This population-based case-control study examines the accumulation of amyloid in adults with childhood-onset epilepsy compared with a group of matched controls.
Key Points
Question
Is uncomplicated childhood-onset epilepsy associated with increased brain amyloid accumulation?
Findings
In this population-based case-control study of Finnish adults who had childhood epilepsy and were followed up prospectively for more than 50 years from their disease onset and a group of matched controls, individuals with childhood-onset epilepsy, and particularly APOE ε4 carriers, had an increased brain amyloid load as measured with positron emission tomography at late middle age.
Meaning
Childhood-onset epilepsy is linked with a biomarker that might be associated with accelerated brain aging and can be considered as a neurobiological predisposition to later-life cognitive disorders.
Abstract
Importance
The effect of childhood epilepsy on later-life cognitive and brain health is an unclear and little-explored issue.
Objective
To determine whether adults with a history of childhood-onset epilepsy exhibit increased brain amyloid accumulation, possibly predisposing to accelerated cognitive impairment or even frank cognitive disorders in later life.
Design, Setting, and Participants
Forty-one adults from a population-based cohort of individuals with childhood-onset epilepsy in southwestern Finland, together with 46 matched population-based controls, underwent amyloid ligand carbon 11–labeled Pittsburgh Compound B (PiB) positron emission tomography after long-term prospective follow-up. The PiB uptake was quantified as a region to cerebellar cortex ratio. Tracer uptake was evaluated visually and analyzed voxel by voxel over the entire brain to investigate the spatial distribution of amyloid deposition. The study was conducted from May 2011 to October 2013; data analysis was performed from January 2014 to October 2016.
Main Outcomes and Measures
Brain amyloid accumulation.
Results
The 41 individuals with epilepsy were originally enrolled in the Turku Adult Childhood Onset Epilepsy study at the mean (SD) age of 5.1 (4.5) years (range, 0-14 years). After a mean 52.5 (4.0) years of follow-up, the participants were evaluated (26 [63%] were women; the mean [SD] age was 56.0 [4.3] years). Nine individuals with childhood-onset epilepsy (22%) and 3 control participants (7%) had a visually abnormal PiB scan showing high cortical uptake in at least 1 of the evaluated brain regions (P = .04). In semiquantitative analyses, there was a significant interaction effect indicating higher prefrontal cortex uptake in apolipoprotein E (APOE) ε4 allele carriers than in noncarriers in participants (mean [SD], 1.66 [0.41] vs 1.43 [0.15]) compared with controls (1.40 [0.26) vs 1.41 [0.12]) (group × APOE interaction, F = 6.8; P = .01). In addition, there was a significant group effect showing higher tracer uptake in participants compared with controls (group effect, F = 8.0; P = .006).
Conclusions and Relevance
Adults with childhood-onset epilepsy, particularly APOE ε4 carriers, have an increased brain amyloid load at late middle age. Thus, epilepsy is linked with a biomarker that might be related to accelerated brain aging and can be considered a neurobiological predisposition to later-life cognitive disorders.
Introduction
Approximately 1 of 100 individuals have epilepsy prior to age 18 years. Somatic comorbidities and cognitive problems are common even among patients with uncomplicated syndromes, and chronic medication-resistant epilepsy is particularly associated with abnormal prospective cognitive trajectories in middle-aged patients.
Population-based studies have shown that prevalence rates of epilepsy and dementia overlap in the elderly. Seizures in individuals with Alzheimer disease become more common after longer disease duration and more advanced neurodegeneration, but they also occur more frequently in patients with young-onset disease or genetic mutations associated with excessive brain β-amyloid (Aβ) accumulation. Translational evidence provides further support for the link between amyloid abnormalities and epilepsy. For instance, in transgenic mouse models of Alzheimer disease that overproduce Aβ, the mice develop seizures along with the increase in Aβ levels and age. However, epilepsy can be considered as a model of chronic neuronal overactivity and stress, which might predispose patients to increased amyloid accumulation and neuronal damage.
Despite the fact that neurologic disorders with amyloid abnormalities are associated with epileptic seizures, human studies investigating amyloid deposition in patients with established epilepsy are scarce. Some neuropathologic examinations in the 1990s of resected brain tissue specimens from patients with treatment-resistant temporal lobe epilepsy suggested a link between epilepsy and abnormalities in localized brain Aβ function. Patients with long-standing, treatment-resistant temporal lobe epilepsy showed increased amyloid production and an abnormally high number of amyloid plaques compared with age-matched individuals without epilepsy (controls) in an age-accelerated fashion. However, it is not known whether amyloid accumulation is linked only to local pathologic brain processes or whether more general amyloid pathologic changes occur in patients with epilepsy because the brain functional abnormalities in epilepsy are not limited to the area of epileptic discharges.
Apolipoprotein E (APOE) is an important factor in neuronal repair; thus, the APOE genotype might be a crucial factor in determining an individual’s capacity to endure neuronal stress related to epileptic seizures. Of the APOE genotypes, the ε4 allele leads to the least effective of isoforms and has been recognized as a major risk factor for increased Aβ accumulation and Alzheimer disease in the general population. The APOE ε4 allele has also been linked to poorer memory performance and increased neurodegeneration in patients with treatment-resistant temporal lobe epilepsy. Thus, the APOE genotype also might be an important determinant of brain aging and vulnerability to excess amyloid accumulation in patients with epilepsy.
In the present study, we investigated brain Aβ accumulation in individuals with childhood-onset epilepsy and a matched control cohort without epilepsy. The study was carried out with a well-known population-based cohort of patients with epilepsy in the Turku Adult Childhood Onset Epilepsy (TACOE) study who have been prospectively followed up from their childhood in the early 1960s until late middle age. Brain amyloid accumulation was investigated using positron emission tomography (PET) with amyloid ligand carbon 11–labeled Pittsburgh Compound B (PiB). We hypothesized that individuals with childhood-onset epilepsy, particularly those with the APOE ε4 allele, would show an increase in brain PiB uptake predisposing them to development of progressive neurodegenerative disorders, such as Alzheimer disease.
Methods
The study was conducted from May 2011 to October 2013; data analysis was performed from January 2014 to October 2016. The study protocol was approved by the institutional review board of Hospital District of Southwest Finland, and the study was conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all study participants; participants received financial compensation.
Participants
Individuals with childhood-onset epilepsy were originally recruited from the Turku University Hospital district area in southwestern Finland (with an approximate total population of 700 000) during the period from 1961 to 1964 and prospectively followed up until 2012. The original cohort included 100 persons with uncomplicated epilepsy (ie, no major neurologic comorbidity, as described in more detail elsewhere). Using stratified random sampling, we randomly assigned controls for the participants with uncomplicated epilepsy, matched for age, sex, and place of domicile, from the general population of the study area in 1992. Although not all participant-control pairs were available at the time of the present study, the remaining controls were included in group-level comparisons.
The population of the present study was white, with a mean (SD) age of 5.1 (4.5) years at baseline and 56.0 (4.3) years at the time of the present study. The mean (SD) length of follow-up of the participants was 52.5 (4.0) years. Recruitment, epilepsy classification, follow-up design, and the 50-year clinical outcome of the TACOE study have been described in detail previously. Overall, 51 of 73 eligible individuals with epilepsy (70%) and 52 of 78 available matched controls (67%) participated in the TACOE study. Of the participants, 84% (41 with epilepsy and 46 controls) underwent PET scanning with PiB and were included in the present study. Thirteen of the original sample of 103 individuals (7 with epilepsy and 6 controls) declined to participate, 2 persons were excluded because structural brain lesions were detected on their magnetic resonance imaging scans, and 1 person discontinued the PET imaging owing to a panic attack in the scanner.
All participants were clinically examined by a consultant neurologist (A.A.). Individual epilepsy syndromes were classified as idiopathic and cryptogenic, as described elsewhere. The neuropsychological examination included 10 validated tests examining episodic memory, semantic memory, language function, executive function, and visuomotor function (Digit Span, Similarities, and Digit Symbol from the Finnish Wechsler Adult Intelligence Scale–Revised; Trail Making Test A and B; Finnish Wechsler Memory Scale–Revised Logical Memory [Story A, immediate and delayed recall]; Controlled Word Association Test; Finnish Boston Naming Test; and Wordlist Learning and Clock Drawing from the Consortium to Establish a Registry for Alzheimer Disease). Neuropsychological test scores were z transformed based on the distribution in the 48 neurologically healthy control participants. The procedure and test outcomes have been described in detail. Cognitive impairment was defined as having a z score of at least 1.5 SDs below the mean in 3 or more tests. The APOE genotyping was performed as described previously.
Imaging
Participants were scanned with a 3-T scanner (Verio; Siemens AG) to evaluate the presence of structural brain abnormalities and provide anatomical reference (3-dimensional T1-weighted magnetic resonance imaging with 1-mm isotropic voxels) for PET image processing. In addition, axial T2 proton density, diffusion, T2*, and fluid-attenuated inversion recovery sequences were obtained for clinical evaluation. One participant with epilepsy and 1 control had a contraindication for magnetic resonance imaging and were therefore scanned only with PET.
Synthesis of carbon 11–labeled PiB (N-methyl-[11C]-2-(4′-methylaminophenyl)-6-hydroxybezothiazole) was described previously. The scanning was performed using the High Resolution Research Tomograph (Siemens AG). The antecubital vein was cannulated for tracer administration, and head motion was controlled using an individually fitted thermoplastic mask during the scanning. A rapid bolus of PiB (mean dose, 444 [100] MBq; range, 206-561 MBq) was administered in the antecubital vein before the scanning. Three 10-minute frames were obtained beginning at 60 minutes after the injection. Head motion tracking was applied during the scanning by using a plastic cap with infrared light detectors (Polaris; Northern Digital Inc) attached on top of the thermoplastic mask. Motion tracking data were available for 33 participants and 40 controls, all of whom were included in the statistical parametric mapping (SPM) analyses.
Image Preprocessing
The images were preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) running in Matlab 2011a (Mathworks Inc; https://www.mathworks.com). First, the between-frame motion was corrected, and the images were coregistered to the individual T1-weighted magnetic resonance images using a mutual information algorithm. The motion-corrected and coregistered images were then normalized to Montreal Neurological Institute space using an SPM unified segmentation algorithm applying the structural information of the T1-weighted image. For the normalization of the 2 participants without brain magnetic resonance imaging scans, a PET template was created using the calculated mean of the normalized images of all other participants. Reference region standardized uptake values were calculated, and specific PiB uptake was quantified as the region to cerebellar cortex ratio from 60 to 90 minutes after injection of the tracer. The images were smoothed using a 10-mm full-width at half maximum gaussian kernel to improve the signal to noise ratio.
As quality control, within-frame head motion was estimated by calculating the amplitude of the transposition within each frame. In addition, displacement from the transmission scan position was estimated by calculating the mean transposition of each frame. The mean values of head motion variables were determined across the analyzed frames for statistical analyses. There were no significant differences in the mean within-frame motion (U = 637.5; P = .98), frame disposition from the transmission position (U = 649.5; P = .91), injected PiB dose per weight (2-tailed, unpaired t test t85 = 0.70; P = .49), or reference region standardized uptake values between the participants and controls (t85 = 0.62; P = .54).
Visual Analysis
Visual analysis was carried out for all 87 participants with anonymized Montreal Neurological Institute space region to cerebellum ratio images by the consensus statement of 2 experienced readers (J.J. and J.O.R.) who were blinded to the clinical data. Because epilepsy-related amyloid accumulation may not follow a similar spatial distribution across all epilepsy syndromes, the PiB scan was deemed abnormal if the tracer uptake in gray matter was higher than or equal to that in the white matter in any of the following regions: frontal lobe, posterior cingulate and precuneus, parietal lobe, or temporal lobe. Thus, in addition to Alzheimer-type pathologic findings, a more focally restricted increase in PiB uptake was evaluated as abnormal.
Statistical Analysis
The demographics were compared between the epilepsy and control cohorts using an independent t test or Mann-Whitney test for continuous variables and the Fisher exact test for categorical variables, as appropriate. For t tests, the normality of the distribution of continuous variables was inspected visually, and the equality of variances was determined using the Levene test. Differences in visual analysis were investigated using the Fisher exact test, where a 1-sided test was chosen based on the a priori hypothesis of increased amyloid accumulation in participants with epilepsy compared with the controls. In addition, differences in visual analysis between participants with and participants without the APOE ε4 allele, between active and remitted epilepsy, between the presence and the absence of cognitive impairment, and between the presence and the absence of cerebrovascular disease were compared using the Fisher exact test.
One individual was excluded from the whole-brain SPM analyses because of an inappropriate field of view excluding part of the cerebellum from the PET image. Thus, the final sample in the SPM analyses included 40 individuals with epilepsy and 46 controls. An analysis mask was created to limit the search volume to the cerebral gray matter with PiB uptake at least at the level of the reference region. The uptake of PiB was examined voxel by voxel over the search volume using the general linear model (GLM) with group and APOE genotype (presence of the ε4 allele) as explanatory variables and age as a covariate of no interest. The effects were investigated on the cluster level using familywise error correction. The findings were confirmed, and magnitudes of the group differences were estimated in the prefrontal cortex (PFC) and whole cerebral cortex by extracting mean ratio values using automated anatomical labeling library regions (excluding nonbrain tissue and white matter from the regions) and using the GLM corresponding to the SPM analyses. The associations between other explanatory variables and cerebral PiB uptake were investigated using the region-of-interest data with the GLM. The model fit in the linear models was inspected visually from the standardized residuals. The statistical analyses, except for voxel-by-voxel SPM analyses, were run in SPSS Statistics, version 21 (IBM Corp). P < .05 was considered to be statistically significant.
Results
Demographics
Although cognitive impairment was more common in the epilepsy cohort, there were no significant differences between the epilepsy and control cohorts in age, body mass index (BMI), family history of dementia, or APOE genotype (Table 1). When comparing the TACOE study participants included vs not included in the PiB PET study, we found that there were no significant differences between the epilepsy and control cohorts (P = .29) with regard to age (t101 = −0.26; P = .80), sex (P = .59), BMI (t101 = 0.17; P = .87), duration of antiepileptic drug therapy (U = 181.5; P = .58), or cumulative years with seizures (U = 192; P = .76). Participants included in the present study were more likely to be cognitively normal than were those not included (presence of cognitive impairment, 24 of 87 [28%] vs 9 of 16 [56%]; P = .04). The epilepsy cohort characteristics are presented in Table 2.
Table 1. Overall Participant Characteristics.
| Characteristic | Epilepsy Cohort (n = 41) |
Control Cohort (n = 46) |
t85 Value | P Value |
|---|---|---|---|---|
| Age, mean (SD) [range], y | 56.0 (4.3) [48-63] | 56.0 (4.3) [49-64] | 0.02 | .99 |
| Sex, No. (%) | ||||
| Male | 15 (37) | 17 (37) | NA | >.99 |
| Female | 26 (63) | 29 (63) | ||
| BMI, mean (SD) [range] | 28.7 (4.8) [18-42] | 27.4 (5.8) [17-42] | −1.1 | .27 |
| APOE ε4 allele, No. (%) | 12 (29) | 13 (28) | NA | >.99 |
| Family history of dementia, No. (%) | 18 (44) | 22 (48) | NA | .83 |
| Cognitive impairment, No. (%)a | 18 (44) | 6 (13) | NA | .002 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable.
Three or more of 10 neuropsychological test scores at least 1.5 SDs below the mean.
Table 2. Epilepsy Cohort Characteristics.
| Characteristic | Participants (n = 41) |
|---|---|
| Age at onset of epilepsy, mean (SD) [range], y | 5.1 (4.5) [0-14] |
| Duration of active epilepsy, mean (SD) [range], y | 17 (15) [1-49] |
| Duration of AED treatment, mean (SD) [range], y | 21 (19) [0-55] |
| Current medication, No. (%) | 9 (22) |
| Epilepsy syndrome, No. (%) | |
| Idiopathic | 24 (59) |
| Cryptogenic | 17 (41) |
| AEDs used, No. (%) | |
| Barbiturates | 33 (80) |
| Phenytoin | 23 (56) |
| Carbamazepine | 9 (22) |
| Succinimide group | 6 (15) |
| Benzodiazepines | 4 (10) |
| Other | 7 (17) |
Abbreviation: AED, antiepileptic drug.
Visual Analysis
Visual analysis revealed 9 participants with epilepsy (22%) and 3 control cohort participants (7%) to have abnormal PiB images showing high cortical uptake in at least 1 of the evaluated brain regions (P = .04). The proportion of the epilepsy cohort with abnormal PiB scans did not reach statistical significance between those with (n = 12) and those without (n = 29) the APOE ε4 allele (5 of 12 [42%] vs 4 of 29 [14%]; P = .06). The cause of epilepsy (idiopathic or cryptogenic), the presence or absence of cognitive impairment, and the presence or absence of cerebrovascular disease were not associated with abnormal PiB scan status (P > .10). Visually, there was no clear correspondence between the epileptic focus and PiB uptake in 12 persons with localizable epilepsy syndromes (ie, temporal, occipital, or frontal lobe epilepsy syndromes).
Semiquantitative Analyses
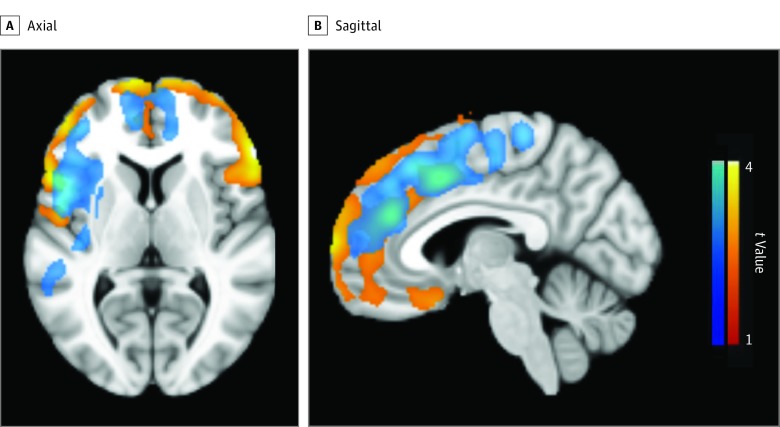
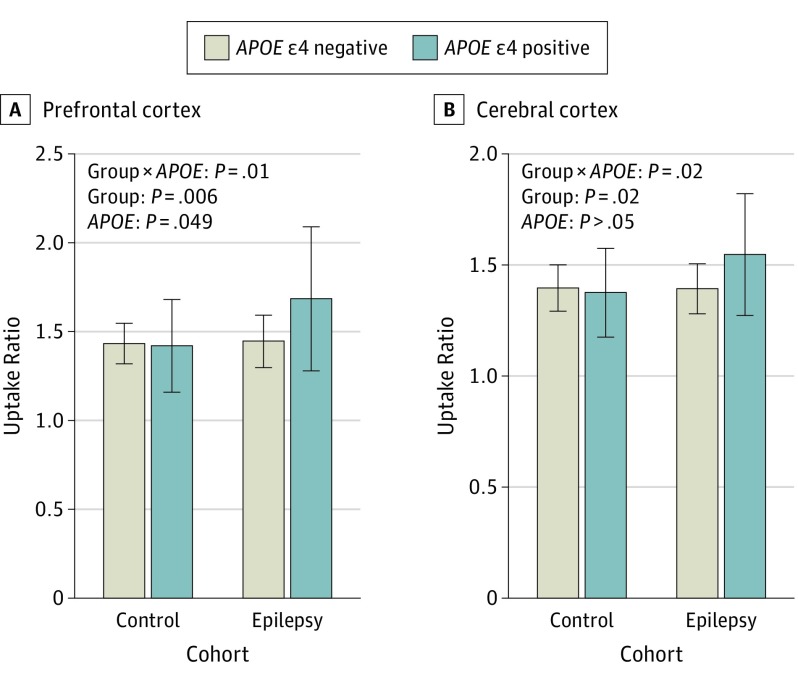
In the whole-brain SPM analysis, there was a significant group × APOE genotype effect showing a higher increase in frontal and insular PiB uptake in APOE ε4 carriers among participants with epilepsy compared with controls (Figure 1; blue clusters). In addition, those with epilepsy showed higher frontal brain region PiB uptake compared with the controls (Figure 1; red-yellow clusters). The findings were confirmed in PFC (control: ε4 noncarrier, 1.41 [0.12] and ε4 carrier, 1.40 [0.26]; epilepsy: ε4 noncarrier, 1.43 [0.15] and ε4 carrier, 1.66 [0.41]; group × APOE F = 6.8; P = .01; group F = 8.0; P = .006; APOE F = 4.0; P = .049) and cerebral cortex (control: ε4 noncarrier, 1.38 [0.11] and ε4 carrier, 1.36 [0.20]; epilepsy: ε4 noncarrier, 1.38 [0.11] and ε4 carrier, 1.53 [0.28]; group × APOE F = 5.9; P = .02; group F = 5.3; P = .02) regions of interest (Figure 2). The group × APOE genotype interaction and the main effect of group remained significant when we excluded outlier values from GLM analyses. However, the main effect of APOE genotype in the PFC was no longer significant.
Figure 1. Increased Carbon 11–Labeled Pittsburgh Compound B (PiB) Uptake in Participants With Childhood-Onset Epilepsy.
Axial (A) and sagittal (B) views. Color scales represent the voxel t values. Blue scale: group × APOE genotype effect showing higher increases in PiB uptake in APOE ε4 allele carriers in participants with epilepsy compared with controls. Red-yellow scale: group effect showing higher PiB uptake in participants with epilepsy compared with controls. The images are thresholded to show only clusters with cluster-level, familywise error–corrected P < .05.
Figure 2. Carbon 11–Labeled Pittsburgh Compound B (PiB) Uptake in Participants and Controls According to the APOE Genotype.
Prefrontal cortex (A) and cerebral cortex (B) PiB uptake. Age-adjusted general linear model analysis P values are presented. The number of participants without and participants with the APOE ε4 allele were 33 and 13 in the control cohort and 28 and 12 in the epilepsy cohort. Error bars indicate SD.
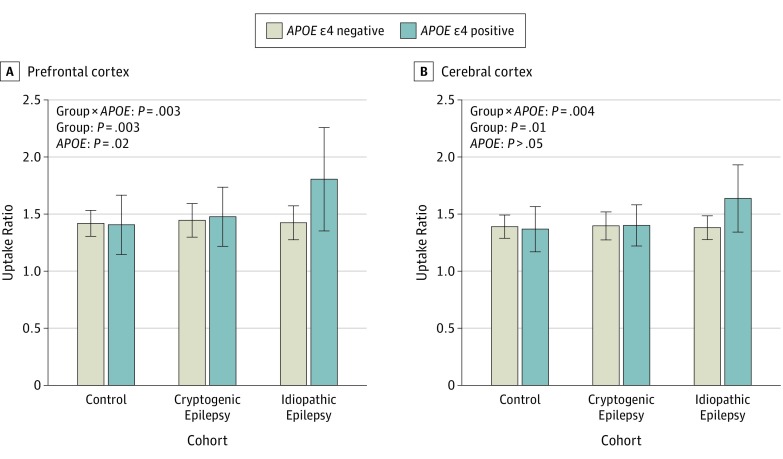
When examining the epilepsy cohort by etiology, we found that the APOE genotype effect on PiB uptake was associated specifically with the idiopathic epilepsy syndromes (Figure 3) in the PFC (cryptogenic: ε4 noncarrier, 1.44 [0.15] and ε4 carrier, 1.47 [0.26]; idiopathic: ε4 noncarrier, 1.42 [0.15] and ε4 carrier, 1.80 [0.46]; group × APOE F = 6.4; P = .003; group F = 6.2; P = .003; APOE F = 5.7; P = .02) and cerebral cortex (cryptogenic: ε4 noncarrier, 1.39 [0.13] and ε4 carrier, 1.39 [0.19]; idiopathic: ε4 noncarrier, 1.37 [0.11] and ε4 carrier, 1.63 [0.30]; group × APOE F = 5.8; P = .004; group F = 4.7; P = .01). However, the number of APOE ε4 carriers in each group was small. Individuals with or without active epilepsy, cognitive impairment, and family history of dementia or cerebrovascular disease did not differ significantly in PFC or whole-brain PiB uptake. In addition, there were no significant associations between PFC or whole-brain PiB uptake and age at epilepsy onset, duration of active epilepsy, or duration of antiepileptic drug therapy. In addition, PFC (r = 0.20, P = .24) or whole-brain PiB uptake (r = 0.15, P = .38) did not correlate with the mean z score or individual scores (all P > .05) in cognitive tests.
Figure 3. Carbon 11–Labeled Pittsburgh Compound B (PiB) Uptake According to Epilepsy Type and APOE Genotype.
Prefrontal cortex (A) and cerebral cortex (B) PIB uptake. Age-adjusted general linear model analysis P values are presented. The number of individuals without and individuals with the APOE ε4 allele were 33 and 13 in the control cohort, 11 and 5 in the cryptogenic epilepsy group, and 17 and 7 in the idiopathic epilepsy group. Error bars indicate SD.
Discussion
To our knowledge, this is the first report on the association between childhood-onset epilepsy and increased brain amyloid deposition at late middle age. We show that increased amyloid deposition can be observed in a sample of unselected individuals with a variety of different epilepsy syndromes who were in remission, with many not having received antiepileptic drug therapy for decades. Thus, the results suggest that increased brain amyloid deposition may be linked to the pathophysiology of epilepsy rather than to seizure control and duration of active epilepsy. Furthermore, individuals with the APOE ε4 allele and idiopathic epilepsy syndromes might be particularly vulnerable to the development of amyloid pathology.
Earlier work with parallel neuroimaging and tissue specimens showed that brain amyloid accumulation can be reliably measured using PiB PET. Brain amyloid accumulation occurs in normal brain aging, and the prevalence of amyloid plaques observed in abnormal PiB imaging of asymptomatic individuals increases in parallel with age. The frequency of abnormal PiB imaging findings has been estimated to be approximately 10% in the age group of 50 to 60 years, which corresponds well to the proportion of abnormal PiB scans in the control cohort (7%) in the present study. However, our epilepsy group exhibited a substantially higher prevalence (22%) of abnormal PiB imaging roughly corresponding to the prevalence estimates of a decade-older population. Thus, the results suggest that individuals with epilepsy show accelerated brain aging in terms of amyloid accumulation compared with controls without epilepsy from the general population.
According to the suggested Alzheimer disease biomarker model, amyloid deposition can be observed as an early presymptomatic phenomenon in Alzheimer disease, and an abnormally high brain amyloid load in asymptomatic individuals may suggest future cognitive impairment. However, the amyloid cascade hypothesis assumes that brain amyloid accumulation is only the initial trigger of a series of molecular events in the brain leading to Alzheimer disease abnormalities. Thus, the increased amyloid accumulation in individuals with epilepsy could be interpreted as a predisposing factor or even an early sign of a neurodegenerative process, such as Alzheimer disease. However, other factors, such as concomitant tau pathology, might also be needed to trigger neurobiological processes leading to clinical Alzheimer disease. For instance, patients with chronic medication-resistant epilepsy due to traumatic brain injury have been shown to have increased tau protein accumulation, which was associated with higher Braak staging of Alzheimer disease at autopsy.
The increase in brain PiB uptake in individuals with childhood-onset epilepsy was strongly associated with the APOE genotype (ε4 allele). Furthermore, although persons with idiopathic epilepsy syndromes tended to have a more favorable clinical outcome compared with individuals with cryptogenic syndromes, those with idiopathic epilepsy seemed to be particularly vulnerable to amyloid accumulation associated with the APOE ε4 allele. It is possible that patients with idiopathic syndromes also have other genetic defects that predispose them to the adverse effects of neuronal stress that are exaggerated by low-functioning neuronal repair mechanisms associated with APOE ε4. It is also of interest that, although the present sample included a substantial proportion of persons (18 of 41) with cognitive impairment, such impairment was not associated with brain amyloid deposition in the cross-sectional analyses. However, epilepsy is known to be associated with marked cognitive impairment even in the absence of comorbid Alzheimer disease, and human postmortem and in vivo studies have shown that amyloid abnormalities can be observed more than a decade before the manifestation of Alzheimer disease. Therefore, the findings indicate that the increased amyloid accumulation here most likely represents a presymptomatic increase in brain amyloid burden when amyloid accumulation is not associated with cognitive performance.
Limitations
There are some limitations in the present study. First, our study was cross-sectional and did not allow for assessing the temporal course of amyloid accumulation. Second, the mean age of the participants was 56.0 years, and the prevalence of Alzheimer disease is low at this age. The sample was also heterogeneous in terms of epilepsy syndromes and medications used, which limits the power to detect associations between individual epilepsy syndromes and/or their associated genetic mutations and amyloid accumulation. We also lacked detailed data about individual seizure types and number of seizures. However, all participants had uncomplicated epilepsy, which reduces the variety of causes. Finally, the sample included in the present imaging study showed some selection bias because participants with cognitive impairment were less likely to be included in the study, which might lead to an underestimation of the group differences and might explain why the APOE ε4 effect on cerebral PiB uptake was not seen in the control group.
Conclusions
Childhood-onset epilepsy appears to be associated with increased amyloid accumulation in late middle age, even among individuals in remission without antiepileptic drug therapy for decades. The findings suggest a link between epilepsy, APOE genotype, and amyloid pathology, prompting more research on brain aging in epilepsy. Further follow-up with frequent amyloid assessments are needed to confirm the findings and test this hypothesis.
References
- 1.Silverberg JI, Joks R, Durkin HG. Allergic disease is associated with epilepsy in childhood: a US population-based study. Allergy. 2014;69(1):95-103. [DOI] [PubMed] [Google Scholar]
- 2.Lin JJ, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet. 2012;380(9848):1180-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermann BP, Seidenberg M, Dow C, et al. . Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60(1):80-87. [DOI] [PubMed] [Google Scholar]
- 4.Hermann B, Seidenberg M, Sager M, et al. . Growing old with epilepsy: the neglected issue of cognitive and brain health in aging and elder persons with chronic epilepsy. Epilepsia. 2008;49(5):731-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larner AJ. Epileptic seizures in AD patients. Neuromolecular Med. 2010;12(1):71-77. [DOI] [PubMed] [Google Scholar]
- 6.Chin J, Scharfman HE. Shared cognitive and behavioral impairments in epilepsy and Alzheimer’s disease and potential underlying mechanisms. Epilepsy Behav. 2013;26(3):343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cirrito JR, Yamada KA, Finn MB, et al. . Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48(6):913-922. [DOI] [PubMed] [Google Scholar]
- 8.Sheng JG, Boop FA, Mrak RE, Griffin WS. Increased neuronal β-amyloid precursor protein expression in human temporal lobe epilepsy: association with interleukin-1α immunoreactivity. J Neurochem. 1994;63(5):1872-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackenzie IR, Miller LA. Senile plaques in temporal lobe epilepsy. Acta Neuropathol. 1994;87(5):504-510. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch LJ. ApoE, MemorE, and EpilepsE. Epilepsy Curr. 2007;7(6):149-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busch RM, Lineweaver TT, Naugle RI, et al. . ApoE-ε4 is associated with reduced memory in long-standing intractable temporal lobe epilepsy. Neurology. 2007;68(6):409-414. [DOI] [PubMed] [Google Scholar]
- 12.Aboud O, Mrak RE, Boop FA, Griffin WS. Epilepsy: neuroinflammation, neurodegeneration, and APOE genotype. Acta Neuropathol Commun. 2013;1:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sillanpää M. Medico-social prognosis of children with epilepsy: epidemiological study and analysis of 245 patients. Acta Paediatr Scand Suppl. 1973;237:3-104. [PubMed] [Google Scholar]
- 14.Sillanpää M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. N Engl J Med. 1998;338(24):1715-1722. [DOI] [PubMed] [Google Scholar]
- 15.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 16.Sillanpää M, Jalava M, Shinnar S. Epilepsy syndromes in patients with childhood-onset seizures in Finland. Pediatr Neurol. 1999;21(2):533-537. [DOI] [PubMed] [Google Scholar]
- 17.Sillanpää M, Anttinen A, Rinne JO, et al. . Childhood-onset epilepsy five decades later: a prospective population-based cohort study. Epilepsia. 2015;56(11):1774-1783. [DOI] [PubMed] [Google Scholar]
- 18.Sillanpää M, Shinnar S. Long-term mortality in childhood-onset epilepsy. N Engl J Med. 2010;363(26):2522-2529. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. Manual of the Finnish Version of WAIS-R. Helsinki, Finland: Psykologien Kustannus; 1992. [Google Scholar]
- 20.Poutiainen E, Kalska H, Laasonen M. Finnish Manual for the Trail Making Test. Helsinki, Finland: Hogrefe Psykologien Kustannus; 2010. [Google Scholar]
- 21.Wechsler D. Manual of the Finnish Version of WMS-R. Helsinki, Finland: Psykologien Kustannus; 1996. [Google Scholar]
- 22.Benton AL, Hamsher K. Controlled Word Association Test. Iowa City, IA: University of Iowa; 1976. [Google Scholar]
- 23.Laine M, Koivuselkä-Sallinen P, Hänninen R, Niemi J. Finnish Version of the Boston Naming Test. Helsinki, Finland: Psykologien Kustannus; 1997. [Google Scholar]
- 24.Pulliainen V, Hokkanen L, Salo J, Hänninen T. Manual of the Finnish CERAD Cognitive Test Battery. Kuopio, Finland: Suomen Alzheimer-tutkimusseura; 1999. [Google Scholar]
- 25.Karrasch M, Tiitta P, Hermann B, et al. . Cognitive outcome in childhood-onset epilepsy: a five-decade prospective cohort study. J Int Neuropsychol Soc. 2017;1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingraham LJ, Aiken CB. An empirical approach to determining criteria for abnormality in test batteries with multiple measures. Neuropsychology. 1996;10(1):120-124. doi: 10.1037/0894-4105.10.1.120 [DOI] [Google Scholar]
- 27.Jänis MT, Siggins S, Tahvanainen E, et al. . Active and low-active forms of serum phospholipid transfer protein in a normal Finnish population sample. J Lipid Res. 2004;45(12):2303-2309. [DOI] [PubMed] [Google Scholar]
- 28.Kemppainen NM, Aalto S, Wilson IA, et al. . Voxel-based analysis of PET amyloid ligand [11C]PIB uptake in Alzheimer disease. Neurology. 2006;67(9):1575-1580. [DOI] [PubMed] [Google Scholar]
- 29.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95-113. [DOI] [PubMed] [Google Scholar]
- 30.Lopresti BJ, Klunk WE, Mathis CA, et al. . Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46(12):1959-1972. [PubMed] [Google Scholar]
- 31.Leinonen V, Alafuzoff I, Aalto S, et al. . Assessment of β-amyloid in a frontal cortical brain biopsy specimen and by positron emission tomography with carbon 11–labeled Pittsburgh Compound B. Arch Neurol. 2008;65(10):1304-1309. [DOI] [PubMed] [Google Scholar]
- 32.Rowe CC, Ellis KA, Rimajova M, et al. . Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275-1283. [DOI] [PubMed] [Google Scholar]
- 33.Morris JC, Roe CM, Grant EA, et al. . Pittsburgh Compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66(12):1469-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR Jr, Knopman DS, Jagust WJ, et al. . Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doraiswamy PM, Sperling RA, Johnson K, et al. ; AV45-A11 Study Group . Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Mol Psychiatry. 2014;19(9):1044-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353-356. [DOI] [PubMed] [Google Scholar]
- 37.Thom M, Liu JY, Thompson P, et al. . Neurofibrillary tangle pathology and Braak staging in chronic epilepsy in relation to traumatic brain injury and hippocampal sclerosis: a post-mortem study. Brain. 2011;134(pt 10):2969-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braak H, Braak E, Bohl J, Reintjes R. Age, neurofibrillary changes, Aβ-amyloid and the onset of Alzheimer’s disease. Neurosci Lett. 1996;210(2):87-90. [DOI] [PubMed] [Google Scholar]
- 39.Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54(11):2072-2077. [DOI] [PubMed] [Google Scholar]