This cohort study compared the performance of amyloid positron emission tomography and 5 β-amyloid cerebrospinal fluid assays for detecting amyloid deposition in the brains of patients with mild cognitive complaints.
Key Points
Question
How well do the core cerebrospinal fluid biomarkers of Alzheimer disease measured using different immunoassays agree with visual amyloid positron emission tomographic assessment?
Findings
In this study of 262 individuals with mild cognitive complaints, the cerebrospinal fluid Aβ42:Aβ40 or Aβ42:tau ratios from several newer assays showed improved accuracy for detection of cortical β-amyloid fibrils as measured by positron emission tomography.
Meaning
These findings support implementation of the cerebrospinal fluid Aβ42:Aβ40 and/or Aβ42:tau ratios as biomarkers of amyloid deposition in clinical practice and trials.
Abstract
Importance
Visual assessment of amyloid positron emission tomographic (PET) images has been approved by regulatory authorities for clinical use. Several immunoassays have been developed to measure β-amyloid (Aβ) 42 in cerebrospinal fluid (CSF). The agreement between CSF Aβ42 measures from different immunoassays and visual PET readings may influence the use of CSF biomarkers and/or amyloid PET assessment in clinical practice and trials.
Objective
To determine the concordance between CSF Aβ42 levels measured using 5 different immunoassays and visual amyloid PET analysis.
Design, Setting, and Participants
The study included 262 patients with mild cognitive impairment or subjective cognitive decline from the Swedish BioFINDER (Biomarkers for Identifying Neurodegenerative Disorders Early and Reliably) cohort (recruited from September 1, 2010, through December 31, 2014) who had undergone flutemetamol F 18 ([18F]flutemetamol)–labeled PET. Levels of CSF Aβ42 were analyzed using the classic INNOTEST and the newer modified INNOTEST, fully automated Lumipulse (FL), EUROIMMUN (EI), and Meso Scale Discovery (MSD) assays. Concentrations of CSF Aβ were assessed using an antibody-independent mass spectrometry–based reference measurement procedure.
Main Outcomes and Measures
The concordance of CSF Aβ42 levels and Aβ42:Aβ40 and Aβ42:tau ratios with visual [18F]flutemetamol PET status.
Results
Of 262 participants (mean [SD] age, 70.9 [5.5] years), 108 were women (41.2%) and 154 were men (58.8%). The mass spectrometry–derived Aβ42 values showed higher correlations with the modified Aβ42-INNOTEST (r = 0.97), Aβ42-FL (r = 0.93), Aβ42-EI (r = 0.93), and Aβ42-MSD (r = 0.95) assays compared with the classic Aβ42-INNOTEST assay (r = 0.88; P ≤ .01). The signal in the classic Aβ42-INNOTEST assay was partly quenched by recombinant Aβ1-40 peptide. However, the classic Aβ42-INNOTEST assay showed better concordance with visual [18F]flutemetamol PET status (area under the receiver operating characteristic curve [AUC], 0.92) compared with the newer assays (AUCs, 0.87-0.89; P ≤ .01). The accuracies of the newer assays improved significantly when Aβ42:Aβ40 (AUCs, 0.93-0.95; P ≤ .01), Aβ42 to total tau (T-tau) (AUCs, 0.94; P ≤ .05), or Aβ42 to phosphorylated tau (P-tau) (AUCs, 0.94-0.95; P ≤ .001) ratios were used. A combination of the Aβ42:Aβ40 ratio and T-tau or P-tau level did not improve the accuracy compared with the ratio alone.
Conclusions and Relevance
Concentrations of CSF Aβ42 derived from the new immunoassays (modified INNOTEST, FL, EI, and MSD) may correlate better with the antibody-independent mass spectrometry–based reference measurement procedure and may show improved agreement with visual [18F]flutemetamol PET assessment when using the Aβ42:Aβ40 or Aβ42:tau ratios. These findings suggest the benefit of implementing the CSF Aβ42:Aβ40 or Aβ42:tau ratios as a biomarker of amyloid deposition in clinical practice and trials.
Introduction
Cerebrospinal fluid (CSF) β-amyloid (Aβ) 42 and amyloid positron emission tomography (PET) have proved to have high diagnostic accuracy for Alzheimer disease (AD) years before the onset of clinical symptoms and are becoming an important part of the diagnostic workup. Both biomarkers have shown correlations with postmortem plaque measurements, and previous studies have indicated 80% to 90% agreement between CSF Aβ42 values and quantitative amyloid PET data. For most immunoassays, the concordance between the 2 biomarker modalities is further improved with the CSF Aβ42:Aβ40 ratio (compared with CSF Aβ42 level alone), probably because the ratio corrects for (1) interindividual variability in the overall Aβ production; (2) interindividual variability in the CSF turnover; (3) changes in global levels of all Aβ isoforms owing to non-AD–related abnormal findings, such as white matter lesions; and (4) variability owing to preanalytical factors.
Several enzyme-linked immunosorbent assays (ELISAs) are commonly used for measurements of Aβ42 levels in CSF. In general, the precision of an immunoassay may be compromised by matrix interference when endogenous biological factors in the sample interact with the analyte of interest or nonspecifically bind to antibodies. Matrix interference affecting Aβ42 quantification has been reported for the initially developed INNOTEST Aβ42 assay but seems to be minimized in the newer Aβ immunoassays from EUROIMMUN, Fujirebio, Meso Scale Discovery, and Roche. A recent study suggested that the diagnostic accuracy of CSF Aβ biomarkers may vary depending on the assays used. More specifically, CSF Aβ42 measured with the classic INNOTEST (Fujirebio) kit performed better in distinguishing patients with early-stage AD from cognitively healthy control individuals than one of the newer immunoassays. At same time, using the CSF Aβ42:Aβ40 ratio improved the diagnostic accuracy of the newer EUROIMMUN (EI; EUROIMMUN) Aβ42 assay but not the classic Aβ42 INNOTEST assay. Another report demonstrated higher concordance between quantitative amyloid PET and the Aβ42:Aβ40 ratio compared with CSF Aβ42 level alone for the EI and Meso Scale Discovery (MSD; Meso Scale Discovery) assays.
In most of the studies that have compared amyloid PET with CSF Aβ42 levels, the quantification of regional and global amyloid PET ligand binding has used semiquantitative image assessments of standardized uptake value ratio (SUVR). In clinical practice, however, the standard approved by regulatory authorities is visual classification of scans on a binary scale as positive or negative for amyloid, with a negative finding indicating that AD is unlikely. Visual readings of PET images agree with PET SUVR values and with brain amyloid burden at autopsy. Nevertheless, concordance between the visual reading of PET and CSF Aβ42 measures from different immunoassays has not been established and may influence the use of amyloid PET and/or CSF analysis in clinical practice and trials. In the present study, we investigated the agreement between visual amyloid PET analysis and CSF Aβ42 measured using 5 different Aβ42 immunoassays or protocols (INNOTEST Aβ1-42 classical procedure, INNOTEST Aβ1-42 modified procedure, fully automated Lumipulse [FL; Fujirebio], EI Aβ1-42, and MSD AβN-42). For each of the 5 Aβ42 immunoassays, the performance of Aβ42 and the Aβ42:Aβ40, Aβ42 to total tau (T-tau), and Aβ42 to phosphorylated tau (P-tau) ratios were determined using visual assessment of flutemetamol F 18–labeled PET images as the criterion standard. Furthermore, an important advancement in the CSF biomarker field was the recent development of a mass spectrometry–based reference measurement procedure (RMP), which allows antibody-independent quantification of absolute CSF concentration of Aβ42 with high accuracy and is now considered to be the criterion standard method for CSF Aβ42 measurement. However, implementation of mass spectrometry in routine clinical laboratories is challenging owing to the complexity of the technology, the need for in-house method development and validation and personal training, and high costs. Herein, we explored correlation between CSF levels of amyloid biomarkers analyzed using immunoassays and antibody-independent mass spectrometry–based RMP.
Methods
Study Participants
The study population included 262 patients with mild cognitive impairment or subjective cognitive decline and no dementia from the prospective and longitudinal Swedish BioFINDER (Biomarkers for Identifying Neurodegenerative Disorders Early and Reliably) cohort (http://www.biofinder.se) who had undergone [18F]flutemetamol PET evaluation from September 1, 2010, through December 31, 2014. The diagnostic criteria and cohort characteristics are described in the eMethods and eTable 1 in the Supplement. The study was approved by the Regional Ethical Review Board in Lund, Sweden, and all study participants gave written informed consent to participate in the study.
CSF Sampling and Analysis
The procedure and analysis of CSF samples were conducted according to the Alzheimer Association Flowchart for CSF biomarkers. Lumbar CSF samples were collected at 3 memory clinics at the Skåne University Hospital in Lund and Malmö and Ängelholm Hospital in Sweden and analyzed according to a standardized protocol.
We measured CSF Aβ42 levels using the EI Aβ1-42 and MSD AβN-42 according to the manufacturer’s instructions. We also measured CSF Aβ42 (1-42) with the INNOTEST kit (Fujirebio). For the latter assay, the classic procedure as described in the product’s package insert and a modified procedure that minimizes matrix interference effects were applied. The modifications included (1) dilution of conjugate 1 in conjugate diluent at 1:10 (classic procedure, 1:100 dilution), (2) addition of only 10 µL of samples or standards to the plate (classic procedure, 25 µL of samples or standards), and (3) incubation of samples or standards for 3 hours (classic procedure, 1 hour incubation). In addition, CSF concentrations of Aβ42 were determined using the fully automated FL assay, which is based on the modified INNOTEST procedure. We analyzed CSF Aβ40 levels with kits from Fujirebio (INNOTEST Aβ1-40), EI (Aβ1-40), and MSD (AβN-40) according to the manufacturer’s instructions, and the ratios with the corresponding CSF Aβ42 values (Aβ42:Aβ40) were calculated. We used the MSD electorchemiluminescence assay (Vplex; MSD) for multiplex detection of Aβ42, Aβ40, and Aβ38 levels. Cerebrospinal fluid T-tau and P-tau(181P) were quantified using EUROIMMUN and INNOTEST ELISA kits, respectively. These tau values and Aβ42 values from 5 different assays (classic INNOTEST, modified INNOTEST, FL, EI, and MSD) were used for the calculations of the CSF Aβ42:tau ratios.
Increasing concentrations of recombinant Aβ40 peptide (1-40 ng/mL) (rPeptide; Bogart) were added to 2 CSF samples with low and high Aβ42 concentrations. We measured Aβ42 levels in spiked samples using the classic and modified INNOTEST procedures and MSD assays. Finally, an antibody-independent mass spectrometry–based method was used to assess CSF concentrations of Aβ42 using an RMP (Joint Committee for Traceability in Laboratory Medicine Database identification number C11RMP9) and Aβ40 in a subgroup of 98 individuals. The [18F]flutemetamol PET imaging is described in the eMethods in the Supplement.
Statistical Analysis
We used SPSS (version 22; IBM), R (version 3.3.1), and MedCalc (version 16.8.4; MedCalc Software bvba) software for statistical analysis. Associations between ELISA and mass spectrometry–based biomarker concentrations were tested using Pearson and Spearman correlation coefficient analyses. Differences between the correlation coefficients were estimated using the Meng test for correlated correlation coefficients. Intermethod agreement between visual and quantitative [18F]flutemetamol PET assessments was estimated with the Cohen κ statistic. The accuracies of CSF biomarkers in detecting [18F]flutemetamol PET status were assessed using receiver operating characteristic (ROC) curve analysis and logistic regression models. Two areas under the ROC curve (AUCs) were compared using the DeLong test. We dichotomized the study participants into groups with normal and increased Aβ deposition based on the previously established [18F]flutemetamol SUVR cutoff of greater than 1.42. Cutoffs for CSF biomarkers were determined using the Youden J index and mixture modeling. P < .05 was considered to be statistically significant.
Results
CSF Aβ Biomarkers: Associations Between ELISA and Mass Spectrometry Measurements
Of 262 participants (mean [SD] age, 70.9 [5.5] years), 108 were women (41.2%) and 154 were men (58.8%). To determine which of the immunoassays are more accurate in quantifying CSF levels of Aβ42, we studied associations between CSF concentrations of Aβ obtained with different immunoassays (classic INNOTEST and the newer modified INNOTEST, FL, EI, and MSD) and the antibody-independent mass spectrometry–based RMP for Aβ42 in a subgroup of 98 individuals. We found correlations between CSF levels of Aβ42 measured using ELISAs and mass spectrometry (r>0.88; Table 1). However, the correlation coefficients between different ELISA and mass spectrometry measurements varied significantly. Correlations with mass spectrometry–derived Aβ42 were greater for the new Aβ42 assays (r = 0.97 for the modified INNOTEST, r = 0.93 for FL, r = 0.93 for EI, and r = 0.95 for MSD) than the classic INNOTEST assay (r = 0.88). In contrast, the correlation between the mass spectrometry–derived Aβ42:Aβ40 ratio and the classic INNOTEST was significantly higher (r = 0.87) compared with the correlations between mass spectrometry–derived Aβ42:Aβ40 and the Aβ42 modified INNOTEST (r = 0.70), Aβ42-FL (r = 0.64), Aβ42-EI (r = 0.73), or Aβ42-MSD (r = 0.74) assays (Table 1).
Table 1. Correlations Between Aβs Measured Using MS and Different Immunoassaysa.
| Immunoassay | MS-Derived Aβ42 Level | MS-Derived Aβ42:Aβ40 Ratio |
|---|---|---|
| Classic Aβ42-INNOTEST | ||
| Pearson correlation | r = 0.88 | r = 0.87 |
| Spearman correlation | ρ = 0.91 | ρ = 0.88 |
| Modified Aβ42-INNOTEST | ||
| Pearson correlation | r = 0.97 | r = 0.70b |
| Spearman correlation | ρ = 0.96b | ρ = 0.74b |
| Aβ42-FL | ||
| Pearson correlation | r = 0.93c | r = 0.64b |
| Spearman correlation | ρ = 0.92 | ρ = 0.70b |
| Aβ42-EI | ||
| Pearson correlation | r = 0.93d | r = 0.73b |
| Spearman correlation | ρ = 0.95d | ρ = 0.78b |
| Aβ42-MSD | ||
| Pearson correlation | r = 0.95b | r = 0.74b |
| Spearman correlation | ρ = 0.96b | ρ = 0.78b |
Abbreviations: Aβ, amyloid β; EI, EUROIMMUN; FL, fully automated Lumipulse; MS, mass spectrometry; MSD, Meso Scale Discovery.
P < .001 for all comparisons except P = .02 for the correlation between MS-derived Aβ42 and Aβ42-INC:Aβ40 ratio. Differences between the correlation coefficients were estimated using the Meng test.
P < .001 compared with classic Aβ42-INNOTEST.
P = .01 compared with classic Aβ42-INNOTEST.
P < .01 compared with classic Aβ42-INNOTEST.
Concordance Between Visual and Quantitative [18F]flutemetamol PET Assessments
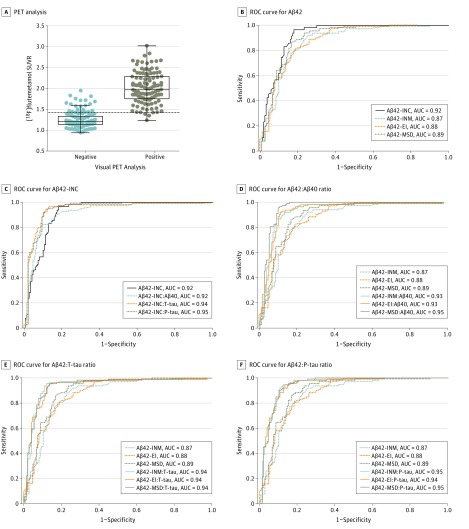
With use of visual readings of [18F]flutemetamol PET, 113 patients (43.1%) were classified as Aβ positive and 149 patients (56.9%) as Aβ negative. When applying the SUVR cutoff, 132 patients (50.4%) showed abnormal amyloid deposition, whereas composite SUVR values were within the reference range in 130 patients (49.6%). The Cohen κ was 0.81, suggesting very good agreement between the visual and quantitative [18F]flutemetamol PET assessment methods. Visual and quantitative [18F]flutemetamol PET data disagreed in 25 cases (9.5%), of which 22 (8.4%) were Aβ positive based on the SUVR cutoff but Aβ negative based on visual analysis (Figure 1A).
Figure 1. Cerebrospinal Fluid (CSF) Alzheimer Disease (AD) Biomarkers and Flutemetamol F 18–Labeled Positron Emission Tomography (PET).
A, Concordance between visual and quantitative amyloid PET analysis. Composite standardized uptake value ratio (SUVR) of [18F]flutemetamol data in patients who were classified as negative (n = 149) or positive (n = 113) for amyloid findings with visual PET assessment are shown. The dotted line represents a cutoff of greater than 1.42 SUVR. Data points indicate individuals. B-F, Correlation of CSF AD biomarkers with [18F]flutemetamol PET status according to visual analysis. Levels of CSF β-amyloid (Aβ) 42 were analyzed with the classic INNOTEST (INC), modified INNOTEST (INM), EUROIMMUN (EI) and Meso Scale Discovery (MSD) assays. To calculate the CSF Aβ42:Aβ40 ratio, we used Aβ40 kits from the assay vendors Fujirebio (INC and INM), EUROIMMUN (EI), and Meso Scale Discovery (MSD). Levels of CSF total tau (T-tau) and phosphorylated tau (P-tau) were measured with the EI and INNOTEST assays, respectively. Receiver operating characteristic (ROC) curves were generated for Aβ42 level (B-F), the Aβ42:Aβ40 ratio (C [INC] and D [INM, EI, and MSD]), the Aβ42:T-tau ratio (C [INC] and E [INM, EI, and MSD]), and the Aβ42:P-tau ratio (C [INC] and F [INM, EI, and MSD]) to determine their accuracy in differentiating Aβ-negative (n = 149) and Aβ-positive (n = 113) visual readings. AUC indicates area under the ROC curve.
Visual Aβ PET vs CSF AD Biomarker Assessments
We investigated how accurately CSF Aβ42 levels could predict visual [18F]flutemetamol PET assessment when using the commercially available classic Aβ42-INNOTEST assay or the newer modified Aβ42-INNOTEST, Aβ42-EI, and Aβ42-MSD asssays. The results of ROC curve analysis are shown in Table 2 and eTable 2 in the Supplement. We found that classic Aβ42-INNOTEST was more accurate than the newer assays (modified Aβ42-INNOTEST, Aβ42-EI, and Aβ42-MSD) in distinguishing individuals with normal and abnormal visual readings (Figure 1B). Next, we studied whether the Aβ42:Aβ40, Aβ42:T-tau, or Aβ42:P-tau ratios improved the accuracy of the 4 assays. For the classic Aβ42-INNOTEST, only the Aβ42:P-tau but not the Aβ42:Aβ40 or the Aβ42:T-tau ratios exhibited a significantly higher AUC than Aβ42 alone in predicting visual classification of [18F]flutemetamol PET (Table 2, eTable 2 in the Supplement, and Figure 1C). For all the newer assays (modified Aβ42-INNOTEST, Aβ42-EI, and Aβ42-MSD), all 3 ratios (ie, Aβ42:Aβ40, Aβ42:T-tau, and Aβ42:P-tau) were comparable and significantly more accurate than Aβ42 levels alone (Table 2, eTable 2 in the Supplement, and Figure 1D-F). The results for the Aβ42-FL assay were comparable to those of the modified Aβ42-INNOTEST assay and are shown in eFigure 1 in the Supplement. Furthermore, using logistic regression analysis, we found that a combination of the Aβ42:Aβ40 ratio and T-tau (AUCs, 0.92-0.95) or P-tau (AUCs, 0.92-0.93) for any of the 4 Aβ42 assays did not have better concordance with visual [18F]flutemetamol PET classification than the Aβ42:Aβ40 ratio alone (AUCs, 0.92-0.95; P > .14 when comparing the AUC of 2 ROC curves). The performance of T-tau and P-tau was similar in all the statistical tests and, therefore, only data for P-tau are presented hereinafter.
Table 2. ROC Analysis of CSF Aβs for Distinguishing Abnormal From Normal Visual Reading Assessments of Flutemetamol F 18–Labeled PET.
| Immunoassaya | AUC (95% CI) | Cutoff (Cutoff for Mixture Modeling Analysis)b | Youden J Index | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Classic Aβ42-INNOTEST | 0.92 (0.89-0.95) | ≤548.00 (524.00) | 0.78 | 0.96 | 0.82 |
| Classic INNOTEST Aβ42:Aβ40 ratio | 0.92 (0.88-0.95) | ≤0.06 (0.05) | 0.73 | 0.91 | 0.82 |
| Classic INNOTEST Aβ42:T-tau ratio | 0.94 (0.91-0.97)c | ≤1.79 (1.23) | 0.84 | 0.96 | 0.87 |
| Classic INNOTEST Aβ42:P-tau ratio | 0.95 (0.92-0.98)c,d | ≤10.30 (8.11) | 0.83 | 0.95 | 0.89 |
| Modified Aβ42-INNOTEST | 0.87 (0.83-0.91)e | ≤1091.00 (1129.00) | 0.67 | 0.92 | 0.74 |
| Modified INNOTEST Aβ42:Aβ40 ratio | 0.93 (0.90-0.96)f | ≤0.12 (0.10) | 0.79 | 0.92 | 0.87 |
| Modified INNOTEST Aβ42:T-tau ratio | 0.94 (0.91-0.97)g | ≤3.30 (2.38) | 0.83 | 0.96 | 0.88 |
| Modified INNOTEST Aβ42:P-tau ratio | 0.95 (0.92-0.97)g | ≤19.60 (16.50) | 0.82 | 0.95 | 0.87 |
| Aβ42-EI | 0.88 (0.84-0.92)e | ≤449.00 (479.00) | 0.63 | 0.82 | 0.80 |
| Aβ42-EI:Aβ40 ratio | 0.93 (0.90-0.96)h | ≤0.10 (0.10) | 0.81 | 0.93 | 0.88 |
| Aβ42-EI:T-tau ratio | 0.94 (0.90-0.97)h | ≤1.44 (1.11) | 0.81 | 0.96 | 0.86 |
| Aβ42-EI:P-tau ratio | 0.94 (0.91-0.96)i | ≤9.59 (7.26) | 0.81 | 0.95 | 0.87 |
| Aβ42-MSD | 0.89 (0.85-0.93)j | ≤506.00 (500.00) | 0.70 | 0.94 | 0.76 |
| Aβ42-MSD:Aβ40 ratio | 0.95 (0.93-0.98)k | ≤0.08 (0.09) | 0.86 | 0.96 | 0.89 |
| Aβ42-MSD:T-tau ratio | 0.94 (0.91-0.97)l | ≤1.39 (1.12) | 0.85 | 0.96 | 0.89 |
| Aβ42-MSD:P-tau ratio | 0.95 (0.92-0.98)k | ≤9.89 (7.84) | 0.83 | 0.96 | 0.87 |
Abbreviations: Aβ, amyloid β; AUC, area under the curve; CSF, cerebrospinal fluid; EI, EUROIMMUN; MSD, Meso Scale Discovery; PET, positron emission tomography; P-tau, phosphorylated tau; ROC, receiver operating characteristic; T-tau, total tau.
Cerebrospinal fluid levels of T-tau and P-tau were quantified with EUROIMMUN and INNOTEST enzyme-linked immunosorbent assay kits, respectively. These tau values and Aβ42 values from the 5 different assays (classic and modified INNOTESTs, fully automated Lumipulse, EI, and MSD) were used for the calculations of the CSF Aβ42:tau ratios.
Cutoffs for Aβ42 are given in pg/mL.
P ≤ .01 compared with classic INNOTEST Aβ42:Aβ40 ratio.
P ≤ .05 compared with classic Aβ42-INNOTEST level.
P ≤ .001 compared with classic Aβ42-INNOTEST level.
P ≤ .01 compared with modified Aβ42-INNOTEST level.
P ≤ .001 compared with modified Aβ42-INNOTEST level.
P ≤ .01 compared with Aβ42-EI level.
P ≤ .001 compared with Aβ42-EI level.
P ≤ .01 compared with classic Aβ42-INNOTEST level.
P ≤ .001 compared with Aβ42-MSD level.
P ≤ .05 compared with Aβ42-MSD level.
Cutoff for the Different Immunoassays
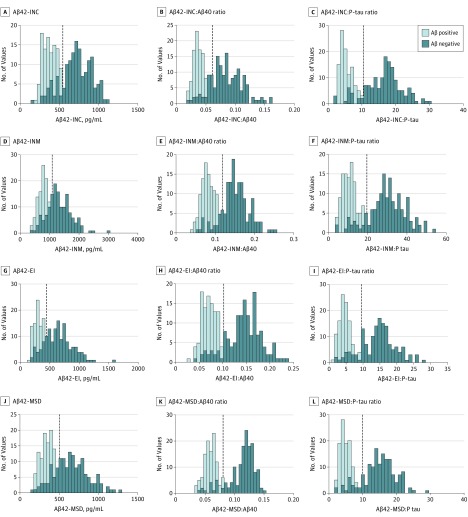
The Aβ42 levels in CSF are most often bimodally distributed, and more reliable cutoffs likely can be derived when biomarker levels show 2 clearly distinct populations. Frequency plots revealed improved bimodal separation of [18F]flutemetamol PET–positive and –negative populations based on the Aβ42:Aβ40 and Aβ42:P-tau ratios compared with the Aβ42 measurement alone when using the modified Aβ42-INNOTEST, Aβ42-EI, and Aβ42-MSD assays (Figure 2D-F, G-I, and J-L). This effect was less pronounced for the classic Aβ42-INNOTEST assay (Figure 2A-C). The cutoffs for each biomarker were determined based on the Youden J index in which visual read of [18F]flutemetamol PET was used as the outcome. Mixture modeling, which is independent of the standard of truth (ie, PET imaging results), produced similar cutoff values (Table 2). Analytical imprecision and within-participant variability may increase the risk for misclassification, especially when sensitivity and/or specificity markedly decrease around cutoff points. In our study, the trends for sensitivity and/or specificity near the cutoff points appeared to be less steep for the Aβ42:Aβ40 and Aβ42:P-tau ratios than for Aβ42 levels alone when Aβ42 was measured using the newer assays (modified Aβ42-INNOTEST, Aβ42-EI, or Aβ42-MSD) (eFigure 2B-D, F-H, and J-L in the Supplement), but this was not the case for the classic Aβ42-INNOTEST assay (eFigure 2A, E, and I in the Supplement).
Figure 2. Frequency Plots of Cerebrospinal Fluid (CSF) β-Amyloid (Aβ) 42 Levels and Aβ42:Aβ40 and Aβ42 to Phosphorylated Tau (P-tau) Ratios.
Histograms of frequency distribution for CSF Aβ42 levels and the Aβ42:Aβ40 and Aβ42:P-tau ratios across groups with Aβ-positive (n = 113) and Aβ-negative (n = 149) visual ratings. Veritical dashed lines indicate cutoff points associated with the Youden J index. EI indicates EUROIMMUN; INC, classic INNOTEST; INM, modified INNOTEST; and MSD, Meso Scale Discovery.
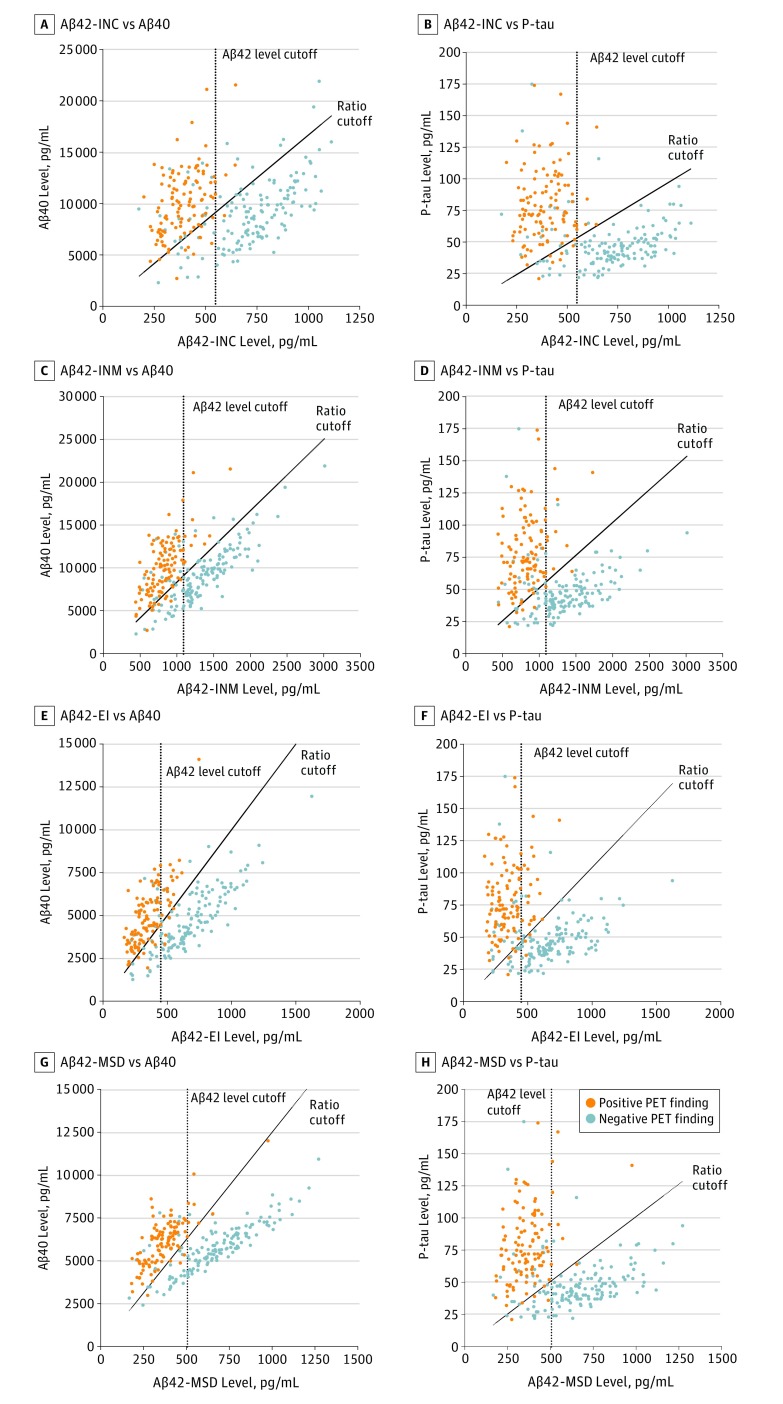
To further assess the concordance between CSF biomarkers and visual PET assessment in terms of classification performance, we dichotomized CSF Aβ variables into abnormal (CSF Aβ-positive) and normal (CSF Aβ-negative) values based on the optimal cutoff points corresponding to the highest Youden J indices. The number of cases with discordant CSF Aβ status compared with visual PET assessments was higher for the newer assays (48 [18.3%] for modified Aβ42-INNITEST; 49 [18.7%] for Aβ42-EI; and 42 [16.0%] for Aβ42-MSD) than for the classic Aβ42-INNOTEST (31 [11.8%]) and consisted of mainly CSF Aβ42-positive and visual PET-negative cases (eTable 3 in the Supplement). However, the number of discordant cases for the newer assays (modified Aβ42-INNOTEST, Aβ42-EI, and Aβ42-MSD) was reduced significantly when using the Aβ42:Aβ40 or Aβ42:P-tau ratios (eTable 3 in the Supplement). As shown in Figure 3, using cutoffs obtained from the 2 ratios (Aβ42:Aβ40 or Aβ42:P-tau) resulted in better separation of visual PET-positive and -negative cases than CSF Aβ42 levels alone, and the ratios mainly reduced the number of cases in the discordant group that were positive for CSF Aβ42 and negative for visual PET findings (eTable 3 in the Supplement). The results were similar for CSF biomarkers measured using antibody-independent mass spectrometry–based RMP and quantitative PET assessment (eResults and eFigure 3 in the Supplement).
Figure 3. Separation of Populations by Visual Positron Emission Tomography (PET) Findings.
Separation of populations with positive and negative PET findings used cutoffs for cerebrospinal fluid (CSF) levels of β-amyloid (Aβ) 42 and the Aβ42:Aβ40 and Aβ42 to phosphorylated tau (P-tau) ratios. Scatterplots of CSF Aβ42 levels against Aβ40 and P-tau levels; Aβ42 was measured using the classic INNOTEST (INC), modified INNOTEST (INM), EUROIMMUN (EI), and Meso Scale Discovery (MSD) assays. Dotted lines indicate Youden J index cutoffs for CSF Aβ42 levels. Solid lines indicated Youden J index cutoffs for the CSF Aβ42:Aβ40 and Aβ42:P-tau ratios.
Spiking of CSF Samples With Aβ40
Addition of recombinant Aβ1-40 peptide to CSF samples caused a spike level–dependent decrease in measured CSF Aβ42 concentrations by as much as 62% when measured using the classic INNOTEST assay (eFigure 4A and D in the Supplement). In contrast, no differences in Aβ42 concentrations were observed when the samples were analyzed using the newer modified INNOTEST procedure (eFigure 4B and E in the Supplement) and MSD assay (eFigure 4C and F in the Supplement).
Discussion
In this study, we showed that the new modified Aβ42-INNOTEST, Aβ42-FL, Aβ42-EI, and Aβ42-MSD assays correlated better with Aβ42 values obtained with antibody-independent mass spectrometry–based RMP compared with the classic Aβ42-INNOTEST assay. The classic Aβ42-INNOTEST instead correlated better with the Aβ42:Aβ40 ratio obtained with mass spectrometry–based RMP compared with the other Aβ42 assays, a phenomenon that might be explained by the fact that the signal in the classic Aβ42-INNOTEST assay is somewhat quenched by Aβ40 levels. Of interest, the classic Aβ42-INNOTEST assay had a higher accuracy to predict visual PET assessment outcome compared with the newer modified Aβ42-INNOTEST, Aβ42-FL, Aβ42-EI, and Aβ42-MSD immunoassays. However, the CSF Aβ42:Aβ40 ratios from these assays performed better than the corresponding Aβ42 levels alone and had comparable performance to the classic Aβ42-INNOTEST level. The accuracy of the classic Aβ42-INNOTEST assay did not improve by using the Aβ42:Aβ40 ratio. For all the Aβ42 assays, the addition of T-tau and P-tau to Aβ42:Aβ40 did not improve the ability to predict cortical Aβ accumulation compared with the Aβ42:Aβ40 ratios alone.
The INNOTEST ELISA is one of the most extensively used assays for assessment of Aβ42 levels in CSF. However, this assay is limited by the matrix interference that may lead to inaccurate estimates of Aβ42 concentrations. The matrix effects are reduced in the newer assays (modified INNOTEST, FL, EI, and MSD), which are based on the analysis of diluted CSF samples. Accordingly, we found that CSF concentrations of Aβ42 derived from antibody-independent mass spectrometry procedure matched better with the data from the newer assays than the classic INNOTEST assay.
Visual rating is the only approved procedure for assessment of amyloid PET scans in the clinic and when selecting AD cases for clinical trials. Although quantitative approaches provide more informative data that might be critical for investigating longitudinal changes in amyloid burden and treatment effects, the concordance between visual and quantitative methods for establishing amyloid status is very high. Similar to previous studies, we found that only 25 cases (9.5%) were discordant according to visual and quantitative PET analysis with very good intermethod agreement (Cohen κ = 0.81). Most discordant cases were found in individuals who had been classified as amyloid negative by the visual analysis but as amyloid positive by SUVR analysis, demonstrating that visual analysis is more conservative than SUVR analysis; this finding is consistent with those of previous studies.
Cerebrospinal fluid Aβ42 is a biomarker of amyloid deposition that is often used interchangeably or in combination with PET imaging in the diagnostic workup in the clinic or in clinical trials. The agreement between CSF Aβ42 and quantitative amyloid PET data has ranged from 80% to 90%, and this agreement might be improved by using the Aβ42:Aβ40 or Aβ42:tau ratios. However, for implementation of CSF AD biomarkers (Aβ42 level, Aβ42:Aβ40 ratio, and/or Aβ42:tau ratio) in routine clinical practice, establishing the concordance with the clinically approved visual amyloid PET ratings, and studying whether the concordance rate is affected by the choice of the immunoassay for CSF Aβ42 measurements are important. Herein, we showed that newer Aβ42-EI and Aβ42-MSD assays have similar performance with respect to association of visual read outcome and exhibit acceptable accuracy with visual [18F]flutemetamol PET status when using the Aβ42:Aβ40 ratio. With the classic INNOTEST assay, the AUC for Aβ42 alone was close to the Aβ42:Aβ40 ratios from the EI and MSD assays and did not increase any further when the classic INNOTEST Aβ42:Aβ40 ratio was used. The modification of the classic INNOTEST assay, which improved the correlation with mass spectrometry–based RMP Aβ42 values, resulted in decreased accuracy of Aβ42 levels alone but improved performance for the Aβ42:Aβ40 ratio, as was the case for other Aβ42 assays evaluated herein. These results could in part be explained by our spiking data, which suggested that Aβ42 concentrations obtained using the classic INNOTEST were influenced by Aβ1-40 and that in individuals with higher CSF levels of Aβ1-40, these effects might be more pronounced, resulting in a lower signal. Further analysis revealed that the CSF Aβ42:Aβ40 ratios from the newer immunoassays (modified INNOTEST, EI, and MSD) had a more pronounced bimodal distribution allowing improved separation of [18F]flutemetamol PET-positive and -negative populations compared with CSF Aβ42 levels alone. Moreover, compared with Aβ42 levels alone, the rate of decrease in sensitivity and specificity values around the cutoff points was less steep when using the Aβ42:Aβ40 ratios. However, we did not observe differences in the distributions and behavior of sensitivity and specificity values near cutoff between Aβ42 level and Aβ42:Aβ40 ratio for the classic INNOTEST assay. These results indicate that the Aβ42:Aβ40 ratio cutoffs for the new assays (modified INNOTEST, EI, and MSD) may be more reliable in terms of performance that is more robust with respect to small changes in cutoff values. In agreement with our findings, the CSF Aβ42:Aβ40 ratio has also been shown to provide a better prediction of prodromal AD compared with Aβ42 level alone when using the Aβ42-EI and Aβ42-MSD assays but not the classic Aβ42-INNOTEST or xMAP AlzBio3 (Fujirebio) assays.
In the present study, the Aβ42:tau ratios were better predictors of visual [18F]flutemetamol PET classification than were Aβ42 levels alone. However, we did not observe improved accuracy when the Aβ42:Aβ40 ratios were combined with T-tau or P-tau compared with the Aβ42:Aβ40 ratios alone. Biochemical mechanisms underlying the better performance of the Aβ42:Aβ40 and Aβ42:tau ratios are not known. Interindividual variability in the production of CSF, the production and secretions of proteins by neurons such as Aβ and tau, and preanalytical factors may affect the accuracy of CSF Aβ42 levels in predicting amyloid status. Although all these effects could be partly compensated by using the ratios to Aβ40 or tau, at present, which of the mechanisms are mainly responsible for the better performance of the ratios and whether these mechanisms differ for ratios to Aβ40 or tau remain unclear. For example, some data suggest that CSF levels of tau are less affected by preanalytical factors than Aβ peptides. Consequently, variations in preanalytical handling should in theory be better compensated for by using Aβ42:Aβ40 ratios than Aβ42:tau ratios, suggesting that the Aβ42:Aβ40 ratios might be more useful in clinical practice as well as for tracking the emergence of amyloid deposition in longitudinal studies.
Limitations
One limitation that could have possibly influenced the result of the present study is that PET images were assessed by a single rater. However, this is unlikely considering that the findings were the same for quantitative measures (SUVR) of amyloid PET.
Conclusions
We showed that the newer Aβ42 assays (modified INNOTEST, EI, and MSD) correlated better with the antibody-free RMP than did the classic INNOTEST assay, possibly because the signal in the classic INNOTEST assay is partly quenched by Aβ1-40. However, the accuracy to correlate with visual [18F]flutemetamol PET status was decreased in the newer Aβ42 assays, a limitation that is overcome by using a Aβ42:Aβ40 or Aβ42:tau ratios. The CSF Aβ42:Aβ40 or Aβ42:tau ratios from the newer assays showed improved accuracy for detection of cortical Aβ fibrils as measured by PET. Moreover, the sensitivities and specificities of these newer assays were less influenced by moderate changes in the cutoffs when Aβ42:Aβ40 or Aβ42:tau ratios were used, a finding that is important when samples will be analyzed consecutively over time. The precision of the Aβ42:Aβ40 ratios in differentiating [18F]flutemetamol PET–positive and –negative visual reads did not improve further when combined with T-tau or P-tau values. Thus, our study provides a comprehensive overview of the correlation of the performance of CSF biomarkers across different immunoassays with amyloid PET status, which may influence the selection of immunoassays and biomarkers in future studies. Furthermore, our findings support implementation of the CSF Aβ42:Aβ40 and/or the Aβ42:tau ratios as biomarkers of amyloid deposition in clinical practice.
eMethods. Participants and [18F]flutemetamol PET
eResults. Visual Aβ PET vs CSF AD Biomarker Measured Using Antibody-Independent MS-based RMP
eTable 1. Biofinder Cohort Characteristics
eTable 2. Comparisons of ROC Analysis of CSF Biomarkers for Distinguishing Abnormal From Normal Visual Reading Assessments of [18F]Flutemetamol PET
eTable 3. Concordance Between CSF Biomarkers and Visual PET Rating
eFigure 1. CSF AD Biomarkers as Predictors of Amyloid PET Status According to Visual Analysis
eFigure 2. Sensitivities and Specificities of CSF Aβ42 and the Aβ42/Aβ40 and Aβ42/P-tau Ratios at Different Cutoffs For Predicting Visual Amyloid PET Assessment
eFigure 3. Agreement Between CSF Aβ Biomarkers and Amyloid PET SUVR
eFigure 4. Spiking of CSF Samples With Increasing Concentrations of Aβ 1-40
References
- 1.Blennow K, Mattsson N, Schöll M, Hansson O, Zetterberg H. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci. 2015;36(5):297-309. [DOI] [PubMed] [Google Scholar]
- 2.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131(pt 6):1630-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strozyk D, Blennow K, White LR, Launer LJ. CSF Aβ 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60(4):652-656. [DOI] [PubMed] [Google Scholar]
- 4.Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66(3):382-389. [DOI] [PubMed] [Google Scholar]
- 5.Wolk DA, Grachev ID, Buckley C, et al. Association between in vivo fluorine 18-labeled flutemetamol amyloid positron emission tomography imaging and in vivo cerebral cortical histopathology. Arch Neurol. 2011;68(11):1398-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landau SM, Lu M, Joshi AD, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann Neurol. 2013;74(6):826-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattsson N, Insel PS, Donohue M, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Independent information from cerebrospinal fluid amyloid-β and florbetapir imaging in Alzheimer’s disease. Brain. 2015;138(pt 3):772-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmqvist S, Zetterberg H, Blennow K, et al. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid β-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol. 2014;71(10):1282-1289. [DOI] [PubMed] [Google Scholar]
- 9.Janelidze S, Zetterberg H, Mattsson N, et al. ; Swedish BioFINDER study group . CSF Aβ42:Aβ40 and Aβ42:Aβ38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol. 2016;3(3):154-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leuzy A, Chiotis K, Hasselbalch SG, et al. Pittsburgh compound B imaging and cerebrospinal fluid amyloid-β in a multicentre European memory clinic study. Brain. 2016;139(Pt 9):2540-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanderstichele HM, Janelidze S, Demeyer L, et al. Optimized standard operating procedures for the analysis of cerebrospinal fluid Aβ42 and the ratios of Aβ isoforms using low protein binding tubes. J Alzheimers Dis. 2016;53(3):1121-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cullen VC, Fredenburg RA, Evans C, Conliffe PR, Solomon ME. Development and advanced validation of an optimized method for the quantitation of Aβ42 in human cerebrospinal fluid. AAPS J. 2012;14(3):510-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bittner T, Zetterberg H, Teunissen CE, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1-42) in human cerebrospinal fluid. Alzheimers Dement. 2016;12(5):517-526. [DOI] [PubMed] [Google Scholar]
- 14.Pan C, Korff A, Galasko D, et al. Diagnostic values of cerebrospinal fluid T-tau and Aβ42 using Meso Scale Discovery assays for Alzheimer’s disease. J Alzheimers Dis. 2015;45(3):709-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandijck M, Moonen R, Andreasson U, et al. Correlation of the modified innotest β-amyloid 1-42 with a lc-ms:ms candidate reference method. Alzheimers Dement. 2015;11(7)(suppl):385.25130659 [Google Scholar]
- 16.Palmqvist S, Zetterberg H, Mattsson N, et al. ; Alzheimer’s Disease Neuroimaging Initiative; Swedish BioFINDER Study Group . Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology. 2015;85(14):1240-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreasen N, Hesse C, Davidsson P, et al. Cerebrospinal fluid β-amyloid(1-42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol. 1999;56(6):673-680. [DOI] [PubMed] [Google Scholar]
- 18.Clark CM, Pontecorvo MJ, Beach TG, et al. ; AV-45-A16 Study Group . Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11(8):669-678. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber S, Landau SM, Fero A, Schreiber F, Jagust WJ; Alzheimer’s Disease Neuroimaging Initiative . Comparison of visual and quantitative florbetapir F 18 positron emission tomography analysis in predicting mild cognitive impairment outcomes. JAMA Neurol. 2015;72(10):1183-1190. [DOI] [PubMed] [Google Scholar]
- 20.Leinenbach A, Pannee J, Dülffer T, et al. ; IFCC Scientific Division Working Group on CSF proteins . Mass spectrometry–based candidate reference measurement procedure for quantification of amyloid-β in cerebrospinal fluid. Clin Chem. 2014;60(7):987-994. [DOI] [PubMed] [Google Scholar]
- 21.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6(3):131-144. [DOI] [PubMed] [Google Scholar]
- 22.Pannee J, Portelius E, Minthon L, et al. Reference measurement procedure for CSF amyloid β (Aβ)1-42 and the CSF Aβ1-42:Aβ1-40 ratio: a cross-validation study against amyloid PET. J Neurochem. 2016;139(4):651-658. [DOI] [PubMed] [Google Scholar]
- 23.A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. http://www.R-project.org. Accessed September 17, 2016.
- 24.Meng XL, Rosenthal R, Rubin DB. Comparing correlated correlation-coefficients. Psychol Bull. 1992;111(1):172-175. [Google Scholar]
- 25.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benaglia T, Chauveau D, Hunter DR, Young DS. mixtools: an R package for analyzing finite mixture models. J Stat Softw. 2009;32(6):1-29. [Google Scholar]
- 27.Altman DG. Practical Statistics for Medical Research. London, England: Chapman & Hall:CRC; 1999. [Google Scholar]
- 28.Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69(1):98-106. [DOI] [PubMed] [Google Scholar]
- 29.De Meyer G, Shapiro F, Vanderstichele H, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67(8):949-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zwan MD, Ossenkoppele R, Tolboom N, et al. Comparison of simplified parametric methods for visual interpretation of 11C-Pittsburgh compound-B PET images. J Nucl Med. 2014;55(8):1305-1307. [DOI] [PubMed] [Google Scholar]
- 31.Wang MJ, Yi S, Han JY, et al. Analysis of cerebrospinal fluid and [11C]PIB PET biomarkers for Alzheimer’s disease with updated protocols. J Alzheimers Dis. 2016;52(4):1403-1413. [DOI] [PubMed] [Google Scholar]
- 32.Hertze J, Minthon L, Zetterberg H, Vanmechelen E, Blennow K, Hansson O. Evaluation of CSF biomarkers as predictors of Alzheimer’s disease: a clinical follow-up study of 4.7 years. J Alzheimers Dis. 2010;21(4):1119-1128. [DOI] [PubMed] [Google Scholar]
- 33.Le Bastard N, De Deyn PP, Engelborghs S. Importance and impact of preanalytical variables on Alzheimer disease biomarker concentrations in cerebrospinal fluid. Clin Chem. 2015;61(5):734-743. [DOI] [PubMed] [Google Scholar]
- 34.Toombs J, Paterson RW, Lunn MP, et al. Identification of an important potential confound in CSF AD studies: aliquot volume. Clin Chem Lab Med. 2013;51(12):2311-2317. [DOI] [PubMed] [Google Scholar]
- 35.Toombs J, Paterson RW, Nicholas JM, Petzold A, Schott JM, Zetterberg H. The impact of Tween 20 on repeatability of amyloid β and tau measurements in cerebrospinal fluid. Clin Chem Lab Med. 2015;53(12):e329-e332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Participants and [18F]flutemetamol PET
eResults. Visual Aβ PET vs CSF AD Biomarker Measured Using Antibody-Independent MS-based RMP
eTable 1. Biofinder Cohort Characteristics
eTable 2. Comparisons of ROC Analysis of CSF Biomarkers for Distinguishing Abnormal From Normal Visual Reading Assessments of [18F]Flutemetamol PET
eTable 3. Concordance Between CSF Biomarkers and Visual PET Rating
eFigure 1. CSF AD Biomarkers as Predictors of Amyloid PET Status According to Visual Analysis
eFigure 2. Sensitivities and Specificities of CSF Aβ42 and the Aβ42/Aβ40 and Aβ42/P-tau Ratios at Different Cutoffs For Predicting Visual Amyloid PET Assessment
eFigure 3. Agreement Between CSF Aβ Biomarkers and Amyloid PET SUVR
eFigure 4. Spiking of CSF Samples With Increasing Concentrations of Aβ 1-40