Abstract
This study examines the effectiveness of transcutaneous stimulation of the vagus nerve for trigeminal autonomic cephalalgias.
Trigeminal autonomic cephalalgias (TACs) are a group of primary headache disorders that are characterized by attacks of unilateral pain of varying duration and prominent, ipsilateral cranial autonomic symptoms, such as lacrimation, conjunctival injection, nasal congestion, rhinorrhea, or periorbital edema. The TACs include cluster headaches, paroxysmal hemicrania, hemicrania continua, and short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing or cranial autonomic symptoms.
Paroxysmal hemicrania (PH) attacks last 2 to 30 minutes and typically occur several times per day. Hemicrania continua (HC) is characterized by continuous pain with exacerbations lasting for hours or days. Both are defined by their response to indomethacin; a high dose of 225 mg daily or greater may be needed and lifelong treatment is frequently required. Unfortunately, indomethacin is often poorly tolerated because of adverse effects, such as nausea, abdominal pain, or gastric bleeding.
Transcutaneous stimulation of the vagus nerve with the GammaCore (electroCore, LLC) has been effective for the acute treatment of episodic cluster headaches in 2 randomized sham-controlled trials, raising the possibility that this treatment might be effective for other TACs including HC. We began treating patients with PH or HC who were unable to tolerate indomethacin or unable to tolerate a sufficient dose to produce an adequate clinical effect with noninvasive vagus nerve stimulation (nVNS).
Methods
We audited the clinical records for all patients with PH or HC seen at King’s College Hospital in London from 2013 to 2017, which was approved by the hospital’s audit registry on September 6, 2016. This process waived patient consent for review of their clinical records. For inclusion, all patients had diagnoses confirmed by a response to intramuscular (100-200 mg) and/or oral indomethacin and were treated with nVNS.
Results
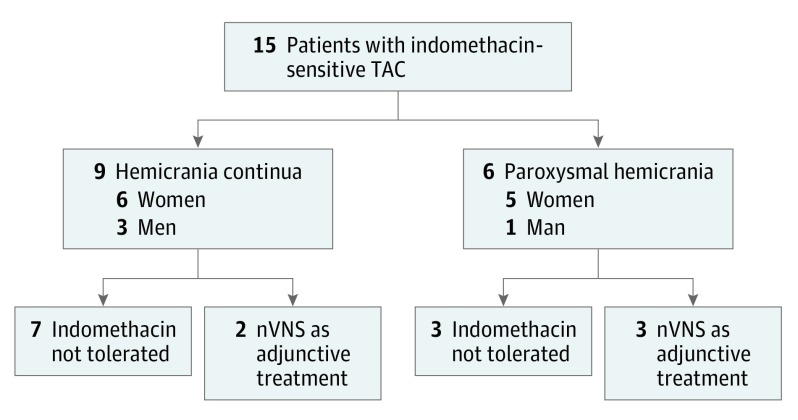
Fifteen patients were identified (Figure), and 9 (60%) had HC (6 women) while 6 (40%) had PH (5 women). The mean (SD) age was 43 (11) years. Noninvasive vagus nerve stimulation was the primary treatment for 10 patients (7 HC, 3 PH) who could not tolerate indomethacin and was used as adjunctive therapy at the maximum tolerated dose (range, 50-225 mg daily) of oral indomethacin for 5 patients (2 HC, 3 PH).
Figure. Flowchart of Patients With Indomethacin-Sensitive Trigeminal Autonomic Cephalalgias (TACs) Treated With Noninvasive Vagus Nerve Stimulation (nVNS).
Among patients with HC, 7 (78%) reported experiencing a reduced severity of continuous pain. Two patients reported experiencing a reduced frequency of exacerbations and a reduced severity and duration of exacerbations with acute treatment. A third reported experiencing a reduced duration of exacerbations. Among patients with PH, 4 (67%) reported receiving benefits from treatment. One patient with PH became attack-free, and among the other 3 patients, 2 reported experiencing a reduced attack frequency, 3 reduced severity, and 1 shorter duration (Table). Effective dosing regimens ranged from 2 to 4 doses twice daily or 2 to 3 doses 3 times daily. The duration of use at last follow-up ranged from 3 months to 5 years.
Table. Patient Demographics and Treatment Response to Noninvasive Vagus Nerve Stimulation.
| Sex/Age, y | Laterality | Treatment Response | Treatment Durationa |
|---|---|---|---|
| Hemicrania Continua | |||
| Male, 49 | Left | Persistent pain reduced by 60% | 2 y and 8 mo |
| Man, 37 | Left | Persistent pain reduced by 40% | 2 y |
| Female, 55 | Left | Persistent pain reduced by 80% | 5 y |
| Female, 27 | Left | Persistent pain reduced by 50% or more, exacerbations reduced from 4 per wk to 2 per mo; acute treatment (1 dose) reduced severity and duration from 48-72 h to 12-24 h | 1 y |
| Female, 42 | Left | Persistent pain reduced by 15%, exacerbation duration reduced from days to 1 h | 6 mo |
| Female, 23 | Left | Persistent pain reduced by 30% | 2 y and 4 mo |
| Female, 53 | Right | Persistent pain reduced by 30%-50%, exacerbations reduced from 15 per mo to 1 every 2 mo; acute treatment (6-8 doses) reduced severity by 40% and duration from >24 h to <24 h | 5 mo |
| Female, 38 | Right | No response | 3 mo |
| Male, 61 | Left | No response | 6 mo |
| Paroxysmal Hemicrania | |||
| Female, 43 | Left | Attack frequency reduced from 12 to 4 per d, reduced severity and duration from 20-40 min to 10 min | 3 mo |
| Female, 47 | Right | “Dramatic” reduction in frequency and severity | 1 y and 8 mo |
| Female, 48 | Left | Complete cessation of attacks | 1 y and 3 mo |
| Female, 29 | Right | Decreased severity | 5 y |
| Male, 54 | Left | No response | 4 mo |
| Female, 38 | Right | No response | 4 mo |
At time of last follow-up.
Discussion
The GammaCore generates a 120-second electrical impulse of adjustable amplitude that stimulates the cervical branch of the vagus nerve when held against the neck. Patients were typically advised to begin with two 120-second doses that were applied ipsilateral to pain twice daily, with the dose titration based on effect and tolerability. Most patients judged nVNS treatment to be useful. However, with the exception of 1 patient (17%) with PH, none of the patients became pain-free.
Whereas all patients in this series experienced prohibitive or dose-limiting adverse effects from indomethacin, only 1 (7%) needed to reduce the nVNS dose (from 10 to 6 daily doses for HC) because of cutaneous irritation at the stimulation site. Our initial experience suggests that nVNS may be an important alternative or adjunctive therapy for patients with these indomethacin-sensitive TACs who are unable to tolerate indomethacin. Conducting a prospective, randomized, and sham-controlled study seems warranted, although given the rarity of the problem, this will be a considerable challenge.
References
- 1.Headache Classification Committee of the International Headache Society The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808. doi: 10.1177/0333102413485658 [DOI] [PubMed] [Google Scholar]
- 2.Cittadini E, Matharu MS, Goadsby PJ. Paroxysmal hemicrania: a prospective clinical study of 31 cases. Brain. 2008;131(pt 4):1142-1155. [DOI] [PubMed] [Google Scholar]
- 3.Cittadini E, Goadsby PJ. Hemicrania continua: a clinical study of 39 patients with diagnostic implications. Brain. 2010;133(pt 7):1973-1986. [DOI] [PubMed] [Google Scholar]
- 4.Silberstein SD, Mechtler LL, Kudrow DB, et al. ; ACT1 Study Group . Non-invasive vagus nerve stimulation for the acute treatment of cluster headache: findings from the randomized, double-blind, sham-controlled ACT1 study. Headache. 2016;56(8):1317-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goadsby PJ, de Coo I, Silver N, et al. . Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: findings from the randomized, double-blind, sham-controlled ACT2 study. Headache. 2017;57(suppl 3):128. doi: 10.1111/head.13102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eren O, Straube A, Schöberl F, Schankin C. Hemicrania continua: beneficial effect of non-invasive vagus nerve stimulation in a patient with a contraindication for indomethacin. Headache. 2017;57(2):298-301. [DOI] [PubMed] [Google Scholar]