Abstract
Macrophages are key cellular components of the innate immunity, acting as the main player in the first-line defence against the pathogens and modulating homeostatic and inflammatory responses. Plasticity is a major feature of macrophages resulting in extreme heterogeneity both in normal and in pathological conditions. Macrophages are not homogenous, and they are generally categorized into two broad but distinct subsets as either classically activated (M1) or alternatively activated (M2). However, macrophages represent a continuum of highly plastic effector cells, resembling a spectrum of diverse phenotype states. Induction of specific macrophage functions is closely related to the surrounding environment that acts as a relevant orchestrator of macrophage functions. This phenomenon, termed polarization, results from cell/cell, cell/molecule interaction, governing macrophage functionality within the hosting tissues. Here, we summarized relevant cellular and molecular mechanisms driving macrophage polarization in “distant” pathological conditions, such as cancer, type 2 diabetes, atherosclerosis, and periodontitis that share macrophage-driven inflammation as a key feature, playing their dual role as killers (M1-like) and/or builders (M2-like). We also dissect the physio/pathological consequences related to macrophage polarization within selected chronic inflammatory diseases, placing polarized macrophages as a relevant hallmark, putative biomarkers, and possible target for prevention/therapy.
1. Introduction
Macrophages belong to the mononuclear phagocyte system (MPS), a family of professional phagocytes that includes monocyte and dendritic cells (DCs). Over the past few decades, classification of the cells within the MPS system has generated considerable controversy given the different, often confusing, nomenclature to identify macrophages in different physio/pathological conditions as a consequence of their plasticity, resulting in very different phenotype/functions.
The first open debate arises already in the definition of macrophage cell of origin. The classic scenario of the MPS stated that monocytes recruited from the periphery, under the influence of specific tissue-local growth factors, developed into macrophages. According to this scenario, macrophages derive from hematopoietic progenitors of bone marrow that differentiate under the influence of specific growth factors within the hosting tissues [1]. These cells primarily enter the blood as monocytes and further infiltrate tissues as macrophages, where they adapt to the local microenvironment to play out specific functions, such Kupffer cells in the liver, microglial cells in the brain [2], and mesangial cells in the kidney [3].
This view has been completely reconsidered over the last decade, and the ontogeny of macrophages has been totally rewritten, based on genetic approaches of cell fate mapping. New evidence demonstrated that macrophages can originate from embryonic precursor cells that colonized developing tissues before birth (foetal tissue macrophages) and that tissue-resident macrophages have self-maintaining abilities in the adulthood. Murine models allow the definition of three main sources for tissue-resident macrophages: (i) the yolk sac in the embryo as a source for progenitor cells by primitive hematopoiesis; (ii) the foetal liver, where the hematopoiesis takes places, shifting form the yolk sac, and (iii) the bone marrow that becomes the elicit hematopoietic centre in late embryos and adult organisms [4–6]. Another intriguing scenario, concerning the origin and persistence of macrophages, has been proposed by Gomez et al. [7]. The model proposed that resident macrophages, developing in the embryo independently of the hematopoietic stem cell (HSC) compartment [2, 8–11], still persist in adults and can coexist with the so termed “passenger” leucocytes that include monocytes and DCs, which originated from bone marrow HSCs and myeloid progenitors [1, 12, 13].
The abundance of macrophages within tissues is finely controlled through the axis colony-stimulating factor-1 or macrophage-colony-stimulating factor (CSF-1 or M-CSF), IL-34, and colony-stimulating factor-1 receptor (CSF-1R) [14].
It has been reported that recruited macrophages differ from the resident tissues in terms of transcriptional profiling. Even if the term “macrophage activation” has been commonly used to describe macrophage activity in response to diverse stimuli, several studies pointed out that the results of cell activation deeply depend on the macrophage location and on the stimulus that triggers their activation.
In vitro and in vivo studies have shown that the phenotypic heterogeneity of macrophages correlates with peculiar functions specific to their local microenvironment [15] and this plasticity enables the appropriate response to pathogen or injury challenge.
Macrophage activation can be obtained in response to a plethora of diverse stimuli, including microbial products, damaged cells, activated lymphocytes, and inflammatory cells, and can result in the acquisition of distinct functional subsets undergoing different phenotypic polarizations.
Macrophage plasticity and heterogeneity give rise to a still opened debate, concerning the nomenclature to identify cell subsets/subtypes undergoing in such different phenotypic, functional (cytokine release), metabolic, regulatory (versus other arms of innate and adaptive immunity) rearrangements.
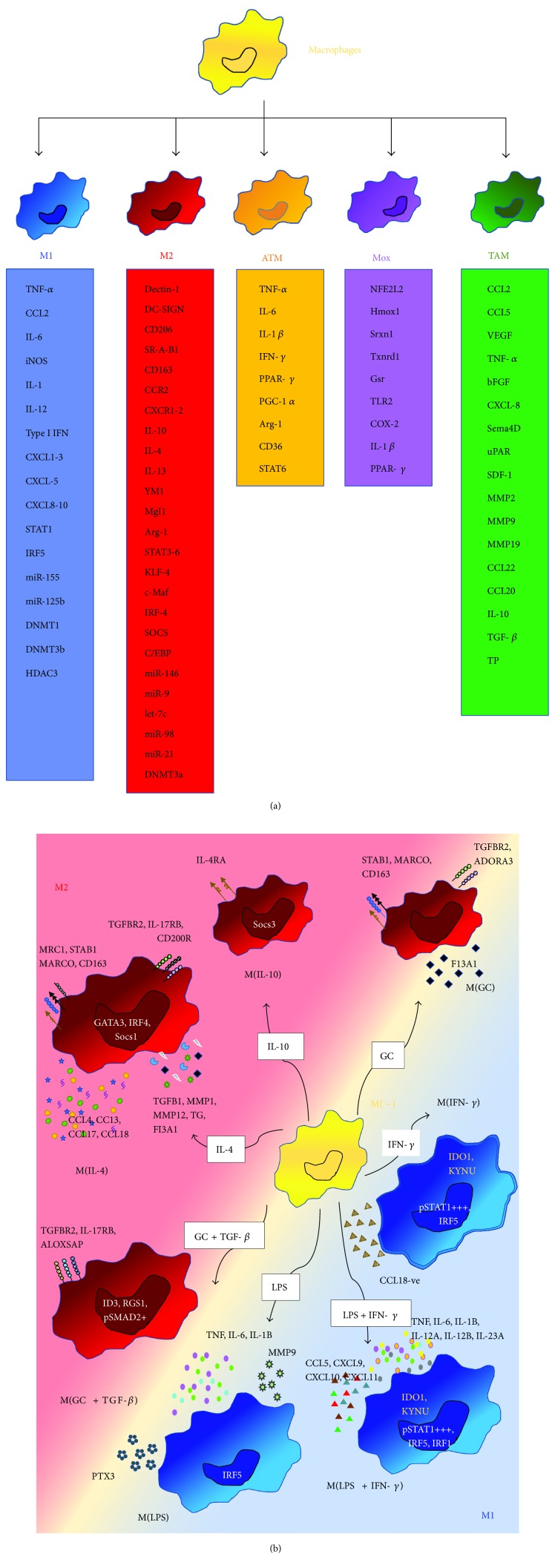
On the basis of the type-1/type-2 helper- T(h-) cell polarization concept [16, 17], phenotypically polarized macrophages have been defined according to two primary activation states, termed classically activated M1 and alternatively activated M2 (Figure 1(a)). M1 and M2 nomenclature has been long and lastly employed to define the “supposed” main subsets of macrophages, which originates in 2000 by Mills et al. [18]. Basically, M1 and M2 responses exemplify the opposing activities of killing (proinflammatory, “killer M1”) and repairing (anti-inflammatory, “builder M2”) [19].
Figure 1.
Past and new concept in macrophage polarization. (a) Schematic overview of the different stimuli that can induce the diverse macrophage polarization state. M1: classically activated phenotype; M2: alternatively activated macrophages; ATM: adipose tissue-derived macrophages; Mox: atherosclerosis-associated macrophages; TAMs: tumour-associated macrophages. (b) The polarization landscape of macrophages. According to the different stimulation conditions, macrophages can acquire peculiar M1 or M2 phenotype, governed by the different surface antigen expressions, including scavenger receptors, chemokine, matrix-associated protein and cytokine release, and different patterns of transcription factors and metabolic pathway activated. The driver stimuli include IL-4, IL-10, glucocorticoids (GC) with TGF-β, glucocorticoids alone, LPS, LPS and IFN-γ, and IFN-γ alone.
However, macrophage polarization in many physiologic and pathologic conditions represents a continuum, involving high plasticity and heterogeneity of these effector cells, and resemble mainly to a spectrum of distinct polarization states that do not fit to the oversimplified M1/M2 classification. Hence, in line with a consensus recommendation, we decide to use “M1” to indicate only IFN-γ and LPS-driven macrophage phenotypes and “M2” to refer to macrophage phenotypes triggered only by IL 4 or IL 13. Furthermore, we use “M1-like” to illustrate diverse signal-induced polarization states that leads to cell cytotoxic function (killer) and antitumour activities and “M2-like” in relation to distinct phenotypes that share the functional capacity of repair, inducing new vessels and remodelling (builder) in parallel with tumour promotion and immunosuppressive ability toward T-cell responses [20] (Figure 1(b)).
In a normal tissue, the ratio of M1-like/M2-like macrophages is highly regulated and increases during the inflammation process [21]. Gene expression profile analysis showed that M1 macrophages can release high levels of proinflammatory cytokines, including tumour necrosis factor-α (TNF-α), CCL2 also known as monocyte chemoattractant protein-1 (MCP-1), IL-6, inducible nitric oxide synthase (iNOS), IL-1, IL-12, type I IFNs, CXCL1–3, CXCL5, and CXCL8–10 [22]. On the contrary, M2 macrophages have been demonstrated to express high levels of dectin-1, DC-SIGN (CD209), mannose receptor (CD206), scavenger receptor A, scavenger receptor B-1, CD163, CCR2, CXCR1, and CXCR2 [23] and to produce a large amount of IL-10, YM1, macrophage and granulocyte inducer-form 1 (MgI1), and arginase-1, highlighting their relevance during tissue remodelling and repair [24].
Macrophage polarization and functions are tightly regulated through the activation of several interconnected pathways. Among all, the balance between activation of STAT1 and STAT3/STAT6 has been demonstrated to play a crucial role; indeed, the predominance of STAT1 activation promotes M1 macrophage polarization, resulting in cytotoxic and proinflammatory functions. In contrast, STAT3 and STAT6 activation by IL-4/IL-13 and IL-10 signaling increases M2 macrophage polarization, associated with active tolerance and tissue repairing [22]. Moreover, the downstream effector of STAT6 and KLF-4 promotes M2 macrophage functions by suppressing the NF-κB/HIF-1α-dependent transcription. IL-10 promotes M2 polarization inducing p50 NF-κB homodimer, c-Maf, and STAT3 activities. In addition, IL-4 induces c-Myc that activates the IRF4 axis that inhibits IRF5-mediated M1 polarization, resulting in the M2 promotion [22]. Bouhlel et al. also demonstrated the relevance of PPAR-γ (peroxisome proliferator-activated receptor gamma) in skewing human monocytes toward an anti-inflammatory M2 phenotype. Indeed, the authors showed that PPAR-γ is highly upregulated in M2 macrophages and PPAR-γ agonists have been demonstrated to induce directly M2-like differentiation of monocytes in vivo and in vitro [25].
In the past decade, a novel class of small noncoding RNAs, termed microRNAs (miRs), has emerged as important regulators in biological processes. Accumulating evidence suggest a relevant role for several miRs in the polarization process (Figure 1(a)). In particular, miR-155 and miR-223 are involved in modulating macrophage activation state by targeting SOCS1, C/EBP (a hallmark of M2 macrophages), and Pknox1 [26]. Overexpression or silencing of miR-155 has been demonstrated to drive macrophages to M1 or M2 phenotype, respectively, confirming that miR-155 plays a central role in regulating Akt-dependent M1/M2 polarization of macrophages. It has been also shown that miR-155 downregulates the expression of IL-13Rα1, suppressing the polarization toward M2 phenotype [27, 28]. Some studies have observed that let-7c was expressed at a higher level in M2 macrophages than in M1 macrophages. Accordingly, the upregulation of let-7c in macrophages diminished M1 phenotype and promotes M2 polarization targeting C/EBP-d [29, 30]. miR-146, miR-125b, miR-155, and miR-9 can inhibit TLR4/IL-1R signaling by regulating IRAK-1, TRAF6, IKKe, p50 NF-κB, and TNF-α [29]. Further, miR-17, miR-20a, and miR-106a reduce the expression level of the signal regulatory protein (SIRPa), an important macrophage differentiation-related marker. miR-98 and miR-21 downregulate the expression of inflammatory genes in monocytes and macrophages via controlling IL-10 level [31].
Emerging data have demonstrated that epigenetic mechanisms, including chromatin remodelling, DNA methylation (DNAm), histone modifications, and regulation of target gene expression, are also involved in the orchestration of macrophage polarization in response to local environmental signals [22, 32, 33]. M1 and M2 macrophages have been shown to express different levels of DNA methyltransferase (DNMT) 1, 3a, and b that are associated with gene silencing [34]. DNMT1 drives the M1 polarization in atherosclerosis by directly targeting the promoter of PPAR-γ in macrophages [35]. The DNMT3b binding of the promoter of PPAR-γ contributes to the M1 phenotype in adipose tissue during inflammatory process [33].
Lund et al. demonstrated that atherogenic lipoproteins can promote global DNA hypermethylation in monocyte [36]. Thus, DNMT inhibition or knockdown could decrease the M1 polarization, providing novel strategies for atherosclerosis prevention and therapy. Accordingly, the treatment with 5-aza-2-deoxycytidine (decitabine), a recognized inhibitor of DNMTs, results in an increased M2 polarization induced by the inhibition of the PPAR-γ promoter, which in turn prevents obesity-induced inflammation, atherosclerosis, and insulin resistance [37, 38]. DNMT3a and DNMT3al expression levels have been shown to be increased significantly in M2 compared to M1 macrophages, and this is associated with AMPK signaling [33]. On the contrary, DNMT3b was significantly lower in M2 compared with M1 adipose macrophages [39]. Histone H3 and H4 acetylations were found to be toughly associated with the maturation of human monocytes [40]. M1 polarization induced by IFN-γ increases histone H4 acetylation at the TNF-α promoter throughout the ERK and p38 mitogen-activated protein kinase (MAPK) signaling pathways [41]. STAT3 and MAPK activation and the simultaneous acetylation of histones H3 and H4 on the SOCS-3 promoter suppress the inflammatory responses in microglial cells and promote M2 polarization [42]. Histone deacetylase 3- (HDAC3-) deficient macrophages showed a decreased expression of IFN-β and Cox-1 showing an M2-like phenotype and thereby ameliorate many inflammatory diseases, such as pulmonary inflammation [43–45].
Such heterogeneity in macrophage phenotypes and functions generated the still open questions of whether they act as killers or builders. During inflammation, macrophages drive in the autoregulatory loop characterizing this process, as they release a wide range of biologically active molecules which participated in both detrimental (killers) and beneficial (builders) in inflammation [46–48]. Therefore, inflammation stands as the typical environmental setting where macrophages show their “Janus” behaviour [46–48]. During the first events occurring during inflammation, macrophages are endowed to kill/remove pathogens and damaged cells, while at the end of the inflammatory process, termed resolution of inflammation, macrophages act as builders that promote damaged tissue regeneration and return to homeostasis [49–51]. Since inflammation represents a shared hallmark from diverse chronic diseases and direct involvement in insurgence and progression of these conditions, here, we discuss whether macrophages can act as killers or builders within the inflammatory landscape of selected and apparently “distant” pathologic conditions.
2. Macrophages in Cancer: Killers or Builders?
Macrophages represent the most abundant tumour infiltrating inflammatory cells [52, 53]. Reflecting their extreme plasticity within healthy tissues, macrophages infiltrating tumours can acquire distinct phenotype and functions resulting in the attenuation of antitumour activity and induction of tumour-supporting functions and have been defined as tumour-associated macrophages (TAMs) with M2-like features (Figure 2). However, in the initial phases of carcinogenesis, macrophages can act as protective killer cells, cooperating with T lymphocytes in the control of early proliferating cancer cells in the immunoediting process [54]. Instead, in developing tumours, compelling evidence indicate that subverted macrophages or TAMs exert a major role in driving tumour progression by different mechanisms and pathways, depending on the types of tumour, tissues, and inflammatory mediators. The builder option of macrophages in the tumour microenvironment (TME) can lie to conditions in which a chronic nonresolving inflammation is established, a feature that has been defined a hallmark of cancer [55] and that points out TAMs as key inflammatory mediators able to link chronic inflammation with cancer development and progression [56, 57].
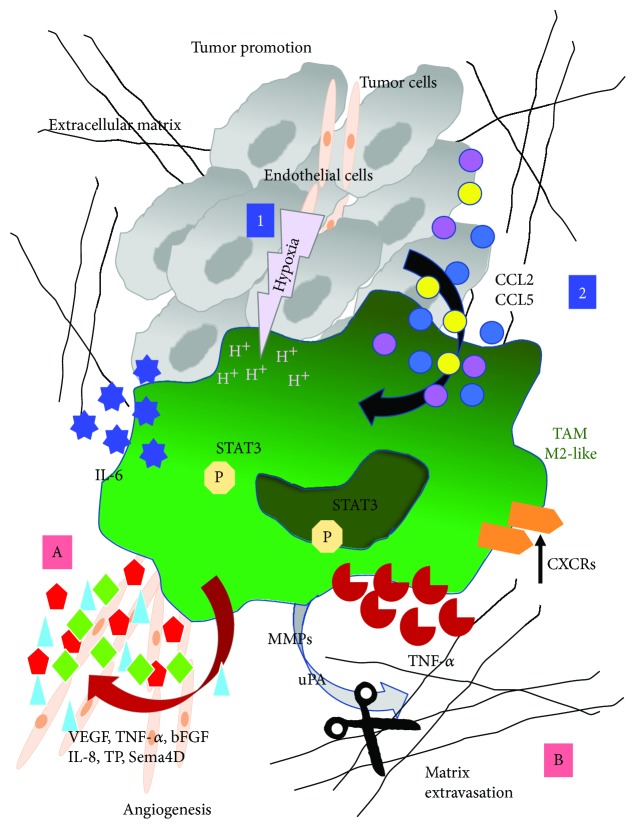
Figure 2.
Macrophage polarization in tumour progression. Macrophage recruitment in tumours and their polarization are regulated by several factors. Among all, hypoxia can induce the differentiation of monocytic myeloid-derived suppressor cells (M-MDSCs) via upregulation of CD45 tyrosine phosphatase activity (1). Further, soluble factors, such as CCL2 and CCL5 that are produced by the cancer cells and stroma cells, can increase macrophage infiltrate (2). In the TME, infiltrating associated to tumours (TAM/M2-like macrophages) can orchestrate tumour progression by several mechanisms including the release of cytokine, chemokines, and tissue remodelling proteins. Hypoxia increases the expression of CXCRs in TAMs and promotes tumour angiogenesis by enhancing the production of VEGF, TNF-α, bFGF, IL-8, TP, and Sema4D that can induce endothelial cell proliferation, sprouting and migration, tube formation, and maturation of new vessel, followed by its stabilization by attaching mural cells (A). TAMs can regulate the extracellular matrix degradation by producing different types of enzymes and proteases, such as matrix metalloproteinases (MMPs), in particular MMP2, MMP9, plasmin, urokinase plasminogen activator (uPA) and cathepsins acting on connective tissue surrounding the tumour, and allow tumour cells to detach from the mass of origin and to disseminate, leading to the formation of distant metastases (B).
Among soluble factors that mediate their displacement, there are CCL2, CCL5, CSF-1, VEGF, and complement elements, which are often produced by the cancer cells and stromal cells in the TME. Moreover, some TAMs can derive from differentiation of monocytic myeloid-derived suppressor cells (M-MDSCs) via upregulation of CD45 tyrosine phosphatase activity in response to tumour hypoxia and following downregulation of STAT3 [58].
Tumour promoting or builder activities exerted by TAMs have been demonstrated by several studies. Elevated TAM infiltration has been correlated with worse clinical outcome in most malignant tumours, such as breast, cervical, ovarian, prostate, and thyroid cancers; Hodgkin's lymphoma; hepatocellular carcinoma; lung carcinoma; and cutaneous melanoma [56, 59–65]. In contrast to these findings, some reports have instead highlighted that tumour infiltrating macrophages correlated to increased survival in colorectal, prostatic, and lung cancer patients [66–70]. The main builder features of TAM include the ability to support tumour angiogenesis as well as lymphangiogenesis, to increase the breakdown of extracellular matrix, to promote tumour cell invasion and migration, and to suppress the antitumour immune responses [56, 62, 71, 72]. These functions are shared with M2-like macrophages that, in a physiological context, are induced during vascular and matrix remodelling, necessary for damage resolution [73–77].
TAM infiltrate is also associated with the onset of resistance to different chemotherapeutic agents through the activation of diverse pathways. In breast cancers, TAMs can induce IL-10/STAT3/Bcl-2 signaling, leading to an inhibition of apoptosis upon paclitaxel treatment [78]. In advanced lung adenocarcinomas, TAMs are also reported to decrease the responsiveness to target therapy based on the epidermal growth factor receptor tyrosine kinase inhibitors [79].
M2-like TAMs support tumour growth directly by producing cytokines able to stimulate the proliferation of tumour cells or indirectly, by fostering endothelial cell (EC) proliferation and angiogenesis (Figure 2). It has been reported that the growth of subcutaneous Lewis lung tumour is impaired in the CSF-1-deficient and macrophage-deficient mice [80]. Furthermore, the treatment of tumour-bearing mice with recombinant CSF-1 reestablished the tumour growth, indicating a role for macrophages in tumour growth. TAMs can produce IL-6, whose release impacts on cell proliferation by a STAT3-dependent mechanism. Inhibition of STAT3 signaling blocks the antiapoptotic activity of IL-6 in human liver cancer cells [81]. TAMs are lower producers of TNF-α, resulting in enhanced tumour growth. Hypoxia significantly impacts on the TAM tumour cell interaction that induces the expression of CXCR4 and its ligand, CXCL12 (SDF-1), further supporting tumour cell dissemination and angiogenesis [82]. The number of TAMs within a tumour has been positively correlated with its metastatic potential, suggesting a role for TAMs in the distant dispersion of tumour cells [52, 83, 84]. By producing different types of enzymes and proteases, such as matrix metalloproteinases (MMPs), in particular MMP2 and MMP9, plasmin, urokinase plasminogen activator (uPA), and cathepsins [85–87] (Figure 2), TAMs can regulate the degradation of the extracellular matrix (ECM) and dictate tumour invasion and the metastatic process [19]. These factors act by relaxing the connective tissue surrounding the tumour, allowing tumour cells to detach from the mass of origin and to disseminate, leading to the formation of distant metastases.
TAMs sustain tumour angiogenesis by producing VEGFA (VEGF), the master growth factor involved in the angiogenic process. Besides VEGF, TAMs release a panel of proangiogenic factors which include TNF-α, basic fibroblast growth factor (bFGF), CXCL8/IL-8, thymidine phosphorylase (TP), adrenomedullin (ADM), and semaphorin 4D (Sema4D) [88–91] (Figure 2). These factors released by TAMs act by inducing endothelial cell proliferation, sprouting and migration of ECs into the tumour, tube formation, and maturation of new vessel, followed by its stabilization by attaching mural cells [92].
It has been recently reported that the expression of Sema3A from tumour cells is able to promote TAM accumulation inside the tumour, particularly in the avascular areas and required neuropilin-1 (NRP-1)-signaling cascade [93]. Macrophages are not only critical regulators of angiogenesis, but also crucial participants in lymphangiogenesis via VEGFC and VEGFD release, both in inflammatory settings and in tumour progression [94]. Thus, TAM-derived factors can link tumour angiogenesis and lymphangiogenesis [95–97].
Among TAMs, a relevant proangiogenic monocyte/macrophage subset, characterized by some distinctive features, has been further identified. These macrophages can express the angiopoietin receptor Tie2, termed TEMs (Tie2-expressing macrophages), and are closely associated with the vasculature [98, 99]. These cells have been implicated in the interference and in the resistance of action of antiangiogenic therapeutics, in particular vascular disrupting agents, and experimental data support the notion that inhibition of TEMs can foster antiangiogenic treatments with higher inhibition of angiogenesis and tumour spreading [100, 101].
Apart from their extreme plasticity, TAMs also sustain an immunosuppressive milieu aiding tumours to escape from immune surveillance [102]. TAM contribution to tumour progression acts also through synergistic interaction with other arms of the innate and adaptive immunity [46–48, 103] within the immunosuppressive TME. TAMs can interact with MDSCs, neutrophils, and DCs [104, 105]. TAMs also orchestrate the recruitment of T regulatory cells, by secreting CCL20 [106, 107] and CCL22 [108], and their activation through a bidirectional interaction by the release of IL-10 and TGF-β [107, 109–111].
Moreover, TAMs represent an important factor for the establishment of the premetastatic niche [112–116].
Different therapeutic strategies have been developed to target TAM physiology with encouraging preclinical and clinical results, either by blocking their tumour recruitment and functions or by redirecting their features to antitumour effector activities [57, 81, 117–121]. In several preclinical experimental models, including prostate, breast, and lung cancer and melanoma, the specific inhibition by antibodies of CCL2 has proven its promising effects, and when they are delivered in combination with chemotherapy shown enhancement of the effectiveness of treatment [122, 123]. However, though in a mouse model of breast cancer, it has been reported that a rebound effect following inhibition of CCL2 pathway resulted in the recruitment of monocytes/macrophages into the tumour and enhancement of lung metastasis [124]; different antibodies targeting CCL2 have been entered phase I and II clinical trials. Regarding the CCL5-CCR5 axis blocking strategies, a CCR5 antagonist has been approved as a treatment for patients with liver metastases of advanced refractory colorectal cancers and preliminary results indicated that this approach can lead to clinical responses [125]. Another interesting TAM-specific therapeutic treatment involves interferences with the CSF-1-CSF-1R axis, and in particular the receptor tyrosine kinase CSF-1R. Several compound and antibody inhibitors have been developed and evaluated in preclinical models and in patients with different types of cancer [120]. Important clinical regressions were obtained from patients with diffuse-type tenosynovial giant-cell tumour, which experienced CSF-1R tumour overexpression [120]. Interestingly, in a mouse glioblastoma multiforme model, CSF-1R blockade did not affect the TAM numbers but instead the M2-like TAM polarization, which is associated with the block of glioma progression and improvement of survival [119]. Also, bisphosphonates, usually used to treat osteoporosis and to prevent bone metastases-related complications, can be used to target macrophages in the tumour context, although their cytotoxic effects have been illustrated initially toward osteoclasts [126]. Combination chemotherapy or hormonal therapy with bisphosphonates in different types of tumour has shown clinical synergistic effects, in particular in postmenopausal women with breast cancer [127]. Another encouraging therapeutic strategy is related to agonistic anti-CD40 antibody and gemcitabine in pancreatic ductal adenocarcinoma patients. This approach revealed clinical responses and importantly demonstrated that in treated mice the CD40 agonist approach is responsible for reeducation of M2-like TAM toward an M1-like phenotype and of effective antitumour responses [128, 129]. Finally, a recently identified compound that found application in soft tissue sarcomas and ovarian cancer patients is trabectedin, which induces selective TRAIL-dependent apoptosis of monocytes, macrophages, and M-MDSCs in the blood, spleens, and tumours with reduction of TAM numbers and angiogenesis [130, 131].
3. Macrophages in Type 2 Diabetes: Killers or Builders?
Type 2 diabetes (T2D) is a metabolic disorder, and its incidence has increased significantly in recent years. T2D is characterized by a peripheral resistance to the action of insulin and a failure of beta cells to compensate, leading to hyperglycaemia. It is now widely accepted that obesity increases the risk of T2D by inducing a chronic low-grade inflammation [132] and progression in local adipose tissue.
Accumulating evidence supports a role for tissue macrophages in a broad spectrum of inflammatory conditions [133], including obesity-associated metabolic diseases, such as insulin resistance and T2D [68, 134].
Macrophages together with other immune cells account almost 10% of the normal adipose tissue and play a key role in maintaining homeostasis. However, diet-induced obesity compromises homeostasis, resulting in an increased infiltration of macrophages representing up to 50% of the cells in adipose tissue [135, 136].
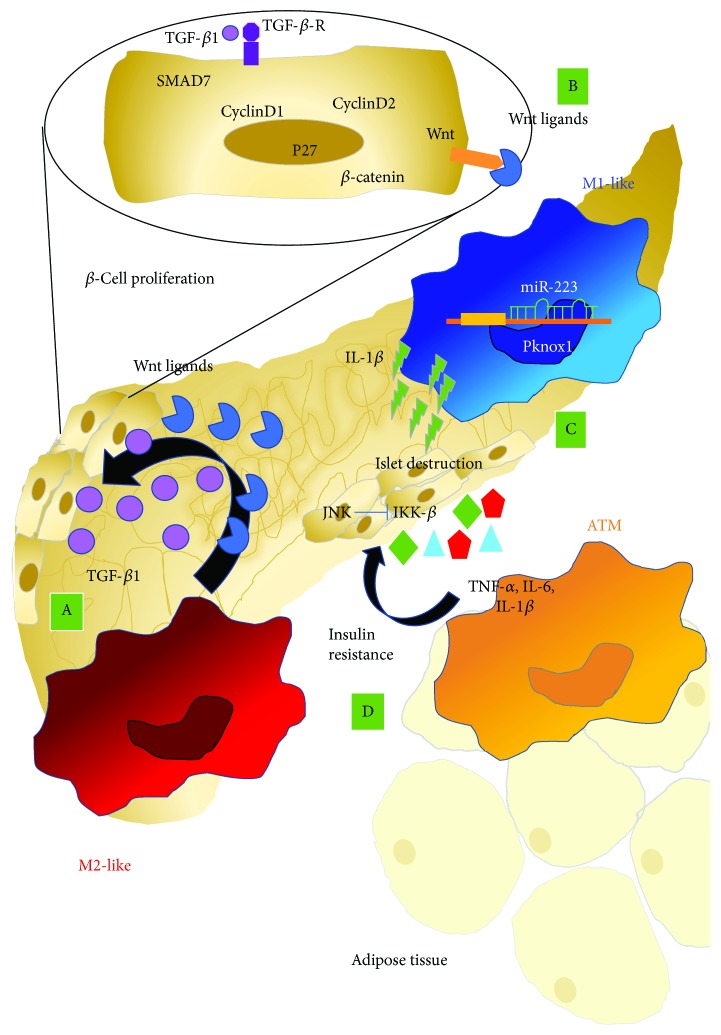
Several studies have established the crucial role of macrophage polarization in the development of T2D. The M1/M2-like polarization of tissue-destructive (killers) versus tissue-reparative (builders) macrophages is of great interest in clinical strategies because of their role in β-cell proliferation [137]. Recent evidence demonstrate that the high plasticity and phenotypic diversity of macrophages promote the cross-talk between β-cells, non-β endocrine cells, endothelial cells, mesenchymal cells, and other circulation-derived blood cells [138–140]. Builder-M2-like macrophages regulate β-cell proliferation through the release of a variety of trophic factors such as TGF-β1, which directly induce upregulation of SMAD7 in β-cells. SMAD7 in turn promotes β-cell proliferation by increasing CyclinD1 and CyclinD2 and by inducing nuclear exclusion of p27 [141] (Figure 3). In addition, M2-like macrophages also secrete Wnt ligands, thus activating the Wnt signaling pathway, and β-catenin, supporting β-cell replication [138] (Figure 3). Conversely, only a few studies investigating the polarization state of macrophages in pancreatic microenvironment have been described in literature [16–19], where an overall increase of macrophages/islets has been detected by immunohistochemistry. Eguchi et al. [142, 143] showed that Ly6c+ M1 macrophage was expanded in the diabetic mouse islet. Ly6c+-killer-M1 macrophage has been shown to secrete IL-1β, resulting in potent inhibition of insulin secretion, followed by islet destruction (Figure 3). The use of IL-1R antagonists and anti-IL-1β-neutralizing antibodies was able to abolish these effects on pancreatic islets [21–24].
Figure 3.
Macrophage polarization in type 2 diabetes. Macrophage within pancreatic tissues can be switched toward different functionalities according to the environment stimuli. M2-like macrophage supports B-cell proliferation by several trophic factors like TGF-β1 which directly induce upregulation of SMAD7 and increases of cyclinD1, cyclinD2, and p27 (A). Moreover, M2-like macrophages release Wnt ligands, thus activating the Wnt signaling pathway, and β-catenin, supporting β-cell replication (B). M1-like macrophage in pancreatic tissues can secrete IL-1b, inhibiting insulin secretion, followed by islet destruction (C). Adipose-derived macrophages (ATM) can release proinflammatory cytokines, including TNF-α, IL-6, and IL-1β that decrease insulin sensitivity through the activation of Jun N-terminal kinase (JNK), inhibitor of IKκB kinase (IKK-β), and other serine kinases in insulin target cells (D).
Several studies in T2D have shown that M1-like macrophages resulted in increased inflammation, obesity, and insulin resistance, while M2-like macrophages are associated with a reduction in both obesity and insulin resistance [144]. M2-like macrophages are reported to not only suppress inflammatory cytokine IL-10 [145] but also provide a niche for preadipocytes to keep the number and quality of them, thus maintaining insulin sensitivity [146].
These data clearly suggest that macrophages play a nonredundant role in the pathogenesis of T2D [147]. An important aspect of diabetes prevention is a better understanding of the underlying mechanisms behind obesity-induced visceral adipose tissue inflammation, crucial for the development of T2D.
Obesity is associated with the accumulation of proinflammatory cells in visceral adipose tissue, which is an important underlying cause of insulin resistance and progression to T2D [148–150]. Establishing the initiating events leading to the switch from an anti-inflammatory M2-like state to M1-like phenotype remains elusive.
Recent studies show that obesity-induced adipocyte hypertrophy results in upregulated surface expression of stress markers. Adipose stress is detected by local sentinels, such as NK cells and CD8+ T cells, which produce IFN-γ, driving M1-like adipose tissue macrophage (ATM) polarization [148–150]. Adipocyte hypertrophy has been reported to create hypoxic area and activates hypoxia-inducible factor-1, which induces inflammatory cytokines and suppresses preadipocyte-related angiogenesis and causes insulin resistance [151].
Normal adipose tissue macrophages phenotypically resemble the alternatively activated M2-like phenotype, expressing the mannose receptor, the CD206 surface antigen, and releasing Arg-1 and IL-10. In contrast, diet-induced obesity leads to a shift toward an M1 classically activated macrophage, characterized by the F4/80, CD11b, and CD11c expression [152] (Figure 3).
Low-grade inflammation in this setting is mediated by the polarization of recruited and resident macrophages to the M1-like phenotype in tissues, such as liver and adipose tissues [153, 154]. In contrast, M2 macrophage activation appears to protect against obesity-associated inflammation and insulin resistance [155, 156]. Several cytokines and chemokines, such as CCL2, interleukin IL-6 and IL-1β, macrophage migration inhibitory factor (MIF), and TNF-α, can be released by both adipocytes and macrophages [157, 158]. Macrophages within adipose tissue are recruited from the bone marrow and are characterized by a wide panel of factors that track with the degree of obesity [136, 159, 160]. Indeed, the paracrine as far as the endocrine activity was exerted by the proinflammatory cytokines, including TNF-α, IL-6, and IL-1β released by ATMs can induce decreased insulin sensitivity through the activation of Jun N-terminal kinase (JNK), inhibitor of IKκB (IKK-β), and other serine kinases in insulin target cells [161, 162].
The unbalance in the ratio between M1-like and M2-like adipose macrophages has been considered to be directly related to the development of insulin resistance [21, 149]. Insulin resistance resulted from a transition in macrophage polarization from the M2-like activation state, induced by STAT6 activation and PPAR, to a classic M1-like activation state, further driven by NF-κB, AP1, and other related factors [163–165].
The network of molecular mediators that regulate M2 polarization in response to hypermetabolism is not fully understood, but peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and PPAR-γ target genes, such as arginase-1 and CD36, are implicated in this process. PPAR-γ has been proven to be essential for macrophage M2 polarization with the function of anti-inflammation and associated with metabolic dysfunction [145, 156, 166]. PPAR-γ was found to be a miR-130b target gene in regulating macrophage polarization insulin tolerance via repression of PPAR-γ [167]. Several studies have shown that PPAR-γ interacts with NF-κB, in the modulation of macrophage polarization. PPAR-γ blocked the proinflammatory pathway of NF-κB and inhibited the expression of relative factors, such as TNF-α [168].
Further, it was shown that IL-6 acts as a Th2-builder cytokine in obesity by stimulating M2-like polarization and local ATM proliferation, presumably due to upregulation of the IL-4 receptor α [169]. Recently, it has been reported that adenosine monophosphate kinase (AMPK) β1 plays an important role in protecting macrophages from inflammation under high lipid exposure resulting in a modulation of obesity-induced insulin resistance (Figure 3). Genetic deletion of the AMPK β1 subunit in mice reduced macrophage AMPK activity, acetyl-CoA carboxylase phosphorylation, and mitochondrial content, resulting in reduced rates of fatty acid oxidation [170].
Inhibition of proinflammatory cytokines and chemokines, such as TNF-α, IL-1β, IL-6, and CCL2, may reduce adipose tissue inflammation and insulin resistance [147, 171, 172]. For instance, several studies have demonstrated that treatment with neutralizing IL-1β antibody or blockage of IL-1β signaling improved glycaemic control in diet-induced obese mice and insulin sensitivity in patients with T2D [173–176]. Other findings suggest that the CCL2-CCR2 signaling pathway disruption reduces adipose tissue macrophage content ameliorating insulin resistance and improves insulin sensitivity [160, 177]. CCL2 knockout mice receiving intact monocytes or mice receiving CCR2-deficient monocytes were both protected from the accumulation of macrophages in adipose tissue and the liver. [178] So far, targeting the CCL2-CCR2 signaling pathway may provide the basis for the development of novel therapies against T2D. In vivo studies have shown that circulating levels of free fatty acid (FFA) promote the generation of M1 macrophages via TLR4 signaling in adipocytes and macrophages in the setting of obesity [179–181]. In this context, adipose tissue inflammation is aggravated by the secretion of TNF-α, which in turn increases lipolysis leading to further production of FFAs establishing a vicious circle. Resistin is another potential target to combat insulin resistance or T2D. In fact, resistin induction which in turn stimulates secretion of several proinflammatory cytokines by increased infiltration of macrophages causes inflammation-induced insulin resistance [182–184].
Several phase II and III clinical trials have been initiated to inhibit key immunological processes of adipose tissue inflammation in T2D patients, such as NF-κB signaling, IL-1β function, or arachidonic acid metabolism, with promising results [148].
A shift in the polarization of adipose tissue macrophages from an M2-like state to an M-like proinflammatory state resulting in insulin resistance favours inflammation and insulin resistance [145]. Thus, targeting of inflammatory M1/M2-like polarization process of obese patients appears to be a promising future strategy for prophylaxis against diabetes development. For instance, adipose tissue macrophages from CCR2 knockout mice are polarized to the M2-like macrophages, even after obesity and CCR2 knockout mice were found to be protected from diet-induced insulin resistance [145, 160]. Furthermore, it has been shown that inhibition of IL-10 secreted by M2-like macrophages enhances the impairment of insulin signaling confirming its protective role in T2D [185].
Insulin-sensitizing thiazolidinediones (TZDs), clinically used for T2D patients [186], target the PPAR-γ that plays a key role in the maturation of M2-like macrophage and insulin sensitivity. PPAR-γ deletion prevents polarization of the monocyte/macrophage to the M2-like phenotype, and PPAR-γ-deficient mice exhibit glucose intolerance and insulin resistance [187]. Therefore, existing and future drug mechanisms may be involved in modulating the phenotypical and functional features of macrophages. For instance, metformin is a drug widely used to treat T2D, to decrease insulin resistance; it has been proposed that the benefit may result, at least in part, from modulating macrophage differentiation and polarization [188, 189]. How metformin can modulate the differentiation of Ly6C monocytes into M2-like macrophages remains the subject of ongoing interesting studies. In addition to glucose-lowering drugs, T2D patients are typically treated with low-dose aspirin (acetylsalicylic acid) that has off-target anti-inflammatory properties. Aspirin exerts its anti-inflammatory effects via inhibition of cyclooxygenase and a subsequent decrease in the proinflammatory prostaglandins [190]. Recently, it has been demonstrated that aspirin-triggered resolvin D1 into a degradable biomaterial after injury was able to significantly increase the accumulation of anti-inflammatory monocytes and M2-like macrophages while limiting the infiltration of neutrophils and increase proregenerative immune subpopulations [191].
Incretin-based treatments and the cannabinoid 1 receptor (CB1) blocker rimonabant have anti-inflammatory effects and may protect the pancreatic islets from IL-1β-driven. However, this anorectic antiobesity and glucose-lowering drug had also psychiatric side effects [164, 192, 193].
Several studies highlight the role of miRs as key regulators of cell fate determination and significant contributors to the pathogenesis of complex diseases, such as inflammatory responses and T2D [194]. It was found that miR-223 inhibits Pknox1, suppressing proinflammatory activation of macrophages, and protects against diet-induced adipose tissue inflammatory response and systemic insulin resistance [195]; miR-130b was found to be a novel regulator of macrophage polarization via repression of PPAR-γ and a promising target for T2D therapy [167]; miR-27a was also proposed as a target of intervention for inflammation and insulin resistance in obesity [196].
In summary, M1/M2-like macrophage polarization and switching hold the key to the regulation of insulin sensitivity and T2D. Macrophage polarization toward the alternative M2-like phenotype may play a preventive role and also be a novel and useful strategy for the treatment of insulin resistance and T2D.
Novel macrophage-targeted strategies that are both tissue-specific and disease-specific hold a promise for the future management of the chronic inflammatory disorders that were covered in this review.
4. Macrophages in Atherosclerosis: Killers or Builders?
Atherosclerosis is a chronic inflammatory disease driven by an imbalance in lipid metabolism and a maladaptive immune response [197]. This disease is characterized by the accumulation of lipids in large- and medium-sized arteries forming plaque deposits that block the flow of the blood. Several factors have been correlated with the development of atherosclerotic diseases, among which the elevated low-density lipoprotein (LDL) cholesterol, hypertension, obesity, and both T2D and T1D. The accumulation of LDL promotes the recruitment of monocytes that lead to the formation of the atherosclerotic plaques [198]. Further, the exposure to CSF-1 and the uptake of oxidized LDL (ox-LDL) induce monocyte differentiation into macrophage and results in foam cell formation with the proliferation of smooth muscle cells [199]. The scavenger receptors lead the ox-LDL recognition, and the intracellular cholesterol is metabolized and transported to exogenous acceptors, such as high-density lipoprotein, through efflux proteins, such as ATP-binding cassette transporters [200] (Figure 4).
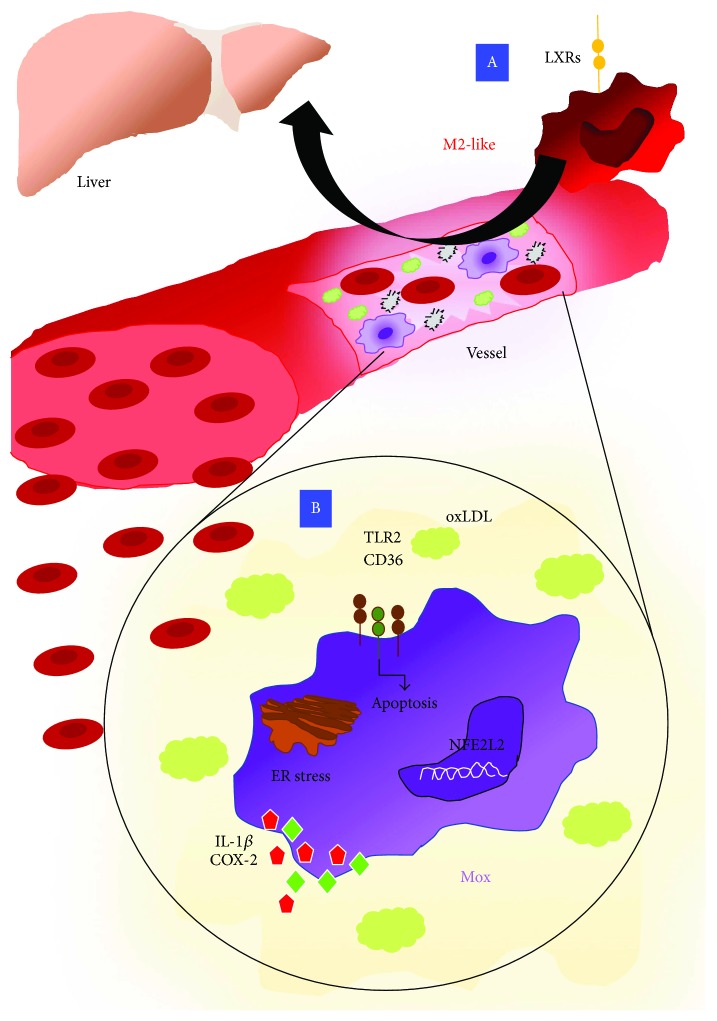
Figure 4.
Macrophage polarization in atherosclerosis. Macrophages are crucial players involved in the atherosclerosis development due to their ability to regulate cholesterol efflux. In this context, the upregulation of LXRs in M2 macrophages has been found to exert a protective role. Indeed, LRXs reduce peripheral tissue excess cholesterol that is returned to the liver by releasing HDL in the plasma (A). Apart from M1 and M2 polarization, a third macrophage state has been described in the atherosclerosis context that is termed Mox. Macrophages exposed to oxidized phospholipids display reduced phagocytic and chemotactic abilities compared with M1- and M2-like macrophages and are characterized by the expression of the transcription factor NFE2L2 as far as Hmox1, Srxn1, Txnrd1, and Gsr genes. Mox macrophages also activate TLR2dependent mechanisms in response to oxidized lipids leading to an increase of IL1β and COX-2 (B).
Macrophage apoptosis has been observed in patients with defects in the Acyl-CoA:cholesterol acyltransferase (ACAT), the enzyme that re-esterificates free cholesterol in cholesteryl fatty acid esters [198]. Seimon et al. showed that oxidized phospholipids, oxidized LDL, saturated fatty acids (SFAs), and lipoprotein(a) can induce apoptosis in ER-stressed macrophages through a CD36- and TLR2-dependent mechanism [201] (Figure 4).
Several in vivo studies have demonstrated macrophage heterogeneity within the atherosclerotic plaque in response to the exposition of lipids and their oxidized derivatives [202]. Indeed, within atherosclerotic microenvironment, macrophages adapt their phenotype activating specific transcriptional programs. Cholesterol crystals that accumulate during the early stages of the atherosclerotic process might be involved in the activation of macrophages [202]. Cholesterol crystals can promote the caspase1-activating NLRP3 inflammasome, which results in the cleavage and secretion of IL-1 and may act as a M1-polarizing stimulus [203]. The proinflammatory M1-like phenotype can also be promoted by a mechanism that involves inhibition of the transcription Kruppel-like factor 2 [204, 205] or the activation of the TLR4-mediated pathway that in turn leads to the activation of NFκB [206]. Conversely, the anti-inflammatory M2-like phenotype is induced by 9-oxononanoyl cholesterol, a major cholesteryl ester oxidation product that can enhance TGFβ secretion [207]. Moreover, sphingolipid metabolites, such sphingosine1phosphate (S1P), promote the switching phenotype of mouse macrophages from M1- to M2-like state, by activating S1P1 receptor [208].
Recently, a third macrophage phenotype has been described in the atherosclerosis context that has been termed Mox (Figure 4) and represents macrophages exposed to oxidized phospholipids [209–211]. In advanced atherosclerotic lesions of mice, Mox macrophages comprise approximately 30% of the total number of macrophages [212]. Mox phenotype can be triggered by the activation of transcription factor NFE2L2 [212, 213]. Mox macrophages display reduced phagocytic and chemotactic abilities compared with M1- and M2-like macrophages. In mice, Mox macrophages typically express NFE2L2-mediated redox regulatory genes, including Hmox1, Srxn1, Txnrd1, and Gsr [212]. Nevertheless, in response to oxidized phospholipids, Mox macrophages activate TLR2-dependent mechanisms that lead to an increase of IL-1β and COX-2 expression [214].
Circulating monocytes in murine models have been classified into two major subsets, described as Ly6Chi and Ly6Clow monocytes. In apolipoprotein E-deficient (ApoE−/−), mice the increase of Ly6Chi subset (corresponding to human M1-like subset) has been observed within atherosclerotic plaques [215].
Several studies have also correlated macrophage polarization with the clinical course of atherosclerosis. Among all, de Gaetano et al. [216] observed a marked difference in a macrophage subset between symptomatic and asymptomatic plaques. Indeed, M1 macrophages were found to be abundant in the developed lipid core of the symptomatic plaque and were rarely found in the intimal regions of the plaque, while M2-like macrophage number was higher in asymptomatic atherosclerotic plaques, suggesting a potential protective role of M2-like macrophages. Moreover, in mouse models, it has been demonstrated that in the regressing plaque a decrease in the number of macrophages occurs and, in some, a switch of their phenotypic characteristics has been observed, with an enrichment in M2-like phenotype, suggesting that this is a common signature of regressing plaques [217].
Despite several current standard therapies for atherosclerosis that may influence general immune responses, including angiotensin-converting enzyme (ACE) inhibitors, β-blockers, aspirin, and corticosteroids, these drugs lack specific macrophage targeting and may only be recognized as mild modifiers of macrophage activity [218]. Several common pharmacological agents have already been proposed to modulate macrophage activity for the prevention as well as the treatment of inflammatory-related diseases, including atherosclerosis. PPAR-γ is a crucial factor involved in the regulation of macrophage lipid metabolism and inflammatory responses and, as already discussed above, is upregulated in M2-like macrophages [25]. PPAR-γ activators might have therapeutic potential, and studies conducted by Bai et al. [219] suggest that mediator 1 (MED1) is required for the PPAR-γ-induced M2 phenotype switch and showed that MED1 in macrophages has an antiatherosclerotic activity via PPAR-γ-regulated transactivation, suggesting MED1 as a promising target for atherosclerosis therapy.
Natural ligands such prostaglandins and some pharmacological agents including anti-TZD that have been demonstrated to activate PPAR-γ have also been shown to decrease atherosclerosis progression. Choi et al. demonstrated that 5-(4-hydroxy-2,3,5-trimethylbenzylidene) thiazolidine-2,4-dione (HMB-TZD) reduced leukotriene B4 (LTB4) production and cytokine production by RAW264.7 macrophages and attenuates atherosclerosis possibly by reducing monocyte recruitment to the lesion [220]. In in vivo studies, selective inactivation of macrophage PPAR-γ impairs M2-like activation exacerbating diet-induced obesity [154], suggesting that PPAR-γ inducer might have a therapeutic potential. Likewise, liver X receptors (LXRs) have been found to be upregulated in M2-like macrophages and exert atheroprotective effects by modulating cholesterol metabolism and M1 macrophage-induced inflammatory genes, including iNOS, COX-2, and IL-6 [221] (Figure 4). Tangirala et al. have observed that in experimental models of atherosclerosis, LXR agonists induced a reduction of preexisting plaque size and this was associated with LXR macrophage activity. Indeed, macrophage-specific loss of LXRs resulted in a statistically significant increase in lesion size [222]. Moreover, the immunomodulatory drug fingolimod (FTY720) that has been described as a S1P1 receptor modulator has been shown to increase the proportion of M2-like macrophages in atherosclerotic lesions and reduce lesion progression in mice [223]. Statins, effective cholesterol-lowering agents, have also been reported to dampen immune responses through inhibition of macrophage inflammatory activity by increasing efferocytosis in vitro in a 3-hydroxyl-3-methylglutaryl coenzyme A (HMG-CoA) reductase-dependent manner, decreasing membrane localization of RhoA and preventing impaired efferocytosis by lysophosphatidic acid, a potent inducer of RhoA [224].
Stimulation of the macrophage autophagy-lysosomal system by the natural sugar trehalose has been reported to reduce the formation of the atherosclerotic plaque by limiting macrophage apoptosis and necrosis in the plaque cores [225].
Finally, some Lactobacillus has been observed to regulate M1/M2-like macrophage ratio by suppressing ox-LDL phagocytosis, thus blocking foam cell formation [226]. These data supported the employment of prebiotic or probiotic in atherosclerosis.
5. Macrophages in Periodontitis: Killers or Builders?
Gingivitis and periodontitis are two common diseases affecting the oral tissues and the health of the supporting structures of a tooth that share inflammation as a common feature. While in gingivitis the inflammatory process is limited to the soft tissues, epithelium, and connective tissue, in periodontitis, the inflammation is extended to the supporting tissues, including the alveolar bone [227].
Chronic periodontitis (CPD) occurs in response to specific bacteria within the oral biofilm and involves the destruction of tooth-supporting tissues. Major features for CPD are accumulation of immune cells in gingival connective tissue, resorption of alveolar bone, and the degradation of periodontal connective tissues, which lead to increased tooth mobility and eventual tooth loss [228, 229].
Chronic periodontitis is strongly associated with the presence of Gram-negative anaerobic bacteria in subgingival plaque, in particular, Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola. Although initiated by bacteria, the bone pathology in CPD is mediated almost entirely by the host response that is thought to be responsible for the local tissue destruction observed in periodontitis [230]. In addition, the response to oral pathogens has systemic consequences. For example, infection and chronic inflammatory conditions, such as periodontitis, may influence the atherogenic process [231, 232].
It has been reported that monocyte/macrophages act as relevant killers in periodontal diseases by contributing to tissue breakdown. Elevated numbers of macrophages/monocytes associated with greater collagen breakdown and higher level of MMPs have been observed in samples from periodontitis [233]. Studies have shown that IL-1 was expressed predominantly by macrophages in the tissue isolated from periodontal patients [234]. In addition, higher levels of Receptor activator of nuclear factor kappa-B ligand (RANKL) protein, associated with macrophages, have been observed in the periodontitis tissues [235].
Activated macrophages have been found in the gingival epithelium, lamina propria, and perivascular tissues and in the blood vessels in human CPD. As lesions are associated with chronic periodontitis progress, increasing numbers of macrophages infiltrate into the gingival tissues [236]. Therefore, the gingival tissue and crevicular fluid of patients with chronic periodontitis have been reported to contain significantly increased amounts of CCL3, also known as macrophage inflammatory protein- (MIP-) 1α and CXCL-8/IL-8, as compared to healthy subjects [237, 238].
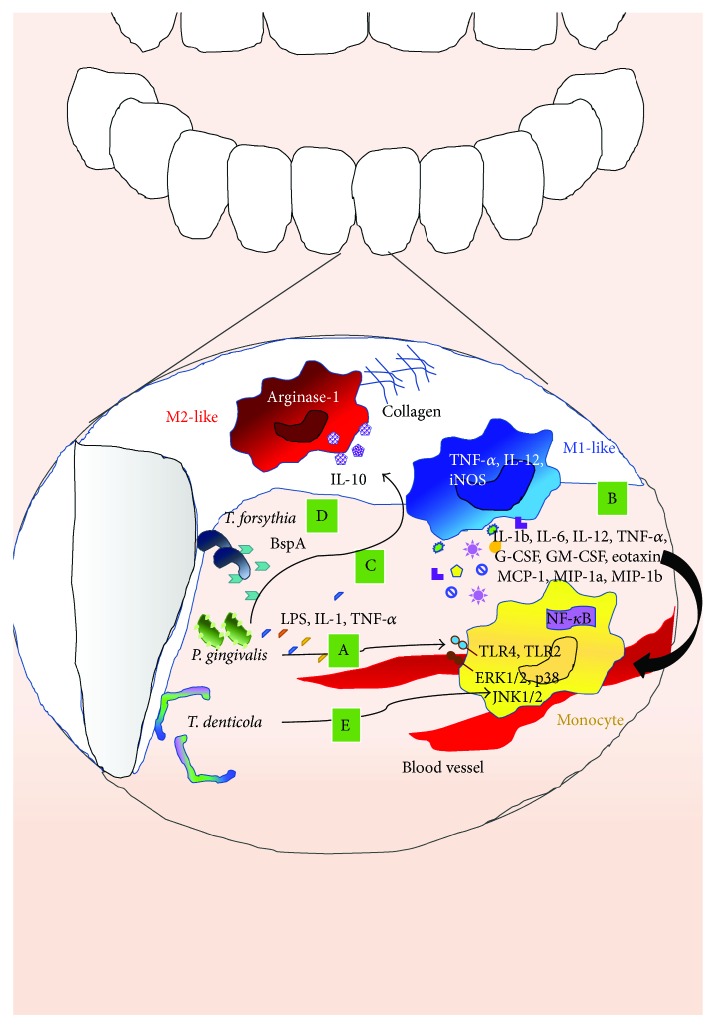
Porphyromonas gingivalis (Pg) is a key periodontal pathogen that promotes dysbiosis between host-and plaque-associated bacteria, thus resulting in both periodontal disease onset and progression [239, 240]. LPS from Pg activates macrophages through both TLR2 and TRL4 [241], and specifically, TLR2 activation by Pg LPS triggers the downstream stimulation of NF-κB, leading to the production of proinflammatory cytokines [242–244] (Figure 5).
Figure 5.
Macrophage polarization in periodontitis. Macrophages that have been found in the gingival epithelium can be activated by several microorganisms able to induce macrophage polarization toward M1- or M2-like phenotype. P. gingivalis releases LPS, IL-1, and TNF-α that promote the proinflammatory M1 macrophage polarization (A). Moreover, Pg infection enhances the secretion of IL-1β, IL-6, IL-12, TNF-α, G-CSF, GM-CSF, and the chemokines eotaxin, MCP1, MIP-1α, and MIP-1β from macrophages, reflecting a M1-like proinflammatory response (B). In spite of this, it has also been reported that Pg infection can also be associated with the increase of IL10, supporting M2 macrophage and increasing arginase-1 production and collagen deposition, leading to periodontitis (C). T. forsythia releases BspA and other ligands that induce TLR2 signaling favouring the development of Th2-type inflammatory responses (D). T. denticola induces TLR2 signaling that stimulates the prolonged activation of both ERK1/2 p38 and JNK1/2 in monocytes (E).
Macrophages are frequently used as the in vitro model cells to define immune cell function in CPD studies. Transfer of TLR2 expressing macrophages to TLR2-deficient mice restored host sensitivity to Pg oral challenge [245] (Figure 5).
Pg LPS, in the presence of IL-1 and TNF-α, has been shown to induce cultured human fibroblasts and epithelial cells to release PGE2, a factor associated with periodontal bone resorption that promotes the proinflammatory M1-like macrophage polarization [229, 246–250] (Figure 5). IL-1 and TNF-α not only enhance inflammation but also promote bone resorption, a major concern in periodontitis [251–253]. Oral infection with Pg in BALB/c and C57BL/6 mice resulted in the influx of M1 macrophages into the submandibular lymph node (SMLN) and gingival tissue, together with an increase in alveolar bone resorption, as compared with untreated mice in a murine model of periodontitis [254, 255]. Selective SMLN macrophage in vivo depletion, using liposomes containing the proapoptotic agent clodronate, resulted in decreased Pg-induced alveolar bone in vivo resorption.
Pg infection enhances the secretion of the cytokines IL-1β, IL-6, IL-12, TNF-α, CSF-3 (G-CSF), and CSF-2 (GM-CSF), in addition to the chemokines eotaxin and CCL2–4 from macrophages, reflecting a M1 proinflammatory response (Figure 5). These cytokines and chemokines are known to act as proinflammatory mediators, to induce monocytes to migrate from the bloodstream into the gingival tissue, and to act synergistically to further stimulate proinflammatory cytokine production [246, 248, 249, 256]. IL-10, which is mainly produced by macrophages, was detected among the wide array of cytokines released during Pg infection [257]. IL-10 strongly supports M2-like macrophage and polarized functions including increased production of arginase-1, higher collagen deposition, and induction of fibrosis in gingival tissue, all common clinical features of chronic periodontitis [258–260].
In a recent study, Lam et al. observed that Pg can persist in naïve and M2-like, but not M1-like, macrophages for 24 hours. Phagocytosis of Pg also induced high levels of TNF-α, IL-12, and iNOS in M1 macrophages, but not in naïve macrophages (MØ) or M2 macrophages [254].
T. forsythia expresses a well-characterized TLR2 ligand, the BspA protein, and N- and O-glycan-linked glycoproteins that comprise its surface- (S-) layer, covering the outer membrane [261]. This S-layer has been shown to be important in delaying the cytokine responses of monocyte and macrophage cells in vitro [262, 263]. BspA and other ligands of T. forsythia induce TLR2 signaling favoring the development of Th2-type inflammatory responses detrimental to the alveolar bone that has been shown to be limited in TLR2−/− mice [242].
T. forsythia whole cells induced significantly greater amounts of IL-6 and IL-10 in wild-type (BALB/c) bone marrow-derived dendritic cells (BM-DCs) and macrophages, markers related to an M2-like polarization, as compared with TLR2−/− cells. The macrophage-inducible C-type lectin receptor (Mincle), a FcγR-coupled pathogen recognition receptor (PRR) [263, 264], has been reported to contribute to macrophage polarization [265]. THP-1 macrophages infected with the purified S-layer on whole wild-type T. forsythia elicit a M2-like polarization (IL-10, TNF-α) that is limited in Mincle knockdown macrophages or where infection is performed with the S-layer TfΔtfsAB-mutated form [265] (Figure 5).
Treponema denticola is among the most frequently isolated oral spirochetal species in patients with periodontitis [266, 267]. Major surface protein complex (MSPc), which is expressed on the envelope of this treponema, plays a key role in the interaction between T. denticola and gingival cells and the related cytopathic effects [268]. Treponema denticola within the periodontium of the host has been reported to be associated with localized inflammation. MSPc has been showed to stimulate the release of the proinflammatory cytokines NO, TNF-α, and IL-1β from murine macrophages, both in LPS-responsive and LPS-nonresponsive murine macrophages [269]. Furthermore, IL-1β, IL-6, and TNF-α secretion by T. denticola-activated macrophages has been shown to exhibit potent bone reabsorption effects due to their proosteoclastic properties [270].
T. denticola-mediated macrophage response is mainly mediated by TLR2 and via MAP kinases [271]. One of the most highly conserved signaling cascades activated in both the innate and the adaptive immune systems involves a family of MAPKs including ERK1/2, p38, and JNK1/2 [272].
T. denticola stimulates the prolonged activation of both ERK1/2 and p38 in monocytes, and pharmacological inhibition of these pathways plays major roles in regulating both pro- and anti-inflammatory cytokine productions by T. denticola-stimulated monocytes [271] (Figure 5).
A study from Miyajima et al. reported a correlation between periodontitis-activated monocytes/macrophages and aortic inflammation in an in vivo ligature-induced experimental model of periodontitis. Gene expression profiling in circulating monocytes in this experimental model showed that periodontitis induced a M1-like specific signature with high levels of TNF-α and IL-6 as compared to controls, indicating that a M1-like phenotype of macrophages is induced by periodontitis [273]. This in turn supports the hypothesis that periodontitis-induced M1-like macrophages are the inflammatory orchestrators driving specific proinflammatory messages to the systemic vasculature [273]. The work from Miyajima et al. also showed that periodontitis-induced M1 macrophages can increase macrophage adhesion to aortic endothelial cells through the NF-κB/VCAM-1 axis [273]. These results clearly suggest that local-tissue alterations of macrophages during periodontitis can impact on circulating monocyte polarization and are associated to vascular alterations involved in apparently distant pathologies that shares inflammatory cell polarization as common features.
6. Conclusion
It is now widely accepted that inflammation represents a host hallmark of diverse chronic diseases, ranging from cancer, diabetes, and metabolic, cardiovascular, and neurological/neurodegenerative disorders. In the same way, inflammation has been recognized as a relevant condition for insurgence, maintenance, and progression of such disorders. Cell plasticity is a key and shared feature of inflammatory cells within the host organism that can potentially acquire killer (M1-like) or builder (M2-like) properties, based on the surrounding environment. Macrophages are the clearest example of immune cells that can be switched from killers to builders and vice versa, and this has been observed in all the inflammatory-based/associated disorders. Here we discussed the cellular and molecular mechanisms involved in macrophage switching to killers or builders in differently and apparently distant disorders, pointing out the attention on how the macrophages/microenvironment reciprocal interaction shape their polarization and distinct functional states.
Further, we discussed some approaches aimed at resolving this process, by interfering with aberrant macrophage killer/builder reciprocal switch. With this knowledge, it is clear that the identification of novel preventive and intervention strategies, along with effective compounds able in targeting/limiting/reverting proinflammatory macrophage polarization, are urgently needed and may represent a relevant tool to shape macrophage function action directly on them or on the hosting/surrounding environment.
Abbreviations
- ACAT:
Acyl-CoA:cholesterol acyltransferase
- ADM:
Adrenomedullin
- AKT:
Protein kinase B
- AMPK:
Adenosine monophosphate kinase
- ATMs:
Adipose tissue macrophages
- Bcl2:
B-cell lymphoma 2
- bFGF:
Basic fibroblast growth factor
- BspA:
Bark storage protein A
- c-Maf:
Avian musculoaponeurotic fibrosarcoma oncogene homolog
- c-Myc:
Avian myelocytomatosis viral oncogene homolog
- C/EBP:
CCAAT-enhancer-binding proteins
- CCR:
Chemokine receptor
- CD:
Cluster of differentiation
- COX:
Cyclooxygenase
- CPD:
Chronic periodontitis
- CSF-1:
Colony-stimulating factor 1
- CSF-1R:
Colony-stimulating factor 1 receptor
- CXCL:
C-X-C chemokine ligand
- CXCR:
C-X-C chemokine receptor
- DC-SIGN:
Dendritic cell-specific ICAM-grabbing nonintegrin
- DC:
Dendritic cells
- DNAm:
DNA methylation
- DNMT:
DNA methyltransferase
- ECM:
Extracellular matrix
- ER:
Endoplasmic reticulum
- ERK:
Extracellular signal-regulated kinase
- G-CSF:
Granulocyte colony-stimulating factor
- GM-CSF:
Granulocyte macrophage colony-stimulating factor
- GSR:
Glutathione-disulfide reductase
- HDAC:
Histone deacetylase
- HIF:
Hypoxia-inducible factor
- HMB-TZD:
5-(4-Hydroxy-2,3,5-trimethylbenzylidene) thiazolidine-2,4-dione
- HMG-CoA:
3-Hydroxyl-3-methylglutaryl coenzyme A
- Hmox1:
Heme oxygenase 1
- IFN:
Interferon
- IKK:
IκB kinase
- IKKβ:
Inhibitor of IKκB kinase
- IL:
Interleukin
- iNOS:
Inducible nitric oxide synthase
- IRAK:
Interleukin receptor-associated kinase
- IRF:
Interferon regulatory factor
- JNK:
Jun N-terminal kinase
- KLF4:
Kruppel-like factor 4
- LDL:
Low-density lipoprotein
- let-7:
Lethal-7
- LPS:
Lipopolysaccharide
- LTB4:
Leukotriene B4
- LXRs:
Liver X receptors
- M-CSF:
Macrophage colony-stimulating factor
- M:
Macrophage
- MAPK:
Mitogen-activated protein kinase
- MCP:
Monocyte chemoattractant protein
- MCP1:
Monocyte chemoattractant protein-1
- MDSCs:
Myeloid-derived suppressor cells
- MED1:
Mediator 1
- MgI1:
Macrophage and granulocyte inducer-form 1
- MIF:
Macrophage migration inhibitory factor
- Mincle:
Macrophage-inducible C-type lectin receptor
- MIP:
Macrophage inflammatory protein
- miRNA/miR:
Micro-RNA
- MMPs:
Metalloproteases
- MØ:
Naïve macrophages
- Mox:
Macrophages exposed to oxidized phospholipids
- MPS:
Mononuclear phagocyte system
- MSPc:
Major surface protein complex
- NF-κB:
Nuclear factor kappa-light-chain-enhancer of activated B-cells
- NFE2L2:
Nuclear factor- (erythroid-derived 2) like 2
- NLRP3:
NLR family pyrin domain containing 3
- NO:
Nitric oxide
- NRP1:
Neuropilin-1
- ox-LDL:
Oxidized LDL
- PD:
Periodontitis
- Pg:
Porphyromonas gingivalis
- PGC-1α:
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PGE2:
Prostaglandin E2
- Pknox1:
PBX/knotted 1 homeobox 1
- PPAR:
Peroxisome proliferator-activated receptor
- PRR:
Pathogen recognition receptor
- RANTES:
Regulated on activation, normal T cell expressed and secreted
- S1P:
Sphingosine1phosphate
- SDF-1:
Stromal cell-derived factor-1
- Sema4D:
Semaphorin 4D
- SFAs:
Saturated fatty acids
- SIRPa:
Signal-regulatory protein
- SMAD:
Small mother against decapentaplegic
- SMLN:
Submandibular lymph node
- SOCS1:
Suppressor of cytokine signaling 1
- Srxn1:
Sulfiredoxin-1
- STAT:
Signal transducers and activators of transcription
- T2D:
Type 2 diabetes
- TAM:
Tumour-associated macrophage
- TEMs:
Tie2-expressing monocytes
- TGF:
Transforming growth factor
- Th:
T helper
- TLR:
Toll-like receptor
- TNF:
Tumour necrosis factor
- TP:
Thymidine phosphorylase
- TRAF:
TNF receptor-associated factor
- Txnrd1:
Thioredoxin reductase 1
- TZD:
Thiazolidinediones
- uPA:
Urokinase-type plasminogen activator
- VCAM:
Vascular cell adhesion molecule
- VEGF:
Vascular endothelial growth factor
- YM1:
Chitinase 3-like 3.
Contributor Information
Luca Parisi, Email: luca.parisi@unimi.it.
Barbara Bassani, Email: babie.bass@gmail.com.
Disclosure
Antonino Bruno was a FIRC (Fondazione Italiana per la Ricerca sul Cancro) fellow and a fellow of Fondazione Umberto Veronesi (FUV).
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Luca Parisi and Elisabetta Gini share equal contribution as first authors. Giampietro Farronato, Antonino Bruno, and Lorenzo Mortara share equal contribution as last authors.
References
- 1.Geissmann F., Manz M. G., Jung S., Sieweke M. H., Merad M., Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginhoux F., Greter M., Leboeuf M., et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon S., Taylor P. R. Monocyte and macrophage heterogeneity. Nature Reviews Immunology. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 4.Davies L. C., Jenkins S. J., Allen J. E., Taylor P. R. Tissue-resident macrophages. Nature Immunology. 2013;14(10):986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dey A., Allen J., Hankey-Giblin P. A. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Frontiers in Immunology. 2014;5:p. 683. doi: 10.3389/fimmu.2014.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheng J., Ruedl C., Karjalainen K. Most tissue-resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity. 2015;43(2):382–393. doi: 10.1016/j.immuni.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Gomez Perdiguero E., Klapproth K., Schulz C., et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epelman S., Lavine K. J., Beaudin A. E., et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40(1):91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto D., Chow A., Noizat C., et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merad M., Manz M. G., Karsunky H., et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nature Immunology. 2002;3(12):1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yona S., Kim K. W., Wolf Y., et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogg D. K., Sibon C., Miled C., et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311(5757):83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 13.Liu K., Victora G. D., Schwickert T. A., et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324(5925):392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garceau V., Smith J., Paton I. R., et al. Pivotal advance: avian colony-stimulating factor 1 (CSF-1), interleukin-34 (IL-34), and CSF-1 receptor genes and gene products. Journal of Leukocyte Biology. 2010;87(5):753–764. doi: 10.1189/jlb.0909624. [DOI] [PubMed] [Google Scholar]
- 15.Bashir S., Sharma Y., Elahi A., Khan F. Macrophage polarization: the link between inflammation and related diseases. Inflammation Research. 2016;65(1):1–11. doi: 10.1007/s00011-015-0874-1. [DOI] [PubMed] [Google Scholar]
- 16.Romagnani S. Th1/Th2 cells. Inflammatory Bowel Diseases. 1999;5(4):285–294. doi: 10.1097/00054725-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Romagnani S. T-cell subsets (Th1 versus Th2) Annals of Allergy, Asthma & Immunology. 2000;85(1):9–18. doi: 10.1016/S1081-1206(10)62426-X. 21. [DOI] [PubMed] [Google Scholar]
- 18.Mills C. D., Kincaid K., Alt J. M., Heilman M. J., Hill A. M. M-1/M-2 macrophages and the Th1/Th2 paradigm. The Journal of Immunology. 2000;164(12):6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Murray P. J., Allen J. E., Biswas S. K., et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujisaka S., Usui I., Bukhari A., et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58(11):2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of Clinical Investigation. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez F. O., Helming L., Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annual Review of Immunology. 2009;27(1):451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 24.Galvan-Pena S., O'Neill L. A. Metabolic reprograming in macrophage polarization. Frontiers in Immunology. 2014;5:p. 420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouhlel M. A., Derudas B., Rigamonti E., et al. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metabolism. 2007;6(2):137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Graff J. W., Dickson A. M., Clay G., McCaffrey A. P., Wilson M. E. Identifying functional microRNAs in macrophages with polarized phenotypes. The Journal of Biological Chemistry. 2012;287(26):21816–21825. doi: 10.1074/jbc.M111.327031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai X., Yin Y., Li N., et al. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. Journal of Molecular Cell Biology. 2012;4(5):341–343. doi: 10.1093/jmcb/mjs044. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Nunez R. T., Louafi F., Sanchez-Elsner T. The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor α1 (IL13Rα1) The Journal of Biological Chemistry. 2011;286(3):1786–1794. doi: 10.1074/jbc.M110.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Litvak V., Ramsey S. A., Rust A. G., et al. Function of C/EBPδ in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nature Immunology. 2009;10(4):437–443. doi: 10.1038/ni.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y. C., Kim I., Lye E., et al. Differential role for c-Rel and C/EBPβ/δ in TLR-mediated induction of proinflammatory cytokines. The Journal of Immunology. 2009;182(11):7212–7221. doi: 10.4049/jimmunol.0802971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang N., Liang H., Zen K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Frontiers in Immunology. 2014;5:p. 614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuch O., Akira S. Epigenetic control of macrophage polarization. European Journal of Immunology. 2011;41(9):2490–2493. doi: 10.1002/eji.201141792. [DOI] [PubMed] [Google Scholar]
- 33.Zhou D., Yang K., Chen L., et al. Promising landscape for regulating macrophage polarization: epigenetic viewpoint. Oncotarget. 2017;8(34):57693–57706. doi: 10.18632/oncotarget.17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoeksema M. A., de Winther M. P. J. Epigenetic regulation of monocyte and macrophage function. Antioxidants & Redox Signaling. 2016;25(14):758–774. doi: 10.1089/ars.2016.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ambade A., Satishchandran A., Saha B., et al. Hepatocellular carcinoma is accelerated by NASH involving M2 macrophage polarization mediated by hif-1αinduced IL-10. OncoImmunology. 2016;5(10, article e1221557) doi: 10.1080/2162402X.2016.1221557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lund G., Andersson L., Lauria M., et al. DNA methylation polymorphisms precede any histological sign of atherosclerosis in mice lacking apolipoprotein E. The Journal of Biological Chemistry. 2004;279(28):29147–29154. doi: 10.1074/jbc.M403618200. [DOI] [PubMed] [Google Scholar]
- 37.Thangavel J., Samanta S., Rajasingh S., et al. Epigenetic modifiers reduce inflammation and modulate macrophage phenotype during endotoxemia-induced acute lung injury. Journal of Cell Science. 2015;128(16):3094–3105. doi: 10.1242/jcs.170258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X., Cao Q., Yu L., Shi H., Xue B., Shi H. Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI Insight. 2016;1(19, article e87748) doi: 10.1172/jci.insight.87748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacKinnon A. C., Farnworth S. L., Hodkinson P. S., et al. Regulation of alternative macrophage activation by galectin-3. The Journal of Immunology. 2008;180(4):2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- 40.Lee J. Y., Kim N. A., Sanford A., Sullivan K. E. Histone acetylation and chromatin conformation are regulated separately at the TNF-α promoter in monocytes and macrophages. Journal of Leukocyte Biology. 2003;73(6):862–871. doi: 10.1189/jlb.1202618. [DOI] [PubMed] [Google Scholar]
- 41.Garrett S., Dietzmann-Maurer K., Song L., Sullivan K. E. Polarization of primary human monocytes by IFN-γ induces chromatin changes and recruits RNA pol II to the TNF-α promoter. The Journal of Immunology. 2008;180(8):5257–5266. doi: 10.4049/jimmunol.180.8.5257. [DOI] [PubMed] [Google Scholar]
- 42.Shakespear M. R., Halili M. A., Irvine K. M., Fairlie D. P., Sweet M. J. Histone deacetylases as regulators of inflammation and immunity. Trends in Immunology. 2011;32(7):335–343. doi: 10.1016/j.it.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Barnes P. J., Adcock I. M., Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. European Respiratory Journal. 2005;25(3):552–563. doi: 10.1183/09031936.05.00117504. [DOI] [PubMed] [Google Scholar]
- 44.Chen X., Barozzi I., Termanini A., et al. Requirement for the histone deacetylase Hdac3 for the inflammatory gene expression program in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(42):E2865–E2874. doi: 10.1073/pnas.1121131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mullican S. E., Gaddis C. A., Alenghat T., et al. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes & Development. 2011;25(23):2480–2488. doi: 10.1101/gad.175950.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruno A., Pagani A., Magnani E., et al. Inflammatory angiogenesis and the tumor microenvironment as targets for cancer therapy and prevention. Cancer Treatment and Research. 2014;159:401–426. doi: 10.1007/978-3-642-38007-5_23. [DOI] [PubMed] [Google Scholar]
- 47.Bruno A., Pagani A., Pulze L., et al. Orchestration of angiogenesis by immune cells. Frontiers in Oncology. 2014;4:p. 131. doi: 10.3389/fonc.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noonan D. M., de Lerma Barbaro A., Vannini N., Mortara L., Albini A. Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer and Metastasis Reviews. 2008;27(1):31–40. doi: 10.1007/s10555-007-9108-5. [DOI] [PubMed] [Google Scholar]
- 49.Koh T. J., DiPietro L. A. Inflammation and wound healing: the role of the macrophage. Expert Reviews in Molecular Medicine. 2011;13, article e23 doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Novak M. L., Koh T. J. Phenotypic transitions of macrophages orchestrate tissue repair. The American Journal of Pathology. 2013;183(5):1352–1363. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zimmermann H. W., Trautwein C., Tacke F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Frontiers in Physiology. 2012;3:p. 56. doi: 10.3389/fphys.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nielsen S. R., Schmid M. C. Macrophages as key drivers of cancer progression and metastasis. Mediators of Inflammation. 2017;2017:11. doi: 10.1155/2017/9624760.9624760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noy R., Pollard J. W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vesely M. D., Kershaw M. H., Schreiber R. D., Smyth M. J. Natural innate and adaptive immunity to cancer. Annual Review of Immunology. 2011;29(1):235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 55.Hanahan D., Weinberg R. A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 56.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 57.Ruffell B., Coussens L. M. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27(4):462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar V., Cheng P., Condamine T., et al. CD45 phosphatase inhibits STAT3 transcription factor activity in myeloid cells and promotes tumor-associated macrophage differentiation. Immunity. 2016;44(2):303–315. doi: 10.1016/j.immuni.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Groblewska M., Mroczko B., Wereszczyńska-Siemiatkowska U., Myśliwiec P., Kedra B., Szmitkowski M. Serum levels of granulocyte colony-stimulating factor (G-CSF) and macrophage colony-stimulating factor (M-CSF) in pancreatic cancer patients. Clinical Chemistry and Laboratory Medicine. 2007;45(1):30–34. doi: 10.1515/CCLM.2007.025. [DOI] [PubMed] [Google Scholar]
- 60.Huang S., Xie K., Bucana C. D., Ullrich S. E., Bar-Eli M. Interleukin 10 suppresses tumor growth and metastasis of human melanoma cells: potential inhibition of angiogenesis. Clinical Cancer Research. 1996;2(12):1969–1979. [PubMed] [Google Scholar]
- 61.Lan C., Huang X., Lin S., et al. Expression of M2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technology in Cancer Research & Treatment. 2013;12(3):259–267. doi: 10.7785/tcrt.2012.500312. [DOI] [PubMed] [Google Scholar]
- 62.Qian B. Z., Pollard J. W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryder M., Ghossein R. A., Ricarte-Filho J. C. M., Knauf J. A., Fagin J. A. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocrine-Related Cancer. 2008;15(4):1069–1074. doi: 10.1677/ERC-08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steidl C., Lee T., Shah S. P., et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. The New England Journal of Medicine. 2010;362(10):875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu J., Escamilla J., Mok S., et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Research. 2013;73(9):2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forssell J., Oberg A., Henriksson M. L., Stenling R., Jung A., Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clinical Cancer Research. 2007;13(5):1472–1479. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 67.Kim S. J., Kim J. S., Papadopoulos J., et al. Circulating monocytes expressing CD31: implications for acute and chronic angiogenesis. The American Journal of Pathology. 2009;174(5):1972–1980. doi: 10.2353/ajpath.2009.080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shoelson S. E., Lee J., Goldfine A. B. Inflammation and insulin resistance. The Journal of Clinical Investigaton. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Welsh T. J., Green R. H., Richardson D., Waller D. A., O'Byrne K. J., Bradding P. Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. Journal of Clinical Oncology. 2005;23(35):8959–8967. doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]
- 70.White E. S., Flaherty K. R., Carskadon S., et al. Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer: role in angiogenesis and prognosis. Clinical Cancer Research. 2003;9(2):853–860. [PubMed] [Google Scholar]
- 71.Pollard J. W. Trophic macrophages in development and disease. Nature Reviews Immunology. 2009;9(4):259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang H., Kim C., Kim M. J., et al. Soluble vascular endothelial growth factor receptor-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Molecular Cancer. 2011;10(1):p. 36. doi: 10.1186/1476-4598-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mescher A. L. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration. 2017;4(2):39–53. doi: 10.1002/reg2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mukwaya A., Peebo B., Xeroudaki M., et al. Factors regulating capillary remodeling in a reversible model of inflammatory corneal angiogenesis. Scientific Reports. 2016;6(1, article 32137) doi: 10.1038/srep32137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ogle M. E., Segar C. E., Sridhar S., Botchwey E. A. Monocytes and macrophages in tissue repair: implications for immunoregenerative biomaterial design. Experimental Biology and Medicine. 2016;241(10):1084–1097. doi: 10.1177/1535370216650293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vannella K. M., Wynn T. A. Mechanisms of organ injury and repair by macrophages. Annual Review of Physiology. 2017;79(1):593–617. doi: 10.1146/annurev-physiol-022516-034356. [DOI] [PubMed] [Google Scholar]
- 77.Wynn T. A., Vannella K. M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang C., He L., He P., et al. Increased drug resistance in breast cancer by tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling pathway. Medical Oncology. 2015;32(2):p. 352. doi: 10.1007/s12032-014-0352-6. [DOI] [PubMed] [Google Scholar]
- 79.Chung F. T., Lee K. Y., Wang C. W., et al. Tumor-associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. International Journal of Cancer. 2012;131(3):E227–E235. doi: 10.1002/ijc.27403. [DOI] [PubMed] [Google Scholar]
- 80.Nowicki A., Szenajch J., Ostrowska G., et al. Impaired tumor growth in colony-stimulating factor 1 (CSF-1)-deficient, macrophage-deficient op/op mouse: evidence for a role of CSF-1-dependent macrophages in formation of tumor stroma. International Journal of Cancer. 1996;65(1):112–119. doi: 10.1002/(SICI)1097-0215(19960103)65:1<112::AID-IJC19>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 81.Ruffell B., Affara N. I., Coussens L. M. Differential macrophage programming in the tumor microenvironment. Trends in Immunology. 2012;33(3):119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ceradini D. J., Kulkarni A. R., Callaghan M. J., et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature Medicine. 2004;10(8):858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 83.Pollard J. W. Tumour-educated macrophages promote tumour progression and metastasis. Nature Reviews Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 84.Sousa S., Maatta J. The role of tumour-associated macrophages in bone metastasis. Journal of Bone Oncology. 2016;5(3):135–138. doi: 10.1016/j.jbo.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagakawa Y., Aoki T., Kasuya K., Tsuchida A., Koyanagi Y. Histologic features of venous invasion, expression of vascular endothelial growth factor and matrix metalloproteinase-2 and matrix metalloproteinase-9, and the relation with liver metastasis in pancreatic cancer. Pancreas. 2002;24(2):169–178. doi: 10.1097/00006676-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 86.Quail D. F., Joyce J. A. Microenvironmental regulation of tumor progression and metastasis. Nature Medicine. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sangaletti S., di Carlo E., Gariboldi S., et al. Macrophage-derived SPARC bridges tumor cell-extracellular matrix interactions toward metastasis. Cancer Research. 2008;68(21):9050–9059. doi: 10.1158/0008-5472.CAN-08-1327. [DOI] [PubMed] [Google Scholar]
- 88.Hildenbrand R., Dilger I., Hörlin A., Stutte H. J. Urokinase and macrophages in tumour angiogenesis. British Journal of Cancer. 1995;72(4):818–823. doi: 10.1038/bjc.1995.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leek R. D., Landers R., Fox S. B., Ng F., Harris A. L., Lewis C. E. Association of tumour necrosis factor alpha and its receptors with thymidine phosphorylase expression in invasive breast carcinoma. British Journal of Cancer. 1998;77(12):2246–2251. doi: 10.1038/bjc.1998.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mantovani A., Marchesi F., Porta C., Sica A., Allavena P. Inflammation and cancer: breast cancer as a prototype. Breast. 2007;16(Supplement 2):S27–S33. doi: 10.1016/j.breast.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 91.Sierra J. R., Corso S., Caione L., et al. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. The Journal of Experimental Medicine. 2008;205(7):1673–1685. doi: 10.1084/jem.20072602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Riabov V., Gudima A., Wang N., Mickley A., Orekhov A., Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Frontiers in Physiology. 2014;5:p. 75. doi: 10.3389/fphys.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Casazza A., Laoui D., Wenes M., et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24(6):695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 94.Ran S., Montgomery K. E. Macrophage-mediated lymphangiogenesis: the emerging role of macrophages as lymphatic endothelial progenitors. Cancer. 2012;4(3):618–657. doi: 10.3390/cancers4030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coffelt S. B., Hughes R., Lewis C. E. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2009;1796(1):11–18. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 96.Gomes F. G., Nedel F., Alves A. M., Nör J. E., Tarquinio S. B. C. Tumor angiogenesis and lymphangiogenesis: tumor/endothelial crosstalk and cellular/microenvironmental signaling mechanisms. Life Science. 2013;92(2):101–107. doi: 10.1016/j.lfs.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scavelli C., Vacca A., di Pietro G., Dammacco F., Ribatti D. Crosstalk between angiogenesis and lymphangiogenesis in tumor progression. Leukemia. 2004;18(6):1054–1058. doi: 10.1038/sj.leu.2403355. [DOI] [PubMed] [Google Scholar]
- 98.De Palma M., Naldini L. Tie2-expressing monocytes (TEMs): novel targets and vehicles of anticancer therapy? Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2009;1796(1):5–10. doi: 10.1016/j.bbcan.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 99.Lewis C. E., Harney A. S., Pollard J. W. The multifaceted role of perivascular macrophages in tumors. Cancer Cell. 2016;30(1):18–25. doi: 10.1016/j.ccell.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mazzieri R., Pucci F., Moi D., et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19(4):512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 101.Wang X., Zhu Q., Lin Y., et al. Crosstalk between TEMs and endothelial cells modulates angiogenesis and metastasis via IGF1-IGF1R signalling in epithelial ovarian cancer. British Journal of Cancer. 2017;117(9):1371–1382. doi: 10.1038/bjc.2017.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chanmee T., Ontong P., Konno K., Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancer. 2014;6(3):1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mortara L., Benest A. V., Bates D. O., Noonan D. M. Can the co-dependence of the immune system and angiogenesis facilitate pharmacological targeting of tumours? Current Opinion in Pharmacology. 2017;35:66–74. doi: 10.1016/j.coph.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 104.Kitamura T., Qian B. Z., Pollard J. W. Immune cell promotion of metastasis. Nature Reviews Immunology. 2015;15(2):73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sica A., Erreni M., Allavena P., Porta C. Macrophage polarization in pathology. Cellular and Molecular Life Sciences. 2015;72(21):4111–4126. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lenz S., Lindenberg S. Is the corpus luteum normal after ovulation induction? Journal of Clinical Ultrasound. 1990;18(3):155–159. doi: 10.1002/jcu.1870180303. [DOI] [PubMed] [Google Scholar]
- 107.Liu J., Zhang N., Li Q., et al. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS One. 2011;6(4, article e19495) doi: 10.1371/journal.pone.0019495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Curiel T. J., Coukos G., Zou L., et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Medicine. 2004;10(9):942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 109.Biswas S. K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature Immunology. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 110.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology. 2002;23(11):549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 111.Marigo I., Dolcetti L., Serafini P., Zanovello P., Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunological Reviews. 2008;222(1):162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 112.Costa-Silva B., Aiello N. M., Ocean A. J., et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature Cell Biology. 2015;17(6):816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gil-Bernabe A. M., Ferjancic S., Tlalka M., et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood. 2012;119(13):3164–3175. doi: 10.1182/blood-2011-08-376426. [DOI] [PubMed] [Google Scholar]
- 114.Kubota K., Moriyama M., Furukawa S., et al. CD163+CD204+tumor-associated macrophages contribute to T cell regulation via interleukin-10 and PD-L1 production in oral squamous cell carcinoma. Scientific Reports. 2017;7(1):p. 1755. doi: 10.1038/s41598-017-01661-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lenz G., Wright G., Dave S. S., et al. Stromal gene signatures in large-B-cell lymphomas. The New England Journal of Medicine. 2008;359(22):2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liguori M., Solinas G., Germano G., Mantovani A., Allavena P. Tumor-associated macrophages as incessant builders and destroyers of the cancer stroma. Cancer. 2011;3(4):3740–3761. doi: 10.3390/cancers3043740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Andon F. T., Digifico E., Maeda A., et al. Targeting tumor associated macrophages: the new challenge for nanomedicine. Seminars in Immunology. 2017 doi: 10.1016/j.smim.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 118.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nature Reviews Clinical Oncology. 2017;14(7):399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pyonteck S. M., Akkari L., Schuhmacher A. J., et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature Medicine. 2013;19(10):1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ries C. H., Cannarile M. A., Hoves S., et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 121.Zheng X., Turkowski K., Mora J., et al. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget. 2017;8(29):48436–48452. doi: 10.18632/oncotarget.17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li X., Yao W., Yuan Y., et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66(1):157–167. doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 123.Loberg R. D., Ying C., Craig M., et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Research. 2007;67(19):9417–9424. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 124.Bonapace L., Coissieux M. M., Wyckoff J., et al. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515(7525):130–133. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- 125.Halama N., Zoernig I., Berthel A., et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell. 2016;29(4):587–601. doi: 10.1016/j.ccell.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 126.Junankar S., Shay G., Jurczyluk J., et al. Real-time intravital imaging establishes tumor-associated macrophages as the extraskeletal target of bisphosphonate action in cancer. Cancer Discovery. 2015;5(1):35–42. doi: 10.1158/2159-8290.CD-14-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Van Acker H. H., Anguille S., Willemen Y., Smits E. L., van Tendeloo V. F. Bisphosphonates for cancer treatment: mechanisms of action and lessons from clinical trials. Pharmacology & Therapeutics. 2016;158:24–40. doi: 10.1016/j.pharmthera.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 128.Beatty G. L., Chiorean E. G., Fishman M. P., et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Beatty G. L., Torigian D. A., Chiorean E. G., et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clinical Cancer Research. 2013;19(22):6286–6295. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Allavena P., Germano G., Belgiovine C., D’Incalci M., Mantovani A. Trabectedin: a drug from the sea that strikes tumor-associated macrophages. OncoImmunology. 2013;2(6, article e24614) doi: 10.4161/onci.24614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Germano G., Frapolli R., Belgiovine C., et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23(2):249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 132.Kammoun H. L., Kraakman M. J., Febbraio M. A. Adipose tissue inflammation in glucose metabolism. Reviews in Endocrine and Metabolic Disorders. 2014;15(1):31–44. doi: 10.1007/s11154-013-9274-4. [DOI] [PubMed] [Google Scholar]
- 133.Chawla A., Nguyen K. D., Goh Y. P. S. Macrophage-mediated inflammation in metabolic disease. Nature Reviews Immunology. 2011;11(11):738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hotamisligil G. S. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 135.Neels J. G., Olefsky J. M. Inflamed fat: what starts the fire? The Journal of Clinical Investigation. 2006;116(1):33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of Clinical Investigation. 2003;112(12):1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xiao X., Gittes G. K. Concise review: new insights into the role of macrophages in β-cell proliferation. Stem Cells Translational Medicine. 2015;4(6):655–658. doi: 10.5966/sctm.2014-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cao X., Han Z. B., Zhao H., Liu Q. Transplantation of mesenchymal stem cells recruits trophic macrophages to induce pancreatic beta cell regeneration in diabetic mice. The International Journal of Biochemistry & Cell Biology. 2014;53:372–379. doi: 10.1016/j.biocel.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 139.Van Gassen N., van Overmeire E., Leuckx G., et al. Macrophage dynamics are regulated by local macrophage proliferation and monocyte recruitment in injured pancreas. European Journal of Immunology. 2015;45(5):1482–1493. doi: 10.1002/eji.201445013. [DOI] [PubMed] [Google Scholar]
- 140.Xiao X., Gaffar I., Guo P., et al. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(13):E1211–E1220. doi: 10.1073/pnas.1321347111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xiao X., Wiersch J., El-Gohary Y., et al. TGFβ receptor signaling is essential for inflammation-induced but not β-cell workload-induced β-cell proliferation. Diabetes. 2013;62(4):1217–1226. doi: 10.2337/db12-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eguchi K., Manabe I., Oishi-Tanaka Y., et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metabolism. 2012;15(4):518–533. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 143.Cucak H., Grunnet L. G., Rosendahl A. Accumulation of M1-like macrophages in type 2 diabetic islets is followed by a systemic shift in macrophage polarization. Journal of Leukocyte Biology. 2014;95(1):149–160. doi: 10.1189/jlb.0213075. [DOI] [PubMed] [Google Scholar]
- 144.Espinoza-Jimenez A., Peon A. N., Terrazas L. I. Alternatively activated macrophages in types 1 and 2 diabetes. Mediators of Inflammation. 2012;2012:p. 10. doi: 10.1155/2012/815953.815953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lumeng C. N., Bodzin J. L., Saltiel A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of Clinical Investigation. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nawaz A., Aminuddin A., Kado T., et al. CD206+ M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nature Communications. 2017;8(1):p. 286. doi: 10.1038/s41467-017-00231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Olefsky J. M., Glass C. K. Macrophages, inflammation, and insulin resistance. Annual Review of Physiology. 2010;72(1):219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 148.Donath M. Y., Shoelson S. E. Type 2 diabetes as an inflammatory disease. Nature Reviews Immunology. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 149.Jung U., Choi M.-S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. International Journal of Molecular Sciences. 2014;15(4):6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nakajima S., Koh V., Kua L. F., et al. Accumulation of CD11c+CD163+ adipose tissue macrophages through upregulation of intracellular 11β-HSD1 in human obesity. The Journal of Immunology. 2016;197(9):3735–3745. doi: 10.4049/jimmunol.1600895. [DOI] [PubMed] [Google Scholar]
- 151.Takikawa A., Mahmood A., Nawaz A., et al. HIF-1α in myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes. 2016;65(12):3649–3659. doi: 10.2337/db16-0012. [DOI] [PubMed] [Google Scholar]
- 152.Zeyda M., Stulnig T. M. Adipose tissue macrophages. Immunology Letters. 2007;112(2):61–67. doi: 10.1016/j.imlet.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 153.Bouloumie A., Curat C. A., Sengenès C., Lolmède K., Miranville A., Busse R. Role of macrophage tissue infiltration in metabolic diseases. Current Opinion in Clinical Nutrition and Metabolic Care. 2005;8(4):347–354. doi: 10.1097/01.mco.0000172571.41149.52. [DOI] [PubMed] [Google Scholar]
- 154.Odegaard J. I., Ricardo-Gonzalez R. R., Red Eagle A., et al. Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. Cell Metabolism. 2008;7(6):496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Odegaard J. I., Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nature Clinical Practice Endocrinology & Metabolism. 2008;4(11):619–626. doi: 10.1038/ncpendmet0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Odegaard J. I., Ricardo-Gonzalez R. R., Goforth M. H., et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Halberg N., Wernstedt-Asterholm I., Scherer P. E. The adipocyte as an endocrine cell. Endocrinology and Metabolism Clinics of North America. 2008;37(3):753–768. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Scherer P. E. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55(6):1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 159.Chen A., Mumick S., Zhang C., et al. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obesity Research. 2005;13(8):1311–1320. doi: 10.1038/oby.2005.159. [DOI] [PubMed] [Google Scholar]
- 160.Weisberg S. P., Hunter D., Huber R., et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. The Journal of Clinical Investigation. 2006;116(1):115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hotamisligil G. S., Arner P., Caro J. F., Atkinson R. L., Spiegelman B. M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of Clinical Investigation. 1995;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Itani S. I., Ruderman N. B., Schmieder F., Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes. 2002;51(7):2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 163.Han M. S., Jung D. Y., Morel C., et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339(6116):218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jourdan T., Godlewski G., Cinar R., et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nature Medicine. 2013;19(9):1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Menghini R., Casagrande V., Menini S., et al. TIMP3 overexpression in macrophages protects from insulin resistance, adipose inflammation, and nonalcoholic fatty liver disease in mice. Diabetes. 2012;61(2):454–462. doi: 10.2337/db11-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Desvergne B. PPARδ/β: the lobbyist switching macrophage allegiance in favor of metabolism. Cell Metabolism. 2008;7(6):467–469. doi: 10.1016/j.cmet.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 167.Zhang M., Zhou Z., Wang J., Li S. MiR-130b promotes obesity associated adipose tissue inflammation and insulin resistance in diabetes mice through alleviating M2 macrophage polarization via repression of PPAR-γ. Immunology Letters. 2016;180:1–8. doi: 10.1016/j.imlet.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 168.Finucane O. M., Reynolds C. M., McGillicuddy F. C., Roche H. M. Insights into the role of macrophage migration inhibitory factor in obesity and insulin resistance. Proceedings of the Nutrition Society. 2012;71(4):622–633. doi: 10.1017/S0029665112000730. [DOI] [PubMed] [Google Scholar]
- 169.Braune J., Weyer U., Hobusch C., et al. IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophages in obesity. The Journal of Immunology. 2017;198(7):2927–2934. doi: 10.4049/jimmunol.1600476. [DOI] [PubMed] [Google Scholar]
- 170.Galic S., Fullerton M. D., Schertzer J. D., et al. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. The Journal of Clinical Investigation. 2011;121(12):4903–4915. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Duncan I. J. H. Designing environments for animals—not for public perceptions. British Veterinary Journal. 1992;148(6):475–477. doi: 10.1016/0007-1935(92)90003-J. [DOI] [PubMed] [Google Scholar]
- 172.Hotamisligil G. S. Inflammatory pathways and insulin action. International Journal of Obesity. 2003;27(Supplement 3):S53–S55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 173.Bleau G., Desaulniers M. High-performance liquid chromatographic assay of benzalkonium in plasma. Journal of Chromatography B: Biomedical Sciences and Applications. 1989;487(1):221–227. doi: 10.1016/S0378-4347(00)83029-7. [DOI] [PubMed] [Google Scholar]
- 174.Dinarello C. A., Donath M. Y., Mandrup-Poulsen T. Role of IL-1β in type 2 diabetes. Current Opinion in Endocrinology, Diabetes, and Obesity. 2010;17(4):314–321. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- 175.Larsen C. M., Faulenbach M., Vaag A., et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. The New England Journal of Medicine. 2007;356(15):1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 176.Thurnau G. R., Scates D. H., Morgan M. A. The fetal-pelvic index: a method of identifying fetal-pelvic disproportion in women attempting vaginal birth after previous cesarean delivery. American Journal of Obstetrics & Gynecology. 1991;165(2):353–358. doi: 10.1016/0002-9378(91)90091-5. [DOI] [PubMed] [Google Scholar]
- 177.Ito A., Suganami T., Yamauchi A., et al. Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. The Journal of Biological Chemistry. 2008;283(51):35715–35723. doi: 10.1074/jbc.M804220200. [DOI] [PubMed] [Google Scholar]
- 178.Oh D. Y., Morinaga H., Talukdar S., Bae E. J., Olefsky J. M. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61(2):346–354. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Saberi M., Woods N. B., de Luca C., et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metabolism. 2009;10(5):419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., Flier J. S. TLR4 links innate immunity and fatty acid-induced insulin resistance. The Journal of Clinical Investigation. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tsukumo D. M. L., Carvalho-Filho M. A., Carvalheira J. B. C., et al. Loss-of-function mutation in toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56(8):1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 182.Mancuso P. The role of adipokines in chronic inflammation. ImmunoTargets and Therapy. 2016;5:47–56. doi: 10.2147/ITT.S73223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Qatanani M., Szwergold N. R., Greaves D. R., Ahima R. S., Lazar M. A. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. The Journal of Clinical Investigation. 2009;119(3):531–539. doi: 10.1172/JCI37273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Suganami T., Nishida J., Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor α. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(10):2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 185.Lainson F. A., Harkins D. C., Wilson C. F., et al. Identification and localization of an iron-regulated 35 kDa protein of Pasteurella haemolytica serotype A2. Journal of General Microbiology. 1991;137(2):219–226. doi: 10.1099/00221287-137-2-219. [DOI] [PubMed] [Google Scholar]
- 186.Consoli A., Formoso G. Do thiazolidinediones still have a role in treatment of type 2 diabetes mellitus? Diabetes, Obesity and Metabolism. 2013;15(11):967–977. doi: 10.1111/dom.12101. [DOI] [PubMed] [Google Scholar]
- 187.Hevener A. L., Olefsky J. M., Reichart D., et al. Macrophage PPARγ is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. The Journal of Clinical Investigation. 2007;117(6):1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hattori Y., Hattori K., Hayashi T. Pleiotropic benefits of metformin: macrophage targeting its anti-inflammatory mechanisms. Diabetes. 2015;64(6):1907–1909. doi: 10.2337/db15-0090. [DOI] [PubMed] [Google Scholar]
- 189.Ohashi W., Hattori K., Hattori Y. Control of macrophage dynamics as a potential therapeutic approach for clinical disorders involving chronic inflammation. The Journal of Pharmacology and Experimental Therapeutics. 2015;354(3):240–250. doi: 10.1124/jpet.115.225540. [DOI] [PubMed] [Google Scholar]
- 190.Feldman M., Jialal I., Devaraj S., Cryer B. Effects of low-dose aspirin on serum C-reactive protein and thromboxane B2concentrations: a placebo-controlled study using a highly sensitive C-reactive protein assay. Journal of the American College of Cardiology. 2001;37(8):2036–2041. doi: 10.1016/S0735-1097(01)01289-X. [DOI] [PubMed] [Google Scholar]
- 191.Sok M. C. P., Tria M. C., Olingy C. E., San Emeterio C. L., Botchwey E. A. Aspirin-triggered resolvin D1-modified materials promote the accumulation of pro-regenerative immune cell subsets and enhance vascular remodeling. Acta Biomaterialia. 2017;53:109–122. doi: 10.1016/j.actbio.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chaudhuri A., Ghanim H., Vora M., et al. Exenatide exerts a potent antiinflammatory effect. The Journal of Clinical Endocrinology & Metabolism. 2012;97(1):198–207. doi: 10.1210/jc.2011-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ferdaoussi M., Abdelli S., Yang J. Y., et al. Exendin-4 protects β-cells from interleukin-1β-induced apoptosis by interfering with the c-Jun NH2-terminal kinase pathway. Diabetes. 2008;57(5):1205–1215. doi: 10.2337/db07-1214. [DOI] [PubMed] [Google Scholar]
- 194.Lodish H. F., Zhou B., Liu G., Chen C. Z. Micromanagement of the immune system by microRNAs. Nature Reviews Immunology. 2008;8(2):120–130. doi: 10.1038/nri2252. [DOI] [PubMed] [Google Scholar]
- 195.Zhuang G., Meng C., Guo X., et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125(23):2892–2903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- 196.Yao F., Yu Y., Feng L., et al. Adipogenic miR-27a in adipose tissue upregulates macrophage activation via inhibiting PPARγ of insulin resistance induced by high-fat diet-associated obesity. Experimental Cell Research. 2017;355(2):105–112. doi: 10.1016/j.yexcr.2017.03.060. [DOI] [PubMed] [Google Scholar]
- 197.Weber C., Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nature Medicine. 2011;17(11):1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 198.Moore K. J., Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Viiri L. E., Full L. E., Navin T. J., et al. Smooth muscle cells in human atherosclerosis: proteomic profiling reveals differences in expression of annexin A1 and mitochondrial proteins in carotid disease. Journal of Molecular and Cellular Cardiology. 2013;54:65–72. doi: 10.1016/j.yjmcc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 200.Tall A. R., Costet P., Wang N. Regulation and mechanisms of macrophage cholesterol efflux. The Journal of Clinical Investigation. 2002;110(7):899–904. doi: 10.1172/JCI0216391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Seimon T. A., Nadolski M. J., Liao X., et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metabolism. 2010;12(5):467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chinetti-Gbaguidi G., Colin S., Staels B. Macrophage subsets in atherosclerosis. Nature Reviews Cardiology. 2015;12(1):10–17. doi: 10.1038/nrcardio.2014.173. [DOI] [PubMed] [Google Scholar]
- 203.Duewell P., Kono H., Rayner K. J., et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hirose K., Iwabuchi K., Shimada K., et al. Different responses to oxidized low-density lipoproteins in human polarized macrophages. Lipids in Health and Disease. 2011;10(1):p. 1. doi: 10.1186/1476-511X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.van Tits L. J. H., Stienstra R., van Lent P. L., Netea M. G., Joosten L. A. B., Stalenhoef A. F. H. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Krüppel-like factor 2. Atherosclerosis. 2011;214(2):345–349. doi: 10.1016/j.atherosclerosis.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 206.Bae Y. S., Lee J. H., Choi S. H., et al. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circulation Research. 2009;104(2):210–218. doi: 10.1161/CIRCRESAHA.108.181040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sottero B., Gamba P., Longhi M., et al. Expression and synthesis of TGFβ1 is induced in macrophages by 9-oxononanoyl cholesterol, a major cholesteryl ester oxidation product. BioFactors. 2005;24(1–4):209–216. doi: 10.1002/biof.5520240125. [DOI] [PubMed] [Google Scholar]
- 208.Hughes J. E., Srinivasan S., Lynch K. R., Proia R. L., Ferdek P., Hedrick C. C. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circulation Research. 2008;102(8):950–8. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Adamson S., Leitinger N. Phenotypic modulation of macrophages in response to plaque lipids. Current Opinion in Lipidology. 2011;22(5):335–342. doi: 10.1097/MOL.0b013e32834a97e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Colin S., Chinetti-Gbaguidi G., Staels B. Macrophage phenotypes in atherosclerosis. Immunological Reviews. 2014;262(1):153–166. doi: 10.1111/imr.12218. [DOI] [PubMed] [Google Scholar]
- 211.De Paoli F., Staels B., Chinetti-Gbaguidi G. Macrophage phenotypes and their modulation in atherosclerosis. Circulation Journal. 2014;78(8):1775–1781. doi: 10.1253/circj.CJ-14-0621. [DOI] [PubMed] [Google Scholar]
- 212.Kadl A., Meher A. K., Sharma P. R., et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circulation Research. 2010;107(6):737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Naito Y., Takagi T., Higashimura Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Archives of Biochemistry and Biophysics. 2014;564:83–88. doi: 10.1016/j.abb.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 214.Kadl A., Sharma P. R., Chen W., et al. Oxidized phospholipid-induced inflammation is mediated by Toll-like receptor 2. Free Radical Biology & Medicine. 2011;51(10):1903–1909. doi: 10.1016/j.freeradbiomed.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Swirski F. K., Libby P., Aikawa E., et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. The Journal of Clinical Investigation. 2007;117(1):195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.de Gaetano M., Crean D., Barry M., Belton O. M1- and M2-type macrophage responses are predictive of adverse outcomes in human atherosclerosis. Frontiers in Immunology. 2016;7:p. 275. doi: 10.3389/fimmu.2016.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Moore K. J., Sheedy F. J., Fisher E. A. Macrophages in atherosclerosis: a dynamic balance. Nature Reviews Immunology. 2013;13(10):709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wilson H. M. Macrophages heterogeneity in atherosclerosis - implications for therapy. Journal of Cellular and Molecular Medicine. 2010;14(8):2055–2065. doi: 10.1111/j.1582-4934.2010.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bai L., Li Z., Li Q., et al. Mediator 1 is atherosclerosis protective by regulating macrophage polarization. Arteriosclerosis, Thrombosis, and Vascular Biology. 2017;37(8):1470–1481. doi: 10.1161/ATVBAHA.117.309672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Choi J. H., Park J. G., Jeon H. J., et al. 5-(4-Hydroxy-2,3,5-trimethylbenzylidene) thiazolidine-2,4-dione attenuates atherosclerosis possibly by reducing monocyte recruitment to the lesion. Experimental & Molecular Medicine. 2011;43(8):471–8. doi: 10.3858/emm.2011.43.8.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Joseph S. B., Castrillo A., Laffitte B. A., Mangelsdorf D. J., Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nature Medicine. 2003;9(2):213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 222.Tangirala R. K., Bischoff E. D., Joseph S. B., et al. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(18):11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nofer J. R., Bot M., Brodde M., et al. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor–deficient mice. Circulation. 2007;115(4):501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- 224.Morimoto K., Janssen W. J., Fessler M. B., et al. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. The Journal of Immunology. 2006;176(12):7657–7665. doi: 10.4049/jimmunol.176.12.7657. [DOI] [PubMed] [Google Scholar]
- 225.Sergin I., Evans T. D., Zhang X., et al. Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis. Nature Communications. 2017;8, article 15750 doi: 10.1038/ncomms15750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ding Y. H., Qian L. Y., Pang J., et al. The regulation of immune cells by Lactobacilli: a potential therapeutic target for anti-atherosclerosis therapy. Oncotarget. 2017;8(35):59915–59928. doi: 10.18632/oncotarget.18346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Page R. C., Schroeder H. E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Laboratory Investigation. 1976;34(3):235–249. [PubMed] [Google Scholar]
- 228.Chapple C. C., Srivastava M., Hunter N. Failure of macrophage activation in destructive periodontal disease. The Journal of Pathology. 1998;186(3):281–286. doi: 10.1002/(SICI)1096-9896(1998110)186:3<281::AID-PATH200>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 229.Page R. C. The role of inflammatory mediators in the pathogenesis of periodontal disease. Journal of Periodontal Research. 1991;26(3):230–242. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 230.Assuma R., Oates T., Cochran D., Amar S., Graves D. T. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. The Journal of Immunology. 1998;160(1):403–409. [PubMed] [Google Scholar]
- 231.Haraszthy V. I., Zambon J. J., Trevisan M., Zeid M., Genco R. J. Identification of periodontal pathogens in atheromatous plaques. Journal of Periodontology. 2000;71(10):1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 232.Li L., Messas E., Batista E. L., Levine R. A., Amar S. Porphyromonas gingivalis infection accelerates the progression of atherosclerosis in a heterozygous apolipoprotein E-deficient murine model. Circulation. 2002;105(7):861–7. doi: 10.1161/hc0702.104178. [DOI] [PubMed] [Google Scholar]
- 233.Seguier S., Gogly B., Bodineau A., Godeau G., Brousse N. Is collagen breakdown during periodontitis linked to inflammatory cells and expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gingival tissue? Journal of Periodontology. 2001;72(10):1398–1406. doi: 10.1902/jop.2001.72.10.1398. [DOI] [PubMed] [Google Scholar]
- 234.Kayal R. A. The role of osteoimmunology in periodontal disease. BioMed Research International. 2013;2013:12. doi: 10.1155/2013/639368.639368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Crotti T. N., Dharmapatni A. A., Alias E., Haynes D. R. Osteoimmunology: major and costimulatory pathway expression associated with chronic inflammatory induced bone loss. Journal of Immunology Research. 2015;2015:13. doi: 10.1155/2015/281287.281287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Charon J., Toto P. D., Gargiulo A. W. Activated macrophages in human periodontitis. Journal of Periodontology. 1981;52(6):328–335. doi: 10.1902/jop.1981.52.6.328. [DOI] [PubMed] [Google Scholar]
- 237.Hanazawa S., Takeshita A., Amano S., et al. Tumor necrosis factor-α induces expression of monocyte chemoattractant JE via fos and jun genes in clonal osteoblastic MC3T3-E1 cells. Journal of Biological Chemistry. 1993;268(13):9526–9532. [PubMed] [Google Scholar]
- 238.Mathur A., Michalowicz B., Castillo M., Aeppll D. Interleukin-1 alpha, interleukin-8 and interferon-alpha levels in gingival crevicular fluid. Journal of Periodontal Research. 1996;31(7):489–495. doi: 10.1111/j.1600-0765.1996.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 239.Hajishengallis G., Darveau R. P., Curtis M. A. The keystone-pathogen hypothesis. Nature Reviews Microbiology. 2012;10(10):717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Socransky S. S., Haffajee A. D., Cugini M. A., Smith C., Kent R. L. Microbial complexes in subgingival plaque. Journal of Clinical Periodontology. 1998;25(2):134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 241.Nichols F. C., Bajrami B., Clark R. B., Housley W., Yao X. Free lipid A isolated from Porphyromonas gingivalis lipopolysaccharide is contaminated with phosphorylated dihydroceramide lipids: recovery in diseased dental samples. Infection and Immunity. 2012;80(2):860–874. doi: 10.1128/IAI.06180-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Burns E., Bachrach G., Shapira L., Nussbaum G. Cutting edge: TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. The Journal of Immunology. 2006;177(12):8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- 243.Hajishengallis G., Wang M., Bagby G. J., Nelson S. Importance of TLR2 in early innate immune response to acute pulmonary infection with Porphyromonas gingivalis in mice. The Journal of Immunology. 2008;181(6):4141–4149. doi: 10.4049/jimmunol.181.6.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Takeuchi O., Hoshino K., Kawai T., et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–451. doi: 10.1016/S1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 245.Papadopoulos G., Weinberg E. O., Massari P., et al. Macrophage-specific TLR2 signaling mediates pathogen-induced TNF-dependent inflammatory oral bone loss. The Journal of Immunology. 2013;190(3):1148–1157. doi: 10.4049/jimmunol.1202511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jandinski J. J., Stashenko P., Feder L. S., et al. Localization of interleukin-1β in human periodontal tissue. Journal of Periodontology. 1991;62(1):36–43. doi: 10.1902/jop.1991.62.1.36. [DOI] [PubMed] [Google Scholar]
- 247.Matsuki Y., Yamamoto T., Hara K. Detection of inflammatory cytokine messenger RNA (mRNA)-expressing cells in human inflamed gingiva by combined in situ hybridization and immunohistochemistry. Immunology. 1992;76(1):42–47. [PMC free article] [PubMed] [Google Scholar]
- 248.Offenbacher S., Katz V., Fertik G., et al. Periodontal infection as a possible risk factor for preterm low birth weight. Journal of Periodontology. 1996;67(10s):1103–1113. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- 249.Offenbacheer S., Odle B. M., Van Dyke T. E. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. Journal of Periodontal Research. 1986;21(2):101–112. doi: 10.1111/j.1600-0765.1986.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 250.Richards D., Rutherford R. B. The effects of interleukin 1 on collagenolytic activity and prostaglandin-e secretion by human periodontal-ligament and gingival fibroblast. Archives of Oral Biology. 1988;33(4):237–243. doi: 10.1016/0003-9969(88)90184-7. [DOI] [PubMed] [Google Scholar]
- 251.Bertolini D. R., Nedwin G. E., Bringman T. S., Smith D. D., Mundy G. R. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986;319(6053):516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- 252.Health J. K., Saklatvala J., Meikle M. C., Atkinson S. J., Reynolds J. J. Pig interleukin 1 (catabolin) is a potent stimulator of bone resorption in vitro. Calcified Tissue International. 1985;37(1):95–97. doi: 10.1007/BF02557686. [DOI] [PubMed] [Google Scholar]
- 253.Merkel K. D., Erdmann J. M., McHugh K. P., Abu-Amer Y., Ross F. P., Teitelbaum S. L. Tumor necrosis factor-α mediates orthopedic implant osteolysis. The American Journal of Pathology. 1999;154(1):203–210. doi: 10.1016/S0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lam R. S., O’Brien-Simpson N. M., Holden J. A., Lenzo J. C., Fong S. B., Reynolds E. C. Unprimed, M1 and M2 macrophages differentially interact with Porphyromonas gingivalis. PLoS One. 2016;11(7, article e0158629) doi: 10.1371/journal.pone.0158629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lam R. S., O’Brien-Simpson N. M., Lenzo J. C., et al. Macrophage depletion abates Porphyromonas gingivalis-induced alveolar bone resorption in mice. The Journal of Immunology. 2014;193(5):2349–2362. doi: 10.4049/jimmunol.1400853. [DOI] [PubMed] [Google Scholar]
- 256.Tam V., O'Brien-Simpson N. M., Chen Y. Y., Sanderson C. J., Kinnear B., Reynolds E. C. The RgpA-Kgp proteinase-adhesin complexes of Porphyromonas gingivalis inactivate the Th2 cytokines interleukin-4 and interleukin-5. Infection and Immunity. 2009;77(4):1451–1458. doi: 10.1128/IAI.01377-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Foey A. D., Habil N., Al-Shaghdali K., Crean S. J. Porphyromonas gingivalis-stimulated macrophage subsets exhibit differential induction and responsiveness to interleukin-10. Archives of Oral Biology. 2017;73:282–288. doi: 10.1016/j.archoralbio.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 258.Chavrier C., Couble M. L., Hartmann D., Grimaud J. A., Magloire H. Immunohistochemical study of types I, III and IV collagen in fibrosis of diseased gingiva during chronic periodontitis: a light and electron microscopic study. Journal of Periodontal Research. 1987;22(1):29–36. doi: 10.1111/j.1600-0765.1987.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 259.Larjava H., Uitto V. J., Haapasalo M., Heino J., Vuento M. Fibronectin fragmentation induced by dental plaque and Bacteroides gingivalis. European Journal of Oral Sciences. 1987;95(4):308–314. doi: 10.1111/j.1600-0722.1987.tb01846.x. [DOI] [PubMed] [Google Scholar]
- 260.Smiley S. T., King J. A., Hancock W. W. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. The Journal of Immunology. 2001;167(5):2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 261.Settem R. P., Honma K., Sharma A. Neutrophil mobilization by surface-glycan altered Th17-skewing bacteria mitigates periodontal pathogen persistence and associated alveolar bone loss. PLoS One. 2014;9(9, article e108030) doi: 10.1371/journal.pone.0108030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Posch G., Sekot G., Friedrich V., et al. Glycobiology aspects of the periodontal pathogen Tannerella forsythia. Biomolecules. 2012;2(4):467–482. doi: 10.3390/biom2040467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sekot G., Posch G., Messner P., et al. Potential of the Tannerella forsythia S-layer to delay the immune response. Journal of Dental Research. 2011;90(1):109–114. doi: 10.1177/0022034510384622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Matsumoto M., Tanaka T., Kaisho T., et al. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. The Journal of Immunology. 1999;163(9):5039–5048. [PubMed] [Google Scholar]
- 265.Chinthamani S., Settem R. P., Honma K., Kay J. G., Sharma A. Macrophage inducible C-type lectin (Mincle) recognizes glycosylated surface (S)-layer of the periodontal pathogen Tannerella forsythia. PLoS One. 2017;12(3, article e0173394) doi: 10.1371/journal.pone.0173394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Cogoni V., Morgan-Smith A., Fenno J. C., Jenkinson H. F., Dymock D. Treponema denticola chymotrypsin-like proteinase (CTLP) integrates spirochaetes within oral microbial communities. Microbiology. 2012;158(Part 3):759–770. doi: 10.1099/mic.0.055939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kurniyati K., Zhang W., Zhang K., Li C. A surface-exposed neuraminidase affects complement resistance and virulence of the oral spirochaete Treponema denticola. Molecular Microbiology. 2013;89(5):842–856. doi: 10.1111/mmi.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Fenno J. C., McBride B. C. Virulence factors of oral treponemes. Anaerobe. 1998;4(1):1–17. doi: 10.1006/anae.1997.0131. [DOI] [PubMed] [Google Scholar]
- 269.Rosen G., Sela M. N., Naor R., Halabi A., Barak V., Shapira L. Activation of murine macrophages by lipoprotein and lipooligosaccharide of Treponema denticola. Infection and Immunity. 1999;67(3):1180–1186. doi: 10.1128/iai.67.3.1180-1186.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Thomas M. V., Puleo D. A. Infection, inflammation, and bone regeneration: a paradoxical relationship. Journal of Dental Research. 2011;90(9):1052–1061. doi: 10.1177/0022034510393967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ruby J., Rehani K., Martin M. Treponema denticola activates mitogen-activated protein kinase signal pathways through Toll-like receptor 2. Infection and Immunity. 2007;75(12):5763–5768. doi: 10.1128/IAI.01117-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Huang G., Shi L. Z., Chi H. Regulation of JNK and p38 MAPK in the immune system: signal integration, propagation and termination. Cytokine. 2009;48(3):161–169. doi: 10.1016/j.cyto.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Miyajima S., Naruse K., Kobayashi Y., et al. Periodontitis-activated monocytes/macrophages cause aortic inflammation. Scientific Reports. 2014;4:p. 5171. doi: 10.1038/srep05171. [DOI] [PMC free article] [PubMed] [Google Scholar]