To the Editor:
Our understanding of transcriptomic responses in acute respiratory distress syndrome (ARDS) is derived, almost exclusively, from studies that have used samples procured from the peripheral circulation as opposed to the alveolar space (1–4). However, it is not known to what degree genomic signatures captured from circulating leukocytes accurately reflect the gene expression patterns of leukocytes in the lung. We hypothesized that the transcriptional signals from peripheral blood monocytes (PBMs) and alveolar macrophages (AMs) would be distinct in ARDS, and that alterations in the transcriptional state of immune cells in the lung would provide new insights into the pathogenesis of ARDS. Some of the results of these studies have been previously reported in the form of an abstract (5).
Methods
Subjects enrolled in the Phase II Randomized Placebo-controlled Trial of Omega-3 Fatty Acids for the Treatment of Acute Lung Injury trial (6) conducted between 2006 and 2008 were included in this study. We performed genome-wide expression analysis of total RNA isolated from paired AM and PBM samples purified from BAL fluid and peripheral blood specimens, respectively, collected from patients within 48 hours of the diagnosis of ARDS. Negative selection for AMs and PBMs was achieved by incubating cells with antibody-labeled microbeads containing the following markers: CD3, CD15, CD19, CD235a, CD294, and CD326. We did not use antibodies for CD294 (eosinophils) and CD326 (epithelial cells) for the blood samples because mononuclear cells were isolated from whole blood before antibody incubation via polyester gel centrifugation. RNA extracted from isolated cells was assessed for purity, and then hybridized to an Illumina HumanRef-8 BeadChip that was inclusive of 18,415 unique genes.
We performed variance stabilization and quantile normalization of the raw microarray data, using the Bioconductor package lumi (7). Detailed microarray information has been deposited at Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE89953). Principal component analysis was performed on the basis of whole-genome gene expression variability between paired AM and PBM samples (8). Differential gene expression between the two cell populations was determined by a Bayesian implementation of the t test (http://cybert.microarray.ics.uci.edu) on log2-transformed probe intensities, using a sliding window size of 101 and a Bayesian confidence estimate value of 10.
To identify transcriptional programs activated in AMs and PBMs in early ARDS and to determine their relationship with patient outcomes, we applied Gene Set Enrichment Analysis (GSEA), using 50 hallmark and 1,329 canonical pathway gene sets curated from the Molecular Signature Database (MSigDB) (9). We used a false discovery rate threshold less than 0.05 to identify significantly enriched pathways. To capture the biological themes that each gene set represented, we compiled their leading-edge subset of genes, and ranked them on the basis of their fold change. For our primary analysis, we compared two groups of subjects dichotomized by a clear bimodal distribution of ventilator-free days (VFDs): the high-VFD subjects had at least 18 VFDs (n = 14) whereas the low-VFD subjects had no more than 7 VFDs (n = 12). GSEA was applied separately in each cell type to patient groups dichotomized by outcome.
Results
Demographic and clinical characteristics of all 26 subjects who had AMs and PBMs collected at study entry are shown in Table 1. Manual inspection of Cytospin slides after negative selection revealed an average purity of 97% for AMs and PBMs.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | VFD High (n = 14) | VFD Low (n = 12) |
|---|---|---|
| Age, yr, mean ± SD | 41 ± 18 | 43 ± 16 |
| Female, n (%) | 4 (29) | 7 (58) |
| White, n (%) | 10 (71) | 12 (100) |
| ARDS risk factor, n (%) | ||
| Sepsis | 8 (57) | 6 (50) |
| Trauma | 6 (43) | 6 (50) |
| Pneumonia | 4 (29) | 3 (25) |
| Other | 2 (14) | 2 (17) |
| Comorbidities, n (%) | ||
| Diabetes | 2 (14) | 2 (17) |
| Cirrhosis | 0 (0) | 1 (8) |
| P/F ratio on admission, mean ± SD | 217.3 ± 54.2 | 170.6 ± 61.9 |
| VFD, median (mean ± SD) | 23.5 (22.5 ± 2.4) | 0 (1.2 ± 2.6) |
| APACHE II, mean ± SD | 19.6 ± 6.2 | 20.9 ± 4.1 |
| 28-d mortality, n (%) | 0 (0) | 3 (25) |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; P/F ratio = ratio of PaO2 to FiO2; VFD = ventilator-free days; VFD high = subjects with ≥18 VFD; VFD low = subjects with ≤7 VFD.
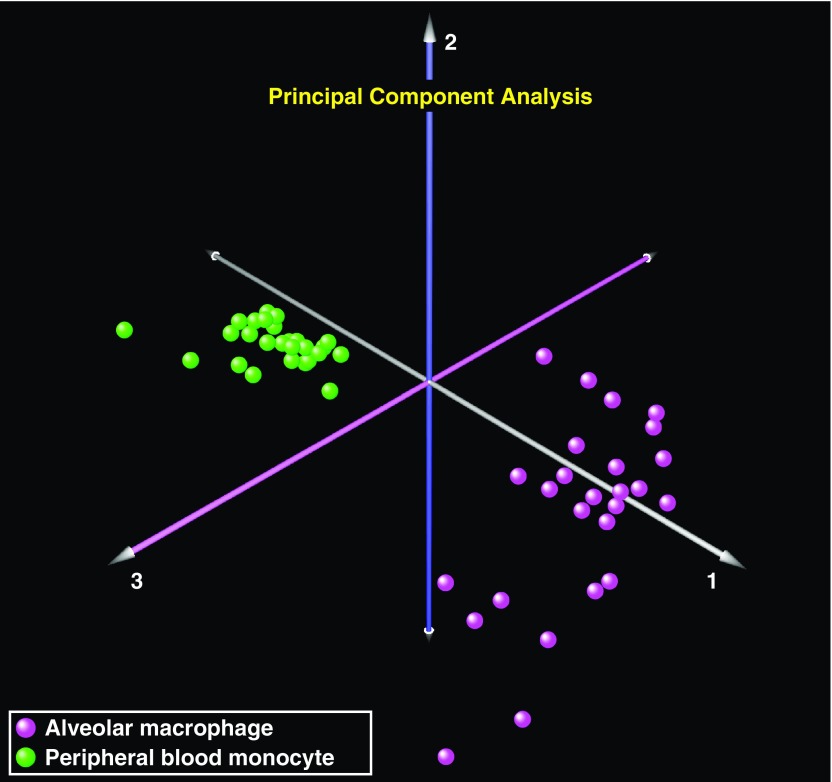
We first performed principal component analysis using the entire microarray data set across the 26 patients with ARDS with both AM and PBM data and observed that the principal driver of gene expression variability was the large-scale differences between cell-specific transcriptomes (Figure 1). We then compared differential gene expression between AMs and PBMs, and found marked cell-specific differences, with 6,099 differentially expressed genes identified between the two cell types (false discovery rate < 0.01).
Figure 1.
Transcriptional landscape of alveolar macrophages (AMs) and peripheral blood monocytes (PBMs) in acute respiratory distress syndrome. Principal component analysis of the AM and PBM transcriptomes in 26 patients at the onset of acute respiratory distress syndrome demonstrated clear segregation between these two cell types, implying that the primary driver of gene expression variability is due to cell-specific differences (AMs, purple spheres; PBMs, green spheres).
After determining that the cell-specific transcriptional profiles between AMs and PBMs were different in early ARDS, we sought to characterize which biological programs were activated by ARDS in each cell type and associate these programs with patient outcomes. In high-VFD subjects (good outcome), there was clear enrichment of immune and proinflammatory gene sets in AMs, whereas there was a much less diverse immune signature in PBMs isolated from those same patients (Table 2). Notably, PBMs collected from low-VFD subjects (poor outcome) were enriched with several of the same immune and inflammatory pathways that were associated with good outcomes in AMs, such as tumor necrosis factor-α and IL-2/Stat5 (signal transducer and activator of transcription 5) signaling. IL6, IL23A, CXCL9 (chemokine [C-X-C motif] ligand 9), and IL1B were among the most up-regulated genes found in the leading-edge enriched hallmark gene sets derived from AMs collected from high-VFD subjects, reinforcing the nature of the immunoinflammatory transcriptional signal associated with AMs from patients with good clinical outcomes (Table 2). Finally, we observed an even more robust pattern of distinctive pathway enrichment between AMs and PBMs when applying GSEA using the more extensive canonical pathway gene sets from MSigDB.
Table 2.
Enriched Gene Sets Based on Cell Type and Clinical Outcome
| VFD ≥ 18 (Good Outcome) | VFD ≤ 7 (Poor Outcome) | ||||||
|---|---|---|---|---|---|---|---|
| Gene Set | FDR | Gene | FC | Gene Set | FDR | Gene | FC |
| Alveolar macrophages | |||||||
| TNFα signaling via NF-κB | <0.01 | CCR7 | 3.1 | MYC targets V1 | <0.01 | CAV1 | 2.5 |
| IFN-γ response | <0.01 | FOSB | 2.6 | Peroxisome | <0.01 | FHL2 | 1.8 |
| Inflammatory response | <0.01 | DUSP2 | 2.5 | UV response down | <0.01 | TFPI | 1.6 |
| IFN-α response | <0.01 | IL6 | 2.3 | Protein secretion | 0.02 | SLC25A4 | 1.6 |
| Allograft rejection | <0.01 | STAT4 | 2.3 | Pancreas β cells | 0.03 | LAMC1 | 1.6 |
| Hypoxia | <0.01 | NR4A2 | 2.1 | ERBB2 | 1.6 | ||
| Complement | <0.01 | IL23A | 2.1 | EFEMP1 | 1.5 | ||
| IL2 STAT5 signaling | 0.01 | SLAMF1 | 2.1 | SCGB1A1 | 1.4 | ||
| IL6 JAK STAT3 signaling | 0.02 | CXCL2 | 2.0 | FBLN5 | 1.4 | ||
| UV response up | 0.03 | DLL1 | 2.0 | TSPAN8 | 1.4 | ||
| Coagulation | 0.03 | DNAJB1 | 2.0 | SLC7A1 | 1.4 | ||
| Apoptosis | 0.04 | MMP9 | 1.9 | FOXA2 | 1.4 | ||
| Notch signaling | 0.04 | TNFAIP3 | 1.9 | GJA1 | 1.3 | ||
| MMP12 | 1.9 | IDH1 | 1.3 | ||||
| C8B | 1.8 | ERH | 1.3 | ||||
| GCH1 | 1.8 | ATP2B4 | 1.2 | ||||
| HAS1 | 1.8 | ICA1 | 1.2 | ||||
| IRAK2 | 1.7 | NFIB | 1.2 | ||||
| CD274 | 1.7 | GNPAT | 1.2 | ||||
| ICOS | 1.7 | CAV2 | 1.2 | ||||
| IL1B | 1.7 | YWHAQ | 1.2 | ||||
| PROK2 | 1.6 | IARS | 1.2 | ||||
| ZC3H12A | 1.6 | MAFB | 1.2 | ||||
| HLA-DQA1 | 1.6 | RPS2 | 1.2 | ||||
| CTSZ | 1.6 | SMARCC1 | 1.2 | ||||
| IL18BP | 1.5 | ACP1 | 1.2 | ||||
| IL1A | 1.5 | PTPRM | 1.2 | ||||
| CXCL9 | 1.5 | APEX1 | 1.2 | ||||
| CXCR7 | 1.5 | CNBP | 1.1 | ||||
| EIF5A | 1.4 | C1QBP | 1.1 | ||||
| Peripheral blood monocytes | |||||||
| IFN-α response | <0.01 | HLA-DQA1 | 1.9 | MYC targets V1 | <0.01 | EGR2 | 2.1 |
| IFN-γ response | <0.01 | XAF1 | 1.3 | Oxidative phosphorylation | <0.01 | FOSB | 1.8 |
| Unfolded protein response | 0.01 | WFS1 | 1.3 | MTORC1 signaling | 0.01 | IL1R2 | 1.6 |
| ISG15 | 1.3 | Adipogenesis | 0.01 | EGR1 | 1.6 | ||
| HLA-DMA | 1.3 | Fatty acid metabolism | 0.01 | CA2 | 1.5 | ||
| DNAJA4 | 1.2 | IL2 STAT5 signaling | 0.03 | CXCL10 | 1.4 | ||
| DHX58 | 1.2 | Peroxisome | 0.03 | AKR1C3 | 1.4 | ||
| SDAD1 | 1.2 | Xenobiotic metabolism | 0.04 | PTGS2 | 1.4 | ||
| PIM1 | 1.2 | TNFα signaling via NF-κB | 0.05 | SH3BGRL2 | 1.3 | ||
| TRIM25 | 1.2 | E2F targets | 0.05 | TUBA4A | 1.3 | ||
| SLC25A28 | 1.2 | TPST1 | 1.3 | ||||
| SAMD9L | 1.2 | ATP1B3 | 1.3 | ||||
| CD86 | 1.2 | FH | 1.3 | ||||
| CD74 | 1.2 | ACTR3 | 1.3 | ||||
| DCP2 | 1.2 | PTGES3 | 1.3 | ||||
| SPCS3 | 1.2 | ME1 | 1.3 | ||||
| SP110 | 1.2 | ID2 | 1.3 | ||||
| PARP12 | 1.2 | CNBP | 1.3 | ||||
| PELI1 | 1.2 | PROS1 | 1.3 | ||||
| SELL | 1.1 | HMGB2 | 1.2 | ||||
| DNAJB9 | 1.1 | GPR65 | 1.2 | ||||
| TNFAIP3 | 1.1 | GSTZ1 | 1.2 | ||||
| ADAR | 1.1 | HSD17B11 | 1.2 | ||||
| CIITA | 1.1 | PPP1R15A | 1.2 | ||||
| MOV10 | 1.1 | SDPR | 1.2 | ||||
| EDC4 | 1.1 | ACOX2 | 1.2 | ||||
| PDE4B | 1.1 | CES1 | 1.2 | ||||
| ARFGAP1 | 1.1 | PSMD14 | 1.2 | ||||
| ATF3 | 1.1 | ALDH2 | 1.2 | ||||
| RBCK1 | 1.1 | ARG2 | 1.2 | ||||
Definition of abbreviations: FC = fold change; FDR = false discovery rate; UV = ultraviolet; VFD = ventilator-free days.
Gene sets are enriched hallmark gene sets curated from the Molecular Signature Database (http://software.broadinstitute.org/gsea/msigdb). Displayed are the significantly enriched gene sets (FDR < 0.05) for each cell type and outcome and a list of the 30 most upregulated genes (fold change) compiled from all the leading-edge genes (9) driving each of the enriched gene sets. These gene lists represent the predominant biological themes that drove each of the gene set associations listed; however, no individual gene met the threshold for statistical significance after multiple hypothesis testing.
Discussion
This report provides the first assessment of paired AM and PBM transcriptional activation in human ARDS. We identified highly divergent patterns of gene expression between AMs and PBMs in subjects with ARDS. Furthermore, we found that the initial transcriptome in AMs procured from patients who ultimately had good outcomes (VFD ≥ 18) was enriched in immunoinflammatory gene sets, whereas enrichment of many of the same pathways in PBMs was associated with poor outcomes (VFD ≤ 7). The broad implication of our finding is that strategies for identifying molecular therapeutic targets in ARDS must take into consideration that organ- and cell-specific responses to this syndrome vary profoundly across different compartments.
Another important implication of this work is that extrapolating transcriptional signals from circulating immune cells to approximate responses in airspace immune cells must be done with caution. Almost all previous studies examining the transcriptome in ARDS have used samples collected from the peripheral circulation as opposed to samples directly procured from the lung (1–4). Our results shed some light on the observed discrepancies between reports based on whole blood transcriptional measurements in ARDS versus studies analyzing products collected directly from the alveolar space. For instance, despite the fact that IL-8 protein levels in BAL fluid have been found to be elevated in early ARDS in multiple studies (10, 11), one of the first genome-wide transcriptional analyses using whole blood RNA of patients with ARDS found decreased expression of IL8 in the acute phase of ARDS compared with the recovery phase (1). Future studies in ARDS that use “omics” approaches should take into consideration that peripheral blood leukocyte gene expression does not accurately reflect the transcriptional patterns of immune cells in the alveolar space.
Footnotes
Supported by NIH grants T32 HL007287 and P50 HL073996.
Author Contributions: S.A.G. and M.M.W. contributed to the conception and design of the work. E.D.M., F.R., A.M.M., C.M., S.A.G., and M.M.W. contributed to the acquisition, analysis, and interpretation of the data for the work. E.D.M., S.A.G., and M.M.W. drafted and revised the manuscript for important intellectual content. E.D.M., F.R., A.M.M., C.M., R.D.S., S.A.G., and M.M.W. significantly contributed to and approved the final version of the manuscript for publication. E.D.M., F.R., A.M.M., C.M., R.D.S., S.A.G., and M.M.W. agree to be accountable for all aspects of the work.
Originally Published in Press as DOI: 10.1164/rccm.201703-0614LE on July 14, 2017
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Wang Z, Beach D, Su L, Zhai R, Christiani DC. A genome-wide expression analysis in blood identifies pre-elafin as a biomarker in ARDS. Am J Respir Cell Mol Biol. 2008;38:724–732. doi: 10.1165/rcmb.2007-0354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kangelaris KN, Prakash A, Liu KD, Aouizerat B, Woodruff PG, Erle DJ, et al. Increased expression of neutrophil-related genes in patients with early sepsis-induced ARDS. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1102–L1113. doi: 10.1152/ajplung.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howrylak JA, Dolinay T, Lucht L, Wang Z, Christiani DC, Sethi JM, et al. Discovery of the gene signature for acute lung injury in patients with sepsis. Physiol Genomics. 2009;37:133–139. doi: 10.1152/physiolgenomics.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gharib SA, Watkins TR, Radella F, Nathens AB, Stapleton RD, Wurfel MM. Prognostic transcriptional signatures in acute respiratory distress syndrome [abstract] Am J Respir Crit Care Med. 2014;189:A5013. [Google Scholar]
- 6.Stapleton RD, Martin TR, Weiss NS, Crowley JJ, Gundel SJ, Nathens AB, et al. A phase II randomized placebo-controlled trial of omega-3 fatty acids for the treatment of acute lung injury. Crit Care Med. 2011;39:1655–1662. doi: 10.1097/CCM.0b013e318218669d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du P, Kibbe WA, Lin SM. lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 8.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 9.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnelly SC, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, et al. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet. 1993;341:643–647. doi: 10.1016/0140-6736(93)90416-e. [DOI] [PubMed] [Google Scholar]
- 11.Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, et al. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]