Abstract
Background
For patients with severe aortic stenosis (AS) at extreme surgical risk, transcatheter aortic valve replacement (TAVR) leads to improved survival and health status when compared with medical therapy. Whether the early health status benefits of TAVR in these patients are sustained beyond 1 year of follow-up is unknown.
Methods and Results
639 patients with severe AS at extreme surgical risk underwent TAVR in the CoreValve US Extreme Risk Pivotal trial. Health status was evaluated at baseline and at 1, 6, 12, 24, and 36 months using the Kansas City Cardiomyopathy Questionnaire (KCCQ), the Short-Form-12, and the EuroQoL-5D. Analyses were performed using pattern mixture models to account for both death and missing data and were stratified by iliofemoral (IF) and non-iliofemoral (non-IF) access. After TAVR, there was substantial health status improvement in disease-specific and generic scales by 6–12 months. Although there were small declines in health status after 12 months, the initial benefits of TAVR were largely sustained through 3 years for both IF and non-IF cohorts (change from baseline in KCCQ Overall Summary score 19.0 points in IF patients and 14.9 points in non-IF patients; p<0.01 for both comparisons). Among surviving patients, clinically meaningful (≥10 point) improvements in the KCCQ Overall Summary Score at 3 years were observed in 85.0% and 83.4% of IF and non-IF patients respectively.
Conclusions
Among extreme risk patients with severe AS, TAVR resulted in large initial health status benefits that were sustained through 3-year follow-up. Although late mortality was high in this population, these findings demonstrate that TAVR offers substantial and durable health status improvements for surviving patients.
Keywords: transcatheter aortic valve implantation, quality of life
Journal Subject Codes: Transcatheter aortic valve replacement, Quality and outcomes
INTRODUCTION
Over the last decade, transcatheter aortic valve replacement (TAVR) has emerged as an alternative treatment option to surgical aortic valve replacement (SAVR) or medical therapy for patients with severe aortic stenosis (AS). In patients with severe AS who are at extreme surgical risk, TAVR has been shown to lead to improved survival compared with medical therapy 1, 2. Despite these benefits, mortality in this population is still high after TAVR (30% at 1 year, 43% at 2 years and 72% at 5 years) 2–4, reflecting the advanced age and multiple co-morbidities present in the extreme risk population. As such, for patients with severe aortic stenosis who are at extreme surgical risk, improvements in quality of life may be as important, or even more important, than improved survival 5, 6.
Several studies have demonstrated improved short-term quality of life after TAVR in the extreme surgical risk population. For example, in the PARTNER B trial, patients treated with TAVR experienced large improvements in both disease-specific and generic health status through 1 year follow-up compared with patients treated with medical therapy alone 7. Similar 1-year results (albeit without a medical therapy control group) were seen in the CoreValve U.S. Extreme Risk Pivotal Trial 8. Although the survival benefit of TAVR for such patients has been found to extend to 5-years 4, 9, few studies have evaluated the effect of TAVR on patient-reported outcomes beyond one year follow-up. To address this gap in knowledge, we sought to assess the durability of health status outcomes among patients with severe AS at extreme surgical risk after implantation of a self-expanding transcatheter aortic valve prosthesis.
METHODS
Study Design and Population
The design and results of the CoreValve U.S. Extreme Risk Trial have been reported previously 1. Briefly, the CoreValve U.S. Extreme Risk Trial was a single arm study that enrolled patients with severe symptomatic aortic stenosis, who were classified as being at extreme risk (i.e., 30 day mortality/morbidity was estimated at ≥ 50%) for traditional surgical AVR by two cardiac surgeons and one interventional cardiologist. After confirmation by the trial oversight committee, patients underwent TAVR using the self-expanding CoreValve system (Medtronic Inc., Minneapolis, MN) via either iliofemoral (IF) access or non-iliofemoral (non-IF) access by either a transaortic or subclavian approach. The study was approved by the institutional review board at each site, and all patients provided written informed consent prior to participation. Only patients who received a transcatheter valve were included in this analysis.
Health Status Instruments
Disease-specific and generic health status were assessed at baseline, and at 1, 6, 12, 24 and 36 months after enrollment using validated instruments. Written questionnaires were administered either during in-person visits or by mail. Disease-specific health status was assessed with the Kansas City Cardiomyopathy Questionnaire (KCCQ). The KCCQ is a 23-item questionnaire that covers 5 domains of health in heart failure patients (physical function, social function, symptoms, self-efficacy, and quality of life). These individual scales may also be converted into a single summary score (KCCQ-Overall Summary [KCCQ-OS]), which was the primary endpoint for this study 10. Values for each of the KCCQ domains and the summary score range from 0 to 100 with higher scores indicating less symptom burden and better quality of life. The KCCQ-OS score generally correlates with New York Heart Association (NYHA) functional class as follows: Class I: KCCQ-OS 75 to 100; Class II: 60 to 74; Class III: 45 to 59; Class IV: 0 to 44 11, 12, and changes in the KCCQ-OS score of 5, 10, and 20 points correspond to small, moderate, or large clinical improvements, respectively 11. The KCCQ has undergone reliability and validity testing among patients with severe aortic stenosis 12 and has been used as a metric of disease-specific health status among patients undergoing TAVR in the pivotal clinical trials 7, 8, 13, 14.
Generic health status was evaluated with the Medical Outcomes Study Short-Form-12 (SF-12) 15 as well as the EuroQol (EQ-5D) 16. The SF-12 is derived from the Medical Outcomes Study Short-Form 36 and provides physical and mental component summary scales, which are scored in a manner that standardizes the mean score in the US population to 50 with a standard deviation of 10. The minimum clinically important difference for the SF-12 summary scores is approximately 2 points 17.
The EuroQoL is a multi-attribute health status classification system that assesses five dimensions of general health (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), using a 3-level scale. These scores can then be converted to utilities using an algorithm developed for the U.S. population 18. Utilities are preference-weighted health status assessments with scores that range from 0 to 1, with 1 representing perfect health and 0 representing worst health 19.
Statistical Analysis
Continuous variables are described as mean ± standard deviation, and categorical variables are described as proportions. In unadjusted analyses, we used paired t-tests to evaluate the change in health status from baseline to each follow up time point among patients with paired data. The unadjusted change in health status between 12 and 36 months among patients with paired data was evaluated in an analogous fashion.
Since there were high rates of mortality as well as modest rates of missing follow-up health status data, we applied pattern mixture model methods to estimate the change in health status over time among surviving patients, while accounting for potentially informative missingness 20. This approach considers the population as a mixture of patients with different missing data patterns and estimates the dependent variable (in this case, QOL scores over time) as a weighted average of estimates across all patterns for each time point. Missing data patterns were defined based on the availability of QOL response data and the reason for missingness (death vs. non-response) at each follow-up time point.
Although we initially specified separate patterns for every unique sequence of available and missing data, several patterns contained too few patients for stable model estimates to be obtained and were therefore combined with others. The ultimate model included 3 missing data patterns: (1) data monotonically missing (i.e., once missing, always missing) due to either patient withdrawal or non-compliance; (2) data missing due to death; and (3) all other surviving patients (including those with no missing data as well as those with non-monotonic missing data). The missingness patterns were then included in a longitudinal random effects model as main effects and as interactions with time. Linear, quadratic and cubic functions of time were considered in the process of model optimization, as were interactions between each time effect and the missingness pattern indicators. The intercept and linear time effects were modeled as both random and fixed effects, whereas quadratic and cubic time effects were modeled as fixed effects to avoid over-parameterization. The models were optimized using a backward elimination process, starting with the interactions between the highest order of time and missing data pattern indicators.
Finally, categorical analyses were performed to further assess changes in health status over time. Among survivors at each time-point, we calculated the proportion of patients who had a small (5 to < 10 points), moderate (10 to < 20 points), or large (≥ 20 points) improvement in the KCCQ-OS score as compared with baseline.
All analyses were stratified according to access site (i.e., IF vs. non-IF), since prior studies in patients undergoing TAVR have demonstrated that access site can be associated with different clinical and health status outcomes 13, 14. No attempt was made to directly compare the health status benefits between the IF and non-IF groups, however, because the “iliofemoral first” design of the CoreValve US Extreme Risk Trial ensured that the groups would be fundamentally non-comparable.
All statistical analyses were performed using SAS software, version 9.3 (SAS institute INC., Cary NC). A 2-sided p-value of < 0.05 was considered statistically significant with no correction for multiple comparisons.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents. This analysis was funded by a research grant from Medtronic Inc.
RESULTS
Study Population
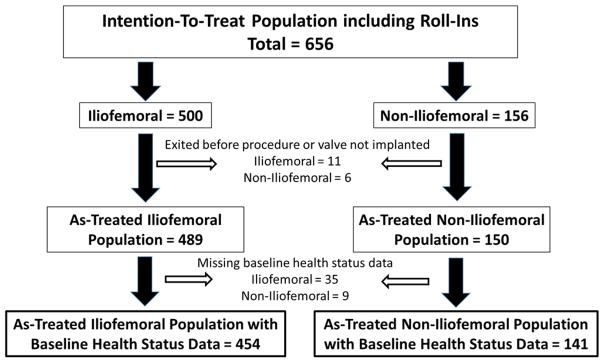
Between February 2011 and August 2012, 656 patients with severe AS who were deemed to be at extreme risk for surgical aortic valve replacement were enrolled in the CoreValve U.S. Extreme Risk Study at 41 U.S. sites (Figure 1). Of these patients, 500 were intended for IF access, and 156 were intended for non-IF access. A total of 17 patients exited the study before the procedure or did not have a transcatheter valve implanted. Of the 639 patients who did undergo valve implantation, 44 did not have baseline health status data and were also excluded. As such, the analytic cohort included a total of 595 patients, of whom 454 underwent TAVR via an IF approach and 141 underwent TAVR via a non-IF approach.
Figure 1. Patient Flow Chart.
Consort diagram showing patient flow in the quality of life substudy for the CoreValve U.S. Extreme Risk Trial
The baseline characteristics of the analytic cohort are summarized in Table 1. In the IF cohort, the mean age was 83.5 years, and 48% were male. Approximately 40% had diabetes, 40% had undergone prior coronary artery bypass surgery, 14% had chronic kidney disease, and 29% had chronic lung disease requiring home oxygen. The mean aortic valve gradient was 47 mmHg, and mean left ventricular ejection fraction was 54.2%. In the non-IF cohort, baseline characteristics were generally similar, although patients tended to be slightly younger (mean age 81.3 years) and were more likely to require home oxygen and to have peripheral artery disease.
Table 1.
Baseline Characteristics of the CoreValve Extreme Risk Cohort
| Iliofemoral (N=454) | Non-Iliofemoral (N=141) | |
|---|---|---|
| Clinical Characteristics | ||
| Age (years) | 83.5 ± 8.6 | 81.3 ± 7.5 |
| Male | 47.8% (217/454) | 46.8% (66/141) |
| STS Risk Score | 10.4 ± 5.6 | 10.7 ± 5.8 |
| NYHA Class III/IV | 91.8% (417/454) | 92.2% (130/141) |
| Diabetes | 40.5% (184/454) | 34.0% (48/141) |
| Previous CABG | 39.4% (179/454) | 41.1% (58/141) |
| Prior Myocardial Infarction | 31.3% (142/454) | 33.3% (47/141) |
| Prior Cerebrovascular Accident | 19.2% (87/454) | 19.3% (27/141) |
| Peripheral Arterial Disease | 35.9% (162/454) | 60.3% (85/141) |
| Prior Atrial Fibrillation/Flutter | 47.6% (215/454) | 48.6% (68/141) |
| Chronic Lung Disease requiring Home Oxygen | 29.3% (133/454) | 42.6% (60/141) |
| Chronic Kidney Disease | 13.6% (61/454) | 14.3% (20/141) |
| Hostile Chest | 21.0% (95/454) | 19.1% (27/141) |
| Wheelchair Bound | 15.6% (71/454) | 9.9% (14/141) |
| Left Ventricular Ejection Fraction (%) | 54.2% ± 14.4 | 54.9% ± 14.2 |
| Mean AV gradient (mmHg) | 47.4 ± 14.6 | 49.7 ± 17.1 |
|
| ||
| Baseline Health Status | ||
| KCCQ Overall Summary | 37.9 ± 22.1 | 42.5 ± 22.3 |
| KCCQ Physical Limitations | 35.3 ± 24.8 | 41.4 ± 24.7 |
| KCCQ Quality of Life | 36.3 ± 24.6 | 38.9 ± 22.5 |
| KCCQ Total Symptoms | 48.2 ± 24.1 | 51.9 ± 24.7 |
| KCCQ Social Limitations | 30.4 ± 28.5 | 38.2 ± 30.1 |
| SF-12 Physical Summary | 28.5 ± 8.3 | 27.9 ± 8.0 |
| SF-12 Mental Summary | 45.8 ± 12.3 | 47.6 ± 12.0 |
| EQ-5D Utilities | 0.65 ± 0.23 | 0.67 ± 0.23 |
Abbreviations: STS – Society of Thoracic Surgeons; NYHA – New York Heart Association; CABG – coronary artery bypass grafting; AV – aortic valve; KCCQ – Kansas City Cardiomyopathy Questionnaire; SF – Short Form; EQ – EuroQoL
Health status was substantially impaired at baseline (Table 1). In the IF cohort, the mean KCCQ-OS score was 37.9 ± 22.1 (roughly comparable to New York Heart Association Class IV), the mean SF-12 physical summary score was 28.5 ± 8.3 (~2 SD below that of the general U.S. population), the mean SF-12 mental summary score was 45.8 ± 12.3; and the mean baseline EQ-5D score was 0.65 ± 0.23. Baseline scores were similarly low in the non-IF cohort.
Health Status over Time
At 3 year follow-up, 317 of the 595 patients in the analytic cohort had died (53.3%). Among surviving patients, any follow-up health status data was available for 73.7% at 6 months, 78.6% at 12 months, 75.6% at 24 months and 66.7% at 36 months after TAVR (Supplementary Table A). Among the individual health status instruments, 36 month response rates ranged from 67.0% for the KCCQ to 61.7% for the SF-12 physical and mental summary scores (Supplementary Tables B–E).
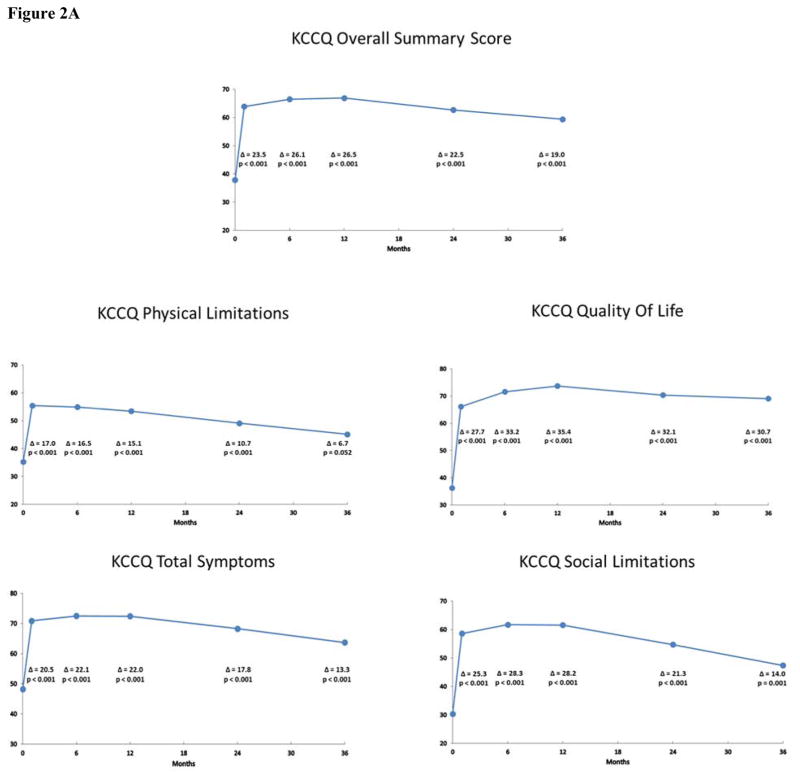
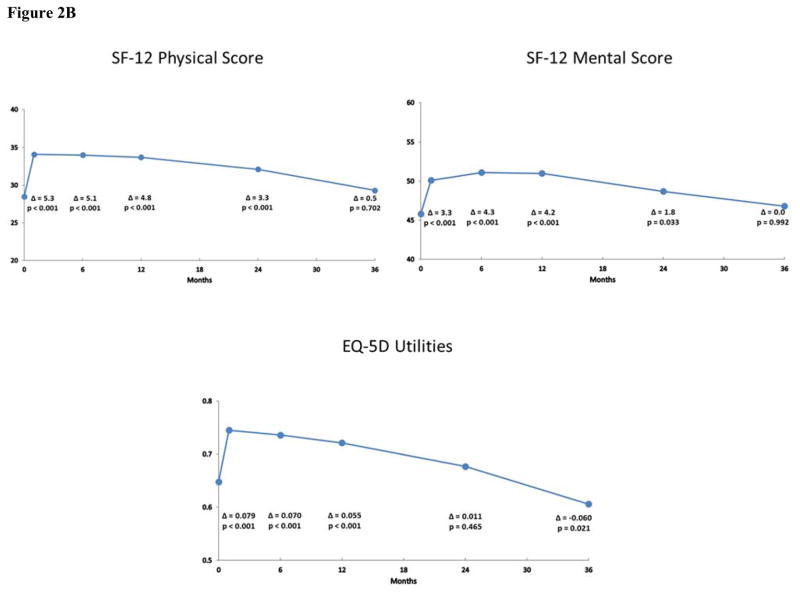
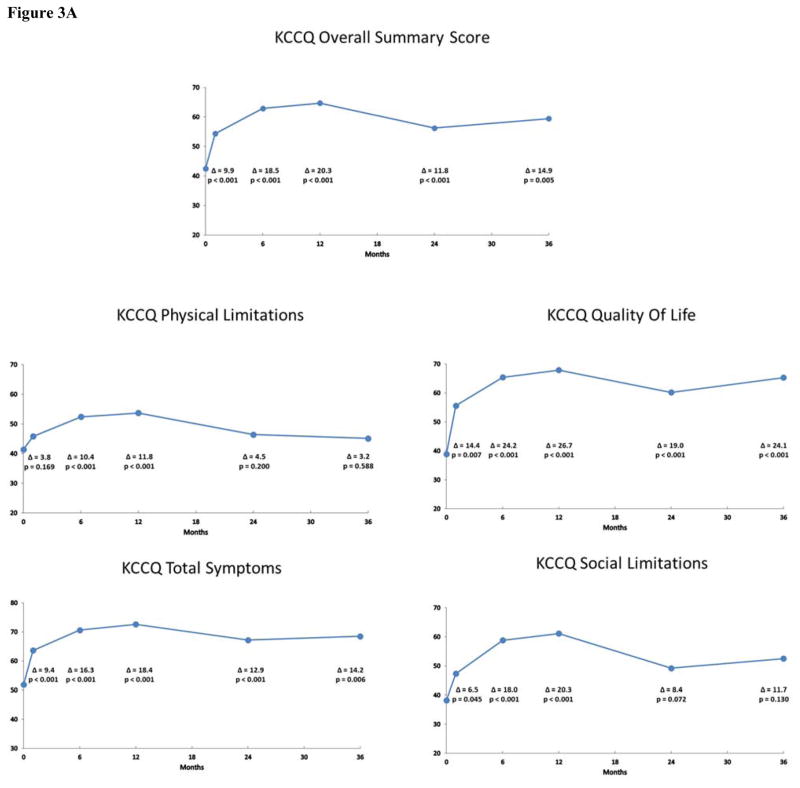
Mean scores and estimated changes from baseline at each follow-up time point according to the pattern mixture models are shown in Figures 2A and 2B for the IF cohort and in Figures 3A and 3B for the non-IF cohort. Overall, patients experienced significant health status improvement after TAVR. For both the KCCQ and SF-12, these differences generally peaked between 6 and 12 months after TAVR and were largely sustained through 3 years of follow-up for both the IF and non-IF cohorts. At 36 months, the change from baseline on the KCCQ-OS score was 19.0 points (95% CI 13.7 to 24.2; p < 0.001) for the IF cohort and 14.9 points (95% CI 4.6 to 25.3; p = 0.005) for the non-IF cohort. In the IF cohort, all subscales of the KCCQ demonstrated significant improvement through 36 months after TAVR. For the non-IF cohort, 3 of 4 subscales of the KCCQ were significantly improved at 36 months.
Figure 2.
Figure 2A: Disease-Specific Health Status after TAVR in the Iliofemoral Cohort. Changes in disease-specific health status according to the KCCQ Overall Summary Scale and selected subscales at 1, 6, 12, 24 and 36 months after TAVR via the iliofemoral approach. Mean values and p-values were derived from pattern mixture models.
Figure 2B. Generic Health Status after TAVR in the Iliofemoral Cohort. Changes in generic health status according to the Short Form-12 and EuroQoL-5D at 1, 6, 12, 24 and 36 months after TAVR via the iliofemoral approach. Mean values and p-values were derived from pattern mixture models.
Figure 3.
Figure 3A: Disease-Specific Health Status after TAVR in the Non-Iliofemoral Cohort. Changes in disease-specific health status according to the KCCQ Overall Summary Scale and selected subscales at 1, 6, 12, 24 and 36 months after TAVR via a non-iliofemoral approach. Mean values and p-values were derived from pattern mixture models.
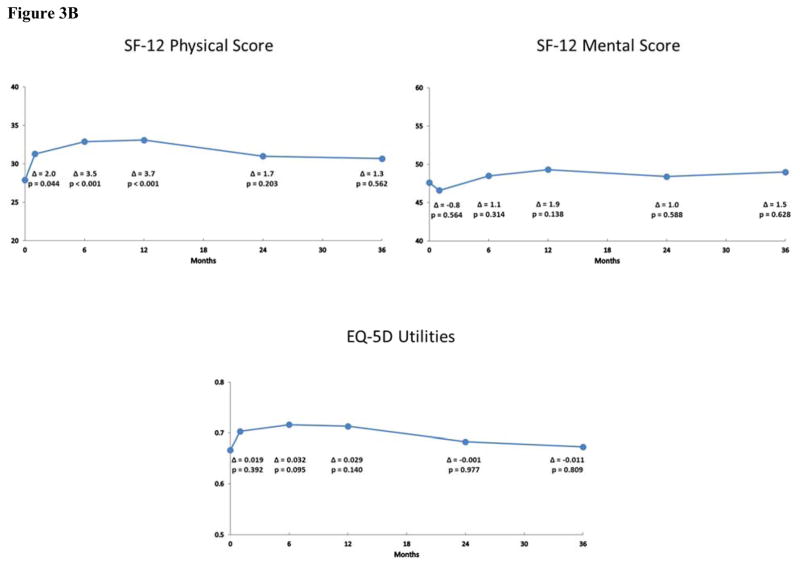
Figure 3B. Generic Health Status after TAVR in the Non-Iliofemoral Cohort. Changes in generic health status according to the Short Form-12 and EuroQoL-5D at 1, 6, 12, 24 and 36 months after TAVR via a non-iliofemoral approach. Mean values and p-values were derived from pattern mixture models.
For the SF-12 physical and mental summary scales, there were significant improvements in the IF cohort through 2 years; these differences were no longer apparent at 3-year follow-up, however (Figure 2B). For the non-IF cohort, there were significant improvements in the physical summary score at 6 and 12 months, but these differences were attenuated and no longer statistically significant at 24 and 36 months (Figure 3B). Moreover, there were no significant differences in the mental summary score among the non-IF cohort at any time point. In unadjusted analyses based on paired t-tests, similar, albeit slightly more robust, results were noted for both disease-specific and general health status scales for the IF and non-IF cohorts (Supplementary Tables F and G).
When paired health status measures at 36 months were compared with those observed at 12 months after TAVR, there was evidence of a decline across both disease-specific and generic measures. In the IF cohort, there were small (2 to 9 points on average), but statistically significant decreases in the KCCQ-OS score (mean decrease 5.9 points, 95% CI 2.6 to 9.1; p < 0.001) and in each of the KCCQ subscales (Table 2). Similar results were seen in the generic health status measures with mean reductions of 4.5 points (95% CI 2.8 to 6.1, p < 0.001) in the SF-12 physical subscale and 2.7 points (95% CI 0.7 to 4.7, p = 0.009) between 12 and 36 months in the SF-12 mental subscale. When these results were expressed in terms of effect size (ratio of the absolute value of change to the standard deviation at baseline), the extent of decline was greater for the SF-12 physical summary scale (0.42) than for the disease specific scales (range 0.15 to 0.35). While similar trends were seen in the non-IF cohort, differences in health status measures between 12 and 36 months were not statistically significant, although the absolute magnitude of change was similar to that seen in the IF cohort (Table 2).
Table 2.
Change in Health Status from 12 Months to 36 Months among Patients with Paired Data
| Health Status Measure | Mean Score at 12 months | Mean Score at 36 months | Change from 12 Months (95% CI) | P-Value | Effect Size * (95% CI) |
|---|---|---|---|---|---|
| Iliofemoral Cohort | |||||
| KCCQ Overall Summary | 73.8 ± 20.2 | 67.9 ± 22.9 | −5.9 ( −9.1, −2.6) | 0.0004 | 0.29 (0.13, 0.45) |
| KCCQ Physical Limitations | 61.3 ± 26.9 | 53.3 ± 28.2 | −8.1 (−11.9, −4.2) | <0.0001 | 0.30 (0.16, 0.44) |
| KCCQ Quality of Life | 79.4 ± 21.9 | 76.1 ± 23.8 | −3.3 ( −6.9, 0.3) | 0.0703 | 0.15 (0.01, 0.32) |
| KCCQ Total Symptoms | 79.5 ± 16.4 | 73.8 ± 20.6 | −5.7 ( −8.8, −2.6) | 0.0004 | 0.35 (0.16, 0.54) |
| KCCQ Social Limitations | 69.5 ± 26.2 | 61.8 ± 30.6 | −7.7 (−13.7, −1.8) | 0.0115 | 0.29 (0.07, 0.52) |
| SF-12 Physical Summary | 36.6 ± 10.7 | 32.1 ± 10.4 | −4.5 ( −6.1, −2.8) | <0.0001 | 0.42 (0.26, 0.57) |
| SF-12 Mental Summary | 53.9 ± 10.1 | 51.3 ± 11.0 | −2.7 ( −4.7, −0.7) | 0.0088 | 0.27 (0.07, 0.47) |
| EQ-5D Utilities | 0.768 ± 0.196 | 0.703 ± 0.218 | −0.065 (−0.106, −0.024) | 0.002 | 0.33 (0.12, 0.54) |
|
| |||||
| Non-Iliofemoral Cohort | |||||
| KCCQ Overall Summary | 73.4 ± 17.3 | 68.8 ± 24.6 | −4.5 (−10.8, 1.8) | 0.1533 | 0.26 (0.10, 0.62) |
| KCCQ Physical Limitations | 61.6 ± 21.6 | 55.1 ± 26.1 | −6.5 (−14.1, 1.0) | 0.0870 | 0.30 (0.05, 0.65) |
| KCCQ Quality of Life | 76.5 ± 21.6 | 73.1 ± 26.0 | −3.3 (−10.1, 3.5) | 0.3274 | 0.15 (0.16, 0.47) |
| KCCQ Total Symptoms | 79.5 ± 14.5 | 74.9 ± 23.1 | −4.5 (−11.1, 2.0) | 0.1708 | 0.31 (0.14, 0.77) |
| KCCQ Social Limitations | 76.6 ± 25.2 | 70.9 ± 32.1 | −5.7 (−14.2, 2.8) | 0.1782 | 0.23 (0.11, 0.56) |
| SF-12 Physical Summary | 36.7 ± 10.4 | 34.5 ± 9.4 | −2.1 ( −5.3, 1.1) | 0.1824 | 0.20 (0.11, 0.51) |
| SF-12 Mental Summary | 52.0 ± 12.1 | 49.4 ± 12.5 | −2.6 ( −5.9, 0.7) | 0.1212 | 0.21 (0.01, 0.49) |
| EQ-5D Utilities | 0.788 ± 0.192 | 0.712 ± 0.240 | −0.076 (−0.137, −0.016) | 0.0145 | 0.40 (0.08, 0.71) |
Effect size: ratio of the absolute value of change to the standard deviation at baseline
Categorical Analyses
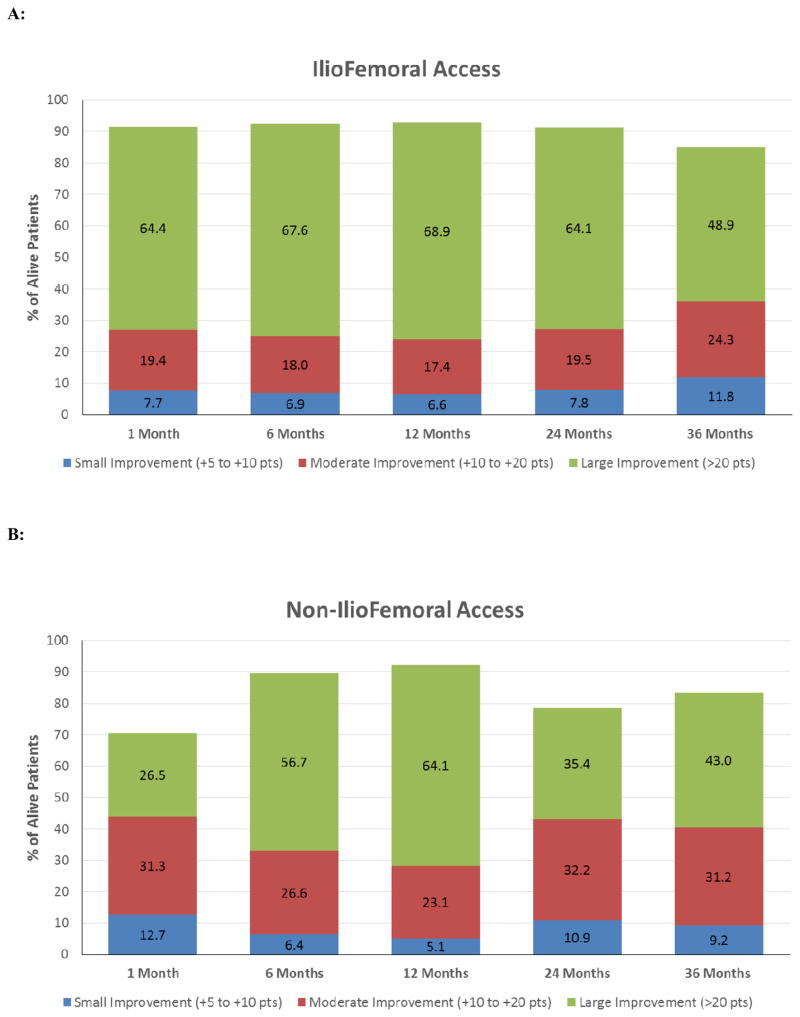
The rates of small, moderate, and large improvements from baseline in the KCCQ-OS over time as determined by pattern mixture models are shown in Figure 4. Among surviving IF patients, 48.9% demonstrated a large clinical improvement 36 months after TAVR, and an additional 36.1% experienced a small or moderate improvement. Similarly, 43.0% of surviving non-IF patients had a large clinical improvement at 36 months, with an additional 41.4% experiencing a small or moderate improvement.
Figure 4. Clinical Improvement in Health Status from Baseline over Time in Surviving Patients.
Proportion of surviving patients with clinically important improvement over time as measured by the KCCQ-Overall Summary Score in the iliofemoral cohort (panel A) and non-iliofemoral cohort (panel B).
DISCUSSION
In this single arm, multi-center study of patients with severe AS at extreme surgical risk, we found that TAVR using the self-expanding CoreValve resulted in both statistically and clinically significant improvements in disease-specific health status among surviving patients through 36 months of follow-up. While there was a clear decline in health status between 12 and 36 month follow-up, the majority of the initial benefit of TAVR was sustained at 36 months such that over 80% of surviving patients continued to experience clinically meaningful improvement in disease-specific health status as compared with their pre-TAVR health status. Long term results were generally similar regardless of whether patients were treated via an IF or a non-IF approach, although results for the non-IF cohort were less certain, owing to the much smaller sample size. These findings of sustained improvement in disease-specific health status further demonstrate the value of TAVR in this population, despite their advanced age and extensive co-morbidities.
This is the first study to rigorously evaluate the long-term effect of TAVR on patient-reported health status among patients at extreme surgical risk. Although previous studies have demonstrated the benefit of TAVR on both disease-specific and generic health status among patients at extreme and high surgical risk, to date these studies have been limited to 1-year follow-up 7, 8, 13, 14. While a few studies have examined the effect of TAVR on functional status in extreme-risk and high-risk patients beyond 1 year, these studies have almost exclusively relied on physician-reported surrogates for health status, such as NYHA classification (which has been shown to be an unreliable measure of functional status due to limited inter-rater as well as intra-rater reliability 21) rather than patient-reported outcomes 4, 9, 22.
To our knowledge, Tamarasso and colleagues have performed the only previous study to assess patient-reported outcomes after TAVR in extreme-risk and high-risk patients. In that study, health status at 2-years after TAVR was evaluated in 100 consecutive patients undergoing TAVR using the 36-item Short-Form health survey (SF-36) and the Minnesota Living with Heart Failure Questionnaire (MLHFQ) 23. Similar to our findings, they observed clinically and statistically significant improvement in health status at 2 years, despite the fact that the MLHFQ has recently been shown to be somewhat unreliable in capturing AS-specific symptoms 24. Our study serves to expand on these findings, by using a disease-specific health status instrument that has been validated in patients with AS undergoing TAVR and by demonstrating that the benefits of TAVR extend to 3-year follow-up in a much larger patient cohort.
The finding that disease-specific health status benefits of TAVR were largely sustained through 36 months has important implications for the clinical utility of TAVR in patients with severe AS and extreme surgical risk. Although all-cause mortality rates were high (> 50%) in this complex, elderly population, nearly half of all surviving patients continued to maintain a large health status benefit (as defined by > 20 point improvement on the KCCQ-OS score) at 3-year follow-up. Although previous studies have shown that health status improves more rapidly with IF access as compared with non-IF access (mainly transapical or transaortic approaches) 13, 14, we found that the 3-year health status benefits of TAVR were similar regardless of access site. While there were no significant differences noted on generic health status scales at 3-year follow-up, there was a trend toward benefit (up to 1.5 points) in both the SF-12 physical and mental summary scales for both the IF and non-IF groups. Since aging has been shown to be associated with a decline in the SF-12 physical summary scale of ~0.5 points/year 25, in the absence of an effective intervention, one might have expected a decline of ~ 1.5 points over 3 years. As such, the absence of sustained improvement in generic health status in our study population most likely reflects general age-related declines in health status rather than deterioration of cardiovascular health, per se. Taken together with other studies demonstrating durable improvement in survival compared with medical therapy 4, these findings suggest that TAVR results in both improved quality of life as well as improved clinical outcomes, even among extreme risk patients with severe, symptomatic AS.
Limitations
This study should be interpreted in light of several limitations. First, the CoreValve U.S. Extreme Risk Study was a single arm trial. As such, there was no control arm to which the effects of TAVR on health status could be compared. While the trial was initially intended to be a randomized comparison against medical therapy, after publication of the results for Cohort B of the PARTNER trial 2, which demonstrated substantial mortality benefit with TAVR as compared with medical therapy in a similar population, randomization was no longer felt to be ethical, and the study design was changed. For this reason, comparisons of health status after TAVR in the CoreValve U.S. Extreme Risk Study could only be made against each individual’s baseline health status.
Second, there was a fair amount of missing health status data (~25–33%) at the 2 and 3-year follow-up, which could have led to biased results. If sicker patients were less likely to respond, then our study may have overestimated the health status benefit for TAVR in this population. We therefore used pattern mixture models for our primary analysis in order to account for non-random missing data.
Finally, the sample size for the non-IF cohort was particularly small at 3 year follow-up (<50 patients), thereby limiting statistical power to detect modest health status benefits in this cohort. This small sample size reflects the fact that only ~20% of patients in the CoreValve Extreme Risk trial required alternative access as well as the relatively high rate of mortality in the extreme risk population.
Conclusions
In this prospective, multicenter study, we found that extreme risk patients with severe AS who were treated with TAVR using the self-expanding CoreValve experienced large improvements in both disease-specific and generic health status that were generally sustained at 24 and 36 months. These benefits were moderate to large in the majority of surviving patients and were observed in patients treated via both IF or non-IF access. Further studies are needed to examine the durability of health status benefits of TAVR beyond 36 months in this population. Moreover, additional studies are needed to compare the long-term health status benefits of TAVR vs. SAVR in patients who are suitable for both procedures given the differences in certain complications (e.g. paravalvular leak, atrial fibrillation, pacemaker implantation) between the two treatments.
Supplementary Material
Acknowledgments
Funding Sources: This study was supported by Medtronic, Inc.
Footnotes
Disclosures: Dr. Baron has received consulting/speaker fees from Edwards LifeSciences and St. Jude Medical Inc. Dr. Arnold has received grant support from the National Heart, Lung and Blood Institute. Dr. Reynolds has received grant support from Medtronic and Edwards LifeSciences as well as consulting/speaker fees from Medtronic. Dr. Reardon has received educational fees from Medtronic. Dr. Hermiller has received consulting/speaker fees from Abbott Vascular, Boston Scientific, Medtronic, St. Jude Medical Inc, Edwards LifeSciences and Astra-Zeneca. Dr. Yakubov has received advisory board fees from Medtronic, Abbott Vascular and Boston Scientific.. Dr. Adams has received grant support from Medtronic as well as intellectual property rights from Medtronic and Edwards LifeSciences. Dr. Popma has received grant support from Abbott Vascular, Boston Scientific, Medtronic and Cook Medical as well as consulting/speaker fees from Abbott Vascular, Boston Scientific and Direct Flow Medical. Dr. Cohen has received research grant support from Medtronic, Edwards Lifesciences, and Boston Scientific as well as consulting fees from Medtronic, Edwards Lifesciences, and St. Jude Medical, Inc.
References
- 1.Popma JJ, Adams DH, Reardon MJ, Yakubov SJ, Kleiman NS, Heimansohn D, Hermiller J, Jr, Hughes GC, Harrison JK, Coselli J, Diez J, Kafi A, Schreiber T, Gleason TG, Conte J, Buchbinder M, Deeb GM, Carabello B, Serruys PW, Chenoweth S, Oh JK CoreValve United States Clinical I. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972–81. doi: 10.1016/j.jacc.2014.02.556. [DOI] [PubMed] [Google Scholar]
- 2.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S Investigators PT. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 3.Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB Investigators PT. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 4.Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, Webb JG, Mack MJ, Douglas PS, Thourani VH, Babaliaros VC, Herrmann HC, Szeto WY, Pichard AD, Williams MR, Fontana GP, Miller DC, Anderson WN, Akin JJ, Davidson MJ, Smith CR investigators Pt. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485–91. doi: 10.1016/S0140-6736(15)60290-2. [DOI] [PubMed] [Google Scholar]
- 5.Tsevat J, Dawson NV, Wu AW, Lynn J, Soukup JR, Cook EF, Vidaillet H, Phillips RS. Health values of hospitalized patients 80 years or older. HELP Investigators. Hospitalized Elderly Longitudinal Project. JAMA. 1998;279:371–5. doi: 10.1001/jama.279.5.371. [DOI] [PubMed] [Google Scholar]
- 6.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–24. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds MR, Magnuson EA, Lei Y, Leon MB, Smith CR, Svensson LG, Webb JG, Babaliaros VC, Bowers BS, Fearon WF, Herrmann HC, Kapadia S, Kodali SK, Makkar RR, Pichard AD, Cohen DJ Placement of Aortic Transcatheter Valves I. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124:1964–72. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- 8.Osnabrugge RL, Arnold SV, Reynolds MR, Magnuson EA, Wang K, Gaudiani VA, Stoler RC, Burdon TA, Kleiman N, Reardon MJ, Adams DH, Popma JJ, Cohen DJ, CoreValve USTI. Health status after transcatheter aortic valve replacement in patients at extreme surgical risk: results from the CoreValve U.S. trial. JACC Cardiovasc Interv. 2015;8:315–23. doi: 10.1016/j.jcin.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, Kodali SK, Makkar RR, Fontana GP, Kapadia S, Bavaria J, Hahn RT, Thourani VH, Babaliaros V, Pichard A, Herrmann HC, Brown DL, Williams M, Akin J, Davidson MJ, Svensson LG investigators Pt. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–84. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 10.Cohen DJ, Becker ER, Culler SD, Ellis S, Green LM, Schnitzler RN, Simon AW, Weintraub WS. Impact of patient characteristics, complications, and facility volume on the costs and time of cardiac catheterization and coronary angioplasty in 70 catheterization laboratories. Am J Cardiol. 2000;86:595–601. doi: 10.1016/s0002-9149(00)01035-3. [DOI] [PubMed] [Google Scholar]
- 11.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS Cardiovascular Outcomes Research C. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–15. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, Smith CR, Reynolds MR, Webb JG, Svensson LG, Cohen DJ. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6:61–7. doi: 10.1161/CIRCHEARTFAILURE.112.970053. [DOI] [PubMed] [Google Scholar]
- 13.Arnold SV, Reynolds MR, Wang K, Magnuson EA, Baron SJ, Chinnakondepalli KM, Reardon MJ, Tadros PN, Zorn GL, Maini B, Mumtaz MA, Brown JM, Kipperman RM, Adams DH, Popma JJ, Cohen DJ CoreValve USPTI. Health Status After Transcatheter or Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Increased Surgical Risk: Results From the CoreValve US Pivotal Trial. JACC Cardiovasc Interv. 2015;8:1207–17. doi: 10.1016/j.jcin.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds MR, Magnuson EA, Wang K, Thourani VH, Williams M, Zajarias A, Rihal CS, Brown DL, Smith CR, Leon MB, Cohen DJ Investigators PT. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A) J Am Coll Cardiol. 2012;60:548–58. doi: 10.1016/j.jacc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 15.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 16.EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Jr, Kosinski M, Bjorner JB, Turner-Bowkes DM, Gandek B, Maruish ME. User’s Manual for the SF-36v2 Health Survery. Lincoln, RI: QualityMetric Incorporated; 2007. [Google Scholar]
- 18.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43:203–20. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes. 2010;8:13. doi: 10.1186/1477-7525-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Post WJ, Buijs C, Stolk RP, de Vries EG, le Cessie S. The analysis of longitudinal quality of life measures with informative drop-out: a pattern mixture approach. Qual Life Res. 2010;19:137–48. doi: 10.1007/s11136-009-9564-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett JA, Riegel B, Bittner V, Nichols J. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung. 2002;31:262–70. doi: 10.1067/mhl.2002.124554. [DOI] [PubMed] [Google Scholar]
- 22.Gurvitch R, Wood DA, Tay EL, Leipsic J, Ye J, Lichtenstein SV, Thompson CR, Carere RG, Wijesinghe N, Nietlispach F, Boone RH, Lauck S, Cheung A, Webb JG. Transcatheter aortic valve implantation: durability of clinical and hemodynamic outcomes beyond 3 years in a large patient cohort. Circulation. 2010;122:1319–27. doi: 10.1161/CIRCULATIONAHA.110.948877. [DOI] [PubMed] [Google Scholar]
- 23.Taramasso M, Latib A, Cioni M, Denti P, Buzzatti N, Godino C, Chieffo A, Alfieri O, Colombo A, Maisano F. Quality of life improvement is maintained up to two years after transcatheter aortic valve implantation in high-risk surgical candidates. EuroIntervention. 2012;8:429–36. doi: 10.4244/EIJV8I4A68. [DOI] [PubMed] [Google Scholar]
- 24.Sandau KE, Boisjolie C, Hodges JS. Use of the Minnesota Living With Heart Failure Questionnaire among elderly patients with aortic stenosis: results from a pilot study. J Cardiovasc Nurs. 2014;29:185–97. doi: 10.1097/JCN.0b013e318279b76f. [DOI] [PubMed] [Google Scholar]
- 25.Ware JKM, Bjorner JB, Turner-Bowkes DM, Gandek B, Maruish ME. How to Score Version 2 of the SF-12 Health Survey (With a Supplement Documenting Version 1) 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.