Key Points
Questions
How safe and efficacious is focused ultrasound thalamotomy for managing medically refractory, tremor-dominant Parkinson disease, and what is the magnitude of the placebo response?
Findings
In this 2-center, double-blind, sham-controlled, pilot randomized clinical trial of 27 patients with tremor-dominant Parkinson disease, on-medication Clinical Rating Scale for Tremor A+B treated hand tremor subscores improved a median of 7 points (62%) at 3 months following focused ultrasound thalamotomy and 2 points (22%) following sham procedures, a statistically significant difference. Two cases of transient hemiparesis occurred owing to unrecognized capsular heating.
Meaning
This initial pilot investigation of focused ultrasound thalamotomy suggests preliminary efficacy for the management of medication-refractory, tremor-dominant Parkinson disease; however, a substantial placebo response was observed.
Abstract
Importance
Clinical trials have confirmed the efficacy of focused ultrasound (FUS) thalamotomy in essential tremor, but its effectiveness and safety for managing tremor-dominant Parkinson disease (TDPD) is unknown.
Objective
To assess safety and efficacy at 12-month follow-up, accounting for placebo response, of unilateral FUS thalamotomy for patients with TDPD.
Design, Setting, and Participants
Of the 326 patients identified from an in-house database, 53 patients consented to be screened. Twenty-six were ineligible, and 27 were randomized (2:1) to FUS thalamotomy or a sham procedure at 2 centers from October18, 2012, to January 8, 2015. The most common reasons for disqualification were withdrawal (8 persons [31%]), and not being medication refractory (8 persons [31%]). Data were analyzed using intention-to-treat analysis, and assessments were double-blinded through the primary outcome.
Interventions
Twenty patients were randomized to unilateral FUS thalamotomy, and 7 to sham procedure. The sham group was offered open-label treatment after unblinding.
Main Outcomes and Measures
The predefined primary outcomes were safety and difference in improvement between groups at 3 months in the on-medication treated hand tremor subscore from the Clinical Rating Scale for Tremor (CRST). Secondary outcomes included descriptive results of Unified Parkinson’s Disease Rating Scale (UPDRS) scores and quality of life measures.
Results
Of the 27 patients, 26 (96%) were male and the median age was 67.8 years (interquartile range [IQR], 62.1-73.8 years). On-medication median tremor scores improved 62% (IQR, 22%-79%) from a baseline of 17 points (IQR, 10.5-27.5) following FUS thalamotomy and 22% (IQR, −11% to 29%) from a baseline of 23 points (IQR, 14.0-27.0) after sham procedures; the between-group difference was significant (Wilcoxon P = .04). On-medication median UPDRS motor scores improved 8 points (IQR, 0.5-11.0) from a baseline of 23 points (IQR, 15.5-34.0) following FUS thalamotomy and 1 point (IQR, −5.0 to 9.0) from a baseline of 25 points (IQR, 15.0-33.0) after sham procedures. Early in the study, heating of the internal capsule resulted in 2 cases (8%) of mild hemiparesis, which improved and prompted monitoring of an additional axis during magnetic resonance thermometry. Other persistent adverse events were orofacial paresthesia (4 events [20%]), finger paresthesia (1 event [5%]), and ataxia (1 event [5%]).
Conclusions and Relevance
Focused ultrasound thalamotomy for patients with TDPD demonstrated improvements in medication-refractory tremor by CRST assessments, even in the setting of a placebo response.
Trial Registration
ClinicalTrials.gov identifier NCT01772693
This randomized clinical trial compares focused ultrasound thalamotomy with sham treatment for relieving tremor in patients with medication-refractory, tremor-dominant Parkinson disease.
Introduction
Tremor is a cardinal motor feature of idiopathic Parkinson disease (PD) and occurs variably during its course. Tremor-dominant PD (TDPD) is a clinical subtype distinct from the akinesia/rigidity (AR) and postural instability/gait disorder subtypes. Compared with those with other subtypes, patients with TDPD may experience slower progression of nonmotor symptoms of PD, but the tremor may be more resistant to dopamine-replacement therapy than bradykinesia or rigidity. Although there are procedural risks with the potential for adverse effects, especially with bilateral procedures, deep-brain stimulation (DBS) and thalamic lesioning are both effective invasive therapies for the treatment of motor symptoms in essential tremor (ET) and PD.
There is renewed interest in focused ultrasound (FUS) lesioning because phased-array transducers allow for precise, incisionless, transcranial delivery of acoustic energy. Lesioning can now be monitored in real time by single-section, 2-dimensional magnetic resonance (MR) thermometry; and future incorporation of volumetric MR thermometry will enable more complete control of the process. Martin et al achieved the first transcranial thalamic ablations in patients with neuropathic pain syndromes. Three subsequent pilot studies targeting the ventral intermediate thalamus for ET demonstrated consistent improvements in contralateral appendicular tremor. Recently, a randomized clinical trial demonstrated the safety and efficacy of FUS ventral intermediate thalamotomy for ET, leading to the first US Food and Drug Administration (FDA) approval of FUS ventral intermediate thalamotomy for use in the brain.
This clinical trial was designed to explore the safety and initial efficacy of unilateral FUS thalamotomy for symptom management in patients with TDPD. Although a pilot study, it incorporates a randomized clinical trial design controlled with sham procedures to account for placebo effects that often confound research on PD treatments.
Methods
Overview
The study was designed as a prospective, sham-controlled randomized clinical trial (randomized 2:1) with double-blinded assessments through the 3-month primary end point analysis at 2 US academic medical centers (Figure 1). Patients assigned to a sham procedure were offered open-label treatment after the 3-month blinded assessment. All treated patients were followed unblinded to 1 year. Study oversight was provided by InSightec (Haifa, Israel). Clinical oversight was provided by one of us (W.J.E., the principal investigator) and an independent data safety monitoring board.
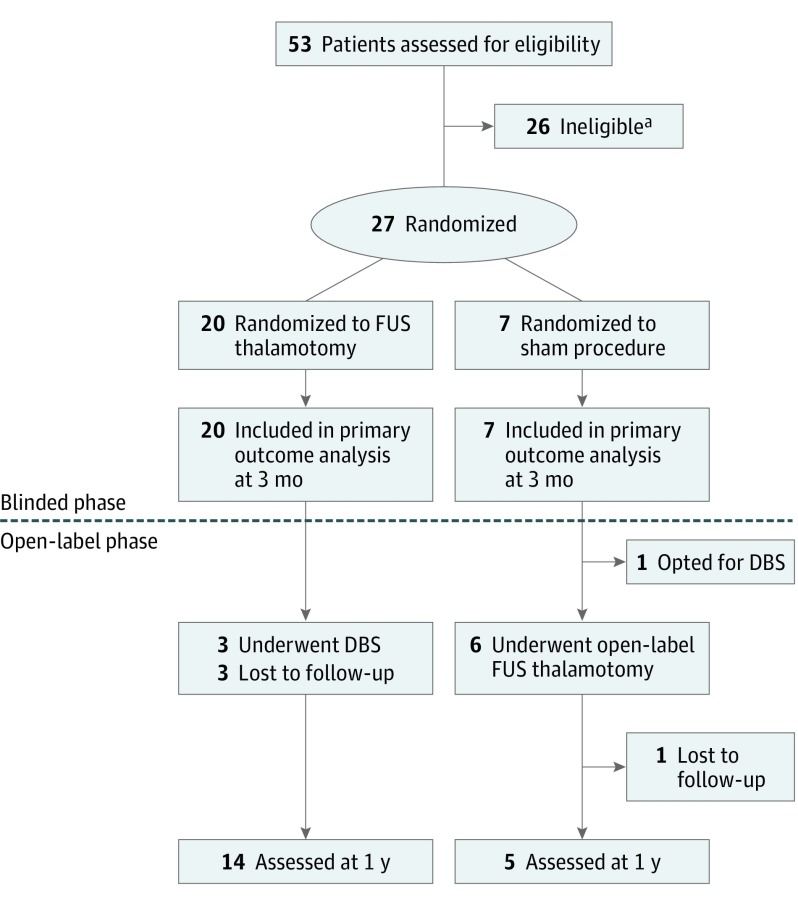
Figure 1. CONSORT Flow Diagram.
DBS indicates deep-brain stimulation; FUS, focused ultrasound.
aScreening failures are listed in eTable 1 in Supplement 2.
Computer randomization was performed by one of us (A.W., study coordinator) and stored electronically. All patients presented on the day of their procedure and were prepared identically with scalp shave, stereotactic frame placement, MR imaging (MRI), and stereotactic planning. Before the initiation of sonications, the treatment team was orally informed of the randomization assignment by the study coordinator. The patient and evaluators remained blinded to the assignment until after the 3-month assessment.
Institutional review board approval of the study protocol (available in Supplement 1) was obtained at both the University of Virginia, Charlottesville, and the Swedish Neuroscience Institute, Seattle, Washington, under an investigational device exemption granted by the FDA. All patients signed informed consent prior to enrollment.
Patients
Adult patients with idiopathic TDPD were included if the disease was deemed medication-refractory, severe, and disabling. The diagnosis was confirmed using the UK Brain Bank Criteria by a movement disorder neurologist. Details of the inclusion and exclusion criteria can be found in eMethods 1 in Supplement 2.
Procedures
The procedure for performing an MR-guided FUS thalamotomy has been described. The detailed steps of the procedure are available in eMethods 2 in Supplement 2. In brief, patients were prepared with scalp shaving and application of a stereotactic head frame under local anesthesia. The patients were positioned supine with a rubber scalp membrane sealed to the midfrequency transducer (InSightec) that operates at 710 kHz with a 3-T MRI system (GE Healthcare).
Initial targeting for FUS thalamotomy was posterior to the midcommissural point by 25% and lateral to midline by 14.0 to 14.5 mm. Therapeutic sonications were administered to the target with incrementally increasing energy. Clinical monitoring of the patient was obtained after each sonication. Tremor was assessed in the resting and postural states as well as with finger-to-nose and drawing tasks. Potential neurologic adverse effects were monitored with sensory and motor testing. Tremor suppression or neurologic signs and symptoms were not typically observed until tissue temperatures exceeded 50°C, at which point the final target ablation was adjusted based on clinical feedback. The goal during treatment was to achieve tremor suppression and an adequate thermal dose to the target. An MRI was performed the following day, after which the patient was discharged.
Patients who received sham treatment were randomized after positioning and stereotactic planning and underwent all stages of treatment, with the sonication power set to zero watts. Patients were clinically assessed at baseline and 1, 3, and 12 months following treatment. Posttreatment MRI was performed at postprocedure day 1, day 30, and 1 year.
Outcomes
All motor assessments were performed in the on-medication state, after FDA review of the protocol, to establish the medication-refractory nature of the tremor. Follow-up assessments were blinded at 1 month and 3 months and unblinded at 1 year. The assessments were timed 1 hour after administration of the patients’ morning dose of PD medications after at least 12 hours without medication.
The primary efficacy outcome was determined by comparing the change from baseline to 3 months in the on-medication treated upper-limb tremor subscore (Clinical Rating Scale for Tremor [CRST] A+B) between FUS thalamotomy and sham procedures using intention-to-treat analysis. The CRST A assesses tremor at rest, posture, and intention; the CRST B assesses tasks including handwriting (dominant hand only), wide and narrow spiral drawings, straight lines, and pouring. The CRST A+B treated hand tremor maximum subscore is 32 points when the dominant hand is treated and 28 points when the nondominant hand is treated. A higher score indicates more severe tremor.
Primary safety outcome was assessed by monitoring the incidence and severity of the procedure-related adverse events from the procedure through 1 year after treatment for all patients. Cognition and mood were monitored with comprehensive neuropsychological assessments, including the Beck Depression Inventory and Montreal Cognitive Assessment at baseline, 3 months, and 12 months.
Predefined, secondary outcomes were also assessed in the on-medication state and included the following: treated hand tremor at rest (Unified Parkinson’s Disease Rating Scale [UPDRS] item 20), treated hand postural or action tremor (UPDRS item 21), UPDRS III motor score (items 18-31), total CRST, level of disability (CRST-C), and quality of life (39-item Parkinson’s Disease Questionnaire [PDQ-39]).
Statistical Analysis
This study was intended to be a pilot study to assess the safety and potential efficacy of FUS thalamotomy in TDPD. A formal power analysis was not performed before initiating the study because we had no information regarding the variability and effect size of the outcome measure. In conjunction with FDA review, a sample size of 30 was planned, with 10 randomized to a sham procedure. A sham procedure arm was implemented to account for potential placebo response in PD.
Baseline characteristics were compared between the 2 groups using the Fischer exact test for categorical variables, and the exact Wilcoxon 2-sample test for continuous variables. Comparative statistics were used for the primary efficacy outcome to gain more insight into the potential treatment and placebo effects in this population. Descriptive statistics were planned for the remainder of the analyses, as this investigation was an early-stage pilot study in 27 patients. For the primary efficacy outcome, the change from baseline in on-medication CRST A+B treated hand tremor subscore between the sham and treatment groups at 3 months, an exact Wilcoxon 2-sample test was performed using intention-to-treat analysis. For safety outcomes, a Fisher exact test was used to assess whether there was a difference in adverse events between the 2 treatment groups.
The statistical analysis was performed using SAS, version 9.4 (SAS Institute Inc) or R 3.4 (The R Foundation for Statistical Computing). Two-sided statistical significance level was set at P < .05. An independent data safety monitoring board reviewed adverse events and severe adverse events throughout the trial.
Results
Twenty-seven patients were enrolled from October 18, 2012, to January 8, 2015, with 20 patients randomized to FUS thalamotomy and 7 patients to sham procedures (Figure 1). There were 26 screening failures, most commonly due to patient withdrawal (8 patients [31%]) and failure to prove medication-refractory status (8 patients [31%]) (eTable 1 in Supplement 2). Twenty-six patients were male (96%), and the median age was 67.8 years (interquartile range [IQR], 62.1-73.8 years). Baseline characteristics between the treatment and sham groups were not statistically different (Table 1).
Table 1. Baseline Demographics and Clinical Characteristicsa.
| Characteristics | FUS Thalamotomy (n = 20) |
Sham Procedure (n = 7) |
|---|---|---|
| Male, No. (%) | 19 (95) | 7 (100) |
| Age, median (IQR), y | 68.1 (63.7-73.3) | 62.4 (50.2-76.2) |
| Dominant hand treated, No. (%) | 18 (90) | 7 (100) |
| Disease duration, median (IQR), y | 5.9 (3.4-9.2) | 6.7 (5.4-8.1) |
| LEDD, median (IQR), mg | 751 (450-950) | 640 (550-1250) |
| CRST A+B treated hand tremor subscore, median (IQR) | 17 (10.5-27.5) | 23 (14-27) |
| CRST disability (part C) score, median (IQR) | 13 (10-18.5) | 17 (13-20) |
| Total CRST score, median (IQR) | 41.5 (28-65) | 48 (43-62) |
| Treated hand UPDRS resting tremor (item 20) score, median (IQR) | 4 (3-4) | 4 (3-4) |
| Treated hand UPDRS postural or action tremor (item 21) score, median (IQR) | 4 (1.5-4) | 4 (2-4) |
| UPDRS motor (part III) score, median (IQR) | 23 (15.5-34) | 25 (15-33) |
| PDQ-39 score, median (IQR) | 21.2 (12.6-32) | 25 (14.8-27.7) |
| MoCA score, median (IQR) | 25.5 (23-27.5) | 27 (23-28) |
| BDI-II score, median (IQR) | 5 (2.5-9) | 5 (4-8) |
Abbreviations: BDI-II, Beck Depression Inventory; CRST, Clinical Rating Scale for Tremor; IQR, interquartile range; LEDD, levodopa-equivalent daily dosage; PDQ-39, 39-item Parkinson’s Disease Questionnaire; MoCA, Montreal Cognitive Assessment; UPDRS, Unified Parkinson’s Disease Rating Scale.
All assessments were performed in the on-medication state.
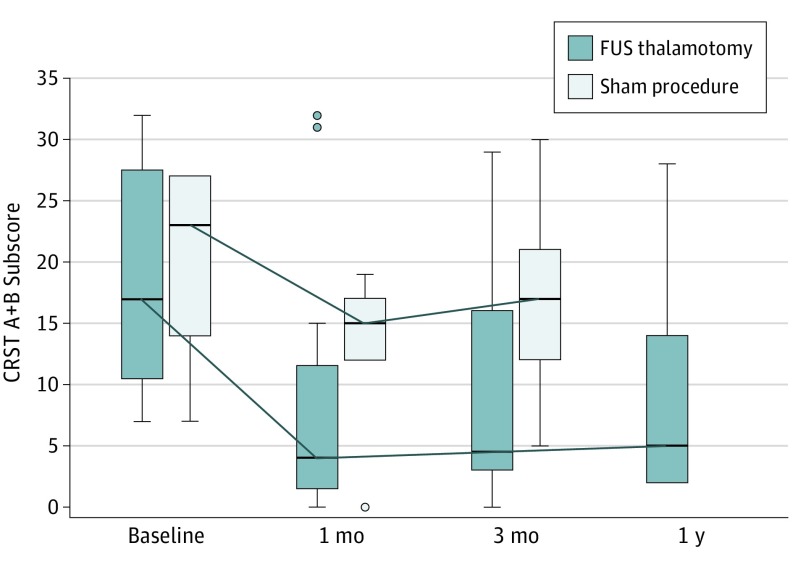
Hand tremor, as measured with the CRST A+B subscores in the on-medication state, improved 62% (IQR, 22%-79%) from a baseline of 17 points (IQR, 10.5-27.5) following FUS thalamotomy and 22% (IQR, −11% to 29%) from a baseline of 23 points (IQR, 14-27) after sham procedures. The between-group difference was significant (exact Wilcoxon 2-sample test between groups, P = .04) (Figure 2 and Table 2).
Figure 2. On-Medication Clinical Rating Scale for Tremor (CRST) A+B Treated Hand Tremor Subscore.
On-medication CRST A+B treated hand tremor subscores for the focused ultrasound (FUS) thalamotomy (n = 20) and sham groups (n = 7) vs time. A notable placebo response was observed, which diminished at 3 months. On-medication median tremor scores improved 62% (interquartile range [IQR], 22%-79%) from a baseline of 17 points (IQR, 10.5-27.5) after FUS thalamotomy and 22% (IQR, −11% to 29%) from a baseline of 23 points (IQR, 14-27) after sham procedures; the between-group difference was significant (Wilcoxon P = .04). The boxes indicate the IQR, the horizontal line in each box, the median; whiskers above and below the boxes, 1.5 times the IQR; and circles, outliers.
Table 2. Clinical Improvements From Baseline to 3 Monthsa.
| Criteria | Median (IQR) | |
|---|---|---|
| FUS Thalamotomy (n = 20) |
Sham Procedure (n = 7) |
|
| CRSTb | ||
| Tremor subscore (CRST A+B), treated handc | 7 (3.5 to 14.0) | 2 (3.0 to 6.0) |
| Tremor subscore (CRST A+B), treated hand, %c | 62 (22.0 to 79.0) | 22 (−11.0 to 29.0) |
| Total CRST | 18 (12.0 to 25.0) | 3 (−4.0 to 17.0) |
| Total CRST, % | 44 (23.0 to 78.0) | 12 (−8.0 to 37.0) |
| UPDRS scores | ||
| Resting tremor (item 20), treated hand | 1.5 (0 to 3.0) | 0 (0 to 0) |
| Postural or action tremor (item 21), treated hand | 1 (0.5 to 2.5) | 0 (0 to 2.0) |
| Motor (part III) subsection | 8 (0.5 to 11.0) | 1 (−5.0 to 9.0) |
| Total UPDRS | 14 (6.5 to 16.0) | 3 (−3.0 to 13.0) |
| Quality of life | ||
| PDQ-39 score | 5.4 (−2.4 to 11.9) | 7.6 (0.9 to 13.0) |
| CRST disability (part C) score | 7.5 (1.0 to 12.5) | 3 (0 to 4.0) |
| Neuropsychological | ||
| MoCA scored | 0 (−1.5 to 2.5) | 1 (−1.0 to 2.0) |
| BDI-II scoree | 0 (−3.0 to 2.0) | 1 (−2.0 to 2.0) |
| LEDD, mg | 0 (0 to 150) | 0 (−200 to 0) |
Abbreviations: BDI-II, Beck Depression Inventory; CRST, Clinical Rating Scale for Tremor; FUS, focused ultrasound; IQR, interquartile range; LEDD, levodopa-equivalent daily dosage; MoCA, Montreal Cognitive Assessment; PDQ-39, 39-item Parkinson’s Disease Questionnaire; UPDRS, Unified Parkinson’s Disease Rating Scale.
All assessments were performed in the on-medication state. Positive improvements represent a decrease in score or dosage from baseline.
Scores for the CRST, UPDRS, and PDQ-39 are explained in the Outcomes subsection of the Methods section.
Predefined primary outcome.
Scores for the MoCA range from 0 to 30, with lower scores indicating impairment.
Scores for the BDI-II range from 0 to 63, with higher scores indicating more depressive symptoms.
Three-month improvements in the predefined secondary outcomes from FUS thalamotomy and sham procedures are reported as descriptive results (Table 2). We observed improvements in all secondary-outcome CRST, UPDRS, and PDQ-39 scores in the treatment group. Following sham procedures, there were lesser improvements in total CRST, UPDRS motor (part III), total UPDRS, and PDQ-39 and no improvements in the UPDRS treated hand resting tremor (item 20) and postural or action tremor (item 21). Montreal Cognitive Assessment and Beck Depression Inventory II score changes were similar in both groups. Complete descriptive results from all assessments can be found in eTable 2 in Supplement 2.
Adverse events are segregated into 2 categories: thalamotomy-related (owing to the creation of a thalamic lesion) and MRI/ultrasound-related (owing to the procedure environment) (Table 3 and eTable 3 in Supplement 2). There were no statistical differences in the adverse events between the blinded thalamotomy and the blinded sham procedure groups (Fisher exact test: all P > .05). The most common thalamotomy-related adverse events for all 26 patients treated (blinded FUS thalamotomy [20 patients] and open-label FUS thalamotomy [6 patients]) were finger paresthesia (10 patients [39%]), ataxia (9 patients [35%]), and orofacial paresthesia (7 patients [27%]). Paresthesia persisted to 1 year in 19% of patients and ataxia, in 4%. Headache (65%) and dizziness/vertigo (42%) were common MRI/ultrasonography-related events, and these resolved by the completion of the procedure. Eight severe adverse events were reported in 4 patients, and 3 were thalamotomy-related. Two patients had persistent mild hemiparesis with gradual improvement almost to their baseline during the study but exhibited tone asymmetries at last follow-up. One of these patients also had an associated persistent mild ataxia. Unrelated serious adverse events included cholecystitis, worsening degenerative knee disease, and a transient ischemic attack. One patient with a history of stable treated depression experienced worsening depressive symptoms that were attributed to the discontinuation of his antidepressant medication within the first month following FUS thalamotomy.
Table 3. Adverse Events in FUS Thalamotomy and Sham Procedure.
| Event | No. (%) | |
|---|---|---|
| FUS Thalamotomy (n = 20) |
Sham Procedure (n = 7) |
|
| Thalamotomy Relateda | ||
| Finger paresthesia | ||
| Transientb | 7 (35) | NA |
| Persistentc | 1 (5) | NA |
| Orofacial paresthesia | ||
| Transient | 1 (5) | NA |
| Persistent | 4 (20) | NA |
| Ataxia | ||
| Transient | 8 (40)d | NA |
| Persistent | 1 (5) | NA |
| Hemiparesis | ||
| Transient | 2 (10) | NA |
| Persistent | 2 (10)e | NA |
| Dysmetria, transient | 1 (5) | NA |
| Mild vocal change, persistent | 1 (5) | NA |
| MRI or Ultrasonography Related, All Transientf | ||
| Scalp numbness | 1 (5) | 0 |
| Headache | 12 (60) | 3 (43) |
| Dizziness or vertigo | 8 (40) | 1 (14) |
| Head pain or heat sensation | 3 (15) | 0 |
| Stomach pain or nausea or emesis | 4 (20) | 1 (14) |
| Tinnitus | 0 | 0 |
| Periorbital swelling | 2 (10) | 0 |
| Neck or back or shoulder pain | 4 (20) | 1 (14) |
| Decline in mental status | 1 (5) | 0 |
| Pin site pain | 1 (5) | 2 (28) |
| Anxiety | 1 (10) | 0 |
| Light headedness | 2 (10) | 0 |
| Right-sided ecchymosis | 1 (5) | 0 |
| Spot in visual field | 1 (5) | 0 |
| Unrelated | ||
| Decline in visuospatial abilities | 1 (5) | 0 |
| Decline in executive function | 0 | 0 |
| Transient ischemic attack | 0 | 0 |
| Brief loss of reality | 1 (5) | 0 |
| Increased daytime sleepiness | 1 (5) | 0 |
| Decreased hand dexterity | 2 (10) | 0 |
| Worsening degenerative knee disease | 1 (5)f | 0 |
| Cholecystitis or cholecystectomy | 1 (5)f | 0 |
| Worsening of depression | 1 (5)f | 0 |
Abbreviations: FUS, focused ultrasound; MRI, magnetic resonance imaging; NA, not applicable.
From the creation of a thalamic lesion.
Transient in all cases was defined as resolved during the 1-year study.
Persistent in all cases was defined as still present at last follow-up.
One patient reported as a serious adverse event.
Reported as a serious adverse event.
From the procedure environment.
Although this pilot study was initially designed for randomization of 30 patients, slow enrollment limited the study to 27 randomized patients. All the patients were available for the primary analysis at 3 months. After unblinding, 6 of the 7 patients who received sham procedures crossed over to undergo open-label treatment and the other patient opted for DBS at a more local institution. Six of 20 patients in the treatment group did not complete 1-year assessments. Two patients had successful outcomes at 3 months but did not return for their 1-year assessment (their on-medication CRST A+B treated hand tremor subscores were reduced from 28 to 0 and from 31 to 1). One patient had a marginal improvement in tremor (30 to 27) and sought treatment at another facility. Three patients had inadequate improvement or worsening tremor scores (their scores changed from 26 to 25, 27 to 29, and 11 to 7 from baseline to 3 months), and underwent DBS (2 unilateral subthalamic nucleus, 1 bilateral ventral intermediate) (Figure 1).
We performed a responder analysis and arbitrarily defined a successful outcome as having a 50% reduction in the on-medication CRST A+B treated hand tremor subscores from baseline to 1 year. Of the 20 patients in the treatment arm, 14 patients were available for unblinded 1-year assessments. With the use of intention-to-treat analysis with the last observation carried forward, 13 patients (65%) had a positive outcome. According to a worst-case analysis that assumes that treatment failed in all 6 patients not assessed at 1 year, 11 patients (55%) would have a successful outcome. Similar results were noted in the open-label crossover group (eTable 4 in Supplement 2).
Discussion
This randomized clinical trial of unilateral FUS thalamotomy for patients with TDPD, controlled with sham procedures and double-blinded assessments, demonstrated a 62% median improvement in contralateral hand tremor CRST subscores in the FUS thalamotomy group and 22% median improvement in the sham group. The between-group difference, predefined as the primary efficacy outcome, was similar to findings from a recent randomized clinical trial of FUS thalamotomy for ET (47%). This TDPD trial measured a placebo effect in the sham group that was not present in the ET study but which is consistent with observations from other sham surgery–controlled trials in PD in which improvements have been shown in UPDRS assessments. As noted, this was a pilot study to assess safety and feasibility and to obtain estimates of variability and effect size of the outcome measures in a TDPD population. Because comparative statistics were only planned for the primary outcome variable, efficacy conclusions are not claimed.
The CRST, originally proposed as a scale for both ET and Parkinson tremor, has been used for the evaluation of hyperkinetic disorders and in some DBS studies of PD. The CRST has not been widely used in Parkinson disease because the UPDRS is a multidomain assessment. We selected the CRST as our primary outcome measure because the TDPD subtype is primarily disabled by tremor, and the use of the CRST provides a good measure of the disability associated with tremor in these patients.
The UPDRS motor subscore (part III) was used as a secondary outcome measure. For completeness, CRST and UPDRS are reported to most fully assess all clinical aspects of patients with TDPD and their response to FUS thalamotomy. On-medication median UPDRS motor scores improved 8 points following FUS thalamotomy and 1 point after sham procedures. This difference between treatment and sham cohorts, although not statistically analyzed, exceeds the minimal clinically important difference of 2.5 for the UPDRS motor scores. Furthermore, median UPDRS tremor scores (items 20 and 21) improved following the treatment but not following sham procedures.
The most common adverse effects of the FUS thalamotomy procedure were finger paresthesia, ataxia, and orofacial paresthesia. Most of these effects were mild or transient, but persistent paresthesia and ataxia occurred in 19% and 4%, respectively. Early in the study, there were 2 patients (8%) with mild hemiparesis caused by unrecognized heating of the internal capsule lateral to the thalamic target. Magnetic resonance thermometry is currently limited to a single-section image where the plane of acquisition is designated before each sonication. We have now implemented frequent temperature measurements in orthogonal planes during the procedure to mitigate the risk for heating outside of the MR thermometry plane.
As an early-stage pilot study, this trial was conducted in a rigorous fashion as a double-blind randomized clinical trial. The control arm involved sham procedures to assess for placebo responses. The cohorts were well matched, with no significant differences in their demographic or baseline characteristics. All patients were available for the primary analysis. Neuropsychological assessments confirmed that there were no significant changes in mental status, global cognitive abilities, or depression from the FUS thalamotomy procedure.
Deep-brain stimulation is the most commonly used procedural treatment in patients with PD owing to its demonstrated safety and efficacy profile; nevertheless, some patients are fearful or avoidant of the invasiveness of the procedure, and its availability to neurologists with programming expertise can be limited. Unilateral FUS thalamotomy does not preclude subsequent internal globus pallidus or subthalamic nucleus DBS if additional symptoms develop with disease progression. There may be cases for which DBS may not be a preferred option. As a therapy dependent on implanted devices, DBS has some issues that do not occur with lesioning, including stimulation tolerance, hardware-related complications, infection, expense, maintenance demands, and other risks. Gamma knife thalamotomy can also suppress tremor, but its widespread acceptance has been limited by its latent radiation effects and the inability to confirm targeting with intraprocedural testing.
The rationale for targeting the thalamus in PD initially with this technology is intended to build on our initial experience with successfully lesioning this structure for ET. We believe that the ventral intermediate thalamus is the safest target to manage PD tremor compared with the internal globus pallidus or the subthalamic nucleus. The future applicability of FUS thalamotomy in the population of patients with PD is likely limited to a well-selected subset of patients in whom unilateral tremor reduction is sufficient to improve quality of life. This can include patients in whom bradykinesia, rigidity, or gait dysfunction due to PD is well controlled with dopamine-replacement therapy but medication-refractory tremor remains problematic, or in patients with advanced PD and comorbid medical conditions in whom palliative tremor reduction and avoidance of general anesthesia is indicated.
Limitations
The trial was limited by small size, and the planned study enrollment of 30 patients was not reached. Medication dose was not fixed during the trial, potentially confounding the results. The trial was not designed to compare FUS thalamotomy with other treatments, such as DBS or gamma knife radiosurgery.
Conclusions
Preliminary results from this randomized clinical trial on the efficacy of unilateral FUS thalamotomy for the treatment of patients with TDPD are encouraging. A notable placebo response was observed with sham procedures, necessitating a larger study to prove efficacy. Adverse events were similar to those of other thalamotomy procedures and will likely further improve as the technology for monitoring the FUS thalamotomy procedure improves.
Trial Protocol
eMethods 1. Inclusion and Exclusion Criteria
eMethods 2. Details of the MR-Guided FUS Thalamotomy Procedure
eTable 1. Screen Failures
eTable 2. Clinical Outcomes
eTable 3. Adverse Events for Open-Label Crossover Patients
eTable 4. Clinical Outcomes of Open-Label, Crossover FUS Thalamotomy
References
- 1.Stochl J, Boomsma A, Ruzicka E, Brozova H, Blahus P. On the structure of motor symptoms of Parkinson’s disease. Mov Disord. 2008;23(9):1307-1312. [DOI] [PubMed] [Google Scholar]
- 2.Zaidel A, Arkadir D, Israel Z, Bergman H. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr Opin Neurol. 2009;22(4):387-393. [DOI] [PubMed] [Google Scholar]
- 3.Marras C. Subtypes of Parkinson’s disease: state of the field and future directions. Curr Opin Neurol. 2015;28(4):382-386. [DOI] [PubMed] [Google Scholar]
- 4.Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med. 2000;342(7):461-468. [DOI] [PubMed] [Google Scholar]
- 5.Pahwa R, Lyons KE, Wilkinson SB, et al. Comparison of thalamotomy to deep brain stimulation of the thalamus in essential tremor. Mov Disord. 2001;16(1):140-143. [DOI] [PubMed] [Google Scholar]
- 6.Huss DS, Dallapiazza RF, Shah BB, Harrison MB, Diamond J, Elias WJ. Functional assessment and quality of life in essential tremor with bilateral or unilateral DBS and focused ultrasound thalamotomy. Mov Disord. 2015;30(14):1937-1943. [DOI] [PubMed] [Google Scholar]
- 7.Hynynen K, McDannold N, Clement G, et al. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain—a primate study. Eur J Radiol. 2006;59(2):149-156. [DOI] [PubMed] [Google Scholar]
- 8.Jolesz FA, Bleier AR, Lauter RS. Laser surgery benefits from guidance by MR. Diagn Imaging (San Franc). 1990;12(9):103-108. [PubMed] [Google Scholar]
- 9.Jolesz FA, Blumenfeld SM. Interventional use of magnetic resonance imaging. Magn Reson Q. 1994;10(2):85-96. [PubMed] [Google Scholar]
- 10.Jolesz FA, McDannold N. Current status and future potential of MRI-guided focused ultrasound surgery. J Magn Reson Imaging. 2008;27(2):391-399. [DOI] [PubMed] [Google Scholar]
- 11.Jolesz FA, Moore GJ, Mulkern RV, et al. Response to and control of destructive energy by magnetic resonance. Invest Radiol. 1989;24(12):1024-1027. [DOI] [PubMed] [Google Scholar]
- 12.Moonen CT, Quesson B, Salomir R, et al. Thermal therapies in interventional MR imaging: focused ultrasound. Neuroimaging Clin N Am. 2001;11(4):737-747, xi. [PubMed] [Google Scholar]
- 13.Salomir R, Delemazure AS, Palussière J, Rouvière O, Cotton F, Chapelon JY. Image-based control of the magnetic resonance imaging-guided focused ultrasound thermotherapy. Top Magn Reson Imaging. 2006;17(3):139-151. [DOI] [PubMed] [Google Scholar]
- 14.Cline HE, Schenck JF, Hynynen K, Watkins RD, Souza SP, Jolesz FA. MR-guided focused ultrasound surgery. J Comput Assist Tomogr. 1992;16(6):956-965. [DOI] [PubMed] [Google Scholar]
- 15.Marx M, Ghanouni P, Butts Pauly K. Specialized volumetric thermometry for improved guidance of MRgFUS in brain. Magn Reson Med. 2017;78(2):508-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol. 2009;66(6):858-861. [DOI] [PubMed] [Google Scholar]
- 17.Elias WJ, Huss D, Voss T, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2013;369(7):640-648. [DOI] [PubMed] [Google Scholar]
- 18.Chang WS, Jung HH, Kweon EJ, Zadicario E, Rachmilevitch I, Chang JW. Unilateral magnetic resonance guided focused ultrasound thalamotomy for essential tremor: practices and clinicoradiological outcomes. J Neurol Neurosurg Psychiatry. 2015;86(3):257-264. [DOI] [PubMed] [Google Scholar]
- 19.Lipsman N, Schwartz ML, Huang Y, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. 2013;12(5):462-468. [DOI] [PubMed] [Google Scholar]
- 20.Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375(8):730-739. [DOI] [PubMed] [Google Scholar]
- 21.Freeman TB, Vawter DE, Leaverton PE, et al. Use of placebo surgery in controlled trials of a cellular-based therapy for Parkinson’s disease. N Engl J Med. 1999;341(13):988-992. [DOI] [PubMed] [Google Scholar]
- 22.Jankovic J, McDermott M, Carter J, et al. ; Parkinson Study Group . Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. Neurology. 1990;40(10):1529-1534. [DOI] [PubMed] [Google Scholar]
- 23.Gibb WRG, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahn STE, Marin C. Clinical rating scale for tremor In: Jankovic JTE, ed. Parkinson’s Disease and Movement Disorders. 2nd ed Baltimore, MD: Williams & Wilkins; 1993:225-234. [Google Scholar]
- 25.Pietracupa S, Bruno E, Cavanna AE, Falla M, Zappia M, Colosimo C. Scales for hyperkinetic disorders: a systematic review. J Neurol Sci. 2015;358(1-2):9-21. [DOI] [PubMed] [Google Scholar]
- 26.Savica R, Matsumoto JY, Josephs KA, et al. Deep brain stimulation in benign tremulous parkinsonism. Arch Neurol. 2011;68(8):1033-1036. [DOI] [PubMed] [Google Scholar]
- 27.Fahn S, Elton R; Members of the UPDRS Development Committee . Unified Parkinson’s Disease Rating Scale In: Fahn SM, Marsden CD, Calne DB, Goldstein M, eds. Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987:153-163. [Google Scholar]
- 28.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson’s disease rating scale. Arch Neurol. 2010;67(1):64-70. [DOI] [PubMed] [Google Scholar]
- 29.Benabid AL, Pollak P, Gervason C, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337(8738):403-406. [DOI] [PubMed] [Google Scholar]
- 30.Lange M, Mauerer J, Schlaier J, et al. Underutilization of deep brain stimulation for Parkinson’s disease? a survey on possible clinical reasons. Acta Neurochir (Wien). 2017;159(5):771-778. [DOI] [PubMed] [Google Scholar]
- 31.Ohye C, Higuchi Y, Shibazaki T, et al. Gamma knife thalamotomy for Parkinson disease and essential tremor: a prospective multicenter study. Neurosurgery. 2012;70(3):526-535. [DOI] [PubMed] [Google Scholar]
- 32.Kooshkabadi A, Lunsford LD, Tonetti D, Flickinger JC, Kondziolka D. Gamma knife thalamotomy for tremor in the magnetic resonance imaging era. J Neurosurg. 2013;118(4):713-718. [DOI] [PubMed] [Google Scholar]
- 33.Elias WJ. Editorial: tremor. J Neurosurg. 2013;118(4):711-712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods 1. Inclusion and Exclusion Criteria
eMethods 2. Details of the MR-Guided FUS Thalamotomy Procedure
eTable 1. Screen Failures
eTable 2. Clinical Outcomes
eTable 3. Adverse Events for Open-Label Crossover Patients
eTable 4. Clinical Outcomes of Open-Label, Crossover FUS Thalamotomy