392 | Antitumer | |
| Isoflavon | 7-Hydroxy-5,6-dimethoxy-2′,3′-methylenedioxy isoflavone | 2.35 | C18H15O7 | 343.0738 | Antioxidant | ||
| Hydroxycinnamic acids (phenolic acid) | p-Coumaroyl glycolic acid | 3.586 | C11H10O5 | 222.194 | 163 | Antioxidant and anti-inflammatory | |
| Hydroxycinnamic acids (phenolic acid) | Caffeic acid | 5.865 | C9H8O4 | 180.157 | 135 | Antimicrobial | |
| Flavonols | Kaempferol 3-O-glucosyl-rhamnosyl-galactoside | 7.066 | C33H40O20 | 756.659 | 609, 143, 285.04, 162 | Antioxidant | |
| Stilbenes | Resveratrol | 7.288 | C14H12O3 | 228.243 | 185, 159 | Phytoalexin, cardioprotective | |
| Flavones | Apigenin 6-C-glucoside | 7.481 | C21H20O10 | 432.378 | 313, 271, 162 | Anti-inflammatory, anticancer, antiadipogenic, antioxidant, hepatoprotective | |
| Flavones | Kaempferol-sinapoyl-di-hexose-pentose | 7.562 | C43H50O25 | 962.259 | 815.2032; 609.1433; 285.0403 | Antioxidant | |
| Hydroxycinnamic acid (phenolic acid) | Caffeic acid-hexoside | 7.574 | C15H18O9 | 341 | 179, 135 | Antioxidant | |
| Flavones | Isorhamnetin–3–0–sophoroside–7–0–glucoside | 7.699 | 801 | 639, 315 | Antioxidant | ||
| Flavones | 5,4′-Dihydroxy-3,3′-dimethoxy-6:7-methylenedioxyflavone-4′-β-d-glucuronide | 7.81 | C24H26O14 | 532 | 357 | Antioxidant | |
| Flavonols | 5,3′,4′-Trihydroxy-3-methoxy-6:7-methylenedioxyflavone 4′-O-glucuronide | 7.888 | C23H20O14 | 520.396 | 343 | Antioxidant | |
| Flavonols | Kaempferol 3-O-galactoside | 8.055 | C21H20O11 | 448.377 | 285, 255, 162 | Antioxidant | |
| Stilbenes | e-Viniferin | 8.172 | C28H22O6 | 454.471 | 435, 411, 369, 359, 347, 333 | Hepatoprotective | |
| Phenolic | Dihydroferulic acid 4-O-glucuronide | 8.538 | C16H20O10 | 372.324 | 309; 195 (glucuronide loss), 175, 112 | Anti-inflammatory, antioxidant | |
| Flavonols | Quercetin | 8.749 | C15H10O7 | 302.236 | 301 (151, 179) | Antioxidant and anti-inflammatory | |
| Flavanols | (+)-Catechin | 8.815 | C15H14O6 | 290.268 | 245, 205, 179 | Antioxidant | |
| Flavonols | Phloretin-2′-O-glucoside | 8.834 | C21H24O11 | 435.358 | 267 | Antioxidant and anticancer activity | |
| Hydroxycinnamic acids (phenolic acid) | Caffeoylquinic acid | 9.142 | C16H18O9 | 246.215 | 191, 179, 173, 135 | Antioxidant, hepatoprotective, antiobesity, anti-inflammatory, antimicrobial | |
| Hydroxycinnamic acids (phenolic acid) | Sinapic acid | 9.344 | C11H12O5 | 224.21 | 193, 134, 149, 179 | Antioxidant | |
| Lignans | Schisandrin B | 9.803 | C23H28O6 | 400.465 | 286, 370, 355, 331, 316, 300, 285 | Antioxidant, anticancer | |
| Flavonoids | 2′,7-Dihydroxy-4′,5′-dimethoxyisoflavone | 10.012 | C17H14O6 | 314.289 | 271, 200, 187, 137, 107 | Antibacterial | |
| Hydroxybenzoic acid (phenolic acid) | Vanillic acid 4-sulfate | 10.127 | C8H8O7S | 248.21 | Antioxidant | ||
| Isoflavon | 5,6-Dimethoxy-2′,3′-methylenedioxy-7-C-β-d-glucopyranosyl isoflavone | 10.518 | C24H25O11 | 489.1317 | 461, 451, 427, 149, 261, 203, 136 | Antioxidant, hepatoprotective | |
| Flavones | Quercetin-3-O-rutinoside | 10.599 | C27H30O16 | 609 | 300, 271, 255 | Antioxidant | |
| Flavanols | (−)-Epigallocatechin 3-O-gallate | 11.388 | C22H18O11 | 458.372 | 169, 331, 305 | Anti-inflammatin, anticancer, antidiabetic | |
| Hydroxybenzoic acids (phenolic acid) | 4-Hydroxyhippuric acid | 12.305 | C9H9NO4 | 195.172 | 176, 150, 136, 100 | Antioxidant activity | |
| Dihydrochalcones (flavonoid) | Phloretin 2′-O-xylosyl-glucoside | 12.721 | C26H32O14 | 568.524 | 437, 275, 169 | Antioxidant, anticancer | |
| Phenolic acid | Isopropyl 3-(3,4-dihydroxyphenyl)-2-hydroxypropanoate | 12.931 | C12H16O5 | 240.252 | 223, 237, 120, 104,76 | Vasodilatory ability | |
| Hydroxybenzoic acid (phenolic acid) | Vanillic acid-hexoside | 13.71 | C14H18O9 | 330 | 167 | Antioxidative | |
| Isoflavon | 7-Hydroxy-4′,5,6-tri-methoxy isoflavone | 13.71 | C18H17O6 | 329.0945[M + H]+ | Antioxidant | ||
| Hydroxybenzoketones (phenolic acid) | 2-Hydroxy-4-methoxy acetophenone 5-sulfate | 15.565 | C9H10O7S | 262.237 | 167, 166, 152, 151, 108, 95, 43 | Analgestic, aesthetic, antibacterial, antioxidative antiviral | |
| Alkaloid | 4′-O-Methyl-epigallocatechin | 15.972 | C16H16O7 | 320.294 | 302 | Antispasmodic, antioxidant and cytotoxic | |
| Furanocoumarins (other polyphenol) | Isopimpinellin | 18.366 | C13H10O5 | 246.215 | 232.0 (M + H-CH3); 217.1 (M + H-2CH3); 188.8 (M + H-2CH3-CO) | Antibacterial antifungal and antioxidant | |
| Isoflavonoids | 5,6,7,3′,4′-Pentahydroxyisoflavone | 21.55 | C15H10O7 | 302.236 | 274, 200, 136, | Anti-inflammatory, cytotoxic and antioxidant |
MS spectral interpretation allowed the identification of 11 phenolic acid and their derivatives (peak 1, 4, 5, 10, 16, 20, 21, 24, 28, 30, 31, 33 and 35). Among them, three compound (peak 25, 29 and 32) were identified as hydroxybenzoic acids such as vanillic acid 4-sulfate, 4-Hydroxyhippuric acid and Vanillic acid-hexoside according to pseudo molecular [M − H]− ion m/z at 248.11, 195.172 and 330 respectively. Like, peaks 1, 4, 5, 10, 20 and 21 were identified as phenolic acid derivative (hydroxycinnamic acid) shows a mass of m/z 337, 163, 135, 341, 246.215 and 224.21 amu, with predicted formula of C16H18O8, C11H10O5, C9H8O4, C15H18O9, C16H18O9 and C11H12O5, annotated as Coumaroylquinic acid, p-Coumaroyl glycolic acid, Caffeic acid, Caffeic acid-hexoside Caffeoylquinic acid and Sinapic acid respectively. Caffeic acid is a key intermediate in the biosynthesis of lignin with potential antioxidant. This polyphenol derivative class has been reported in different fruit and vegetable species which reported exerting antioxidant activity (Lidija et al. 2007).
Peak 33 was assigned to hydroxybezoketones; 2-Hydroxy-4-methoxy acetophenone 5-sulfate (m/z at 262.237) according to an analysis of the molecular ion peak and MS2 fragment ion peaks described in the previous report (Atta-ur-Rahman et al. 1995). The molecular ion peaks and MS2 fragment peak of 2-Hydroxy-4-methoxy acetophenone 5-sulfate are 167, 166, 152, 151, 108, 95 and 43. Peak 35 which present a Furanocoumarins, [M − H]− ion at m/z 246.215 and daughter ion at 232.0 (M + H-CH3); 217.1 (M + H-2CH3); 188.8 (M + H-2CH3-CO) based on a comparison with a reported compound, was annotated as Isopimpinellin.
Peak 16 was identified as a dihydroferulic acid 4-O-glucuronide according to its pseudo molecular [M − H]− ion (m/z at 372.324) and the release of fragments of m/z 195 [p-coumaric acid-H]− (− 176 amu, glucuronide). Peak 30 showed a pseudo molecular [M − H]− ion (m/z at 240.252) and MS2 gave a fragment at m/z 223.4 [M–1–OH]–, m/z 137.6 [M–OH–COOCH(CH3)2]–, m/z 120.1 [M–OH–COOCH(CH3)2–OH]– The compound was thus identified as an Isopropyl 3-(3,4-dihydroxy phenyl)-2-hydroxy propanoate. These phenolic acid have been previously reported from novel metabolite extracted from Salvia miltiorrhiza (Wang et al. 2008) with vasorelaxant activity.
As indicated in Fig. 1 and Table 2 MS spectral analysis presented alkaloid (peak 34) showing fragment ion at m/z 320.298 with a molecular formula of C16H16O7 annotated as 4′-O-Methyl-epigallocatechin. Schisandrin B annotated (peak 22) was identified as lignan. The precursor ion at 400.465and a predicted formula C23H28O6. This lignan class has been reported into exhibit a potential antioxidant, anti-aging and anticancer property (Hou et al. 2015).
As shown in Fig. 1 and Table 2 peak 7 and 15 reveal the presence of two stilbenes according to the MS spectral analysis, [M − H]− molecular ion at m/z 254.471 produced fragment ion at (m/z at 435, 411, 369, 359, 347, 333) which is similar to the mass of e-Viniferin and while Resveratrol shows an [M − H]− at m/z 228.243 for C14H12O3 and fragment ion at (m/z 185, 159) that previously described in previously describe in Sun et al. (2007).
MS/MS spectral analysis revealed the presence of isoflavones (peak 3, 23 25, 32 and 36), flavonols (peak 11, 12, 13, 18 and 27), Kaempferol derivative (peak 6, 9 and 14), Phloretin (peak 19 and 29), Quercetin (peak 17) and Apigenin (peak 8) (Fig. 1; Table 2). MS spectra have provided a sufficient information regarding the aglycone structure, the attachment point of substituents and the monosaccharide units of the glycan sequence. Fragmentation pathway of O-glycosylated flavonoids starts with the cleavage of the glycosidic bonds and elimination of the sugar moieties with charge retention on the aglycone or sugar fragments (Stobiecki 2000). In detail, deprotonated flavonol glycosides, ions corresponding to the deprotonated aglycones, [M]− at m/z 285, 271, 273 and 301 generated by the loss of sugar unit, furthermore the following fragment ions for aglycones are detected: [M–H]− at m/z 286, 272, 274 and 302, [M–H–CO2–H]− at m/z 256, 242, 244 and 272 and [M–H–2CO–H]− at m/z 228, 214, 216 and 244 for kaempferol, Apigenin, Phloretin and quercetin respectively.
Several detected flavonoid were di-, tri- and tetra flavonol glycosides of Phloretin, quercetin apigenin, and kaempferol based on their spectral data with peaks (6, 8, 9, 14, 19, 29 and 26) and masses shows a sequential loss of 162 amu [hexose; glucose or galactose]−, 146 amu [dexyhexose]− or 132 amu [pentose]− and 176 amu [glucoronic acid]−. Apigenin 6-C-glucoside (peak 8; m/z at 432.378) have been reported to be present in the various medicinal plant which is receiving an increasing attention due to their wide range of pharmacological activity such as antioxidant, anti-inflammatory, anti-cancer, antihyperalgesic, and neuroprotective effects.(He et al. 2016). From peaks, it is clear that there is a high ratio of [M − H]− and [M]− ion derived from homolytic and heterolytic cleavage of the sugar moieties from apigenin, quercetin, phloretin, and kaempferol. This could be affected by the length of the saccharide substituent (Lu et al. 2010).
As shown in Fig. 1 and Table 2 the aglycones quercetin (peak 17; m/z at 302.236) and quercetin-3-O-rutinoside (peak 27; m/z at 609), produced fragment ion at m/z 151 and 179 respectively, which obtained from a cleavage of the heterocyclic C-ring by RDA (Bravo et al. 2006). In the case of quercetin-3-O-rutinoside spectra showed the deprotonated [M − H]− a molecule of the glycoside and the [α-H]− ion corresponding to the deprotonated aglycone. The latter ion is formed by losing the rutinose, galactose and rhamnose moiety from the corresponding glycosides.
Peak 3, 23, 25, 32 and 36 were identified as isoflavones presented pseudo molecular [M − H]− ion at 343.738, 314.289, 489.1317, 329.09 and 302.23 amu,with predicted molecular formula C18H15O7, C17H14O6, C24H25O11, C18H17O6 and C15H10O7, annotated as 7-hydroxy-5,6-dimethoxy-2′,3′-methylenedioxy isoflavone, 5,6-dimethoxy-2′,3′-methylenedioxy-7-C-β-d-glucopyranosyl isoflavone, 7-hydroxy-4′,5,6-tri-methoxy isoflavone and 7-hydroxy-4′,5,6-tri-methoxy isoflavone, respectively. Sequential loss of 15 amu [methyl group], 162 amu [hexose; glucose or galactose]−, and 176 amu [glucoronic acid] was also evident in isorhamnetin–3–0-sophoroside–7–0–glucoside (peak 11; m/z at 801), 5,4′-dihydroxy-3,3′-dimethoxy-6:7-methylenedioxy flavone-4′-β-d-glucuronide (peak 12 m/z at 532) and 5,3′,4′-Trihydroxy-3-methoxy-6:7-methylenedioxy flavone 4′-O-glucuronide (peak 13, m/z at 520.396).
As indicated in Fig. 1 and Table 2 MS spectral analysis presented (peak 18) showing fragment ion at m/z 290.268 with a molecular formula of C15H14O6 annotated as (+)-Catechin, exhibit fragment ions at m/z 179, 205, 245. The [M − H-44]− fragment ion at m/z 245 in (+)-catechin was produced by the loss of a (CH)2OH group as described by Sun et al. (2007). Similarly, (−)-Epigallocatechin 3-O-gallate (peak 27; [M − H]− m/z at 458.372) exhibit the fragment ions at m/z 169, 331 and 305 which is consistent when compared with the previous report (Sun et al. 2007). It shows that the fragment ion at m/z 169 that corresponds to gallic acid and the fragment ion at m/z 305 corresponds to the (−)-gallocatechin or (−)-epigallocatechin units.
Many of the bioactive compounds identified are known to possess various functional properties. Especially the identified classes such as flavones, flavanols, Isoflavones, phenolic acids, lignans, and alkaloids are known antioxidant and anti-inflammatory agents. Boots et al. (2008) explained the antioxidant potential of quercetin, a flavonol present in the extract. Another compound found in the extracts, epigallocatechin gallate, was identified as the most effective chemopreventive polyphenol in green tea (Du et al. 2012). Chen and Chen (2014) reported that kaempferol has a significant role in reducing the risk of chronic diseases, especially cancer by augmenting the body’s antioxidant defense against free radicals. Caffeoylquinic acid is another compound of interest found in the extract and is known for many functional properties including antioxidant, anti-inflammatory, antimicrobial etc. (Miyamae et al. 2011; Coulibaly et al. 2014). Most of the compounds identified were observed as efficient antioxidants and therefore, it is essential to analyze its potential to act as antioxidant and anti-inflammatory agents.
In vitro anti-inflammatory activity
Inflammation is a very complex biological response of cellular tissue to injurious stimuli. It is mainly classified into acute and chronic. In acute inflammation, the body initiates an early response to the harmful stimuli by accelerating the movement of plasma and leukocytes from the blood into harmful tissue. Whereas chronic inflammation is also known as prolonged inflammation which exhibits series of a biochemical reaction that involves the immune and vascular system (Kadam and Lele 2017). In the last few decades, many researchers have been described the anti-inflammatory potential of phenolic and flavonoid content in plants and their potency in risks reduction of diseases and improve overall health. Further, studies proved that anti-inflammatory activity of one compound or extracts is complex and different from another. In the present study, the anti-inflammatory potential of the extract estimated by HRBC membrane stabilization assay and protein denaturation inhibition assay. Human red blood cell (HRBC) membranes or erythrocyte and lysosomal membrane are possessed similar structure (Saleem et al. 2011; Kadam and Lele 2014). Therefore, inhibition of hypotonicity of erythrocyte membrane lysis can taken as a measure of the anti-inflammatory activity of LSS-C extract. The percent membrane stabilization for LSS-C extracts was studied at different concentration (50–250 μg/ml). The results are tabulated (Table 3). Since protein denaturation is the one major factor causes inflammation, few anti-inflammatory drugs work on their ability to inhibit protein denaturation. Results of the study show that LSS-C extract is able to restrain the production of autoantigen and inhibits denaturation of protein in rheumatic disease (Table 3). The results are in line with the LC–MS results, which showed that the extract is comprised of compounds such as Coumaroylquinic acid, Apigenin 6-C-glucoside, quercetin and Caffeoylquinic acid. The anti-inflammatory potential of these compounds have been studied and proved by various researchers and this supports the current in vitro assay results (Gattuso et al. 2007; Miyamae et al. 2011; Liang and Kitts 2015).
Table 3.
Antioxidant and metal chelating activity and in vitro anti-inflammatory of LSS-C extract
| Sample | Antioxidant activity (IC50) | Anti-inflammatory activity (IC50) | ||||
|---|---|---|---|---|---|---|
| DPPH (μg/ml) | ABTS (μg/ml) | Superoxide scavenging activity (μg/ml) | Metal (iron) chelating property (μg/ml) | Stabilization of HRBC (μg/ml) | Inhibition of protein denaturation (μg/ml) | |
| LSS-C extract | 162.4 ± 2.3 | 35.29 ± 1.02 | 187.12 ± 3.4 | 119.32 ± 1.5 | 173.72 ± 1.23 | 121.78 ± 1.03 |
| Standard | 8.12 ± 0.54 (AA) | 10.563 ± 0.91 (AA) | 12.87 ± 0.63 (AA) | 2.4 ± 0.2 (AA) | 89.3 ± 2.13 (ASA) | 78.41 ± 1.37 (ASA) |
Each value is the mean ± SD of three independent measurements
AA ascorbic acid; ASA acetylsalicylic acid
In vitro antioxidant activity
Many plant-based phytonutrients including phenolic compounds are increasingly viewed as a potent antioxidant. In plants, these compounds limit a wide variety of environmental stresses while, in humans, phenolic compound play significant role in the prevention of degenerative diseases, particularly cancers, neurodegenerative diseases and cardiovascular diseases (Lu et al. 2010; Carciochi and Dimitrov 2014). The L. sativum seed cake extract was screened for the antioxidant property of 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethyl-benzothiazoline-6-sulphonic acid (ABTS) and superoxide anion radical scavenging activity method as shown in Table 3.
The effect of antioxidant on DPPH· is due to their hydrogen donating ability (Prathapan et al. 2011). DPPH is a stable free-radical molecule, exhibit trap for other radical and a strong inhibitor of radical-mediated polymerization often used in the evaluation of natural antioxidant. It shows strong absorption band at 517 nm, where radicals with deep violet color solution become colourless or pale yellow upon neutralized. The IC50 value of DPPH radical scavenging activity for LSS-C extract was found to be 162.4 ± 2.3 μg/ml. Similar, results were reported by Annie Shirwaikar et al. (2011) (IC50 value of 171.13 μg/ml).
Table 3 showed the antioxidant property of LSS-C extract as the ability to scavenge 50% of free radical ABTS·+ (IC50). The ABTS radical cation is highly reactive towards most antioxidant such as phenolic compound. Assay determines the antioxidant activity in terms of hydrogen donating and chain-breaking activity and therefore compounds with a greater number of hydrogen group shows higher antioxidant capacity, which is expressed as the lowest IC50. The different relative scavenging activity of LSS-C extract against different tested radical, mainly because of different mechanisms involved in the radical-antioxidant reactions.
The superoxide anion radicals (O2−) exhibit significant importance in the biological system since it is produced in large quantities by the enzyme NADPH oxidase as a product of one-electron reduction of dioxygen O2, which occurs widely in nature. Superoxide considers as the main cause of oxidative stress since they play important role in initiate lipid peroxidation of unsaturated fatty acids and also reacts with carbonyl compounds and halogenated carbons to create toxic peroxy radicals (Prathapan et al. 2011). Therefore, it is important to study the scavenging effect of the LSS-C extract on superoxide radical in order to understand the mechanisms of antioxidant activity. The estimated IC50 value of the extract in superoxide anion radical scavenging activity was found to be 187.12 ± 3.4 μg/ml. Indumathy and Ajithadas (2013) had reported that the methanolic extract of L. sativum seeds showed an IC50 value of 110 and 85 μg/ml for DPPH and superoxide radical scavenging activity. Ayedemir and Becerik (2011) also reported the IC50 value of ethanolic extracts of L. sativum seed (172 μg/ml). In addition, the ethanol fraction of L. sativum showed 11.63 ± 0.3% inhibition for DPPH radical scavenging activity at 100 μg/ml concentration (Linn et al. 2014). The results of the DPPH, (ABTS) and superoxide anion radical scavenging method were in agreement with the phenolic and flavonoid content, indicating L. sativum seed cake having a compelling antioxidant activity.
The LC–MS profile of the extract claimed the presence of many bioactive compounds including quercetin, Catechin, Sinapic acid, Lignans, Epigallocatechin and many more. The compounds listed were studied widely and reported to have significant antioxidant activity (Du et al. 2012; Chen and Chen 2014). Therefore, the in vitro antioxidant assay results confirms the presence of these bioactive compounds in the extract.
Metal (iron) chelating property
Oxidation of biomolecules through metal ions is significant as they can catalyze the formation of earlier radicals which lead to the radical chain reaction of lipid peroxidation (Decker and Barbara 1990). The chelating property of extracts on Fe+2 ions shows the protection against oxidative damage. Chelating agents inhibit the radical-mediated oxidative chain reactions in food and biological systems and thus improves the human health and quality, stability and safety of food (Sonawane and Arya 2017). The metal chelating effect of ethanolic extract of LSS-C was found to be IC50 119.32 ± 1.5 μg/ml (Table 3). Ayedemir and Becerik (2011) reported an IC50 value for methanol, ethanol and water extract of L. sativum seed cake and was found to be 137.19, 480, 341 μg/ml respectively. In addition, IC50 value for the chelating effect of L. sativum seed extract was 128 ± 94 μg/ml.
Conclusion
The present study reveals an important data regarding the nutraceutical potential of LSS-C. Liquid chromatography coupled with selectivity and sensitivity of Q-TOF-MS significantly identified the various phenolic acid, flavonoid and another phytonutrient with mass accuracy. Also, LSS-C exhibited excellent antioxidant and anti-inflammatory property. Results indicate that LSS-C might have possible health benefits and be helpful in preventing various oxidative stress associated diseases. This study provides a basis for further investigation into this plant and further, possible applications in food.
Acknowledgements
The authors are grateful to UGC-BSR (Government of India) for providing financial assistance during the course of this investigation.
Compliance with ethical standards
Conflict of interest
Authors have no conflicts of interest.
References
- Atta-ur-Rahman Malik S, Hasan S, et al. Nigellidine—a new indazole alkaloid from the seeds of Nigella sativa. Tetrahedron Lett. 1995;36:1993–1996. doi: 10.1016/0040-4039(95)00210-4. [DOI] [Google Scholar]
- Ayala-zavala JF, Vega-vega V, Rosas-domínguez C, et al. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res Int. 2011;44:1866–1874. doi: 10.1016/j.foodres.2011.02.021. [DOI] [Google Scholar]
- Ayedemir T, Becerik S. Phenolic content and antioxidant activity of different extracts from Ocimum basilicum, Apium graveolens and Lepidium sativum seeds. J Food Biochem. 2011;35:62–79. doi: 10.1111/j.1745-4514.2010.00366.x. [DOI] [Google Scholar]
- Belwal T, Dhyani P, Bhatt ID, et al. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM) Food Chem. 2016;207:115–124. doi: 10.1016/j.foodchem.2016.03.081. [DOI] [PubMed] [Google Scholar]
- Boots AW, Haenen GRMM, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Bravo MN, Silva S, Coelho AV, et al. Analysis of phenolic compounds in Muscatel wines produced in Portugal. Anal Chim Acta. 2006;563:84–92. doi: 10.1016/j.aca.2005.11.054. [DOI] [Google Scholar]
- Carciochi RA, Dimitrov K. Optimization of antioxidant phenolic compounds extraction from quinoa (Chenopodium quinoa) seeds. J Food Sci Technol. 2014;52:4396–4404. doi: 10.1007/s13197-014-1514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2014;138:2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulibaly AY, Hashim R, Sulaiman SF, et al. Bioprospecting medicinal plants for antioxidant components. Asian Pac J Trop Med. 2014;7:S553–S559. doi: 10.1016/S1995-7645(14)60289-3. [DOI] [PubMed] [Google Scholar]
- Decker EA, Barbara W. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem. 1990;38:674–677. doi: 10.1021/jf00093a019. [DOI] [Google Scholar]
- Diwakar BT, Dutta PK, Lokesh BR, Naidu KA. Physicochemical properties of garden cress (Lepidium sativum L.) seed oil physicochemical properties of garden cress. J Am Oil Chem Soc. 2017;87:539–548. doi: 10.1007/s11746-009-1523-z. [DOI] [Google Scholar]
- Doshi P, Adsule P, Banerjee K, Oulkar D. Phenolic compounds, antioxidant activity and insulinotropic effect of extracts prepared from grape (Vitis vinifera L) byproducts. J Food Sci Technol. 2015;52:181–190. doi: 10.1007/s13197-013-0991-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du GJ, Zhang Z, Wen XD, et al. Epigallocatechin gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients. 2012;4:1679–1691. doi: 10.3390/nu4111679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattuso G, Barreca D, Gargiulli C, et al. Flavonoid composition of citrus juices. Molecules. 2007;12:1641–1673. doi: 10.3390/12081641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Min JW, Kong WL, et al. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia. 2016;115:74–85. doi: 10.1016/j.fitote.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Hou W, Gao W, Wang D, et al. The protecting effect of deoxyschisandrin and schisandrin B on HaCaT cells against UVB-induced damage. PLoS ONE. 2015 doi: 10.1371/journal.pone.0127177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indumathy R, Ajithadas A. Free radical scavenging activities, total phenolic and flavonoid content of Lepidium sativum (Linn.) Int J Pharm Pharm Sci. 2013;5:634–637. [Google Scholar]
- Kadam D, Lele SS. Anti-inflammatory activity of the fruit extract of Benincasa hispida. J Nutr Ther. 2014;3:178–182. doi: 10.6000/1929-5634.2014.03.04.6. [DOI] [Google Scholar]
- Kadam D, Lele SS. Extraction, characterization and bioactive properties of Nigella sativa seedcake. J Food Sci Technol. 2017 doi: 10.1007/s13197-017-2853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang N, Kitts DD. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients. 2015;8:1–20. doi: 10.3390/nu8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidija J, Mariian S, Martina M-K, Lvana N. Anthocyanin content and antioxidant activity of various red fruit juices. Dtsch Leb. 2007;2:58–64. [Google Scholar]
- Linn L, Vanmathi JS, Chairman K, Balasubramanian A. Antioxidative activity of different parts of the plant. Biotechnol Rep. 2014;3:8–11. doi: 10.1016/j.btre.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Song F-R, Tsao R, et al. Letter to the editor. Rapid Commun Mass Spectrom. 2010;24:169–172. doi: 10.1002/rcm.4368. [DOI] [PubMed] [Google Scholar]
- Miyamae Y, Kurisu M, Han J, et al. Structure–activity relationship of caffeoylquinic acids on the accelerating activity on ATP production. Chem Pharm Bull (Tokyo) 2011;59:502–507. doi: 10.1248/cpb.59.502. [DOI] [PubMed] [Google Scholar]
- Perez-Ramirez IF, Castano-Tostado E, Leon R-D, et al. Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa L.) beverage. Food Chem. 2015;172:885–892. doi: 10.1016/j.foodchem.2014.09.126. [DOI] [PubMed] [Google Scholar]
- Prathapan A, Lijo Cherian O, Nampoothiri SV, et al. In vitro antiperoxidative, free radical scavenging and xanthine oxidase inhibitory potentials of ethyl acetate fraction of Saraca ashoka flowers. Nat Prod Res. 2011;25:298–309. doi: 10.1080/14786419.2010.510472. [DOI] [PubMed] [Google Scholar]
- Ravi S, Shanmugam B, Subbaiah GV, et al. Identification of food preservative, stress relief compounds by GC–MS and HR-LC/Q-TOF/MS; evaluation of antioxidant activity of Acalypha indica leaves methanolic extract (in vitro) and polyphenolic fraction (in vivo) J Food Sci Technol. 2017;54:1585–1596. doi: 10.1007/s13197-017-2590-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem TM, Azeem AK, Dilip C, et al. Anti-inflammatory activity of the leaf extracts of Gendarussa vulgaris Nees. Asian Pac J Trop Biomed. 2011;1:147–149. doi: 10.1016/S2221-1691(11)60014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirwaikar A, Patel B, Kamariya Y, et al. In vitro free radical scavenging potential of defatted ethanolic extract of the seeds of lepidium sativum linn. Chin J Nat Med. 2011;9:435–440. [Google Scholar]
- Shirzad H, Niknam V, Taheri M, Ebrahimzadeh H. Ultrasound-assisted extraction process of phenolic antioxidants from Olive leaves: a nutraceutical study using RSM and LC–ESI–DAD–MS. J Food Sci Technol. 2017;54:2361–2371. doi: 10.1007/s13197-017-2676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane SK, Arya SS. Citrullus lanatus protein hydrolysate optimization for antioxidant potential. J Food Meas Charact. 2017 [Google Scholar]
- Sonmezdag AS, Kelebek H, Selli S. Characterization of aroma-active and phenolic profiles of wild thyme (Thymus serpyllum) by GC–MS-Olfactometry and LC-ESI-MS/MS. J Food Sci Technol. 2016;53:1957–1965. doi: 10.1007/s13197-015-2144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobiecki M. Application of mass spectrometry for identification and structural studies of flavonoid glycosides. Phytochemistry. 2000;54:237–256. doi: 10.1016/S0031-9422(00)00091-1. [DOI] [PubMed] [Google Scholar]
- Sun J, Liang F, Bin Y, et al. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules. 2007;12:679–693. doi: 10.3390/12030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh S, Bekhit AE, Birch J. Antioxidative polyphenols from defatted oilseed cakes: effect of solvents. Antioxidants. 2014;3:67–80. doi: 10.3390/antiox3010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira C, Fatibello-filho O. Amperometric biosensor for the determination of phenols using a crude extract of sweet potato (Ipomoea Batatas (L.) Lam.) Anal Lett. 1997;30:895–907. doi: 10.1080/00032719708002304. [DOI] [Google Scholar]
- Wang S, Zang W, Kong S, et al. Vasorelaxant effect of isopropyl 3-(3,4-dihydroxyphenyl)-2-hydroxypropanoate, a novel metabolite from Salvia miltiorrhiza, on isolated rat mesenteric artery. Eur J Pharmacol. 2008;579:283–288. doi: 10.1016/j.ejphar.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Zia-Ul-Haq M, Ahmad S, Calani L, et al. Compositional study and antioxidant potential of Ipomoea hederacea Jacq. and Lepidium sativum L. Seeds. Mol Biotechnol. 2012;17:10306–10321. doi: 10.3390/molecules170910306. [DOI] [PMC free article] [PubMed] [Google Scholar]