This longitudinal cohort study assesses peripheral inflammatory markers, tracks immune changes, and correlates these changes with disease progression in patients with amyotrophic lateral sclerosis.
Key Points
Question
Are changes in the number or activation of specific immune cells in peripheral blood correlated with rapid progression of amyotrophic lateral sclerosis?
Finding
In this longitudinal cohort study of peripheral blood leukocytes from 119 patients with amyotrophic lateral sclerosis and 35 control individuals, multivariate regression showed that changes in neutrophil and CD4 T-cell numbers were correlated with rapid disease progression. In addition, transient bursts of a CD11b+CD14−CD16− myeloid cell population were detected.
Meaning
Specific immune populations may serve as viable biomarkers or therapeutic targets in amyotrophic lateral sclerosis.
Abstract
Importance
Amyotrophic lateral sclerosis (ALS) has an immune component, but previous human studies have not examined immune changes over time.
Objectives
To assess peripheral inflammatory markers in participants with ALS and healthy control individuals and to track immune changes in ALS and determine whether these changes correlate with disease progression.
Design, Setting, and Participants
In this longitudinal cohort study, leukocytes were isolated from peripheral blood samples from 35 controls and 119 participants with ALS at the ALS Clinic of the University of Michigan, Ann Arbor, from June 18, 2014, through May 26, 2016. Follow-up visits occurred every 6 to 12 months. Fifty-one participants with ALS provided samples at multiple points. Immune cell populations were measured and compared between control and ALS groups. Surface marker expression of CD11b+ myeloid cells was also assessed. Changes over time were correlated with disease progression using multivariate regression.
Main Outcomes and Measures
The number of immune cells per milliliter of blood and the fold expression of cell surface markers. Multivariate regression models were used to correlate changes in immune metrics with changes on the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R).
Results
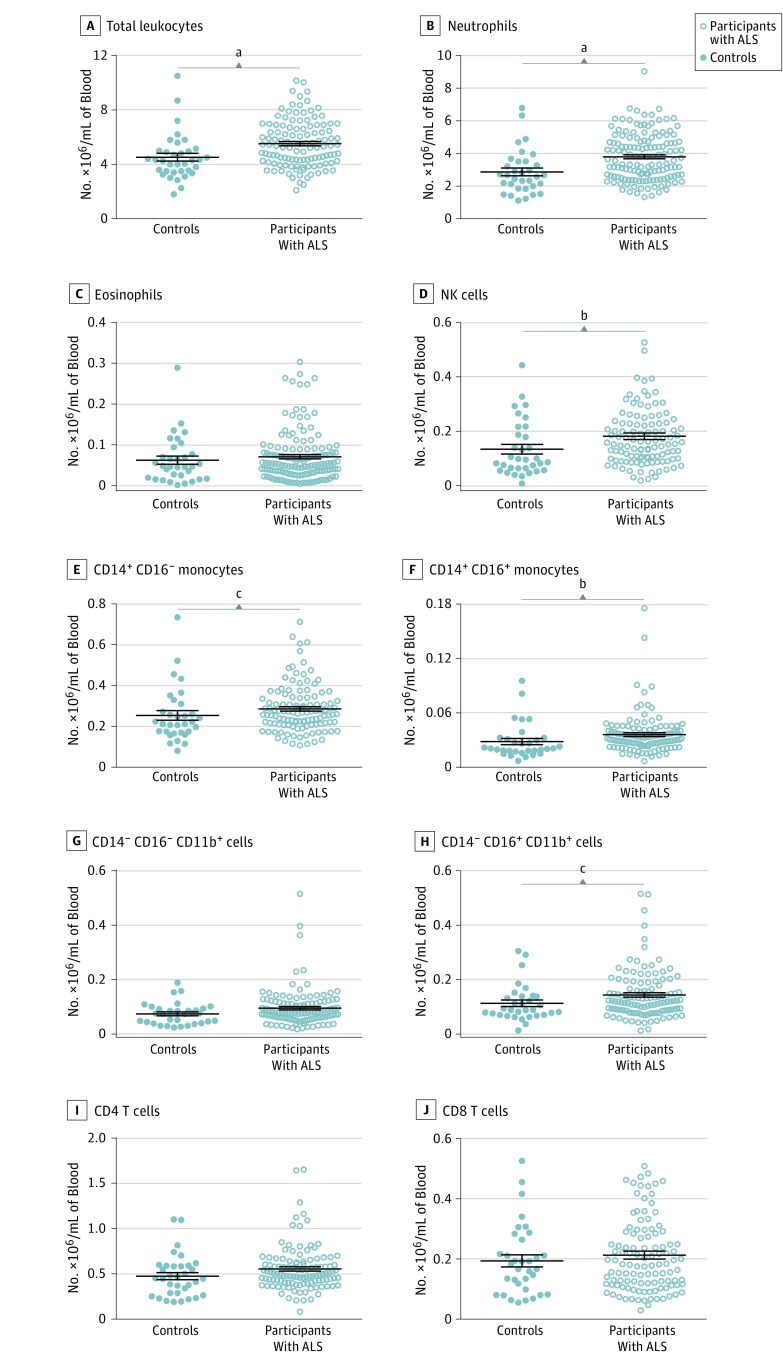
Thirty-five controls (17 women [48.6%] and 18 men [51.4%]; mean [SD] age, 63.5 [9.9] years) and 119 participants with ALS (50 women [42.0%] and 69 men [68.0%]; mean [SD] age, 61.4 [11.5] years) were enrolled. Compared with controls, participants with ALS had increased mean (SEM) counts ( × 106/mL) of total leukocytes (4.57 [0.29; 95% CI, 3.94-5.11] vs 5.53 [0.16; 95% CI, 5.21-5.84]), neutrophils (2.87 [0.23; 95% CI, 2.40-3.35] vs 3.80 [0.12; 95% CI, 3.56-4.04]), CD16+ monocytes (0.03 [0.003; 95% CI, 0.02-0.04] vs 0.04 [0.002; 95% CI, 0.03-0.04]), CD16− monocytes (0.25 [0.02; 95% CI, 0.21-0.30] vs 0.29 [0.01; 95% CI, 0.27-0.31]), and natural killer cells (0.13 [0.02; 95% CI, 0.10-0.17] vs 0.18 [0.01; 95% CI, 0.16-0.21]). We also observed an acute, transient increase in a population of CD11b+ myeloid cells expressing HLA-DR, CD11c, and CX3CR1. Finally, early changes in immune cell numbers had a significant correlation with disease progression measured by change in ALSFRS-R score, particularly neutrophils (−4.37 [95% CI, −6.60 to −2.14] per 11.47 × 104/mL [SD, 58.04 × 104/mL] per year) and CD4 T cells (−30.47 [95% CI, −46.02 to −14.94] per −3.72 × 104/mL [SD, 26.21 × 104/mL] per year).
Conclusions and Relevance
Changes in the immune system occur during ALS and may contribute to the pathologic features of ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is an adult-onset disease characterized by progressive motor neuron degeneration that results in muscle atrophy, spasticity, hyperreflexia, paralysis, and eventually death. The average patient lifespan ranges from 3 to 5 years after diagnosis. Numerous potential factors may contribute to disease, including genetics, exposure to environmental toxins, and trauma to the central nervous system (CNS), but in the sporadic form of ALS, a single cause of disease has yet to be identified. Instead, sporadic ALS may result from multiple factors that each increase disease susceptibility.
Neuroinflammation is a hallmark of ALS, and regardless of the source of disease, all ALS forms may ultimately funnel through the immune system. The role of immunity in ALS, however, is complex, with multiple cell types influencing disease progression; often the same cell type can play a positive or negative role depending on the stage of disease. This process likely explains why previous clinical attempts to use immune suppression in patients with ALS had no positive effect or exacerbated disease. Previous studies demonstrated that peripheral immune changes occur in patients with ALS. Moreover, disease progression in murine models of ALS can be modulated by manipulating individual immune cell populations. This modulation makes blood-borne leukocytes attractive as biomarkers and targets for ALS therapies because such strategies would be less invasive than those targeting the CNS.
We hypothesize that specific leukocyte populations or myeloid subpopulations accelerate the rate of ALS progression. In the present study, cellular populations and myeloid surface marker expression from participants with ALS were obtained longitudinally, and multivariate regression analysis was used to correlate changes in immune cell populations with changes in disease scores.
Methods
Study Population
Participants with ALS who met a diagnosis of possible, probable laboratory-supported, probable, or definite ALS based on revised El Escorial criteria were recruited from the ALS Clinic of the University of Michigan, Ann Arbor, from June 18, 2014, through May 26, 2016. Disease progression, measured using the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R; range, 0-48, with lower scores indicating more advanced disease), was abstracted from medical records and collected prospectively. Control participants, recruited through the university, were excluded if they had a family history of ALS, previous diagnosis of a neurodegenerative disorder, chronic inflammatory disease, collagen vascular disease, use of immunomodulatory medication, or any fever or sickness at the time of blood sample obtainment. The study was approved by the institutional review board of the University of Michigan; all participants provided oral and written informed consent.
Blood Samples
Blood samples were collected by peripheral venipuncture into a sodium heparin tube (BD Vacutainer; BD Biosciences), placed at 4°C, and processed within 2 hours of collection. A 1-mL aliquot of whole blood was lysed with red blood cell–lysing buffer (0.8% ammonium chloride, 0.098% potassium hydrogen carbonate, 0.1mM EDTA, and 13.8mM Hepes), and washed twice with flow buffer (phosphate-buffered saline, 4% fetal bovine solution, and 0.1% sodium azide). An aliquot was counted using a hemocytometer (Hausser Scientific).
Flow Cytometry
Cells were incubated with blocking solution (TruStain FcX; Biolegend). A cocktail of titrated, monoclonal, fluorochrome-labeled antibodies (eTable 1 in the Supplement) was added to each well and incubated in the dark at 4°C for 30 minutes. Cells were washed with flow buffer, spun down, and resuspended in stabilizing fixative (BD Biosciences). Phenotyping was performed on a cytometer (BD FACS Canto II; BD Biosciences). Compensation for each color used cells stained with a single fluorochrome-labeled antibody. Isotype control gates were set at no greater than 1.0%.
Leukocyte Population Gating
Myeloid populations were identified using CD11b, CD14, and CD16 (eFigure 1 in the Supplement). Neutrophil (CD16high) and eosinophil (CD16low) gating was confirmed with CD15 and CCR3 expression, respectively. Lymphocyte and lymphoid populations were identified using antibodies for CD3, CD4, CD8, CD56, and CD16 (eFigure 2 in the Supplement).
Myeloid Surface Marker Expression
Surface marker expression within the 2 CD14+ monocyte groups was calculated using median fluorescence intensity (MFI). For each myeloid population, the MFI of each surface marker was calculated as well as that of the corresponding IgG isotype control. Surface marker MFI expression was divided by the isotype MFI expression, and data were assessed based on the MFI fold increase. CD14− myeloid cell subpopulations were identified based on positive and negative surface marker expression (eFigure 3 in the Supplement). Expression and function are outlined in eTable 2 in the Supplement.
Statistical Analysis
To compare controls and participants with ALS, data for each participant with ALS were pooled from multiple visits to generate a single value for each immune metric. This procedure prevented participants with multiple visits from being overrepresented in the data set. Data sets were analyzed using a D’Agostino and Pearson omnibus normality test to assess normality. We found that all the data sets comparing controls and participants with ALS were nonnormally distributed; therefore, we used the Mann-Whitney test to compare control and ALS data.
Multivariate Regression
Correlation between cellular population changes and disease progression was assessed for participants visiting initially within the first 3 years after disease onset. The gap time between disease onset and the first visit among the 119 participants with ALS varied significantly (Table 1); thus, restricting the analysis to participants whose first visit was within 3 years of disease onset reduced patient heterogeneity in terms of disease progression. A 2-step process assessed the association between cellular population changes and disease progression. First, linear regression models of the cell population counts and ALSFRS-R scores on time were fitted to estimate the participant-specific rate of change in these variables. Median fluorescence intensity expression and CD14− cell population counts were transformed by taking the logarithm or the square root to improve normality. Second, a multiple linear regression model was fitted to assess whether the rate of change in cellular population counts was associated with disease progression after controlling for potential confounders (ie, baseline biomarker value, age at onset, and sex). A separate model was fitted for each biomarker to assess its association with disease progression. Analyses were performed using R software (https://www.r-project.org/).
Table 1. Demographic Characteristics of the Study Population.
| Characteristic | Control Participants (n = 35) |
Participants With ALS (n = 119) |
|---|---|---|
| Sex, No. (%) | ||
| Women | 17 (48.6) | 50 (42.0) |
| Men | 18 (51.4) | 69 (68.0) |
| Age, mean (SD) [range], y | 63.5 (9.9) [46.3-86.3] | 61.4 (11.5) [30.4-86.6] |
| ALSFRS-R score, mean (SD) [range]a | NA | 27.0 (7.0) [9-44] |
| Time from onset to first blood sample obtainment, mean (SD) [range], y | NA | 2.32 (2.77) [0.55-19.18] |
| Participants with multiple visits to the clinic, No. (%) | NA | 51 (42.9) |
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSFRS-R, Revised Amyotrophic Lateral Sclerosis Functional Rating Scale; NA, not applicable.
Scores range from 0 to 48, with lower scores indicating more advanced disease.
Results
Population Demographics
The study population included 35 healthy controls (17 women [48.6%] and 18 men [51.4%]; mean [SD] age, 63.5 [9.9] years) and 119 participants with ALS (50 women [42.0%] and 69 men [58.0]; mean [SD] age, 61.4 [11.5] years) (Table 1). The mean (SD) ALSFRS-R score for participants with ALS was 27.0 (7.0), and 51 (42.9%) provided multiple clinical samples.
Immune Cell Kinetics and Correlation With Disease Progression
Neutrophils, eosinophils, CD14+CD16+ monocytes, CD14+CD16− monocytes, natural killer (NK) cells, CD4 T cells, CD8 T cells, and 2 CD11b+CD14− myeloid cell populations were identified using flow cytometry in participant peripheral blood samples based on surface marker expression. Compared with healthy controls, participants with ALS had increased mean (SEM) numbers ( × 106/mL) of total leukocytes (4.57 [0.29; 95% CI, 3.94-5.11] vs 5.53 [0.16; 95% CI, 5.21-5.84]) (Figure 1); in addition, the numbers of neutrophils (2.87 [0.23; 95% CI, 2.40-3.35] vs 3.80 [0.12; 95% CI, 3.56-4.04]), CD16+ monocytes (0.03 [0.003; 95% CI, 0.02-0.04] vs 0.04 [0.002; 95% CI, 0.03-0.04]), CD16− monocytes (0.25 [0.02; 95% CI, 0.21-0.30] vs 0.29 [0.01; 95% CI, 0.27-0.31]), CD11b+CD14−CD16− myeloid cells (0.07 [0.01; 95% CI, 0.06-0.09] vs 0.10 [0.01; 95% CI, 0.08-0.11]), and NK cells (0.13 [0.02; 95% CI, 0.10-0.17] vs 0.18 [0.01; 95% CI, 0.16-0.21]) were increased (Table 2).
Figure 1. Total Number of Peripheral Immune Cells in Participants With Amyotrophic Lateral Sclerosis (ALS).
The total number of leukocytes and numbers of individual cell populations were calculated for each participant with ALS by calculating the mean population numbers at each visit to create a single value per patient. Cell populations in participants with ALS were compared with those of healthy control participants. Horizontal lines indicate mean; error bars, SEM for each cell population. NK indicates natural killer. aP < .001, Mann-Whitney test; bP < .01, Mann-Whitney test; cP < .05, Mann-Whitney test.
Table 2. Statistical Comparison of Immune Cell Populations and Subpopulationsa.
| Cell | Immune Cell Population, Mean (SEM), ×106/mL | Surface Marker Expression, Mean (SEM), Fold MFI | Cell Subpopulation, Mean (SEM), ×106/mL | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD14+CD16+ Monocytes | CD14+CD16− Monocytes | CD14−CD16+ Cells | CD14−CD16− Cells | ||||||||||||||||
| Control | ALS | P Value | Marker | Control | ALS | P Value | Marker | Control | ALS | P Value | Marker | Control | ALS | P Value | Marker | Control | ALS | P Value | |
| All leukocytes | 4.52 (0.23) | 5.53 (0.16) | <.001 | HLADR | 21.07 (2.15) | 17.51 (0.91) | .13 | HLA-DR | 5.47 (0.37) | 5.11 (0.21) | .27 | HLADR | 0.03 (0.004) | 0.02 (0.001) | .78 | HLADR | 0.04 (0.004) | 0.05 (0 .004 | .16 |
| Neutrophils | 3.52 (0.20) | 4.01 (0.13) | <.001 | CD11c | 14.55 (2.39) | 15.00 (1.01) | .24 | CD11c | 7.99 (1.17) | 9.30 (0.91) | .44 | CD11c | 0.03 (0.004) | 0.04 (0.003) | .24 | CD11c | 0.01 (0.002) | 0.02 (0.002) | .15 |
| Eosinophils | 0.06 (0.01) | 0.07 (0.01) | .39 | CD40 | 3.40 (0.17) | 3.22 (0.08) | .44 | CD40 | 2.17 (0.12) | 2.05 (0 .06) | .38 | CD40 | 0.02 (0.003) | 0.01 (0.001) | .24 | CD40 | 0.02 (0.003) | 0.02 (0.001) | .59 |
| CD14+CD16+ | 0.03 (0.003) | 0.04 (0.002) | .002 | CD62L | 2.27 (0.24) | 1.96 (0 .10) | .66 | CD62L | 12.54 (1.38) | 9.77 (0 .44) | .15 | CD62L | 0.04 (0.01) | 0.04 (0.003) | .01 | CD62L | 0.06 (0.01) | 0.07 (0.004) | .15 |
| CD14+CD16− | 0.25 (0.02) | 0.29 (0.01) | .04 | CD86 | 6.14 (0.89) | 7.26 (0.49) | .32 | CD86 | 4.73 (0.74) | 5.00 (0.41) | .93 | CD86 | 0.02 (0.01) | 0.02 (0.002) | .10 | CD86 | 0.01 (0.001) | 0.003 (0.001) | .15 |
| CD14−CD16+ | 0.11 (0.01) | 0.14 (0.01) | .03 | CCR2 | 5.99 (1.22) | 4.93 (0.44) | .95 | CCR2 | 39.43 (5.99) | 50.17 (7.65) | .26 | CX3CR1 | 0.09 (0.01) | 0.11 (0.01) | .23 | BDCA-1 | 0.01 (0.002) | 0.01 (0.001) | .09 |
| CD14−CD16− | 0.07 (0.01) | 0.10 (0.01) | .10 | CX3CR1 | 8.44 (0 .79) | 9.62 (0.35) | .12 | CX3CR1 | 4.77 (0.40) | 5.09 (0 .18) | .42 | TLR2 | 0.03 (0 .003) | 0.03 (0.002) | .94 | CCR2 | 0.02 (0.002) | 0.03 (0.002) | .04 |
| NK cells | 0.13 (0.02) | 0.18 (0.01) | .007 | TLR2 | 6.85 (0 .40) | 6.56 (0.14) | .83 | TLR2 | 6.45 (0 .38) | 6.69 (0 .16) | .69 | NA | NA | NA | NA | CX3CR1 | 0.03 (0 .004) | 0.04 (0.01) | .17 |
| CD4 T cells | 0.48 (0.04) | 0.55 (0.02) | .08 | TLR4 | 2.64 (0.27) | 2.32 (0 .09) | .61 | TLR4 | 2.36 (0 .24) | 2.19 (0 .10) | .72 | NA | NA | NA | NA | TLR2 | 0.01 (0.001) | 0.01 (0.001) | .32 |
| CD8 T cells | 0.19 (0.02) | 0.21 (0.01) | .54 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Abbreviations: ALS, amyotrophic lateral sclerosis; MFI, median fluorescence intensity; NA, not applicable; NK, natural killer.
P values were determined by Mann-Whitney test measuring significance of direction of change.
Next, the number of immune cells in the peripheral blood samples from each participant was plotted over time (eFigure 4A in the Supplement) or in association with the ALSFRS-R score (eFigure 4B in the Supplement) for participants who had enrolled in the study within 3 years of disease onset. Consistent with previous reports, leukocyte numbers were highly variable over time, even within the same participant. However, we found increasing total numbers of leukocytes, neutrophils, and NK cells; conversely, the total number of CD4 cells appeared to decrease during the first years of disease progression (Table 3). We also observed large but transient increases in CD11b+CD14−CD16− cell count.
Table 3. Correlation Between Changes in Cell Populations and ALSFRS-R Score.
| Leukocyte Population | No. of Participants | Leukocyte Change, Mean (SD), ×104/mL per Year | ALSFRS-R Score Change/y per Unit of Leukocyte Change (95% CI) | P Value |
|---|---|---|---|---|
| All leukocytes | 24 | 6.34 (47.91) | −3.68 (−5.45 to −1.92) | <.001 |
| Neutrophils | 23 | 11.47 (58.04) | −4.37 (−6.60 to −2.14) | <.001 |
| Eosinophils | 23 | −5.61 (27.96) | 8.06 (−28.83 to 44.96) | .65 |
| CD14+CD16+ | 23 | −1.37 (10.92) | 68.18 (−134.13 to 270.49) | .49 |
| CD14+CD16− | 23 | 2.05 (12.49) | −2.40 (−50.34 to 45.53) | .92 |
| CD14−CD16+ | 23 | −4.69 (17.44) | −45.66 (−103.69 to 12.35) | .12 |
| CD14−CD16− | 23 | 1.27 (18.05) | −2.66 (−102.08 to 96.67) | .96 |
| NK cells | 21 | 8.04 (18.64) | −19.23 (−64.83 to 26.36) | .38 |
| CD4 T cells | 23 | −3.72 (26.21) | −30.47 (−46.02 to −14.94) | <.001 |
| CD8 T cells | 21 | 0.82 (23.19) | −29.62 (−63.33 to 4.07) | .08 |
Abbreviations: ALSFRS-R, Revised Amyotrophic Lateral Sclerosis Functional Rating Scale; NK, natural killer.
SI conversion factor: To convert leukocyte counts to ×109/L, divide by 100.
To determine whether changes in any cell populations might play a role in disease progression, we created a multivariate linear model of regression using data from participants whose first visit to the clinic occurred within 3 years of disease onset. Changes in the immune cell population counts were correlated with changes in ALSFRS-R score between time points. We found a significant correlation between a decrease in ALSFRS-R score and changes in the total number of immune cells (−3.68 [95% CI, −5.45 to −1.92] per 6.34 × 104/mL [SD, 47.91 × 104/mL] increase per year; P < .001) (Table 3). Changes in the number of neutrophils were also significantly correlated with a decrease in ALSFRS-R score (−4.37 [95% CI, −6.60 to −2.14] per 11.47 × 104/mL [SD, 58.04 × 104/mL] increase per year; P < .001). On average, the total numbers of leukocytes and neutrophils increased over time, indicating that early increases in total leukocyte and neutrophil numbers are correlated with disease progression. We also observed a correlation between changes in ALSFRS-R score and changes in the number of CD4 T cells (−30.47 [95% CI, −46.02 to −14.94] per −3.72 × 104/mL [SD, 26.21 × 104/mL] decrease per year; P < .001); however, CD4 T-cell levels decreased over time, and the rate of decrease was correlated with a drop in ALSFRS-R score. Other cell types showed no significant correlation between early population changes and ALSFRS-R score. Although NK cell changes were not directly correlated with changes in ALSFRS-R scores, these cell levels increased by a mean of 80 000/mL of blood per year in participants with ALS in the initial stages of disease (a 60% yearly increase vs controls).
Myeloid Subgroups
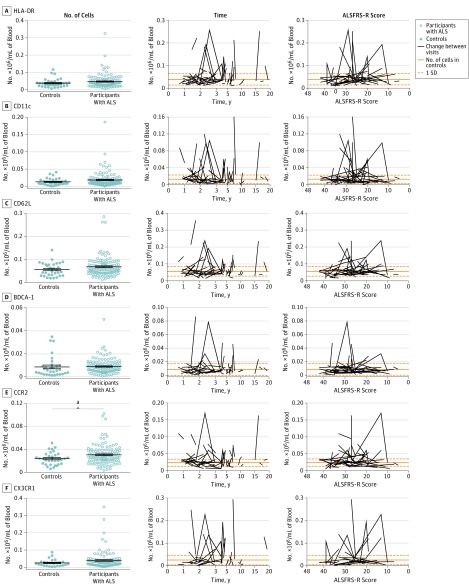
Multiple studies in humans and mice have suggested that monocytes may be involved in ALS. By gating on nongranulocytic CD11b+ myeloid cells, we detected CD16+ and CD16− monocytes and 2 additional CD14− populations composed of heterogeneous subpopulations of cells (eFigure 3 in the Supplement). For each of these 4 myeloid populations (CD14+CD16+, CD14+CD16−, CD14−CD16+, and CD14−CD16−), surface markers were analyzed to assess activation, differentiation, adhesion, antigen presentation, and environmental sensing (eTable 2 in the Supplement). Some of these markers were expressed by all myeloid groups, whereas others were expressed by only certain subpopulations (eFigure 3 and eTable 2 in the Supplement).
Using the same approach used with total cell numbers, we measured the mean surface marker expression on CD16+ and CD16− monocytes and changes over time (eFigures 5 and 6 in the Supplement). In both populations of monocytes, we found no significant change in surface marker expression (Table 2). Using multivariate regression, we attempted to determine whether changes in surface marker expression are correlated with changes in disease score. In CD14+CD16+ monocytes, we found a significant correlation between CX3CR1 expression and ALSFRS-R score (4.12 [95% CI, 0.50-7.79] per 0.586 MFI ratio increase per year [normalized]; P = .03), with increased CX3CR1 expression being associated with better disease progression (eTable 3 in the Supplement). We observed a similar correlation in CD14+CD16− monocytes (P = .06).
Within the heterogeneous CD14− populations, we found increases in the number of CD14−CD16+CD62L+ cells (Table 2 and eFigure 7 in the Supplement) and total number of CD14−CD16−CCR2+ cells (Figure 2). Using multivariate regression, we found that decreases in CD14−CD16+CD62L+ were correlated with disease progression (ALSFRS-R score, −58.52 [95% CI, −108.93 to −8.11] per −0.0065 × 104/mL decrease per year [normalized]; P = .03), but we found no correlation between CD14−CD16−CCR2+ cells and ALSFRS-R score (eTable 3 in the Supplement). However, we found a significant correlation between decreasing ALSFRS-R score and decreasing numbers of CD14−CD16−CX3CR1+ cells (61.41 [95% CI, 10.51-112.30] per −0.0272 × 104/mL decrease per year [normalized]; P = .03).
Figure 2. Total Number and Changes in CD11b+CD14−CD16− Cell Subpopulations in Participants With Amyotropic Lateral Sclerosis (ALS).
Total numbers of cells in CD11b+CD14−CD16− cell subpopulations for each participant with ALS were calculated using the mean population numbers at each visit to create 1 value per patient (left). Horizontal lines indicate mean; error bars, SEM for each population. Time from ALS onset (middle) and the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) score (range, 0-48; lower scores indicate more advanced disease) (right) for participants with ALS were calculated for each visit and plotted vs subpopulation number for multiple visits. Lines are for reference and do not indicate change over time because controls did not make multiple clinic visits. Participants with ALS with 1 clinic visit are not included in the kinetic data. aP < .05, Mann-Whitney test.
Finally, we attempted to identify a cellular subpopulation that could account for the transient bursts of CD14−CD16− cells observed in some participants (eFigure 4 in the Supplement). By analyzing the kinetics of individual cell subpopulations within the CD14−CD16− group, we observed that several cell populations had nearly identical kinetic patterns (Figure 2). In particular, CD14−CD16− cells expressing HLA-DR, CD11c, and CX3CR1 had acute changes in total cell numbers. To determine whether these cells might be the same population of cells, we enriched leukocytes from a healthy control and examined the surface marker expression of CD14−CD16− cells that expressed HLA-DR (eFigure 8 in the Supplement). These HLA-DR+ cells were capable of expressing CD11c and CX3CR1. In addition, these cells expressed other myeloid surface markers, such as CD32, and low levels of TLR4. Together, these data indicate that, although changes in surface marker expression or specific myeloid subpopulations correlate with disease progression, brief bursts of nonmonocyte myeloid cells may also play a role in disease course.
Discussion
This study examined long-term changes in the peripheral immune system of participants with ALS and correlated these changes with disease progression. Leukocytes were isolated from the peripheral blood samples of participants with ALS and healthy controls; the total number of numerous leukocyte populations, the surface marker expression of monocytes, and the number of myeloid subpopulations was tracked in participants with ALS during multiple clinical visits. These numbers were compared with levels seen in controls and were also correlated with changes in ALSFRS-R score using multivariate regression analysis. The study was performed for several reasons. First, it has become increasingly clear that ALS has an immunologic component: immune changes have been detected in humans with ALS, and altering the function of specific immune cells can alter disease progression in mouse models. Second, the immune system can alter the immune environment within the CNS, polarizing resident cells to a more destructive phenotype, making it an attractive target for therapeutics. Finally, many previous clinical studies examining differences between participants with ALS and controls have focused on single clinical visits; few have focused on changes over time.
In the peripheral blood of participants with ALS, we found a significant increase in the total number of leukocytes per milliliter of blood; numbers of neutrophils, CD16+ monocytes, CD16− monocytes, and NK cells also increased. Using a multivariate regression model that accounted for baseline values, time from onset, and sex, we found that changes in total numbers of leukocytes, neutrophils, and CD4 T cells were significantly correlated with disease progression, although whether these cells are playing a pathogenic role, positive or negative, in disease progression remains unclear. These analyses were further extended to nongranulocytic myeloid cell populations. We found few differences between participants with ALS and controls in monocytes, although subpopulations of CD14− nonmonocytic cells showed small but significant differences. Multivariate regression suggested that CX3CR1 expression in various myeloid populations is correlated with disease progression. We observed a CD14−CD16− myeloid cell population that showed transient but acute expansion during disease and likely expressed HLA-DR, CD11c, and CX3CR1. For all these significantly correlated metrics, we observed that participants often had immune changes that were contrary to findings for most of the patients. These discrepancies are likely attributable to inherent immune fluctuation because no obvious common factors were found among these participants.
Our observations are consistent with and expand on previous studies examining the role of the immune system in ALS. Modest increases in neutrophil levels have been previously reported. However, we demonstrate herein a significant correlation between increases in neutrophils in the peripheral blood and disease progression. These cells could play a protective and/or destructive role; neutrophil infiltrate is associated with enhanced tissue damage, but studies also indicate that neutrophils contribute to neuronal repair.
Similarly, monocyte populations may also play a role in disease pathogenesis. Previous studies indicate that CD14+CD16+ monocytes are protective in ALS, whereas the murine analogs of CD16− monocytes (Ly6C+ monocytes) can exacerbate disease. In addition, numerous reports show increased transcripts associated with monocyte infiltration in human and mouse studies, although to what extent, if any, leukocytes infiltrate the CNS during ALS remains unclear. Herein, we found that CD16+ and CD16− monocyte populations increased in participants with ALS, but changes in these populations were not correlated with disease progression. We found, however, that increased CX3CR1 expression on CD16+ monocytes and CD16− monocytes was associated with slower disease progression. Previous studies found that CX3CR1 is essential for myeloid cell trafficking to the CNS during infection; thus, it may play a similar trafficking role in ALS.
We also found that NK cells were similarly upregulated in participants with ALS compared with controls. This finding is consistent with previous studies that observed NK cells in the CNS of mice with ALS, changes in NK cell methylation, or involvement of other innate lymphoid cells, such as NK T cells, in disease. Although NK cell levels were not significantly correlated with disease progression during early disease, the mean NK cell numbers in blood samples from participants with ALS increased over time. These cells may have an effect on disease progression because they are capable of producing proinflammatory cytokines that can skew the immune system toward a destructive response. More directly, NK cells lyse infected, oncogenic, and apoptotic cells. They also target cells lacking major histocompatibility complex I expression, and motor neurons have recently been shown to lack major histocompatibility complex I expression in ALS. However, NK cells can also have a protective effect in the CNS. In multiple sclerosis, NK cells dampen the immune response in the CNS, and CX3CR1 expression is needed for NK-mediated protection.
Conversely, almost all information to date indicates that CD4 T cells are protective during ALS. Although we did not observe a significant change in CD4 T cell counts between participants with ALS and healthy controls, we found that decreasing CD4 T-cell levels were correlated with disease progression. This correlation is likely attributable to reduced numbers of regulatory T cells within the CD4 population because regulatory T cells skew the immune system toward a protective response and delay ALS progression in mouse models. Moreover, human studies have found that reduced regulatory T-cell levels are associated with rapid ALS progression. We did not examine CD4 T cells for regulatory function in the present study, but such an examination may form the basis of future investigation.
Finally, we observed a nongranulocytic myeloid population that displayed acute, transient increases in participants with ALS. Within the CD11b+CD14−CD16− populations, subpopulations of HLA-DR+, CD11c+, and CX3CR1+ cells briefly increased before returning to baseline levels. Based on the staining profiles and kinetics of these subpopulations, we believe that a single myeloid population expresses HLA-DR, CD11c, and CX3CR1 together. At present, however, what role these cells may play remains unclear. The lack of CD14 expression indicates that these cells are not monocytes, but the expression of HLA-DR, CD11c, and CX3CR1 indicates that they are a mature cell type rather than a myeloid precursor.
Limitations
This study is correlative. Although the data are consistent with previous clinical reports and mouse models of ALS, changes in peripheral immunity may be a consequence rather than a cause of disease. In addition, participants in the study did not provide samples until 6 to 12 months after the initial diagnosis of ALS, meaning that the earliest stages of disease were often not examined in the study. Finally, subpopulations of CD4 T cells were not examined; decreases in ALSFRS-R may correlate with decreases in specific CD4 T-cell subpopulations rather than CD4 T cells as a whole.
Conclusions
In this study, we demonstrate that changes in the number of immune cells, particularly neutrophils and CD4 T cells, are correlated with disease progression. Other cell populations, such as NK cells and a population of CD11b+CD14−CD16− myeloid cells, may also play a role; thus, further investigations are needed in these areas.
eTable 1. Titrated Monoclonal Fluorochrome-Labeled Antibodies Used
eTable 2. Expression and Function of Surface Markers
eTable 3. Regression Analysis of CD11b+ Nongranulocyte Myeloid Cells
eFigure 1. Myeloid Cell Population Gating Strategy
eFigure 2. Lymphoid Cell Population Gating Strategy
eFigure 3. Myeloid Cell Subpopulation Identification
eFigure 4. Changes in Immune Cell Subpopulations in Participants With ALS
eFigure 5. Total Number and Changes in CD11b+CD14+CD16+ Cell Subpopulations in Participants With ALS
eFigure 6. Total Number and Changes in CD11b+CD14+CD16− Cell Subpopulations in Participants With ALS
eFigure 7. Total Number and Changes in CD11b+CD14−CD16+ Cell Subpopulations in Participants With ALS
eFigure 8. Surface Marker Expression of CD14−CD16−HLA-DR+ Myeloid Cells
References
- 1.Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369(9578):2031-2041. [DOI] [PubMed] [Google Scholar]
- 2.Mehta P, Antao V, Kaye W, et al. ; Division of Toxicology and Human Health Sciences, Agency for Toxic Substances and Disease Registry; Centers for Disease Control and Prevention (CDC) . Prevalence of amyotrophic lateral sclerosis—United States, 2010-2011. MMWR Suppl. 2014;63(7):1-14. [PubMed] [Google Scholar]
- 3.Renton AE, Chiò A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci. 2014;17(1):17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su FC, Goutman SA, Chernyak S, et al. Association of environmental toxins with amyotrophic lateral sclerosis. JAMA Neurol. 2016;73(7):803-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Richard M, Sandler DP, Umbach DM, Kamel F. Head injury and amyotrophic lateral sclerosis. Am J Epidemiol. 2007;166(7):810-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology. 2012;79(19):1970-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Chalabi A, Calvo A, Chio A, et al. Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol. 2014;13(11):1108-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooten KG, Beers DR, Zhao W, Appel SH. Protective and toxic neuroinflammation in amyotrophic lateral sclerosis. Neurotherapeutics. 2015;12(2):364-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murdock BJ, Bender DE, Kashlan SR, et al. Increased ratio of circulating neutrophils to monocytes in amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3(4):e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murdock BJ, Bender DE, Segal BM, Feldman EL. The dual roles of immunity in ALS: injury overrides protection. Neurobiol Dis. 2015;77:1-12. [DOI] [PubMed] [Google Scholar]
- 11.Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57(7):1282-1289. [DOI] [PubMed] [Google Scholar]
- 12.Beers DR, Zhao W, Liao B, et al. Neuroinflammation modulates distinct regional and temporal clinical responses in ALS mice. Brain Behav Immun. 2011;25(5):1025-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cudkowicz ME, Shefner JM, Schoenfeld DA, et al. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol. 2006;60(1):22-31. [DOI] [PubMed] [Google Scholar]
- 14.Meininger V, Drory VE, Leigh PN, Ludolph A, Robberecht W, Silani V. Glatiramer acetate has no impact on disease progression in ALS at 40 mg/day: a double-blind, randomized, multicentre, placebo-controlled trial. Amyotroph Lateral Scler. 2009;10(5-6):378-383. [DOI] [PubMed] [Google Scholar]
- 15.Gordon PH, Moore DH, Miller RG, et al. ; Western ALS Study Group . Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6(12):1045-1053. [DOI] [PubMed] [Google Scholar]
- 16.Meininger V, Asselain B, Guillet P, et al. ; Pentoxifylline European Group . Pentoxifylline in ALS: a double-blind, randomized, multicenter, placebo-controlled trial. Neurology. 2006;66(1):88-92. [DOI] [PubMed] [Google Scholar]
- 17.Kawamata T, Akiyama H, Yamada T, McGeer PL. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am J Pathol. 1992;140(3):691-707. [PMC free article] [PubMed] [Google Scholar]
- 18.Engelhardt JI, Tajti J, Appel SH. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch Neurol. 1993;50(1):30-36. [DOI] [PubMed] [Google Scholar]
- 19.Iida A, Takahashi A, Kubo M, et al. A functional variant in ZNF512B is associated with susceptibility to amyotrophic lateral sclerosis in Japanese. Hum Mol Genet. 2011;20(18):3684-3692. [DOI] [PubMed] [Google Scholar]
- 20.Henkel JS, Engelhardt JI, Siklós L, et al. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55(2):221-235. [DOI] [PubMed] [Google Scholar]
- 21.Turner MR, Cagnin A, Turkheimer FE, et al. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004;15(3):601-609. [DOI] [PubMed] [Google Scholar]
- 22.Zondler L, Feiler MS, Freischmidt A, et al. Impaired activation of ALS monocytes by exosomes. Immunol Cell Biol. 2017;95(2):207-214. [DOI] [PubMed] [Google Scholar]
- 23.Zondler L, Müller K, Khalaji S, et al. Peripheral monocytes are functionally altered and invade the CNS in ALS patients. Acta Neuropathol. 2016;132(3):391-411. [DOI] [PubMed] [Google Scholar]
- 24.Seksenyan A, Ron-Harel N, Azoulay D, et al. Thymic involution, a co-morbidity factor in amyotrophic lateral sclerosis. J Cell Mol Med. 2010;14(10):2470-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henkel JS, Beers DR, Wen S, et al. Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol Med. 2013;5(1):64-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantovani S, Garbelli S, Pasini A, et al. Immune system alterations in sporadic amyotrophic lateral sclerosis patients suggest an ongoing neuroinflammatory process. J Neuroimmunol. 2009;210(1-2):73-79. [DOI] [PubMed] [Google Scholar]
- 27.Butovsky O, Siddiqui S, Gabriely G, et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122(9):3063-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu IM, Chen A, Zheng Y, et al. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc Natl Acad Sci U S A. 2008;105(46):17913-17918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee R, Mosley RL, Reynolds AD, et al. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS One. 2008;3(7):e2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finkelstein A, Kunis G, Seksenyan A, et al. Abnormal changes in NKT cells, the IGF-1 axis, and liver pathology in an animal model of ALS. PLoS One. 2011;6(8):e22374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases . El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293-299. [DOI] [PubMed] [Google Scholar]
- 32.Höchstetter R, Dobos G, Kimmig D, Dulkys Y, Kapp A, Elsner J. The CC chemokine receptor 3 CCR3 is functionally expressed on eosinophils but not on neutrophils. Eur J Immunol. 2000;30(10):2759-2764. [DOI] [PubMed] [Google Scholar]
- 33.D’Agostino RB. Transformation to normality of the null distribution of g1. Biometrika. 1970;57(3):679-681. [Google Scholar]
- 34.Pearson ES. Note on tests for normality. Biometrika. 1931;22(3/4):423-424. [Google Scholar]
- 35.Casazza JP, Betts MR, Picker LJ, Koup RA. Decay kinetics of human immunodeficiency virus–specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J Virol. 2001;75(14):6508-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao W, Beers DR, Appel SH. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J Neuroimmune Pharmacol. 2013;8(4):888-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keizman D, Rogowski O, Berliner S, et al. Low-grade systemic inflammation in patients with amyotrophic lateral sclerosis. Acta Neurol Scand. 2009;119(6):383-389. [DOI] [PubMed] [Google Scholar]
- 38.Nagata T, Nagano I, Shiote M, et al. Elevation of MCP-1 and MCP-1/VEGF ratio in cerebrospinal fluid of amyotrophic lateral sclerosis patients. Neurol Res. 2007;29(8):772-776. [DOI] [PubMed] [Google Scholar]
- 39.Zhang R, Gascon R, Miller RG, et al. MCP-1 chemokine receptor CCR2 is decreased on circulating monocytes in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol. 2006;179(1-2):87-93. [DOI] [PubMed] [Google Scholar]
- 40.Desport JC, Preux PM, Magy L, et al. Factors correlated with hypermetabolism in patients with amyotrophic lateral sclerosis. Am J Clin Nutr. 2001;74(3):328-334. [DOI] [PubMed] [Google Scholar]
- 41.Chiò A, Calvo A, Bovio G, et al. ; Piemonte and Valle d’Aosta Register for Amyotrophic Lateral Sclerosis . Amyotrophic lateral sclerosis outcome measures and the role of albumin and creatinine: a population-based study. JAMA Neurol. 2014;71(9):1134-1142. [DOI] [PubMed] [Google Scholar]
- 42.Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train. 2006;41(4):457-465. [PMC free article] [PubMed] [Google Scholar]
- 43.Henkel JS, Beers DR, Siklós L, Appel SH. The chemokine MCP-1 and the dendritic and myeloid cells it attracts are increased in the mSOD1 mouse model of ALS. Mol Cell Neurosci. 2006;31(3):427-437. [DOI] [PubMed] [Google Scholar]
- 44.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10(12):1538-1543. [DOI] [PubMed] [Google Scholar]
- 45.Mildner A, Schmidt H, Nitsche M, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10(12):1544-1553. [DOI] [PubMed] [Google Scholar]
- 46.Menasria R, Canivet C, Piret J, Gosselin J, Boivin G. Protective role of CX3CR1 signalling in resident cells of the central nervous system during experimental herpes simplex virus encephalitis. J Gen Virol. 2017;98(3):447-460. [DOI] [PubMed] [Google Scholar]
- 47.Lam L, Chin L, Halder RC, et al. Epigenetic changes in T-cell and monocyte signatures and production of neurotoxic cytokines in ALS patients. FASEB J. 2016;30(10):3461-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rentzos M, Evangelopoulos E, Sereti E, et al. Alterations of T cell subsets in ALS: a systemic immune activation? Acta Neurol Scand. 2012;125(4):260-264. [DOI] [PubMed] [Google Scholar]
- 49.Morandi B, Bougras G, Muller WA, Ferlazzo G, Münz C. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion. Eur J Immunol. 2006;36(9):2394-2400. [DOI] [PubMed] [Google Scholar]
- 50.Song S, Miranda CJ, Braun L, et al. Major histocompatibility complex class I molecules protect motor neurons from astrocyte-induced toxicity in amyotrophic lateral sclerosis. Nat Med. 2016;22(4):397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segal BM. The role of natural killer cells in curbing neuroinflammation. J Neuroimmunol. 2007;191(1-2):2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hertwig L, Hamann I, Romero-Suarez S, et al. CX3CR1-dependent recruitment of mature NK cells into the central nervous system contributes to control autoimmune neuroinflammation. Eur J Immunol. 2016;46(8):1984-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci U S A. 2008;105(40):15558-15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devigili G, Uçeyler N, Beck M, et al. Vasculitis-like neuropathy in amyotrophic lateral sclerosis unresponsive to treatment. Acta Neuropathol. 2011;122(3):343-352. [DOI] [PubMed] [Google Scholar]
- 55.Zhao W, Beers DR, Liao B, Henkel JS, Appel SH. Regulatory T lymphocytes from ALS mice suppress microglia and effector T lymphocytes through different cytokine-mediated mechanisms. Neurobiol Dis. 2012;48(3):418-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beers DR, Henkel JS, Zhao W, et al. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain. 2011;134(pt 5):1293-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Titrated Monoclonal Fluorochrome-Labeled Antibodies Used
eTable 2. Expression and Function of Surface Markers
eTable 3. Regression Analysis of CD11b+ Nongranulocyte Myeloid Cells
eFigure 1. Myeloid Cell Population Gating Strategy
eFigure 2. Lymphoid Cell Population Gating Strategy
eFigure 3. Myeloid Cell Subpopulation Identification
eFigure 4. Changes in Immune Cell Subpopulations in Participants With ALS
eFigure 5. Total Number and Changes in CD11b+CD14+CD16+ Cell Subpopulations in Participants With ALS
eFigure 6. Total Number and Changes in CD11b+CD14+CD16− Cell Subpopulations in Participants With ALS
eFigure 7. Total Number and Changes in CD11b+CD14−CD16+ Cell Subpopulations in Participants With ALS
eFigure 8. Surface Marker Expression of CD14−CD16−HLA-DR+ Myeloid Cells