Key Points
Question
Does the severity of incident depression influence a patient’s risk of developing epilepsy?
Findings
When used as a surrogate for depression severity, analyses stratified by antidepressant treatment type demonstrate a clear, statistically significant gradient in the hazard of epilepsy and the odds of seizure outcomes.
Meaning
Using depression treatment as a surrogate of severity, we found that worsening depression appears to augment the risk of epilepsy and the odds of worse seizure outcomes. Future prospective studies designed to address causation between depression and epilepsy are warranted.
Abstract
Importance
A bidirectional relationship exists between epilepsy and depression. However, any putative biological gradient between depression severity and the risk of epilepsy, and the degree to which depression mediates the influence of independent risk factors for epilepsy, has yet to be examined.
Objective
To determine the effect of depression on the risk of epilepsy and seizure outcomes.
Design, Setting, and Participants
An observational study of a population-based primary care cohort (all patients free of prevalent depression and epilepsy at 18-90 years of age who were active after the Acceptable Mortality Reporting date in The Health Improvement Network database) and a prospectively collected tertiary care cohort (all patients whose data were prospectively collected from the Calgary Comprehensive Epilepsy Programme). The analyses were performed on March 16, 2016.
Main Outcome and Measures
The hazard of developing epilepsy after incident depression and vice versa was calculated. In addition, a mediation analysis of the effect of depression on risk factors for epilepsy and the odds of seizure freedom stratified by the presence of depression were performed.
Results
We identified 10 595 709 patients in The Health Improvement Network of whom 229 164 (2.2%) developed depression and 97 177 (0.9%) developed epilepsy. The median age was 44 years (interquartile range, 32-58 years) for those with depression and 56 years (interquartile range, 43-71 years) for those with epilepsy. Significantly more patients with depression (144 373 [63%] were women, and 84 791 [37%] were men; P < .001) or epilepsy (54 419 [56%] were women, and 42 758 [44%] were men; P < .001) were female. Incident epilepsy was associated with an increased hazard of developing depression (hazard ratio [HR], 2.04 [95% CI, 1.97-2.09]; P < .001), and incident depression was associated with an increased hazard of developing epilepsy (HR, 2.55 [95% CI, 2.49-2.60]; P < .001) There was an incremental hazard according to depression treatment type with lowest risk for those receiving counselling alone (HR, 1.84 [95% CI, 1.30-2.59]; P < .001), an intermediate risk for those receiving antidepressants alone (HR, 3.43 [95% CI, 3.37-3.47]; P < .001), and the highest risk for those receiving both (HR, 9.85 [95% CI, 5.74-16.90]; P < .001). Furthermore, depression mediated the relationship between sex, social deprivation, and Charlson Comorbidity Index with incident epilepsy, accounting for 4.6%, 7.1%, and 20.6% of the total effects of these explanatory variables, respectively. In the Comprehensive Epilepsy Programme, the odds of failing to achieve 1-year seizure freedom were significantly higher for those with depression or treated depression.
Conclusions and Relevance
Common underlying pathophysiological mechanisms may explain the risk of developing epilepsy following incident depression. Treated depression is associated with worse epilepsy outcomes, suggesting that this may be a surrogate for more severe depression and that severity of depression is associated with severity of epilepsy.
This study reports on the effect of depression on the risk of epilepsy and seizure outcomes.
Introduction
Psychiatric comorbidities are common in people with epilepsy. In particular, depression has a prevalence of 23.1% (95% CI, 20.6%-28.3%) in community-based studies of epilepsy. There also appears to be a bidirectional relationship between epilepsy and depression because the incidence rate ratios and prevalence ratios for depression are significantly elevated both before and after an epilepsy diagnosis.
It has therefore been postulated that there may be shared pathophysiological mechanisms between epilepsy and psychiatric disease. Overlapping neuroanatomical regions, such as the temporal, orbitofrontal, and inferior prefrontal areas, and altered expression and function of specific neurotransmitters have been identified as potential substrates for depression and epilepsy
Despite this, prior studies have not addressed the hazard of developing epilepsy following an incident diagnosis of depression, or whether there is a biological gradient in the risk of epilepsy according to depression severity. In addition, mediation analyses of depression and epilepsy have yet to be performed. Finally, the effect of depression on seizure outcomes has yet to be explored in detail. We postulated a priori that depression severity is associated with an incremental hazard for epilepsy and that depression mediates the interaction between other risk factors and epilepsy.
Methods
The Health Improvement Network
The Health Improvement Network (THIN) database contains valid, complete prospective data that are broadly representative of the general population of the United Kingdom. It currently contains electronic medical records collected from 562 general practices in the United Kingdom. The database contains anonymized longitudinal demographic, medical, and prescription information on individual patients that are processed and validated by Cegedim Strategic Data–Medical Research UK. The Health Improvement Network has been used for scientific research since approval from the NHS South-East Multicentre Research Ethics Committee in 2003. Ethics approval for this study was obtained through both the University of Calgary’s Conjoint Health Research Ethics Board and the Cegedim Strategic Data–Medical Research UK Scientific Research Committee in December 2015.
The data are pseudoanonymized. However, patients can request that their general practitioner not allow their medical records to be used for medical research. Patients were provided with opt-out forms, and their data were excluded from research purposes at their request. Despite this, the database has been demonstrated to be representative of the overall UK population.
Study Population
We required that all patients were active after the Acceptable Mortality Reporting date, the year in which mortality reporting was deemed complete and accurate for each practice, thus functioning as an indicator for high-quality data collection. We used a 5-year washout period from first medical code after the Acceptable Mortality Reporting date to exclude patients with prevalent epilepsy and depression. All patients between 18 and 90 years of age at first medical code entry who met these conditions were included.
Patients developing presumed incident epilepsy were identified using a previously published pediatric epilepsy Read code definition designed for THIN. This algorithm has been demonstrated to be 92% accurate. After review by 2 epileptologists (C.B.J. and S.W.), the algorithm is expected to perform similarly in an adult population. The case definition requires a diagnosis of an epilepsy syndrome or 2 symptoms of epilepsy (ie, codes for nonfebrile seizures on 2 or more occasions) and a prescription for an antiepileptic drug repeated within a 4-month period.
A consensus-driven case definition of depression was developed by 2 coauthors with expertise in psychiatry (I.V. and S.P.). The definition requires the presence of any Read code for a diagnosis of depression. Read codes associated with only symptoms of depression, bipolar disorder, mania, or hypomania were excluded (eAppendix 1 in the Supplement).
Determining the Hazard of Depression Following Incident Epilepsy
We first sought to confirm the anticipated increased hazard of depression among patients meeting the incident case definition for epilepsy. We observed patients from the time of the first medical code designation after the washout period until an incident diagnosis of depression, death, loss to follow-up, or end of follow-up. Epilepsy was treated as a time-varying covariate in this analysis. Patients were coded as “0” until the date they met the case definition for epilepsy (at which point they were coded as “1”) and were considered to have the disease thereafter for the remainder of the study.
Determining the Hazard of Epilepsy Following Incident Depression
We next sought to determine whether those meeting the incident case definition of depression had an increased hazard of developing incident epilepsy. We observed patients until an incident diagnosis of epilepsy, death, loss to follow-up, or end of follow-up. Depression was treated as a time-varying covariate for this analysis. Again, patients were considered to have the disease for the remainder of the study after meeting the case definition.
We also explored the effect of depression treatment, using it as a proxy for depression severity, by selecting Read codes and Multilex codes for antidepressants and counselling (eAppendix 2 in the Supplement). Depression treatment was treated as a time-varying covariate in a survival analysis of the time to develop incident epilepsy. To assess whether depression treatment was associated with an increased risk of epilepsy, the hazard of epilepsy was compared with a composite group (comprising those without depression and those with untreated depression).
Statistical Analysis of the Association Between Epilepsy and Depression
Survival analyses were used to compare the hazard of depression following epilepsy and vice versa. Nelson-Aalen cumulative hazard plots were generated in which the index date (time zero) was the time of the patient’s first medical code designation of any kind following the 5-year washout period for depression and epilepsy after the Acceptable Mortality Reporting date. To avoid immortal time bias, depression, depression treatment, and epilepsy were analyzed as time-varying covariates. Patients were coded as “0” prior to the diagnosis of depression, epilepsy, or depression treatment, and then as “1” on the date they met the case definition that incorporated the 5-year washout period. Patients were censored at the date of death, the date of last follow-up, or the end of the analysis period if no outcome occurred.
We also performed a sensitivity analysis in which we treated each form of therapy (counselling and antidepressant medications) as separate time-varying covariates and as an interaction term in a Cox proportional hazards regression model to delineate the individual and combined effect of each intervention. We anticipated that this would function as a surrogate measure of depression severity, with counselling being reserved for milder cases and antidepressants (with or without counselling) being reserved for more severe cases. The hazard for each treatment stratum was compared with a composite-group hazard for those without depression and those with untreated depression.
We identified a priori the following independent variables for the survival analyses: age at index date, sex, Charlson Comorbidity Index (CCI), and socioeconomic status (as defined by the Townsend Deprivation Index [TDI]). These variables are associated with a risk of mental illness and epilepsy. The TDI was recorded at the most recent census date for the region in which the patient was last residing. We calculated the CCI after 5 years of THIN follow-up (starting from the patient’s first medical code) to permit sufficient time for concurrent conditions to develop. We separately evaluated the influence of the CCI calculated over the last 5 years of follow-up in THIN, as well as over the entire follow-up period, in a sensitivity analysis. We then evaluated the role of depression in the relationship between known independent risk factors and the development of epilepsy in a mediation analysis for time-to-event outcomes using the entire cohort (those free of depression and epilepsy at index date).
Evaluating the Effect of Depression on Seizure Outcome
We also sought to determine whether depression was associated with worse seizure outcomes. We used the Calgary Comprehensive Epilepsy Programme (CEP) database, a registry of adult (≥18 years of age) outpatient encounters that draws from a population of 1.3 million people. Prospective data collection began in 2007 using standardized forms completed by patients and staff epileptologists pertaining to demographics, seizure and epilepsy characteristics, medical and psychiatric history, physical examination findings, the results of diagnostic investigations, multidisciplinary consultations, use of antiepileptic drugs, and surgical history. Epilepsy-specific indices (eg, epilepsy syndrome, seizure types, and diagnostic results) are captured by the staff epileptologists. All epilepsy diagnoses are made by 1 of 6 core staff epileptologists initially using the 2005 International League Against Epilepsy consensus definition and then the updated 2014 criteria.
Depression was identified by both patients and staff epileptologists. If there was no history of a formal diagnosis of depression, staff epileptologists further explored the issue by asking a series of questions pertaining to the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria for major depression.
Our analyses herein use data collected between 2007 and October 2015. All data used in this study were derived from the first-visit evaluation forms. The CEP data are collected, managed, stored, and extracted using Research Electronic Data Capture tools hosted at the University of Calgary’s Clinical Research Unit.
Multiple logistic regression modeling was used to evaluate the association between depression and 1-year seizure freedom. First, we used the presence of depression as the exposure variable and controlled for age, sex, lesional epilepsy (as determined through 1.5- or 3-T magnetic resonance imaging), history of generalized tonic-clonic seizures, and whether the patient was taking an antiepileptic drug (evaluated as a dichotomous variable to account for the psychotropic properties of certain medications). We then selected only those patients with depression and used current depression-specific therapy (antidepressants and/or counselling) as the exposure and controlled for the same variables. The predictive accuracy of each model was assessed using the C statistic.
All analyses using both THIN and CEP data were conducted using Stata version 13.1 and R. Statistical significance was set at P ≤ .05.
Results
THIN Analyses
We identified 10 595 709 patients in THIN of whom 229 164 (2.2%) developed depression and 97 177 (0.9%) developed epilepsy. The median age was 44 years (interquartile range, 32-58 years) for those with depression and 56 years (interquartile range, 43-71 years) for those with epilepsy. Significantly more patients with depression (144 373 [63%] were women, and 84 791 [37%] were men; P < .001) or epilepsy (54 419 [56%] were women, and 42 758 [44%] were men; P < .001) were female. Overall median follow-up was 5.6 years (interquartile range, 1.9-11.7 years).
The Risk of Depression Following Incident Epilepsy
When controlling for age, sex, CCI (calculated over the first 5 years of follow-up), and TDI, the hazard of incident depression was higher for those with incident epilepsy (hazard ratio [HR], 2.04 [95% CI, 1.97-2.09]; P < .001) than for those who did not develop epilepsy (eTable in the Supplement).
The Risk of Epilepsy Following Incident Depression
When controlling for age, female sex, TDI, and CCI, the hazard of developing epilepsy was significantly higher for those developing incident depression (HR, 2.54 [95% CI, 2.48-2.60]; P < .001) than for those free of depression (Table 1). Furthermore, in the mediation analysis, occurrence of depression influenced the relationship between sex, TDI, CCI, and incident epilepsy, accounting for 4.6%, 7.1%, and 20.6% of the total effects of these explanatory variables, respectively.
Table 1. Hazard of Developing Epilepsy When Controlling for Known Risk Factorsa.
| Risk Factor | HR (95% CI) | P Value |
|---|---|---|
| Incident depression | 2.54 (2.48-2.60) | <.001 |
| Age at index date | 1.02 (1.02-1.02) | <.001 |
| Female sex | 1.11 (1.10-1.13) | <.001 |
| Townsend Deprivation Index | 1.11 (1.10-1.12) | <.001 |
| Charlson Comorbidity Index | 1.32 (1.31-1.34) | <.001 |
Incident depression is treated as a time-varying covariate in this Cox proportional hazards regression analysis. The hazard ratio (HR) for age, Townsend Deprivation Index, and Charlson Comorbidity Index is for each incremental increase in value. Higher values indicate more social deprivation and a larger number of comorbidities.
The HR for developing epilepsy in those with incident depression (compared with those without) did not appreciably change whether the analysis was performed controlling for CCI accrued over the first 5 years of follow-up (HR, 2.54 [95% CI, 2.48-2.60]; P < .001), over the last 5 years of follow-up (HR, 2.40 [95% CI, 2.35-2.45]; P < .001), or for the full period of follow-up (HR, 2.51 [95% CI, 2.46-2.57]; P < .001).
The association with incident epilepsy was even stronger for those with treated depression (HR, 3.42 [95% CI, 3.37-3.47]; P < .001) (Table 2) compared with a combination of those who did not develop depression and those who developed depression not requiring therapy. The hazard did not change appreciably when controlling for CCI accrued over the last 5 years of follow-up (HR 3.23 [95% CI, 3.19-3.27]; P < .001) or for the full period of follow-up (HR, 3.45 [95% CI, 3.40-3.50]; P < .001).
Table 2. Hazard of Developing Epilepsy Among Those Patients Treated for Depression (Counselling and/or Medical Treatment)a.
| Risk Factor | HR (95% CI) | P Value |
|---|---|---|
| Treatment for depression | 3.42 (3.37-3.47) | <.001 |
| Age at index date | 1.02 (1.02-1.02) | <.001 |
| Female sex | 1.01 (1.01-1.03) | .03 |
| Townsend Deprivation Index | 1.09 (1.08-1.10) | <.001 |
| Charlson Comorbidity Index | 1.30 (1.29-1.31) | <.001 |
Treatment for major depressive disorder was considered as a time-varying covariate in this Cox proportional hazards regression analysis. The hazard ratio (HR) for age, Townsend Deprivation Index, and Charlson Comorbidity Index is for each incremental increase in value. Higher values indicate more social deprivation and a larger number of comorbidities.
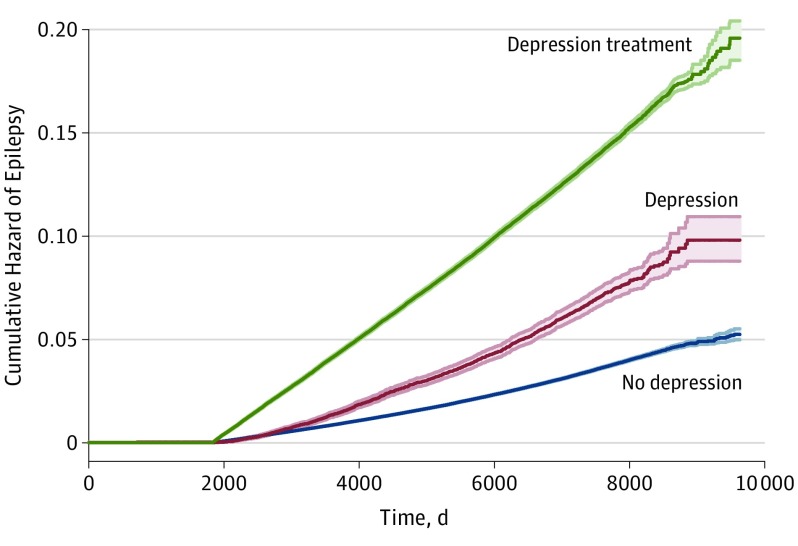
In a sensitivity analysis controlling for age, sex, TDI, and CCI, counselling (HR, 1.84 [95% CI, 1.30-2.59]; P < .001), antidepressant medications (HR, 3.43 [95% CI, 3.37-3.47]; P < .001), and a combination of counselling and antidepressant medications (HR, 9.85 [95% CI, 5.74-16.90]; P < .001) were all associated with an incrementally greater hazard of epilepsy (Figure).
Figure. Cumulative Hazard of Epilepsy According to Depression Status.
Nelson-Aalen cumulative hazard plot depicting a gradient in the hazard of an epilepsy diagnosis between those without depression, those with depression, and those who are receiving depression therapy. Depression is treated as a time-varying covariate in this analysis. The shaded areas indicate 95% CI.
The Association Between Depression and Seizure Outcome
We identified 2573 patients in the CEP cohort of whom 504 (20%) achieved 1-year seizure freedom over the year prior to their first clinic visit. The presence of past or current depression was associated with higher odds of failing to achieve seizure freedom over the previous year compared with those free of depression (odds ratio, 1.41 [95% CI, 1.03-1.96]; P = .03; C statistic, 0.62) (Table 3).
Table 3. Odds of Failing to Achieve 1-Year Seizure Freedom Among Those With Depression Identified Through a Tertiary Care Cohort of Patients With Epilepsy When Controlling for Potential Confounders.
| Potential Confounder | OR (95% CI) | P Value |
|---|---|---|
| Depression | 1.41 (1.03-1.96) | .04 |
| Age at seizure onset | 1.02 (1.01-1.02)a | .001 |
| Female sex | 1.01 (0.78-1.30) | .95 |
| Normal MRI finding | 0.99 (0.76-1.27) | .91 |
| Generalized tonic-clonic seizures | 0.82 (0.60-1.11) | .20 |
| Taking an AED | 0.34 (0.20-0.57) | <.001 |
Abbreviations: AED, antiepileptic drug; MRI, magnetic resonance imaging; OR, odds ratio.
The OR or age is for each 1-year increment.
When focusing only on those with current or past depression (n = 738), current depression treatment (antidepressants and/or counselling) was associated with a statistically significant higher odds of failing to achieve 1-year seizure freedom (odds ratio, 1.75 [95% CI, 1.06-2.94]; P = .03; C statistic, 0.64) after controlling for age at onset of epilepsy, sex, lesional epilepsy detected by magnetic resonance imaging, a history of generalized tonic-clonic seizures, and current antiepileptic drug use (Table 4).
Table 4. Odds of Failing to Achieve 1-Year Seizure Freedom Among Those With Epilepsy and Depression Stratified by Antidepressant Therapy in a Tertiary Care Cohort of Patients With Epilepsy When Controlling for Potential Confoundersa.
| Potential Confounder | OR (95% CI) | P Value |
|---|---|---|
| Depression therapy | 1.75 (1.06-2.94 | .03 |
| Age at seizure onsetb | 1.01 (0.99-1.02) | .27 |
| Female sex | 1.44 (0.90-2.33) | .13 |
| Normal MRI finding | 0.92 (0.52-1.64) | .77 |
| Generalized tonic-clonic seizures | 1.19 (0.73-1.92) | .48 |
| Taking an AED | 0.23 (0.07-0.70) | .02 |
Abbreviations: AED, antiepileptic drug; MRI, magnetic resonance imaging; OR, odds ratio.
Antidepressant treatment includes counselling and/or antidepressant medications.
The OR or age is for each 1-year increment.
Discussion
This study used 2 independent sources of data to explore the relationship between depression and epilepsy. First, using electronic medical records data derived from a population-based general practice cohort, we demonstrated a strong association between the severity of an incident diagnosis of depression and developing subsequent epilepsy. In addition, using more detailed clinical data, we demonstrated that depression is associated with not only an increased hazard of epilepsy but worse seizure outcomes compared with those free from depression.
This study benefits from the statistical power conferred by THIN database and by the systematic collection of high-quality clinical data in the CEP database. In THIN, we evaluated an unselected group of patients who, apart from their age, are not restricted by demographic, clinical, or diagnostic indices. The internal and external validity of the study should be high because THIN contains valid and complete prospective primary care data that are broadly representative of the UK population. The reliability of the data are enhanced through the UK’s health care model in which each patient can only be registered with a single general practitioner who is responsible for virtually all specialist referrals. All information resulting from specialist evaluation and emergent medical care is forwarded to the general practitioner, who recorded it in THIN using the Read code system.
Our incidence estimate for depression (2.2%), although lower than the anticipated incidence of 7% to 8%, is consistent with that reported in prior analyses using THIN (2.5%). Underascertainment of depression is expected in THIN database owing to the vagaries of the reporting system. We used a 5-year washout period to increase the chance that each diagnosis was incident, thus adhering to recommendations for chronic, relapsing conditions such as epilepsy and depression, and to maintain consistency with other studies using population-based UK electronic medical records to evaluate depression and epilepsy.
Limitations
This study also has limitations. The source data did not allow us to control for etiology, epilepsy subtype, or other conditions that could cause both epilepsy and depression. Also, we cannot exclude the potential for inadvertently including patients with nonepileptic events in our cohort, although the reported accuracy (92%) and the comprehensive nature of the definition do help mitigate the risk of producing misleadingly inflated estimates. We were unable to determine whether patients continued with antidepressant therapy, and we could not control for dose, duration, or frequency of therapy sessions. Nonetheless, the simple need for antidepressant treatment significantly enhanced the hazard of epilepsy. In addition, epilepsy and depression could be underreported in THIN, which could lead to nondifferential misclassification bias and underestimation of the overall point estimates. Furthermore, differential misclassification bias is a possibility if mild depression was disproportionately underreported by general practitioners owing to its perceived lack of severity. If that is the case, one would anticipate dilution of the overall gradient effect due to the relative preponderance of patients with mild depression in the untreated stratum. Finally, depression treatment may not be predicated on severity but, instead, on patient or physician preference and resource availability. However, this risk is likely minimal because counselling is recommended as first-line treatment for mild depression, whereas combination therapy (antidepressants and counselling) is reserved for severe depression according to primary care guidelines.
We are unable to definitively establish a cause-effect relationship because of the study design. However, the strong, specific, and temporal association between the exposure (depression) and the outcome (epilepsy) provides compelling evidence suggestive of a causal association. If one assumes that depression treatment is a surrogate marker for depression severity, then there also appears to be a biological gradient. This assertion is corroborated by the incremental hazard of epilepsy among those receiving counselling alone (lowest hazard), antidepressants alone (intermediate hazard), and a combination of counselling and antidepressants (highest hazard). Another interpretation, though, could be that antidepressant treatment itself augments the hazard of epilepsy or that patients are more severely depressed because they have not achieved 1-year seizure freedom. However, the idea that depression treatment increases the hazard of epilepsy or enhances the odds of a poor outcome is less biologically plausible because counselling alone was associated with an elevated risk of seizures. Furthermore, counselling interacted with antidepressant exposure and resulted in a 3-fold increase in the hazard of epilepsy over that of medications alone. This association is clearly counterintuitive from a pathophysiological perspective. Rather, it is entirely more congruent with the notion that treatment is acting as a proxy for depression severity and is thus consistent with the existence of a biological dose-response relationship. Finally, a recent review has indicated that virtually all antidepressants are safe for patients with epilepsy, thereby debunking the notion that these medications increase the risk of seizures.
Conclusions
Our study adds to the existing knowledge by demonstrating a temporal association and an apparent severity gradient in the association between depression and epilepsy. Furthermore, we provide novel evidence that depression partially mediates the effects of sex, socioeconomic deprivation, and CCI on the risk of developing epilepsy. This suggests that depression-specific interventions might reduce the overall effect of each of these variables on epilepsy. Finally, we provide corroborating evidence that depression is associated with worse epilepsy outcomes.
Additional studies building on these results are warranted. Specifically, prospective studies designed to address causation are needed. These studies should also stratify outcomes to determine whether the risk is equivalent between epilepsy subtypes (generalized vs focal and/or temporal vs extratemporal). Genetic and functional neuroimaging studies are required to determine whether genomics, proteomics, and connectomics vary according to the presence or absence of depression in those with epilepsy. These preliminary results, however, indicate a shared relationship between depression and epilepsy, with each appearing to act as a risk factor for the other, and, therefore, should have a direct effect on counselling and management.
eTable. Hazard of developing depression when adjusting for known risk factors. Incident epilepsy is treated as a time-varying covariate in this Cox proportional hazards regression analysis.
eAppendix 1. Read codes identified through a consensus driven process for depression
eAppendix 2. Read codes and Multilex codes used to identify treatment for depression
References
- 1.Fiest KM, Dykeman J, Patten SB, et al. . Depression in epilepsy: a systematic review and meta-analysis. Neurology. 2013;80(6):590-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hesdorffer DC, Ishihara L, Mynepalli L, Webb DJ, Weil J, Hauser WA. Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Ann Neurol. 2012;72(2):184-191. [DOI] [PubMed] [Google Scholar]
- 3.Mayberg HS. Frontal lobe dysfunction in secondary depression. J Neuropsychiatry Clin Neurosci. 1994;6(4):428-442. [DOI] [PubMed] [Google Scholar]
- 4.Kanner AM. Depression in epilepsy: a neurobiologic perspective. Epilepsy Curr. 2005;5(1):21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourke A, Dattani H, Robinson M. Feasibility study and methodology to create a quality-evaluated database of primary care data. Inform Prim Care. 2004;12(3):171-177. [DOI] [PubMed] [Google Scholar]
- 6.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of The Health Improvement Network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16(4):393-401. [DOI] [PubMed] [Google Scholar]
- 7.Szatkowski L, Lewis S, McNeill A, Huang Y, Coleman T. Can data from primary care medical records be used to monitor national smoking prevalence? J Epidemiol Community Health. 2012;66(9):791-795. [DOI] [PubMed] [Google Scholar]
- 8.THIN database. University College London website. https://www.ucl.ac.uk/pcph/research-groups-themes/thin-pub/database. Accessed January 24, 2017.
- 9.Meeraus WH, Petersen I, Chin RF, Knott F, Gilbert R. Childhood epilepsy recorded in primary care in the UK. Arch Dis Child. 2013;98(3):195-202. [DOI] [PubMed] [Google Scholar]
- 10.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492-499. [DOI] [PubMed] [Google Scholar]
- 11.Mula M, Monaco F. Antiepileptic drugs and psychopathology of epilepsy: an update. Epileptic Disord. 2009;11(1):1-9. [DOI] [PubMed] [Google Scholar]
- 12.Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115(3):424-443. [DOI] [PubMed] [Google Scholar]
- 13.Saxena S, Jané-Llopis E, Hosman C. Prevention of mental and behavioural disorders: implications for policy and practice. World Psychiatry. 2006;5(1):5-14. [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367(9516):1087-1100. [DOI] [PubMed] [Google Scholar]
- 15.Lange T, Hansen JV. Direct and indirect effects in a survival context. Epidemiology. 2011;22(4):575-581. [DOI] [PubMed] [Google Scholar]
- 16.Fisher RS, van Emde Boas W, Blume W, et al. . Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46(4):470-472. [DOI] [PubMed] [Google Scholar]
- 17.Fisher RS, Acevedo C, Arzimanoglou A, et al. . ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475-482. [DOI] [PubMed] [Google Scholar]
- 18.Kwan P, Arzimanoglou A, Berg AT, et al. . Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069-1077. [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stata Statistical Software [computer program]. Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 21.Team RDC R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 22.Chisholm J. The Read clinical classification. BMJ. 1990;300(6732):1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King M, Walker C, Levy G, et al. . Development and validation of an international risk prediction algorithm for episodes of major depression in general practice attendees: the PredictD study. Arch Gen Psychiatry. 2008;65(12):1368-1376. [DOI] [PubMed] [Google Scholar]
- 24.Rait G, Walters K, Griffin M, Buszewicz M, Petersen I, Nazareth I. Recent trends in the incidence of recorded depression in primary care. Br J Psychiatry. 2009;195(6):520-524. [DOI] [PubMed] [Google Scholar]
- 25.Lewis JD, Bilker WB, Weinstein RB, Strom BL. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2005;14(7):443-451. [DOI] [PubMed] [Google Scholar]
- 26.Weihs K, Wert JM. A primary care focus on the treatment of patients with major depressive disorder. Am J Med Sci. 2011;342(4):324-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institute for Health and Care Excellence (NICE). Depression. NICE website. https://www.nice.org.uk/guidance/conditions-and-diseases/mental-health-and-behavioural-conditions/depression. Updated April 2016. Accessed January 20, 2017.
- 28.Kanner AM. Most antidepressant drugs are safe for patients with epilepsy at therapeutic doses: a review of the evidence. Epilepsy Behav. 2016;61:282-286. [DOI] [PubMed] [Google Scholar]
- 29.Kanner AM, Byrne R, Chicharro A, Wuu J, Frey M. A lifetime psychiatric history predicts a worse seizure outcome following temporal lobectomy. Neurology. 2009;72(9):793-799. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Hazard of developing depression when adjusting for known risk factors. Incident epilepsy is treated as a time-varying covariate in this Cox proportional hazards regression analysis.
eAppendix 1. Read codes identified through a consensus driven process for depression
eAppendix 2. Read codes and Multilex codes used to identify treatment for depression