Abstract
This study examines the safety and efficacy of bortezomib in patients with highly relapsing neuromyelitis optica spectrum disorder.
In neuromyelitis optica spectrum disorder (NMOSD), enhanced plasma cell activity contributes to antiaquaporin-4 autoantibody (AQP4-ab) production. Longitudinal data indicate that 25% to 66% of patients with NMOSD who are treated with azathioprine, rituximab, and other immune-modifying therapies still experience relapses. Here we assess the safety and efficacy of bortezomib, a selective inhibitor of the 26S proteasome subunit, among patients with highly relapsing NMOSD.
Methods
This is a registered longitudinal study from the National Institutes of Health (NCT02893111) that was conducted from December 2015 to January 2017. Five Chinese female patients who satisfied the 2015 diagnostic criteria of NMOSD were enrolled. The study protocol and supporting documentation were approved by the ethical committee of Tianjin Medical University General Hospital. Written informed consent was obtained from each patient or a legally acceptable surrogate. The characteristics of patients and their responsiveness to prior therapies were collected and illustrated in the Table. Study patients received 4 cycles of subcutaneous bortezomib at a dosage of 1 mg/m2 of body surface area on days 1, 4, 8, and 11 per cycle followed by a 10-day treatment-free interval. This intervention was concurrent with an oral corticosteroid or azathioprine regimen.
Table. Characteristics of Patients With Neuromyelitis Optica Spectrum Disorder Receiving Bortezomib Therapy.
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Before BTZ treatment | |||||
| Age at disease onset, y | 41 | 27 | 43 | 36 | 43 |
| Age at BTZ initiation, y | 42 | 43 | 43 | 37 | 53 |
| Anti-AQP4-ab | + | + | + | + | + |
| Duration of disease at BTZ initiation, y | 1.7 | 16.5 | 0.4 | 1.8 | 10.9 |
| Relapses, No. | 5 | 14 | 2 | 4 | 15 |
| Coexisting autoimmune disease | None | None | None | Myasthenia gravis | Sjögren syndrome |
| Coexisting autoantibodies | ANA, SSA | ANA, SSA, Ro-52, RF, and nRNP | ANA | ANA and AChR | ANA, SSA, Ro-52, and SSB |
| EDSS score at BTZ initiation | 6.5 | 5.5 | 6.0 | 2.5 | 5.0 |
| VAS score at BTZ initiation | 6.0 | 6.0 | 6.5 | 4.0 | 7.0 |
| Attacks in the year before BTZ treatment, No. | 3 (1 attack of corpus callosum, 1 of ON, and 1 of LETM) | 3 (1 attack of brain stem attack, 1 of ON, and 1 of LETM) | 2 (1 attack of ON and 1 of LETM) | 3 (2 attacks of LETM and1 of ON) | 3 (2 Attacks of ON and 1 brain stem and subcortical attack) |
| Attack prevention 2 y before BTZ treatment | Prednisone,15-20 mg/d; rituximab to deplete B cell countsa | IV cyclophosphamide, 0.4 g/w, total 10.0 g; prednisone, 15 mg/d; AZA, 3 mg/kg/d | Prednisone, 15 mg/d; AZA, 3 mg/kg/d | Prednisone,15-20 mg/d; AZA, 3 mg/kg/d | Prednisone, 20-30 mg/d; AZA, 3 mg/kg/d; rituximab to deplete B cell countsa |
| Responses to prior therapies 2 y before BTZ treatment | Poor; 1 attack experienced while using rituximab | Poor; 1 attack experienced even while using a combination of prednisone and AZA | Poor; 1 attack experienced when using a combination of prednisone and AZA (unable to tolerate AZA)b | Poor; 2 attacks experienced when combination of prednisone and AZA, but unable to tolerate AZAb | Poor; 2 attacks experienced while using rituximab, or 2 relapses experienced while using a combination of prednisone and AZA |
| Peripheral CD19+ B cells, counts/μLc | 230 | 270 | 374 | 194 | 156 |
| Peripheral CD138+ plasma cells, counts/μL | 6 | 11 | 6 | 7 | 14 |
| 1-Year follow-up after initial BTZ treatment | |||||
| Attacks, No. | 1 (Myelitis) | 0 | 0 | 0 | 0 |
| EDSS score | 3.5 | 4.0 | 1.0 | 0.5 | 3.5 |
| VAS score | 3.5 | 3.0 | 2.0 | 2.5 | 4.0 |
| Peripheral CD19+ B cells, counts/μL | 41 | 25 | 211 | 105 | 16 |
| Peripheral CD138+ plasma cells, counts/μL | 2 | 0 | 3 | 2 | 1 |
| Attack prevention after discontinuation of BTZ | Prednisone, 15 mg/d | AZA, 3 mg/kg/d | Prednisone, 15 mg/d | Prednisone, 15 mg/d | Prednisone, 20 mg/d |
| BTZ-related adverse effects | Headache, common cold | Headache, enterocolitis | Macula-papular rash at the injection site | Common cold | Macula-papular rash at the injection site |
Abbreviations: AChR+, antiacetylcholine receptor antibody; ANA, anti-nuclear antibody; AQP4-ab, antiaquaporin-4 autoantibody; AZA, azathioprine; BTZ, bortezomib; CD, cluster of differentiation; EDSS, Expanded Disability Status Scale; IV, intravenous; LETM, longitudinal extensive transverse myelitis; nRNP, antinuclear ribonucleoprotein antibody; ON, optica neuritis; RF, rheumatic factor; Ro-52, anti-Ro-52 antibody; SSA, anti–Sjögren syndrome A antibody; SSB, anti–Sjögren syndrome B antibody; VAS, visual analog scale.
The patient had ever received rituximab treatment, total 400 mg/cycle, 2 cycles to ensure that CD19+ and CD27+ memory B cells were less than 0.05% of the peripheral blood mononuclear cell count.
The reason for discontinuation of AZA treatment was hepatic dysfunction.
Before receiving a bortezomib infusion, the peripheral blood CD19+ B cells counts of all the 5 patients were in the normal range.
Relapses, Expanded Disability Status Scale scores, and pain levels (visual analog scale) were assessed by 2 experienced neurologists who were blinded to the study. Serum AQP4-ab titers were tested by a green fluorescent protein–AQP4 fluorescence immunoprecipitation assay. Peripheral B cells and plasma cell counts were measured by flow cytometry with anticluster of differentiation (CD) 19 (allophycocyanin; Biolegend) and anti-CD138 (fluorescein isothiocyanate; Biolegend), respectively. Descriptive values are given as medians (interquartile range [IQR]) for continuous variables.
Results
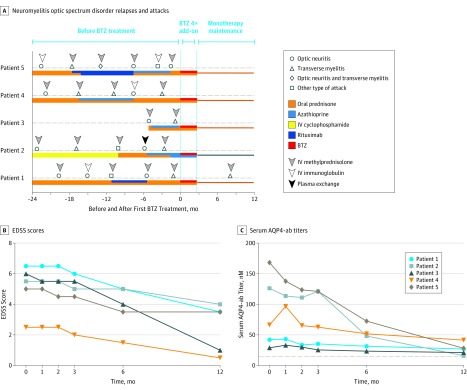
Despite undergoing vigorous therapies (Table), all the patients experienced at least 2 relapses in the previous 6 months or 3 relapses throughout the years (Figure, A). After initiating bortezomib treatment, 4 of the 5 patients, including patient 2, were relapse-free during the 1-year follow-up. A myelitis relapse was only observed in patient 1 when the patient experienced slight paraplegia 10 months following the onset of bortezomib. Magnetic resonance imaging with gadolinium enhancement showed a new lesion in the 12th thoracic–first lumbar segment of the spinal cord. Prompt treatment with intravenous methylprednisolone (500 mg/d) for 5 days ameliorated her symptoms.
Figure. Clinical Course of Patients Before and After Starting Bortezomib (BTZ) as an Escalation Therapy.
A, Neuromyelitis optica spectrum disorder relapses and attacks among patients before, during, and after receiving BTZ treatment. The zero on the x-axis represents the first subcutaneous injection of BTZ. On the line of dashes, different clinical courses or relapses are shown. The colored thread under the dashes represents different medications used throughout patient therapy. The arrowheads indicate the treatment option in acute relapse. After receiving 4 cycles of BTZ treatment, all patients continued to receive the treatment of oral prednisone or azathioprine. IV indicates intravenous. Alteration of Expanded Disability Status Scale (EDSS) scores (B) and serum antiaquaporin-4 autoantibody (AQP4-ab) titers (C) among patients with neuromyelitis optica spectrum disorder receiving BTZ treatment during 1-year follow up. The 0 month represents baseline before BTZ was administered. The cutoff of the negative value of the AQP4-antibody, shown by the dotted line, was set at 15.0nM in serum.
None of the 5 patients experienced further neurological deterioration at the conclusion of the study. The median Expanded Disability Status Scale scores reduced from a median (IQR) of 5.50 (3.75-6.25) at baseline to 3.50 (0.75-4.25) after 1-year follow-up (Figure, B). Their visual analog scale scores also declined from a median (IQR) of 6.0 (5.0-6.75) on study entry to 3.0 (2.25-3.75) after 12 months. Compared with the baseline, serum AQP4-ab titers reduced from a median (IQR) of 66.4nM (35.7-147.3) to 27.1nM (18.7-34.9) after 1 year (median reduction, 59.2%) (Figure, C). Peripheral CD19+ B cell counts declined from a median (IQR) of 230/μL (175-390) to 41/μL (21-158) and CD138+ plasma cell counts decreased from 7/μL (6-13) to 2/μL (1-3) after 1 year. Finally, the observed adverse effects related to bortezomib were mild and transient (Table).
Discussion
The reason that a proportion of patients with NMOSD treated with rituximab still experience attacks may be derived from resistance to CD20 devoid of long-lived plasma cells that are resistant to depletion. Bortezomib can deplete long-lived plasma cells. Our results support this notion and suggest that bortezomib could serve as a promising escalation therapy for highly active NMOSD that does not respond well to or does not tolerate current immunosuppressive treatments. In addition, clinical improvements among the 5 patients were closely associated with the reduction of autoimmune activity, reflected by a decrease in serum AQP4-ab titers, peripheral plasma cell count, and precursor B cells with proteasome inhibition. However, this study is limited by a small and heterogeneous sample size of Asian women. Large-scale randomized clinical trials are further required to generate definitive evidence.
References
- 1.Chihara N, Aranami T, Oki S, et al. . Plasmablasts as migratory IgG-producing cells in the pathogenesis of neuromyelitis optica. PLoS One. 2013;8(12):e83036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papadopoulos MC, Bennett JL, Verkman AS. Treatment of neuromyelitis optica: state-of-the-art and emerging therapies. Nat Rev Neurol. 2014;10(9):493-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wingerchuk DM, Banwell B, Bennett JL, et al. ; International Panel for NMO Diagnosis . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damato V, Evoli A, Iorio R. Efficacy and safety of rituximab therapy in neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. JAMA Neurol. 2016;73(11):1342-1348. [DOI] [PubMed] [Google Scholar]
- 5.Halliley JL, Tipton CM, Liesveld J, et al. . Long-lived plasma cells are contained within the CD19(-)CD38(hi)CD138(+) subset in human bone marrow. Immunity. 2015;43(1):132-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neubert K, Meister S, Moser K, et al. . The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14(7):748-755. [DOI] [PubMed] [Google Scholar]