This cohort study assesses whether retinal layer volumes correlate with immune cell subsets and immunoglobulin indices in cerebrospinal fluid and are associated with disease activity in patients with relapsing-remitting multiple sclerosis.
Key Points
Question
Are retinal layer volumes as measured by optical coherence tomography associated with immunologic variables in the cerebrospinal fluid and with distinct aspects of disease activity in patients with relapsing-remitting multiples sclerosis?
Findings
In this cohort study of 312 patients, atrophy of the ganglion cell layer was associated with increased intrathecal B-cell fractions and was a strong and independent risk factor for confirmed worsening of disability, whereas thickening of the inner nuclear layer correlated with enhanced brain activity at magnetic resonance imaging.
Meaning
Retinal optical coherence tomography might be a convenient tool to estimate different disease activity patterns during multiple sclerosis and might support stratification of patients for different therapies.
Abstract
Importance
Biomarkers to estimate long-term outcomes in patients with multiple sclerosis (MS) and to assign patients to individual treatment regimens are urgently needed.
Objective
To assess whether retinal layer volumes are correlated with immune cell subsets and immunoglobulin indices in the cerebrospinal fluid and whether retinal layer volumes alone or in combination with intrathecal variables are associated with worsening of disease in patients with relapsing-remitting MS.
Design, Setting, and Participants
This observational cohort study included 312 patients with relapsing-remitting MS in 2 independent cohorts (72 patients with short disease duration [cohort 1] and 240 patients with longer disease duration [cohort 2]) treated at a single German university hospital from April 15, 2013, through November 11, 2015.
Main Outcomes and Measures
The common ganglion cell and inner plexiform layer (GCIPL) and inner nuclear layer (INL) volumes were tested for association with the immunoglobulin indices and the frequencies of immune cells in the cerebrospinal fluid (including B cells, T cells, and natural killer cells) (cohort 1). Volumes of GCIPL alone (cohorts 1 and 2) or GCIPL corrected for intrathecal B-cell frequencies (cohort 1) were tested for their association with worsening disability.
Results
A total of 312 patients (212 women [67.9%] and 100 men [32.1%]; median age, 34.0 years [interquartile range (IQR), 28.0-42.0 years]) were available for analysis. In cohort 1 (50 women [69.4%] and 22 men [30.6%]; median age, 31.0 years [IQR, 26.3-38.3 years]), with short disease durations (median, 1.0 months [IQR, 1.0-2.0 months]), low GCIPL volumes were associated with increased intrathecal B-cell frequencies (median, 1.96% [IQR, 1.45%-4.20%]) and intrathecal IgG synthesis (median cerebrospinal fluid/serum IgG index, 0.78 [IQR, 0.53-1.07]). The INL volumes correlated with the frequencies of intrathecal CD56bright natural killer cells (r = 0.28; P = .007). Individuals with low GCIPL volumes (<1.99 mm3) had a 6.4-fold risk for worsening disability during follow-up compared with patients with higher GCIPL values (95% CI, 1.7-24.2; P = .007). This finding was reproduced in cohort 2 (162 women [67.5%] and 78 men [32.5%]; median age, 34.0 years [IQR, 29.0-42.0 years]) consisting of patients with longer disease durations (median, 36.0 months [IQR, 21.0-60.0 months]) (hazard ratio, 2.4; 95% CI, 1.2-4.8; P = .02). In both cohorts, INL volumes correlated with the prospective increase in T2 lesion load and the number of gadolinium-enhancing lesions.
Conclusions and Relevance
Retinal layers reflect different aspects of disease activity during MS. Loss of GCIPL is associated with intrathecal B-cell immunity and constitutes an independent risk factor for worsening disability, whereas high INL volumes are associated with activity on magnetic resonance imaging in the brain parenchyma. Thus, retinal optical coherence tomography might be a means to support stratification of patients with MS for different therapeutic regimens.
Introduction
With the amendment of the therapeutic armamentarium in multiple sclerosis (MS) by novel therapies, stratification of patients for individual therapies becomes an urgent need. Therefore, reliable prognostic markers for assigning patients to individual therapeutic regimens are essential. Cerebral and spinal magnetic resonance imaging (MRI) have long been the primary tool for the evaluation of paraclinical disease activity in MS. Whereas common MRI measures within the white matter reflect inflammatory disease activity, the value of currently used MRI variables to estimate future worsening disability is limited. Imaging techniques for gray matter pathologic features were proposed to better reflect and estimate disability. However, technical and methodologic issues prevent widespread use of this technique beyond experimental settings.
Retinal optical coherence tomography (OCT) is a fast and well-tolerated imaging technique that allows accurate and reproducible quantification of different retinal structures. Optical coherence tomography might be a powerful technique for estimating inflammatory disease activity in MS that could also reflect responses to immunotherapeutic interventions in MS. Recently, reduced peripapillary retinal nerve fiber layer (RNFL) thickness was linked to confirmed worsening of disability in patients with progressive and relapsing-remitting MS in a large multicenter cohort study. In the present study, we investigated whether retinal OCT measures are associated with cellular and humoral inflammatory patterns in the cerebrospinal fluid (CSF) and whether OCT measures alone or in combination with CSF variables are associated with worsening disability in relapsing-remitting MS.
Methods
Study Design
In this prospective longitudinal cohort study, patients with relapsing-remitting MS aged 18 to 60 years and treated in the Department of Neurology, Klinikum Rechts der Isar, Munich, Germany, were included from April 15, 2013, through November 11, 2015. Relapsing-remitting MS was defined using the 2010 McDonald criteria. Exclusion criteria were substantial eye disease, a refractive error of greater than 6 diopters, and neurologic comorbidities, which could affect disability. We excluded eyes with poor OCT quality or with anamnestic or subclinical optic neuritis before enrollment in the study. We followed STROBE statements for reporting cohort studies. The study was approved by the ethics commission of the Technische Universität München and followed the Declaration of Helsinki. All patients provided written informed consent.
Two different longitudinal observational patient cohorts underwent analysis. Cohort 1 consisted of patients with very early relapsing-remitting MS, who had their first clinical relapse within the 3 months before baseline and underwent CSF analysis before inclusion in the study. Patients from cohort 2 did not undergo lumbar puncture before study enrollment and had longer disease durations (≥12 months) (eFigure 1 in the Supplement). At baseline, all patients underwent cerebral MRI, retinal OCT scanning, and clinical examination with recording of the Expanded Disability Status Scale (EDSS score) (range, 0-10, with higher scores indicating a higher grade of disability).
Patients from cohort 1 underwent lumbar puncture before baseline and prebaseline OCT scanning after lumbar puncture. Baseline visits took place at least 30 days after the last clinical relapse and last corticosteroid administration. Follow-up visits were scheduled depending on the clinical presentation. All patients underwent MRI and evaluation of the EDSS score at least once per year. The patients’ disease-modifying therapies (DMTs) were categorized into first-line DMT, including interferon beta-1a/b, glatiramer acetate, dimethyl fumarate, or teriflunomide, and second-line DMT, including natalizumab, fingolimod, alemtuzumab, or rituximab (according to the licensing of immunotherapies in Germany).
The primary clinical outcome variable was confirmed worsening of disability as measured by EDSS. Worsening EDSS was defined as an increase in the EDSS score of at least 1.0 points or at least 0.5 points in patients with an EDSS score of less than 5.5 or at least 5.5, respectively, and confirmed by a visit at least 3 months later. Additional outcome variables were annualized relapse rate, annualized increase in T2 lesion load, annualized number of gadolinium-enhancing (Gd+) lesions, and violation of the no evidence of disease activity 3 (NEDA-3) status, defined as the absence of relapses, confirmed EDSS worsening, Gd+ lesions, and new T2 lesions on cerebral MRI.
Imaging Studies
All MRI scans were acquired on a 3-T scanner (Achieva; Philips) as previously described. Two blinded raters (C.W. and L.A.) manually counted numbers of lesions. For OCT images, we used a spectral domain OCT device (Spectralis; Heidelberg Engineering) as previously described. In brief, evaluation of the peripapillary RNFL was performed using a 3.4-mm ring scan centered on the optic nerve head (automatic real time [ART], 100). The macular area underwent 61 vertical B-scans (scanning angle, 30° × 25°) focusing on the fovea centralis (ART, 13). We checked all examinations for sufficient quality using OSCAR-IB (obvious problems, poor signal strength, centration of scan, algorithm failure, retinal pathologic feature other than MS related, illumination, and beam placement) criteria. According to the OSCR-IB criteria, a signal strength greater than 15 dB was considered as sufficient for retinal segmentation and volume assessment. Every B-scan was segmented automatically into different layers using Eye Explorer software (version 6.0.9.0; Heidelberg Engineering). We checked segmentations manually and corrected in a blinded manner if necessary. Layer volumes were calculated by the software’s segmentation algorithm (6-mm-diameter circle around the fovea).
CSF and Blood Analysis
We obtained CSF and blood samples before initiation of any immunomodulatory therapy. Samples were processed immediately after collection. Concentrations of albumin, IgG, IgM, and IgA were measured in cell-free CSF and serum by using a commercially available analyzer (BNProSpec; Siemens). For cellular analyses, fresh CSF and blood cells (after red blood cell lysis) were washed with 2% fetal calf serum and phosphate-buffered saline and directly processed on ice. Surface staining was performed using CD14-FITC, CD138-PE, CD19-ECD, CD56-APC, CD8-PE-Cy7 (all from Beckman Coulter), CD45-V450, CD4-PerCP, and CD3-APC-Cy7 (all from BD Bioscience) for 30 minutes at 4°C. Samples were analyzed directly after the staining procedure using an advanced digital processing analyzer (CyAn ADP; Beckman Coulter) and FlowJo software (version 10.1; FlowJoj, LLC). Gating strategies and analyzed cell populations are shown in eFigure 2 in the Supplement.
Statistical Analysis
For statistical analyses, we used R software (version 3.2.3; R Core Team 2015) with the survival (version 2.39-5), dynpred, and ppcor (version 1.1) packages. For OCT analysis, we used a previously described statistical approach: mean values of both eyes were calculated and used as an individual data point when both eyes were available for analysis. If one eye was excluded owing to our study criteria, OCT values of the remaining eye were used. For demographic and OCT layer differences at baseline, we performed bivariate analyses with Fisher exact test for categorical and Mann-Whitney test for quantitative variables. Associations of OCT measurements with CSF variables and disease activity were analyzed using partial correlations by Kendall τ rank correlation analysis corrected for age, sex, EDSS score at baseline, disease duration, and treatment group. For disability worsening, Kaplan-Meyer analysis with robust multivariate Cox proportional hazards regression analysis were performed. We systematically corrected for the influence of therapy, age, sex, disease duration, and EDSS values at baseline for each Cox proportional hazards regression model. Values are given as median (25% to 75% interquartile range [IQR]) if not stated otherwise. Statistical significance was established at P < .05.
Results
Study Population
In total, 322 patients with relapsing-remitting MS were recruited for this study from the Department of Neurology, Klinikum Rechts der Isar, Munich, Germany. The mean OCT signal strength of the ring and macular scans in every patient was at least 23 dB. Ten patients and 126 eyes were excluded from the analysis for various reasons (eFigure 1 in the Supplement). Thus, 312 patients were included in the analysis (212 women [67.9%] and 100 men [32.1%]; median age, 34.0 years [IQR, 28.0-42.0 years]). Seventy-two patients with MS (113 eyes) were studied in cohort 1 and 240 patients with MS (385 eyes) in cohort 2. Data from 87 patients have been reported previously.
Patients from cohort 1 had very early MS (median disease duration, 1.0 month [IQR, 1.0-2.0 months]), whereas patients from cohort 2 were older and had a longer disease duration (median, 36.0 months [IQR, 21.0-60.0 months]) (Table 1). A larger fraction of patients from cohort 2 received second-line DMTs. Both groups showed similar EDSS values and annualized relapse rates. Patients from cohort 1 tended to exhibit higher paraclinical disease activity as measured by the increase in T2 lesion load at brain MRI and tended to have higher rates of disability worsening during follow-up (Table 1). The OCT measures at baseline were similar in both cohorts. In both groups, peripapillary RNFL, macular RNFL, and the common ganglion cell and inner plexiform layer (GCIPL) correlated positively with each other, whereas the total macular volume correlated positively with all retinal layers (eTable 1 in the Supplement). In both cohorts, GCIPL volume correlated positively with the inner nuclear layer (INL) (eTable 1 in the Supplement). In summary, our cohorts were representative of patients with MS and mild clinical affection. The fraction of patients with confirmed disability worsening was within the range of recently reported cohorts receiving treatment.
Table 1. Baseline Characteristics of 312 Patients With Relapsing-Remitting Multiple Sclerosis .
| Characteristic | Study Cohorta | P Valueb | |
|---|---|---|---|
| Cohort 1 (n = 72) |
Cohort 2 (n = 240) |
||
| Female, No. (%) | 50 (69.4) | 162 (67.5) | .78 |
| Age, y | 31.0 (26.3-38.3) | 34.0 (29.0-42.0) | .04 |
| Disease duration, mo | 1.0 (1.0-2.0) | 36 (21.0-60.0) | <.001 |
| EDSS scorec | 1.0 (1.0-1.9) | 1.0 (0-2.0) | .85 |
| Disease activity during follow-up | |||
| Duration of follow-up, mo | 17.0 (12.0-25.0) | 23.5 (14.0-25.8) | .02 |
| No. of relapses/y | 0 (0-0.4) | 0 (0-0.4) | .81 |
| No. of new T2 lesions/y | 0.9 (0-2.0) | 0 (0-1.4) | .08 |
| No. of Gd+ lesions/y | 0 (0-0) | 0 (0-0) | .54 |
| Confirmed EDSS worsening, No. (%) | 18 (25) | 37 (15.4) | .08 |
| Disease-modifying therapies, No. (%) | |||
| None | 11 (15.3) | 34 (14.2) | .85 |
| First-line | 54 (75) | 151 (62.9) | .07 |
| Second-line | 7 (9.7) | 55 (22.9) | .02 |
| OCT measurement | |||
| Peripapillary RNFL thickness, µm | 99.0 (92.0-103.8) | 100.5 (92.5-106.5) | .51 |
| TMV, mm3 | 8.7 (8.4-8.9) | 8.7 (8.5-9.0) | .44 |
| Macular RNFL volume, mm3 | 0.88 (0.82-0.96) | 0.86 (0.80-0.93) | .22 |
| GCIPL volume, mm3 | 1.99 (1.88-2.10) | 2.0 (1.88-2.10) | .98 |
| INL volume, mm3 | 0.99 (0.95-1.03) | 0.98 (0.94-1.03) | .42 |
| OPL volume, mm3 | 0.80 (0.76-0.86) | 0.81 (0.77-0.85) | .48 |
| ONL volume, mm3 | 1.78 (1.68-1.90) | 1.82 (1.70-1.95) | .09 |
| PR volume, mm3 | 2.22 (2.19-2.27) | 2.24 (2.20-2.27) | .21 |
Abbreviations: EDSS, Expanded Disability Status Scale; GCIPL, common ganglion cell and inner plexiform layer; Gd+, gadolinium-enhancing; INL, inner nuclear layer; OCT, optical coherence tomography; ONL, outer nuclear layer; OPL, outer plexiform layer; PR, photoreceptor layer; RNFL, macular retinal nerve fiber layer; TMV, total macular volume.
Data are presented as median value (interquartile range) unless otherwise indicated.
Calculated using the Fisher exact test or the Mann-Whitney test.
Scores range from 0 to 10, with higher scores indicating a higher grade of disability.
Correlation of OCT Variables With Patterns of Inflammatory Cells in the CSF
The OCT analysis was performed a median of 23.5 days (IQR, 0-40 days) after lumbar puncture. Twenty of 72 patients in cohort 1 underwent OCT scanning on the day of the lumbar puncture and thus before corticosteroid therapy. Forty-nine of the remaining 52 patients received high-dose methylprednisolone therapy before OCT analysis. Forty-three of these patients underwent an additional OCT scan at least 30 days after corticosteroid pulse therapy. We observed no differences in retinal layer architecture between OCT measurements obtained during and 30 days after corticosteroid pulse therapy. Analysis of CSF samples was available for all 72 patients with MS in cohort 1. Patients showed an inflammatory CSF phenotype with mild pleocytosis (median, 6.0/µL [IQR, 3.0/µL to 12.0/µL]; to convert to 109 per liter, multiply by 0.001), intrathecal IgG synthesis (median CSF/serum IgG index, 0.78 [IQR, 0.53-1.07]), and increased B-cell (median, 1.96% [IQR, 1.45%-4.20%]) and plasmablast (median, 0.52% [IQR, 0.14%-0.94%]) frequencies (eTable 2 in the Supplement).
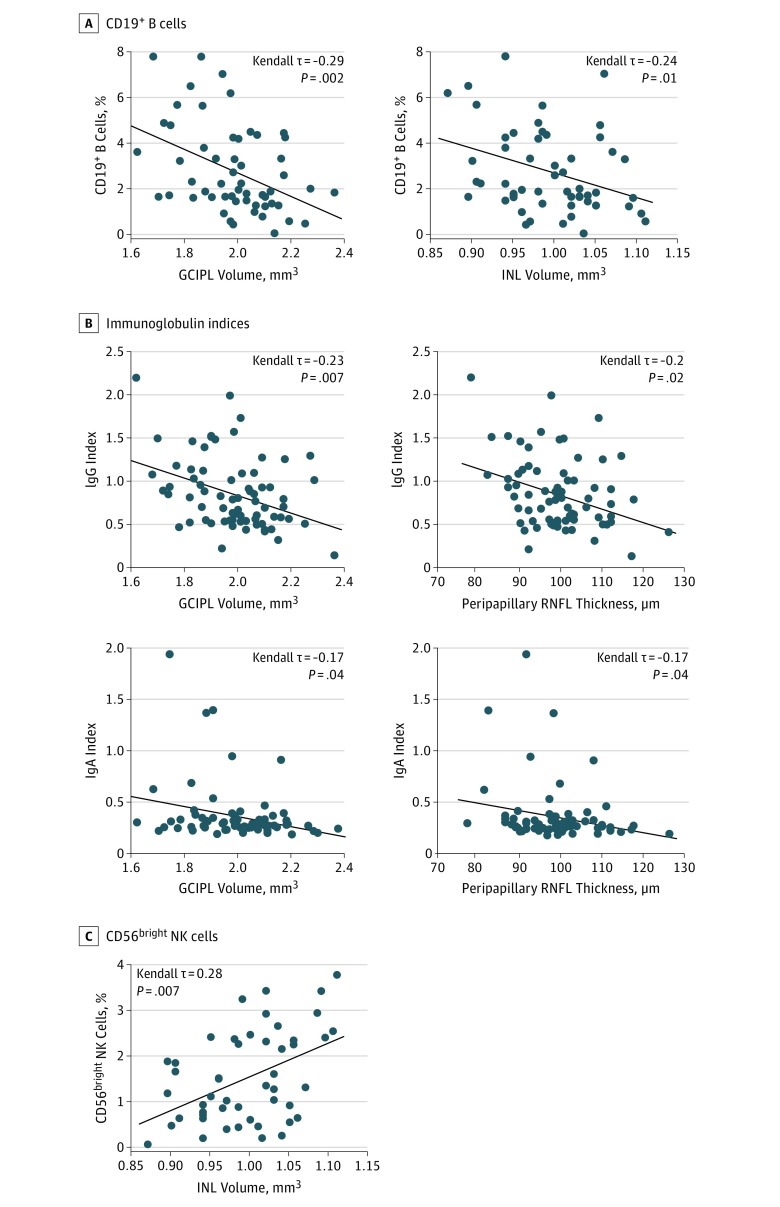
Distinct patterns of CSF immune cells have previously been reported to be associated with disease worsening in MS. Herein, we sought to test whether specific OCT variables correlated with distinct immune cell populations in the CSF. The GCIPL and INL volumes at baseline correlated negatively with the fraction of CD19+ B cells in the CSF (Figure 1A). Low GCIPL and peripapillary RNFL values were associated with higher CSF/serum indices for IgG and IgA (Figure 1B). Total macular volumes correlated negatively with the frequency of intrathecal CD19+ B cells and the CSF/serum IgG index. No further correlations were recognized between OCT and CSF measures (eFigure 3 in the Supplement). Thus, low values for GCIPL and peripapillary RNFL are linked to a pronounced B-cell response in the CSF. Large INL volumes, which have previously been correlated with parenchymal central nervous system (CNS) inflammation as assessed by MRI, were associated with low frequencies of B cells but high frequencies of CD56bright natural killer (NK) cells in the CSF (Figure 1A and C). Together, small GCIPL volumes might indicate an inflammatory milieu in the CSF space with increased fractions of B cells.
Figure 1. Correlation of Retinal Layer Volumes With Cerebrospinal Fluid (CSF) Variables in Early Multiple Sclerosis (MS).
A, Plot of CD19+ B-cell frequency in the CSF and corresponding common ganglion cell and inner plexiform layer (GCIPL) volume or inner nuclear layer (INL) volume in 65 patients (103 eyes) with relapsing-remitting MS. B, Plot of CSF/serum IgG and CSF/serum IgA indices and corresponding GCIPL volume and peripapillary retinal nerve fiber layer (RNFL) thickness in 72 patients (113 eyes) with relapsing-remitting MS. C, Plot of CD56bright natural killer (NK) cell frequency in the CSF and corresponding INL volume in 65 patients (103 eyes) with relapsing-remitting MS. Kendall-rank correlation analysis corrected for age, sex, Expanded Disability Status Scale (EDSS) score at baseline, disease duration, and treatment group. Diagonal line indicates median regression slope.
Correlation of OCT Variables With Subsequent Disease Activity in Early MS
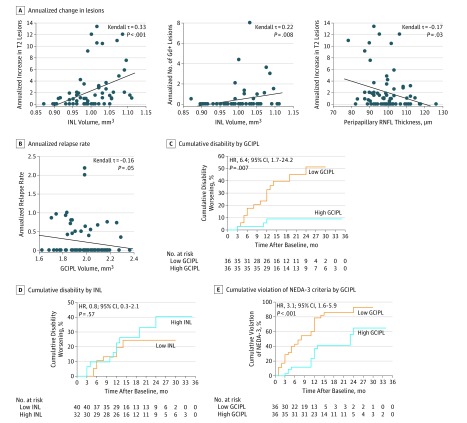
To test whether retinal layers were correlated with worsening of MS, we evaluated baseline retinal layer measurements for their associations with different variables of clinical and paraclinical disease activity in patients with early MS (cohort 1) during follow-up. As expected, INL volumes correlated positively with the increase in T2 lesion load and the number of Gd+ lesions during the 3-year follow-up (Figure 2A). Low peripapillary RNFL values were associated with high annualized numbers of new T2 lesions (Figure 2A). We noticed a weak negative correlation of GCIPL volumes with the annualized relapse rate (Figure 2B). When further assessing clinical variables, we noticed that patients with worsening of EDSS scores had lower GCIPL volumes compared with patients with stable EDSS scores as a unique feature (Table 2). To further test the predictive value of OCT measures for disability worsening, we divided all patients into 2 groups according to the median of their retinal layer measurements. In this analysis, low GCIPL volumes at baseline were associated with an increased risk for confirmed worsening of EDSS scores during the subsequent 3 years. Patients with GCIPL volumes of 1.99 mm3 or lower had a 6.4-fold (Harrell C, 0.752) increased risk for disease worsening compared with patients with higher GCIPL volumes (Figure 2C). This cutoff value revealed a sensitivity of 83.3% and a specificity of 61.1% (area under the curve [AUC], 0.70; positive predictive value [PPV], 42.0%; negative predictive value [NPV], 92.0%; accuracy, 68.1%). This finding even remained robust when correcting for intrathecal B-cell frequency and CSF/serum IgG index (hazard ratio [HR], 7.4; 95% CI, 1.5-36.8; P = .01; Harrell C, 0.747). When combining intrathecal B-cell frequencies and GCIPL volumes, patients with low GCIPL volumes (≤1.99 mm3) and high B-cell frequencies (≥1.96%) had a 5.4-fold increased risk for disease worsening compared with patients who did not meet both criteria (HR, 5.4; 95% CI, 1.9-15.2; P = .002; Harrell C, 0.702; sensitivity, 52.4%; specificity, 84.1%; PPV, 61.1%; NPV, 78.7%; accuracy, 73.8%). Patients with low peripapillary RNFL thicknesses (≤99 µm) tended to have a higher risk for disease worsening than patients with high peripapillary RNFL values (HR, 2.6; 95%, CI 0.9-7.5; P = .07; Harrell C, 0.721). When dividing the cohort into 3 tertiles, patients with the lowest peripapillary RNFL values (≤96 µm) were 4.1-fold more likely to develop disability progression than patients in the highest peripapillary RNFL tertile (>103 µm) (HR, 4.1; 95% CI, 1.1-15.9; P = .04; Harrell C, 0.72). Except for GCIPL, no other retinal layer correlated with worsening of MS (Figure 2D). Furthermore, patients with low GCIPL volumes (≤1.99 mm3) had a 3.1-fold higher risk for violating NEDA-3 criteria than patients with high GCIPL volumes (Harrell C, 0.718; AUC, 0.65; sensitivity, 66.0%; specificity, 80.0%; PPV, 86.1%; NPV, 55.6%; accuracy, 75.4%) (Figure 2E). Thus, low GCIPL volumes represent an independent risk factor for confirmed disability worsening.
Figure 2. Correlation of Baseline Retinal Layer Measures With Prospective Disease Activity Patterns in Early Multiple Sclerosis.
Analysis includes 72 patients (113 eyes) in cohort 1. A, Annualized increase in T2 lesions and number of gadolinium-enhancing (Gd+) lesions as a function of corresponding inner nuclear layer (INL) volumes or peripapillary retinal nerve fiber layer (RNFL) thickness. B, Plot of annualized relapse rate during follow-up vs common ganglion cell and inner plexiform layer (GCIPL) volume at baseline. Kendall rank correlation analysis corrected for age, sex, Expanded Disability Status Scale (EDSS) score at baseline, disease duration, and treatment group (A and B). Diagonal line indicates median regression slope. C, Cumulative fraction of patients with disability worsening (as measured by EDSS scores) by GCIPL volume measurements at baseline. GCIPL values were divided into groups according to the median: 1.62 to 1.99 mm3 (low) and 2.00 to 2.36 mm3 (high). D, Cumulative fraction of patients with disability worsening (as measured by EDSS score) by INL volume measurements at baseline. INL values were divided into groups according to the median: 0.87 to 0.99 mm3 (low) and 1.00 to 1.11 mm3 (high). E, Cumulative fraction of patients with ongoing disease activity (as measured by violation of the no evidence of disease activity 3 [NEDA-3] criteria) in patients stratified according to their GCIPL volume measurements at baseline. GCIPL values were divided into groups according to the median as described in part C. Kaplan-Meier analysis with robust multivariate Cox regression analysis corrected for the covariates age, sex, EDSS score at baseline, disease duration, and treatment group (C-E). HR indicates hazard ratio.
Table 2. Variables Associated With Confirmed Disability Worsening as Measured by EDSS Score in Cohort 1.
| Variable | EDSS Scorea | P Valueb | |
|---|---|---|---|
| Stable (n = 54) |
Worse (n = 18) |
||
| Female, No. (%) | 34 (63) | 16 (88.9) | .07 |
| Age, y | 31.0 (26.0-41.3) | 32.0 (26.5-37.3) | .77 |
| Disease duration, mo | 1.5 (1.0-2.0) | 1.0 (0-2.0) | .21 |
| EDSS scorec | 1.0 (1.0-2.0) | 1.0 (0-1.6) | .38 |
| Disease activity during follow-up | |||
| No. of relapses/y | 0 (0-0) | 0.2 (0-0.9) | .006 |
| No. of new T2 lesions/y | 0.7 (0-2.1) | 1.0 (0-2.4) | .38 |
| No. of Gd+ lesions/y | 0 (0-0) | 0 (0-0.6) | .28 |
| Disease-modifying therapies, No. (%) | |||
| None | 10 (18.5) | 1 (5.6) | .27 |
| First-line | 38 (70.41) | 16 (88.9) | .21 |
| Second-line | 6 (11.1) | 1 (5.6) | .67 |
| OCT measurement | |||
| Peripapillary pRNFL thickness, µm | 99.8 (93.8-108.0) | 96.3 (91.6-100.5) | .09 |
| TMV, mm3 | 8.7 (8.5-9.0) | 8.5 (8.3-8.7) | .06 |
| Macular RNFL volume, mm3 | 0.89 (0.82-0.96) | 0.86 (0.80-0.89) | .14 |
| GCIPL volume, mm3 | 2.01 (1.91-2.10) | 1.92 (1.82-1.98) | .01 |
| INL volume, mm3 | 0.99 (0.96-1.02) | 1.01 (0.93-1.06) | .69 |
| OPL volume, mm3 | 0.79 (0.76-0.85) | 0.81 (0.78-0.86) | .34 |
| ONL volume, mm3 | 1.80 (1.69.1.90) | 1.72 (1.67-1.93) | .50 |
| PR volume, mm3 | 2.23 (2.18-2.27) | 2.22 (2.20-2.25) | .80 |
Abbreviations: See Table 1.
Data are presented as median value (interquartile range) unless otherwise indicated.
Calculated using the Fisher exact test or the Mann-Whitney test.
Scores range from 0 to 10, with higher scores indicating a higher grade of disability.
Correlation of OCT Variables With Subsequent Disease Activity in Longer-Duration MS
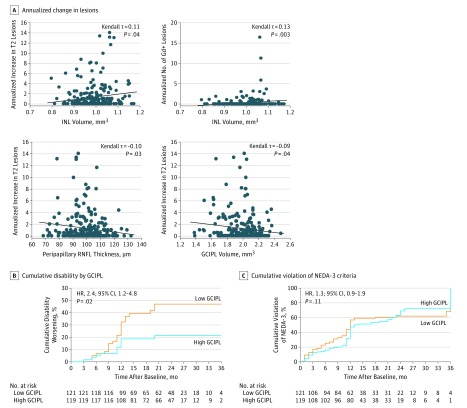
Because we identified GCIPL thinning as a risk factor for disease worsening independently of CSF variables, we also checked the predictive value of GCIPL measures in a second cohort of patients with MS and longer disease durations (cohort 2). Again, peripapillary RNFL thickness and GCIPL volumes correlated negatively with the number of annualized new T2 lesions, and INL volumes correlated positively with the annualized increase in T2 lesion load and the annualized number of Gd+ lesions during follow-up (Figure 3A). Patients whose disease worsened revealed higher annualized relapse rates, more annualized Gd+ lesions, and a greater increase in T2 lesion load (eTable 3 in the Supplement). As in the cohort with early MS, patients with GCIPL volumes of 2.00 mm3 or lower had a 2.4-fold increased risk for worsening of disease (Harrell C, 0.637; AUC, 0.61; sensitivity, 64.9%; specificity, 53.7%; PPV, 20.7%; NPV, 89.9%; accuracy, 55.0%) compared with patients with GCIPL values higher than 2.00 mm3 (Figure 3B). Again, neither stratification above or below the median INL value (HR, 0.8; 95% CI, 0.4-1.4; P = .39; Harrell C, 0.661) nor stratification above or below the median peripapillary RNFL value segregated with prospective confirmed disability worsening (HR, 1.4; 95% CI, 0.8-2.4; P = .27; Harrell C, 0.663). Unlike patients with early MS, low GCIPL volumes were not a risk factor for violating NEDA-3 criteria during the subsequent 3-year follow-up in cohort 2 patients (Harrell C, 0.658) (Figure 3C).
Figure 3. Correlation of Baseline Retinal Layer Measures With Subsequent Disease Activity in Patients With Longer Duration of Multiple Sclerosis.
Analysis includes 240 patients (385 eyes) in cohort 2. A, Plot of annualized increase in T2 lesion numbers and number of gadolinium-enhancing (Gd+) lesions as a function of corresponding inner nuclear layer (INL) volume at baseline (top left and top right), and annualized increase in T2 lesion numbers as a function of corresponding peripapillary retinal nerve fiber layer (RNFL) thickness or common ganglion cell and inner plexiform layer (GCIPL) volume at baseline (bottom left and bottom right). Kendall rank correlation analysis corrected for age, sex, Expanded Disability Status Scale score at baseline, disease duration, and treatment group. Diagonal line indicates median regression slope. B, Cumulative fraction of patients with disability worsening (as measured by EDSS score) in patients stratified according to their GCIPL volume measurements at baseline. GCIPL values were divided into groups according to the median: 1.38 to 2.00 mm3 (low) and 2.01 to 2.48 mm3 (high). C, Cumulative fraction of patients with ongoing disease activity (as measured by violation of no evidence of disease activity [NEDA-3] criteria) in patients stratified according to their GCIPL volume measurements at baseline. GCIPL values were divided into groups according to the median: 1.38 to 2.00 mm3 (low) and 2.01 to 2.48 mm3 (high). Kaplan-Meier analysis with robust multivariate Cox regression analysis corrected for the covariates age, sex, EDSS score at baseline, disease duration, and treatment group. HR indicates hazard ratio.
Discussion
In the present study, we show that retinal layers as measured by OCT are associated with distinct facets of intrathecal immunity and reflect different aspects of disease activity in MS. Low GCIPL volumes are associated with increased frequencies of intrathecal CD19+ B cells and higher rates of intrathecal immunoglobulin synthesis. In addition, low GCIPL values are a risk factor for future confirmed disability worsening. In contrast, high INL measures correlate with frequencies of intrathecal CD56bright NK cells and paraclinical inflammatory disease activity as measured by MRI.
Increased B-cell frequencies in the CSF were previously reported to be linked with disease worsening in patients with MS. Patterns of intrathecal immunoglobulin production were associated with a worse disease course and brain atrophy. More recently, meningeal inflammation has been considered as a main driver of cortical demyelination, which appears to be associated with disability worsening in MS. Meningeal lymphocyte aggregates, which are evident in progressive MS, consist mainly of B cells and might be directly associated with cortical pathologic findings. Whether mechanisms of meningeal immunity directly affect ganglion cells in the retina is not clear. However, cortical lesions in patients with primary progressive MS have recently been shown to be linked to GCIPL atrophy.
Low GCIPL volumes in the absence of former optic neuritis are frequently found in patients with MS, also at the earliest disease stages. In our study, GCIPL atrophy was linked to atrophy patterns in other parts of the CNS. Progressive GCIPL loss is associated with increased disease activity in MS, which correlates with atrophy of brain gray and white matter. Furthermore, low GCIPL measures reflect upper spinal cord atrophy, and spinal cord atrophy is associated with the EDSS score worsening in patients with a clinically isolated syndrome. Our study now links GCIPL loss with B-cell immunity in the CSF. However, GCIPL thinning remained an independent risk factor for subsequent disease worsening when correcting for B-cell frequencies and CSF/serum IgG index. Thus, whether B-cell responses in the CSF and GCIPL atrophy are mechanistically linked needs to be tested in future studies.
The predictive value of GCIPL atrophy for subsequent disease activity was different for both MS cohorts. The effect of GCIPL loss on EDSS worsening was higher in patients with early MS compared with patients with longer disease durations. In early MS stages, GCIPL might even be associated with violation of NEDA-3 criteria, whereas we found no correlation in patients with a longer MS history. Whether higher rates of immunomodulatory treatments in the advanced MS cohort interfere with the predictive value of GCIPL for disease worsening is unclear. A recent study has shown that successful anti-inflammatory treatments in MS translate into decreasing INL volumes. In that study, higher INL volumes were associated with MRI variables indicating active inflammation in the CNS, which is in line with previous reports. However, low INL values do not appear to protect against confirmed disability worsening. This observation is consistent with the clinical-radiologic paradox in MRI: T2 and Gd+ lesions might reflect inflammation in the CNS parenchyma but have a rather poor predictive value for long-term outcomes of disability. Of note, high INL values were associated with high frequencies of CD56bright NK cells in the CSF. CD56bright NK cells are believed to have immunoregulatory functions. Whether high frequencies of CD56bright NK cells in the CSF counterregulate enhanced CNS tissue inflammation remains to be observed. Because INL and GCIPL volumes are positively correlated in patients with MS, distinct combined patterns of B-cell and CD56bright NK–cell frequencies in the CSF might be identifiable that can guide the generation of hypotheses as to specific underlying immunologic mechanisms.
In a recent study, peripapillary RNFL atrophy has already been described as a risk factor for confirmed EDSS worsening in MS. In that study, mainly older patients (40.6 years) with longer disease durations (6.5 years) have been investigated. With use of the top and bottom ranks of a tertile split of the peripapillary RNFL, the bottom peripapillary RNFL values in our study were associated with EDSS scores worsening.
Limitations
Our study has several limitations. First, only a small fraction of patients underwent OCT scanning on the day of the spinal tap. Thus, we cannot exclude an influence of corticosteroid therapy on retinal architecture. However, several patients underwent an additional OCT examination, and we did not see changes in the retinal architecture irrespective of whether the OCT scan was performed before or after corticosteroid therapy. Furthermore, our patients are representative of early disease stages, and our results might not be able to be expanded to progressive disease. However, prognostic markers are particularly valuable in patients with MS at early disease stages to allot them to individual therapies when the therapeutic options are still diverse.
Conclusions
Our study shows that different retinal layers are associated with different patterns of intrathecal immunity and that those OCT measures might be associated with various aspects of disease activity patterns in MS. In particular, we identified GCIPL layer thinning as an independent risk factor for confirmed disability worsening. With a still increasing arsenal of DMTs in MS, OCT could help to identify patients who might benefit most from early therapeutic interventions with compounds that have the highest potential to halt disease worsening. Finally, associations of OCT variables with immune cell populations in the CSF might help to raise informed hypotheses on pathophysiologic links between the immune response in MS and retinal pathologic findings that will have to be tested in future studies.
eFigure 1. Flow Diagram for Recruitment, Inclusion, and Exclusion of Patients
eFigure 2. Gating Strategy for Analysis of Cerebrospinal Fluid Cells by Flow Cytometry
eFigure 3. Correlation of Additional Retinal Layer Volumes With Cerebrospinal Fluid Parameters
eTable 1. Retinal Layer Correlations at Baseline in Cohorts 1 and 2
eTable 2. Cerebrospinal Fluid Parameters Cohort 1
eTable 3. Parameters Associated With Confirmed Disability Worsening as Measured by EDSS in Cohort 2
References
- 1.Housley WJ, Pitt D, Hafler DA. Biomarkers in multiple sclerosis. Clin Immunol. 2015;161(1):51-58. [DOI] [PubMed] [Google Scholar]
- 2.Geurts JJ, Calabrese M, Fisher E, Rudick RA. Measurement and clinical effect of grey matter pathology in multiple sclerosis. Lancet Neurol. 2012;11(12):1082-1092. [DOI] [PubMed] [Google Scholar]
- 3.Biberacher V, Schmidt P, Keshavan A, et al. Intra- and interscanner variability of magnetic resonance imaging based volumetry in multiple sclerosis. Neuroimage. 2016;142:188-197. [DOI] [PubMed] [Google Scholar]
- 4.Balk LJ, Cruz-Herranz A, Albrecht P, et al. Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol. 2016;263(7):1323-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saidha S, Sotirchos ES, Ibrahim MA, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11(11):963-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012;135(pt 6):1786-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knier B, Schmidt P, Aly L, et al. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis [published online August 30, 2016]. Brain. doi: 10.1093/brain/aww219 [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Lapiscina EH, Arnow S, Wilson JA, et al. ; IMSVISUAL consortium . Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15(6):574-584. [DOI] [PubMed] [Google Scholar]
- 9.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7(4):e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petzold A, Wattjes MP, Costello F, et al. The investigation of acute optic neuritis: a review and proposed protocol. Nat Rev Neurol. 2014;10(8):447-458. [DOI] [PubMed] [Google Scholar]
- 12.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 13.Mühlau M, Buck D, Förschler A, et al. White-matter lesions drive deep gray-matter atrophy in early multiple sclerosis: support from structural MRI. Mult Scler. 2013;19(11):1485-1492. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al. ; IMSVISUAL consortium . The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86(24):2303-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gold R, Kappos L, Arnold DL, et al. ; DEFINE Study Investigators . Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098-1107. [DOI] [PubMed] [Google Scholar]
- 16.Montalban X, Comi G, Antel J, et al. Long-term results from a phase 2 extension study of fingolimod at high and approved dose in relapsing multiple sclerosis. J Neurol. 2015;262(12):2627-2634. [DOI] [PubMed] [Google Scholar]
- 17.Cepok S, Jacobsen M, Schock S, et al. Patterns of cerebrospinal fluid pathology correlate with disease progression in multiple sclerosis. Brain. 2001;124(pt 11):2169-2176. [DOI] [PubMed] [Google Scholar]
- 18.Knier B, Berthele A, Buck D, et al. Optical coherence tomography indicates disease activity prior to clinical onset of central nervous system demyelination. Mult Scler. 2016;22(7):893-900. [DOI] [PubMed] [Google Scholar]
- 19.Caroscio JT, Kochwa S, Sacks H, Makuku S, Cohen JA, Yahr MD. Quantitative cerebrospinal fluid IgG measurements as a marker of disease activity in multiple sclerosis. Arch Neurol. 1986;43(11):1129-1131. [DOI] [PubMed] [Google Scholar]
- 20.Magraner MJ, Bosca I, Simó-Castelló M, et al. Brain atrophy and lesion load are related to CSF lipid-specific IgM oligoclonal bands in clinically isolated syndromes. Neuroradiology. 2012;54(1):5-12. [DOI] [PubMed] [Google Scholar]
- 21.Magliozzi R, Howell OW, Reeves C, et al. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010;68(4):477-493. [DOI] [PubMed] [Google Scholar]
- 22.Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134(pt 9):2755-2771. [DOI] [PubMed] [Google Scholar]
- 23.Petracca M, Cordano C, Cellerino M, et al. Retinal degeneration in primary-progressive multiple sclerosis: a role for cortical lesions? Mult Scler. 2017;23(1):43-50. [DOI] [PubMed] [Google Scholar]
- 24.Seigo MA, Sotirchos ES, Newsome S, et al. In vivo assessment of retinal neuronal layers in multiple sclerosis with manual and automated optical coherence tomography segmentation techniques. J Neurol. 2012;259(10):2119-2130. [DOI] [PubMed] [Google Scholar]
- 25.Syc SB, Saidha S, Newsome SD, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135(pt 2):521-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberwahrenbrock T, Ringelstein M, Jentschke S, et al. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler. 2013;19(14):1887-1895. [DOI] [PubMed] [Google Scholar]
- 27.Ratchford JN, Saidha S, Sotirchos ES, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology. 2013;80(1):47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol. 2015;78(5):801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh J, Sotirchos ES, Saidha S, et al. Relationships between quantitative spinal cord MRI and retinal layers in multiple sclerosis. Neurology. 2015;84(7):720-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brownlee WJ, Altmann DR, Alves Da Mota P, et al. Association of asymptomatic spinal cord lesions and atrophy with disability 5 years after a clinically isolated syndrome [published online July 1,2016]. Mult Scler. doi: 10.1177/1352458516663034 [DOI] [PubMed] [Google Scholar]
- 31.Bar-Zohar D, Agosta F, Goldstaub D, Filippi M. Magnetic resonance imaging metrics and their correlation with clinical outcomes in multiple sclerosis: a review of the literature and future perspectives. Mult Scler. 2008;14(6):719-727. [DOI] [PubMed] [Google Scholar]
- 32.Michel T, Poli A, Cuapio A, et al. Human CD56bright NK cells: an update. J Immunol. 2016;196(7):2923-2931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow Diagram for Recruitment, Inclusion, and Exclusion of Patients
eFigure 2. Gating Strategy for Analysis of Cerebrospinal Fluid Cells by Flow Cytometry
eFigure 3. Correlation of Additional Retinal Layer Volumes With Cerebrospinal Fluid Parameters
eTable 1. Retinal Layer Correlations at Baseline in Cohorts 1 and 2
eTable 2. Cerebrospinal Fluid Parameters Cohort 1
eTable 3. Parameters Associated With Confirmed Disability Worsening as Measured by EDSS in Cohort 2