This study assesses the ability of neurofilament light chain to serve as a diagnostic biomarker in amyotrophic lateral sclerosis and the prognostic value of cerebrospinal fluid neurofilament light chain in patients with amyotrophic lateral sclerosis.
Key Points
Question
Is neurofilament light chain a reliable diagnostic and prognostic biomarker in amyotrophic lateral sclerosis?
Finding
In this longitudinal study, neurofilament light chain levels were significantly increased in patients with amyotrophic lateral sclerosis compared with those with frontotemporal dementia, those with motor neuropathies, and controls. Among amyotrophic lateral sclerosis subtypes, higher levels of neurofilament light chain were found in patients with upper motor neuron involvement.
Meaning
Neurofilament light chain levels may help discriminate amyotrophic lateral sclerosis from other diseases and different phenotypes of amyotrophic lateral sclerosis; low neurofilament light chain levels in patients with lower motor neuron signs could be prognostic of milder phenotypes of disease.
Abstract
Importance
A clearer definition of the role of neurofilament light chain (NFL) as a biomarker in amyotrophic lateral sclerosis (ALS) is needed.
Objectives
To assess the ability of NFL to serve as a diagnostic biomarker in ALS and the prognostic value of cerebrospinal fluid NFL in patients with ALS.
Design, Setting, and Participants
In this single-center, retrospective, longitudinal study, disease progression was assessed by the ALS Functional Rating Score–Revised and the ALS Milano-Torino Staging system at baseline and 6, 12, 24, and 36 months. Cerebrospinal fluid samples were obtained from 176 patients admitted to the Department of Neurosciences of the University of Padua, Padova, Italy, from January 1, 2010, through February 29, 2016. Patients with ALS underwent ambulatory follow-up at the same department.
Main Outcomes and Measures
Levels of NFL.
Results
The study included 94 patients with ALS (64 men [36.4%] and 30 women [17.0%]; median age, 62.5 years), 20 patients with frontotemporal dementia (FTD) (8 men [4.5%] and 12 women [6.8%]; median age, 65 years), 18 patients with motor neuropathies (14 men [8.0%] and 4 women [2.3%]; median age, 63 years), and 44 controls (24 men [13.6%] and 20 women [11.4%]; median age, 54 years). Log-transformed NFL (log[NFL]) concentrations were higher in the ALS and FTD groups compared with the motor neuropathies and control groups (hazard ratio [HR], 2.45; 95% CI, 1.66-3.61; P < .001). Patients with typical ALS (HR, 1.0 [reference]), progressive bulbar palsy (HR, 1.48; 95% CI, 0.58-3.75; P = .41), and upper motor neuron dominant ALS (HR, 0.12; 95% CI, 0.02-0.61; P = .01) had higher levels of NFL than did those with flail arm or leg syndrome (HR, 0.28; 95% CI, 0.08-0.10; P = .049) and progressive muscular atrophy (HR, 0.17; 95% CI, 0.22-1.36; P = .10). There was an inverse correlation between log[NFL] concentration and overall survival (HR, 2.45; 95% CI, 1.66-3.61; P < .001). There was no evidence of different log[NFL] concentrations and survival in genetic ALS.
Conclusions and Relevance
This study confirms the role of NFL as a biomarker in ALS. Elevation in NFL levels in patients with upper motor neuron involvement and FTD might reflect the corticospinal tract degeneration. Low NFL levels in patients with lower motor neuron signs might be a prognostic indicator of milder phenotypes of disease.
Introduction
Substantial efforts have been made to discover biomarkers in amyotrophic lateral sclerosis (ALS) that could act as reliable indicators for early diagnosis, prognosis, and disease progression. Because neurofilaments are the most important scaffolding proteins of the cytoskeleton of the axon, they have been indicated as possible markers of neuronal injury. Despite neurofilament levels having been extensively recognized as promising biomarkers of neuroaxonal breakdown in miscellaneous conditions, the elevation appears to be more significant in patients with ALS.
Amyotrophic lateral sclerosis is a neurodegenerative disorder that affects the upper and lower motor neurons, with a median survival of 3 years after the onset of symptoms. The estimated incidence of ALS in Europe is 2 to 16 per 100 000 person-years. Peak age at onset is 58 to 63 years for sporadic disease. Approximately 5% to 10% of cases are familial. Effective treatments are not currently available. Within ALS, variants of motor neuron disease are described, with distinct clinical presentation and rate of progression. Subtypes of ALS with predominant or exclusive involvement of upper or lower motor neuron are associated with better prognosis.
Patients with ALS may develop cognitive dysfunctions, ranging from impaired frontal executive skills (20%-40% of patients) to definite frontotemporal dementia (FTD) in 5% of cases. Both FTD and ALS belong to the same spectrum of neurodegenerative diseases because they share clinical, histopathologic, and genetic features. Neurofilament levels are higher in patients with FTD compared with those with other dementias. The elevation of neurofilament levels might contribute to the definition of the pathologic continuum of FTD and ALS.
Neurofilament accumulation is reported in several neurodegenerative diseases. The precise mechanism by which neurofilaments accumulate is still unclear. A failure of the transport of neurofilaments through axons has been described as an early pathologic event in several transgenic models of ALS. A possible explanation for the disruption of this process is the phosphorylation state, because it may contribute to abnormal neurofilament accumulation. Exposure to glutamate was associated with an increase in phosphorylation of neurofilaments in cell bodies, resulting in their pathologic accumulation. This finding emphasizes the concept of glutamate-induced excitotoxicity in some neurodegenerative diseases. In accordance with that finding, the mutant neurofilament peptide, light polypetide (NFL) gene (OMIM 162280) in which serine 55 is converted to aspartate to mimic permanent phosphorylation leads to abnormal accumulation of neurofilaments in motor neurons once transfected into mammalian cells, and mutations of the tail domain of neurofilament heavy chain (NFH), responsible for the hyperphosphorylation of the lysine-serine-proline motifs, are reported in patients with ALS. A key role of the ubiquitin proteasome system has been demonstrated too, although other players in the neurofilament turnover have yet to be identified. Of interest, among neurodegenerative diseases manifesting with neurofilament accumulation, neuronal intermediate filament inclusion disease configures a distinct neuropathologic pattern of FTD characterized by intracellular inclusions of neurofilament light chain (NFL), neurofilament medium chain, NFH, and α-internexin and negative for τ, synuclein, and TAR DNA-binding protein 43. Nonetheless, neurofilaments have been proven to be markers of disease stage, indicating a potential prognostic role.
The aim of our study was to assess the role of NFL as a diagnostic biomarker in ALS, testing the discriminatory capacity between ALS mimics (motor neuropathies [MNs]), FTD, and controls and among different subtypes of motor neuron disease. Another objective was to verify the prognostic value of cerebrospinal fluid (CSF) NFL in patients with ALS by retrospective analysis of longitudinal assessments and survival in distinctive phenotypes of motor neuron disease.
Methods
Participants and Clinical Characterization
This was a single-center, retrospective, longitudinal study of 176 inpatients who underwent a lumbar puncture at the Department of Neurosciences of the University of Padua, Padova, Italy, from January 1, 2010, through February 29, 2016. This study included 94 patients with ALS, 20 patients with FTD, 18 patients with MNs (3 multifocal MNs and 15 chronic inflammatory demyelinating polyneuropathies [2 with motor variants]), and 44 controls (mononeuritis, primary headaches, and patients who, based on extensive diagnostic evaluation, had no objective signs of a neurologic disease). The ALS group comprised 94 patients, further subdivided by clinical subtype into 58 patients with typical ALS (clinically definite or probable ALS according to the revised El Escorial diagnostic criteria), 6 with flail arm syndrome, 5 with flail leg syndrome, 9 with progressive muscular atrophy (PMA), 9 with progressive bulbar palsy (PBP), and 7 with upper motor neuron dominant (UMND) ALS. The ALS group comprised 30 women and 64 men. The study was approved by the local ethics committee (Ethics Committee for Clinical Trials [Comitato Etico per la Sperimentazione Clinica], Azienda Ospedaliera di Padova, Padova, Italy). All participants provided written informed consent, and all data were deidentified.
After CSF analysis and diagnosis, patients with ALS were followed up as outpatients in the ALS Clinic at the Department of Neurosciences, General Hospital of Padua, Padova, Italy. DNA was available from 91 patients with motor neuron disease and was screened for mutations in chromosome 9 opening reading frame 72 (C9orf72) (OMIM 614260), fused in sarcoma (FUS) (OMIM 137070), TAR DNA-binding protein (TARDBP) (OMIM 605078), and superoxide dismutase 1 (SOD1) (OMIM 147450) according to established protocols. Eight patients carried a C9orf72 GGGGCC-repeat expansion.
Demographic and clinical characteristics were obtained from medical records and directly from patients and controls and included age, disease duration, phenotype of motor neuron disease, cognitive impairment, overall survival, and disease extension assessed by the ALS Functional Rating Score–Revised (ALSFRS-R) and the ALS Milano-Torino Staging (MITOS) system. Progression rate (∆FS) until CSF analysis was calculated as follows: ∆FS = (48 − ALSFRS-R Score at Time of Diagnosis)/Duration From Onset to Diagnosis (Months).
Sample Collection and Analysis
Cerebrospinal fluid samples were obtained by lumbar puncture, centrifuged, aliquoted, and stored at −80°C. Standard CSF analysis was performed within 1 hour after sample collection, and findings were unremarkable in all patients. The NFL levels were measured in April 2016.
An NFL enzyme-linked immunosorbent assay (UmanDiagnostics AB) was used to quantify NFL. Samples from the ALS, FTD, MNs, and control groups were evenly distributed on each plate and measured in duplicate; only CSF samples of patients with ALS were diluted 1:2. Each plate contained calibrators (0-10 000 pg/mL) and quality controls. The interassay and mean intra-assay coefficients of variance were all below 10%. Linearity of the NFL assay was established (0-50 000 pg/mL).
Statistical Analysis
The NFL concentrations in CSF revealed an extremely left-skewed, nonnormal distribution (Shapiro-Wilk test for normality P < .001). After natural logarithm transformation, data appeared to be normally distributed (Shapiro-Wilk test for normality P = .43) (eFigure 1 in the Supplement). Thus, the log-transformed NFL (log[NFL]) concentration was used for graphical representation of data and analysis of covariance (ANCOVA) models.
The NFL levels were compared among the ALS, FTD, MNs, and control groups and among different subtypes of ALS using the Mann-Whitney test. Correlations between log[NFL] and quantitative variables (CSF conservation time, disease duration, ∆FS) were assessed using Pearson correlation coefficient. Influence of age and sex on log[NFL] within each group was tested by ANCOVA. The ANCOVA was used within the ALS group with subtype, age, sex, and disease duration as covariates as well. Among patients with typical ALS, the NFL levels were compared between sporadic and genetic ALS, using the Mann-Whitney test and the t test with log-transformed data.
Survival was analyzed with death or tracheostomy as events, recording time in months from the lumbar puncture. Patients who were alive and had not undergone tracheostomy by the last follow-up were censored. A Cox proportional hazards regression model was devised with the following covariates: log[NFL], ALS subtypes, age at onset, sex, cognitive impairment, and ∆FS. Hazard ratios (HRs) were calculated for each quantitative covariate and each level of categorical covariates (subtype with typical ALS as reference and sex with female sex as reference), with 95% CIs. Reference levels were assigned HRs of 1.0.
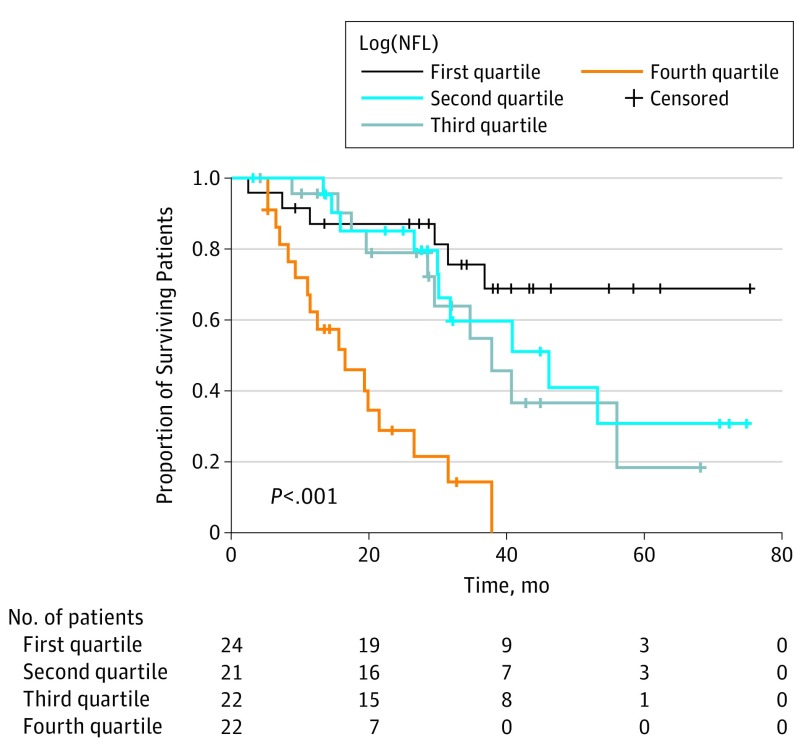
Ninety-two patients were divided into 4 groups corresponding to NFL concentration quartiles (2 observations deleted because of missing survival data). These groups were used to calculate median survival within NFL concentration ranges and graphically represent survival data in relation to NFL concentration. Kaplan-Meier curves were also estimated for genetic vs sporadic ALS.
Longitudinal changes in the ALSFRS-R and MITOS score were assessed in repeated-measures ANCOVA models, testing for concurrent effects of the following covariates: time elapsed from baseline (0, 6, 12, 24, and 30 months), log[NFL] concentration, age at onset, ALS subtype, sex, cognitive impairment, and ∆FS.
Receiver operating characteristic (ROC) curves were used for a sensitivity analysis of NFL cutoff values for ALS vs all other samples, ALS vs controls, and ALS vs mimics (MNs and FTD). Overall sensitivity and specificity were assessed as areas under the curve (AUCs). We selected optimal cutoff values corresponding to the point in the ROC curve nearest to the upper-left corner of the plot (least sum of the squares of 1 – sensitivity and 1 – specificity).
R version 3.3.0 (R Foundation for Statistical Computing) was used for analyses. The pROC package was used for sensitivity analysis. Statistical significance was set at 2-sided P < .05.
Results
The study included 94 patients with ALS (64 men [36.4%] and 30 women [17.0%]; median age, 62.5 years), 20 patients with FTD (8 men [4.5%] and 12 women [6.8%]; median age, 65 years), 18 patients with MNs (14 men [8.0%] and 4 women [2.3%]; median age, 63 years), and 44 controls (24 men [13.6%] and 20 women [11.4%]; median age, 54 years). There was no statistically significant correlation between CSF conservation time and NFL levels. Patient characteristics are summarized in Table 1. The CSF conservation time was similar in the 4 groups, whereas a significant difference was found in sex and age distribution, which was accounted for in the ANCOVA models, as mentioned below. The ALS group characteristics are given in the eTable in the Supplement.
Table 1. Characteristics of the Study Groups.
| Group | No. of Patients | Male/Female | Median (IQR) | |
|---|---|---|---|---|
| Age at Lumbar Puncture, y | CSF Conservation Time, mo | |||
| ALS | 94 | 64/30 | 62.5 (52.3-70.0) | 42.0 (25.0-57.5) |
| Control | 44 | 24/20 | 54.0 (37.0-69.3) | 40.0 (34.0-53.0) |
| FTD | 20 | 8/12 | 65.0 (61.0-72.3) | 37.0 (7.5-65.8) |
| MNs | 18 | 14/4 | 63.0 (57.0-71.5) | 39.5 (14.3-48.5) |
| P value across groups | NA | .04a | .009b | .54 |
Abbreviations: ALS, amyotrophic lateral sclerosis; CSF, cerebrospinal fluid; FTD, frontotemporal dementia; IQR, interquartile range; MNs, motor neuropathies; NA, not applicable.
P ≤ .05.
P ≤ .01.
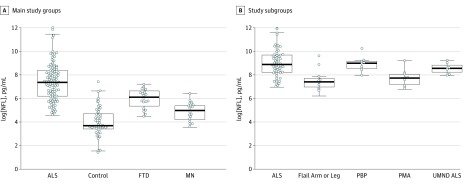
The NFL concentrations were higher in the ALS (median, 5741.51 pg/mL; interquartile range [IQR], 2494.26-11 911.51 pg/mL) and FTD groups (median, 2297.50 pg/mL; IQR, 1529.75-3184.97 pg/mL) compared with the MNs (median, 1009.72 pg/mL; IQR, 596.78-1360.81 pg/mL) and control groups (median, 395.53 pg/mL; IQR, 320.44-804.84 pg/mL). Figure 1A shows the levels of NFL in the ALS, FTD, MNs, and control groups after natural logarithm transformation. Sex did not influence NFL levels in the ALS group.
Figure 1. Median Log-Transformed Neurofilament Light Chain (log[NFL]) Concentrations in the Study Groups.
A, Log[NFL] concentrations in the 4 main study groups; B, log[NFL] concentrations in the amyotrophic lateral sclerosis (ALS) group divided into clinical subtypes. The central location and scatter and dispersion of the observations are shown. Error bars indicate SD. FTD indicates frontotemporal dementia; MNs, motor neuropathies; PBP, progressive bulbar palsy; PMA, progressive muscular atrophy; and UMND, upper motor neuron dominant.
The ANCOVA did not reveal significant effects of sex and age on NFL concentration. The ANCOVA was repeated within the ALS groups to investigate the effect of age, ALS subtype, disease duration, and sex. Patients with typical ALS, PBP, or UMND ALS had higher NFL concentrations than did patients with flail arm syndrome, flail leg syndrome, or PMA (Figure 1B).
Pairwise comparisons of the typical ALS, flail arm syndrome, flail leg syndrome, and PMA groups were statistically significant, whereas there was no difference in log[NFL] levels among the typical ALS, UMND ALS, and PBP groups. The ANCOVA revealed that this subtype effect was statistically significant (F = 7.78, P < .001), as was that of disease duration (F = 5.89, P = .02) and age (F = 9.28, P = .003) but not of sex.
Cox proportional hazards regression analysis confirmed a significant inverse correlation between log[NFL] level and overall survival (HR, 2.45; 95% CI, 1.66-3.61; P < .001), adjusting for the concurrent effects of subtype, age at onset, ∆FS score, sex, and cognitive impairment (Table 2 and Figure 2).
Table 2. Measures for the Cox Proportional Hazards Regression Analysis of Effects of CSF Log(NFL) Concentration, ALS Subtype, Age, Sex, Cognitive Impairment, and ∆FS on Overall Survival.
| Covariates | HR (95% CI) | SE | P Value |
|---|---|---|---|
| Log[NFL] concentration | 2.45 (1.66-3.61) | 0.20 | <.001a |
| Age at ALS onset | 1.08 (1.04-1.13) | 0.02 | <.001a |
| ∆FS | 1.16 (0.75-1.82) | 0.23 | .499 |
| Subtype | |||
| Typical ALS | 1 [Reference] | NA | NA |
| Flail arm or leg | 0.28 (0.08-0.10) | 0.65 | .049b |
| PBP | 1.48 (0.58-3.75) | 0.48 | .41 |
| PMA | 0.17 (0.02-1.36) | 1.05 | .10 |
| UMND ALS | 0.12 (0.02-0.61) | 0.83 | .01b |
| Sex | |||
| Male | 1 [Reference] | NA | NA |
| Female | 0.62 (0.30-1.26) | 0.36 | .18 |
| Cognitive impairment | |||
| No | 1 [Reference] | NA | NA |
| Yes | 0.93 (0.38-2.26) | 0.45 | .88 |
Abbreviations: ALS, amyotrophic lateral sclerosis; CSF, cerebrospinal fluid; HR, hazard ratio; NA, not applicable; PBP, progressive bulbar palsy; PMA, progressive muscular atrophy; UMND, upper motor neuron dominant; ∆FS, progression rate of disease from onset to CFS analysis.
P ≤ .001.
P ≤ .05.
Figure 2. Kaplan-Meier Plots of the Proportion of Surviving Patients Relative to Time From Baseline Grouped by Log-Transformed Neurofilament Light Chain (Log[NFL]) Concentrations.
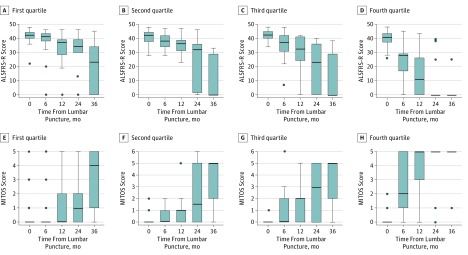
Log[NFL] levels correlated inversely with the time from onset to lumbar puncture in patients with ALS (r = −0.42, P < .001) and directly with the ∆FS score (r = 0.53, P < .001). Log[NFL] concentrations were a prognostic indicator of disease progression assessed by longitudinal ALSFRS-R at baseline and 6, 12, 24, and 36 months (Figure 3). Repeated-measures ANCOVA proved that this effect was statistically significant (F = 62.56, P < .001), as was that of subtype (F = 19.78, P < .001), age at onset (F = 19.71, P < .001), ∆FS score (F = 15.10, P < .001), and sex (F = 8.81, P = .003), whereas cognitive impairment had no significant effect. In correspondence with these findings, the disease progression assessed by the MITOS score was significantly influenced by log[NFL] level (F = 52.40, P < .001) (Figure 3). Effects of subtype, age at onset (F = 14.10, P < .001), ∆FS score (F = 7.27, P = .007), and sex (F = 4.48, P = .04) were also significant but not that of cognitive impairment.
Figure 3. Amyotrophic Lateral Sclerosis (ALS) Functional Rating Score–Revised (ALSFRS-R) and the ALS Milano-Torino Staging (MITOS) Score Change Relative to Time From Lumbar Puncture by Log-Transformed Neurofilament Light Chain (Log[NFL]) Quartiles.
Error bars indicate SD. A-D, ALSFRS-R changes; E-H, MITOS score changes.
In a group of 54 patients with typical ALS who underwent genetic tests, different log[NFL] concentrations were found between those with sporadic (mean [SD], 33 372.71 [51 400.69] pg/mL) and those with genetic ALS (mean [SD], 12 471.20 [16 263.44] pg/mL; t test P = .08). There was no evidence of different survival rates between patients with genetic and those with sporadic ALS.
An NFL cutoff of 1843.52 pg/mL provided the optimal discrimination between patients with ALS and all other patients at a sensitivity of 81.9% (95% CI, 74.5%-89.4%) and a specificity of 80.5% (95% CI, 71.9%-89%), with an AUC of 0.91 (95% CI, 0.87-0.95). A lower cutoff of 1380.48 pg/mL was most appropriate for differentiation between patients with ALS and controls at a sensitivity of 88.7% (95% CI, 79.5%-97.7%) and a specificity of 89.4% (95% CI, 83%-96%), with an AUC of 0.96 (95% CI, 0.92-0.99). Conversely, a higher cutoff of 3113.03 pg/mL was optimal for differentiating between ALS and FTD or MNs at a sensitivity of 70.2% (95% CI, 60.6%-78.7%) and a specificity of 86.8% (95% CI, 76.3%-97.4%), with an AUC of 0.85 (95% CI, 0.79-0.92). The ROC curves are shown in eFigure 2 in the Supplement.
Discussion
Our data confirm the role of NFL as a biomarker in ALS, supportive of diagnosis and prognosis. Consistent with previous observations, the NFL concentration was stable over time and independent of age. The NFL levels were considerably higher in the ALS group compared with the FTD, MNs, and control groups (Figure 1A). We observed that elevation in NFL concentration in patients with ALS significantly correlates with median survival, even after adjustment for sex, age, ALS subtype, disease duration, and cognitive impairment. The detection of high CSF NFL levels in the early stages of disease would therefore be prognostic of severe progression and worse outcome.
We noticed an inverse correlation between the levels of NFL and the time from onset of disease (eFigure 3 in the Supplement). One could object that NFL concentrations are not stable during the disease course and that this finding could be the result of a decrease in NFL levels over time. Investigators previously performed serial measurements of phosphorylated NFH and NFH in serum samples from patients with ALS and registered a decrease in values in the later stage of disease, when few motor neurons remain to degenerate. On the other hand, a steady blood expression of NFL over time was reported by Lu et al. Because NFL levels are higher in patients with more severe disease, we believe that those patients with faster progression seek medical attention earlier and exhibit higher values of NFL. In addition, patients with high values of ∆FS, which reflect the disease progression rate from onset to baseline, also presented with higher levels of NFL (eFigure 4 in the Supplement). Additional longitudinal studies with serial measurements of NFL levels are needed to untie this knot.
Although executive impairment has been linked to worse prognosis in ALS, we did not find a significant correlation between NFL levels and cognitive dysfunction, which is consistent with previous findings. This discordance may be attributable to the small number of patients. Of interest, our results indicate that NFL levels tend to be higher in subtypes of motor neuron disease with upper motor neuron involvement (ie, typical ALS, PMA, and PBP) compared with those that primarily affect the lower motor neuron (ie, PMA, flail arm syndrome, and flail leg syndrome) (Figure 1B). These data are consistent with previous observations and could unravel the reason why NFL concentrations are increased in patients with ALS. Brettschneider et al postulated that the damage to the upper motor neuron would result in wallerian degeneration of large-caliber axons along their entire spinal cord pathway, resulting in the release of a large amount of neurofilament into the CSF. Menke et al found a correlation between elevation in NFL concentration and the corticospinal tract degeneration captured by neuroimaging. The motor neurons that constitute the corticospinal tract are believed to be rich in neurofilament; thus, their damage would result in the release of a high amount of NFL in the CSF.
Of note, we found that NFL levels are significantly higher in patients with FTD compared with those with MNs and controls (Figure 1A). The elevation of neurofilament concentrations in patients with FTD has been previously reported and started a debate on the role of neurofilaments in defining the clinical and histopathologic continuum between ALS and FTD. To our knowledge, this is the first study to find a significant elevation of NFL levels in patients with ALS and those with FTD compared with other groups.
The involvement of the corticospinal tract could be a missing piece in this puzzle. Lillo et al examined the gray and white matter differences and commonalities across the ALS-FTD continuum. Patients with ALS, ALS-FTD, or behavioral FTD and controls underwent structural imaging using voxel-based morphometry and diffusion tensor magnetic resonance imaging of the brain. Of interest, corticospinal tract degeneration was documented in patients with behavioral FTD; moreover, patients with ALS had more white matter changes compared with patients with behavioral FTD and ALS-FTD, with a predominant involvement of corticospinal tracts. In addition, Burrell et al documented that patients with FTD may have a prolongation of the central motor conduction time, presumably because of axonal loss and consistent with the degeneration of corticospinal tracts. A further consideration is that the FTD-ALS phenotype is more frequently characterized by upper motor neuron signs rather then lower motor neuron.
Moreover, ALS and FTD share disease mechanisms of proteostasis, and neurofilament accumulations are reported as hallmarks of neurodegenerative disease. Whether the abnormal accumulation of neurofilaments might contribute to the definition of the pathologic continuum of FTD and ALS needs to be established. It would be interesting to explore whether pathologic accumulation of neurofilaments particularly affects the corticospinal tracts.
The prognostic role of NFL is corroborated by our longitudinal assessments with disability scores. The ALSFRS-R and the MITOS score worsen more rapidly in patients with ALS with high concentrations of NFL at baseline (Figure 3).
With the exception of a few cases, all our patients were receiving riluzole treatment; thus, we cannot postulate on the effect it might exert on NFL levels during the disease. Mc Combe et al did not find any influence of riluzole on serial measurements of serum phosphorylated NFH levels.
The main discrepancy of our data is the great increase of NFL in patients with UMND ALS, despite the widely proven best prognosis associated with this subtype. As expected, data from our cohort of patients with ALS confirmed a slower progression and disability accumulation in the UMND subtype (eFigure 5 in the Supplement). A possible response to this issue is that NFL may reflect the corticospinal tract breakdown, whereas the combination of upper and lower motor neuron degeneration dictates the prognosis, with a main contribution of the respiratory involvement.
We found that NFL concentrations are particularly low in patients with flail arm and flail leg syndrome compared with other subtypes of motor neuron disease. If these data are confirmed in larger cohorts of patients, the effect on prognosis definition will be profound because the detection of low NFL concentrations in patients with lower motor neuron signs, especially when confined to the upper or the lower limbs, would be a strong support to the diagnosis of these milder phenotypes. In addition, we could assume that patients with lower motor neuron involvement and high NFL concentrations have a worse prognosis. This approach would have strong consequences on clinical trial enrollment too because it would allow the selection of homogeneous populations of patients at the early stage of disease with minimal disability and a consistent amount of viable motor neurons. Despite the small number of patients, to our knowledge, this is the first study to analyze NFL levels in different subtypes of motor neuron disease.
On the basis of our data, NFL levels are helpful to discriminate between patients with ALS and controls in the diagnostic assessment. However, the test has a weaker diagnostic capability in differentiating between ALS from MNs and FTD, with a higher probability of overlap and a higher false-positive rate. We therefore believe that NFL might be supportive of diagnosis, which still remains eminently clinical. The discriminatory capacity is more relevant if NFL values greatly exceed the cutoff level.
Blood detection of NFL, although less sensitive and sensible compared with CSF measurement, is obviously minimally invasive and will hugely enlarge the cohorts of eligible patients. Moreover, long-term serial measurements will provide more information about NFL expression over time.
Limitations
The main limitation of our study, beyond its retrospective design, was the small number of patients, especially if divided into different clinical subtypes. In addition to the prevalence of disease, the invasive detection of NFL further decreased the sample size.
Conclusions
As mentioned before, NFL measurement is probably heading to the ALS clinic, and our data strongly support this perspective. Despite the diagnosis of ALS being primarily based on clinical examination, further evidence of the diagnostic and prognostic role of NFL will have a profound effect on the selection of patients to enroll in clinical trials, strengthened by the potential discriminatory capacity among different subtypes of motor neuron disease.
eFigure 1. Natural Logarithm Transformation of CSF NFL Concentration With Subsequent Normal Distribution of Data
eFigure 2. ROC Curves of NFL Concentrations for Discrimination Between ALS and Controls/FTD/MN, Between ALS and Controls, and Between ALS and MN/FTD
eFigure 3. Effect of Time From Onset to Lumbar Puncture on the Concentration of NFL in the ALS Group
eFigure 4. Effect of Disease Progression Rate From Onset to Lumbar Puncture (∆FS) on NFL Concentration in the ALS Group
eFigure 5. ALSFRS-R (A-B-C-D) and MITOS (E-F-G-H) Change Relative to Time (Months From Lumbar Puncture) by Subtypes
eTable. Characteristics of the ALS Group
References
- 1.Petzold A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci. 2005;233(1-2):183-198. [DOI] [PubMed] [Google Scholar]
- 2.Gentil BJ, Tibshirani M, Durham HD. Neurofilament dynamics and involvement in neurological disorders. Cell Tissue Res. 2015;360(3):609-620. [DOI] [PubMed] [Google Scholar]
- 3.Herrmann DN, Griffin JW. Intermediate filaments: a common thread in neuromuscular disorders. Neurology. 2002;58(8):1141-1143. [DOI] [PubMed] [Google Scholar]
- 4.Norgren N, Rosengren L, Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain Res. 2003;987(1):25-31. [DOI] [PubMed] [Google Scholar]
- 5.Singh P, Yan J, Hull R, et al. . Levels of phosphorylated axonal neurofilament subunit H (pNfH) are increased in acute ischemic stroke. J Neurol Sci. 2011;304(1-2):117-121. [DOI] [PubMed] [Google Scholar]
- 6.Lewis SB, Wolper RA, Miralia L, Yang C, Shaw G. Detection of phosphorylated NF-H in the cerebrospinal fluid and blood of aneurysmal subarachnoid hemorrhage patients. J Cereb Blood Flow Metab. 2008;28(6):1261-1271. [DOI] [PubMed] [Google Scholar]
- 7.Gaiottino J, Norgren N, Dobson R, et al. . Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One. 2013;8(9):e75091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brettschneider J, Petzold A, Süssmuth SD, Ludolph AC, Tumani H. Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology. 2006;66(6):852-856. [DOI] [PubMed] [Google Scholar]
- 9.Lu CH, Petzold A, Topping J, et al. . Plasma neurofilament heavy chain levels and disease progression in amyotrophic lateral sclerosis: insights from a longitudinal study. J Neurol Neurosurg Psychiatry. 2015;86(5):565-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu CH, Macdonald-Wallis C, Gray E, et al. . Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;84(22):2247-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCombe PA, Pfluger C, Singh P, Lim CY, Airey C, Henderson RD. Serial measurements of phosphorylated neurofilament-heavy in the serum of subjects with amyotrophic lateral sclerosis. J Neurol Sci. 2015;353(1-2):122-129. [DOI] [PubMed] [Google Scholar]
- 12.Tortelli R, Copetti M, Ruggieri M, et al. . Cerebrospinal fluid neurofilament light chain levels: marker of progression to generalized amyotrophic lateral sclerosis. Eur J Neurol. 2015;22(1):215-218. [DOI] [PubMed] [Google Scholar]
- 13.Steinacker P, Feneberg E, Weishaupt J, et al. . Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. J Neurol Neurosurg Psychiatry. 2016;87(1):12-20. [DOI] [PubMed] [Google Scholar]
- 14.Chiò A, Logroscino G, Traynor BJ, et al. . Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology. 2013;41(2):118-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiernan MC, Vucic S, Cheah BC, et al. . Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942-955. [DOI] [PubMed] [Google Scholar]
- 16.Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis. 2009;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007;6(11):994-1003. [DOI] [PubMed] [Google Scholar]
- 18.Neumann M, Sampathu DM, Kwong LK, et al. . Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130-133. [DOI] [PubMed] [Google Scholar]
- 19.Brettschneider J, Van Deerlin VM, Robinson JL, et al. . Pattern of ubiquilin pathology in ALS and FTLD indicates presence of C9ORF72 hexanucleotide expansion. Acta Neuropathol. 2012;123(6):825-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennion Callister J, Pickering-Brown SM. Pathogenesis/genetics of frontotemporal dementia and how it relates to ALS. Exp Neurol . 2014;262(pt B):84-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petzold A, Keir G, Warren J, Fox N, Rossor MN. A systematic review and meta-analysis of CSF neurofilament protein levels as biomarkers in dementia. Neurodegener Dis. 2007;4(2-3):185-194. [DOI] [PubMed] [Google Scholar]
- 22.Collard JF, Côté F, Julien JP. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995;375(6526):61-64. [DOI] [PubMed] [Google Scholar]
- 23.Al-Chalabi A, Miller CC. Neurofilaments and neurological disease. Bioessays. 2003;25(4):346-355. [DOI] [PubMed] [Google Scholar]
- 24.Ackerley S, Grierson AJ, Brownlees J, et al. . Glutamate slows axonal transport of neurofilaments in transfected neurons. J Cell Biol. 2000;150(1):165-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibb BJ, Robertson J, Miller CC. Assembly properties of neurofilament light chain Ser55 mutants in transfected mammalian cells. J Neurochem. 1996;66(3):1306-1311. [DOI] [PubMed] [Google Scholar]
- 26.Al-Chalabi A, Andersen PM, Nilsson P, et al. . Deletions of the heavy neurofilament subunit tail in amyotrophic lateral sclerosis. Hum Mol Genet. 1999;8(2):157-164. [DOI] [PubMed] [Google Scholar]
- 27.Balastik M, Ferraguti F, Pires-da Silva A, et al. . Deficiency in ubiquitin ligase TRIM2 causes accumulation of neurofilament light chain and neurodegeneration. Proc Natl Acad Sci U S A. 2008;105(33):12016-12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Song F, Zhang C, et al. . Carboxyl-terminus of Hsc70 interacting protein mediates 2,5-hexanedione-induced neurofilament medium chain degradation. Biochem Pharmacol. 2011;81(6):793-799. [DOI] [PubMed] [Google Scholar]
- 29.Peth A, Kukushkin N, Bossé M, Goldberg AL. Ubiquitinated proteins activate the proteasomal ATPases by binding to Usp14 or Uch37 homologs. J Biol Chem. 2013;288(11):7781-7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Josephs KA, Holton JL, Rossor MN, et al. . Neurofilament inclusion body disease: a new proteinopathy? Brain. 2003;126(pt 10):2291-2303. [DOI] [PubMed] [Google Scholar]
- 31.Cairns NJ, Grossman M, Arnold SE, et al. . Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology. 2004;63(8):1376-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehnert S, Costa J, de Carvalho M, et al. . Multicentre quality control evaluation of different biomarker candidates for amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(5-6):344-350. [DOI] [PubMed] [Google Scholar]
- 33.Norgren N, Karlsson JE, Rosengren L, Stigbrand T. Monoclonal antibodies selective for low molecular weight neurofilaments. Hybrid Hybridomics. 2002;21(1):53-59. [DOI] [PubMed] [Google Scholar]
- 34.Weydt P, Oeckl P, Huss A, et al. . Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann Neurol. 2016;79(1):152-158. [DOI] [PubMed] [Google Scholar]
- 35.Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases . El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293-299. [DOI] [PubMed] [Google Scholar]
- 36.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. . Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hübers A, Marroquin N, Schmoll B, et al. . Polymerase chain reaction and Southern blot-based analysis of the C9orf72 hexanucleotide repeat in different motor neuron diseases. Neurobiol Aging. 2014;35(5):1214.e1-1214.e6. [DOI] [PubMed] [Google Scholar]
- 38.Chiò A, Hammond ER, Mora G, Bonito V, Filippini G. Development and evaluation of a clinical staging system for amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2015;86(1):38-44. [DOI] [PubMed] [Google Scholar]
- 39.Kimura F, Fujimura C, Ishida S, et al. . Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology. 2006;66(2):265-267. [DOI] [PubMed] [Google Scholar]
- 40.Petzold A, Altintas A, Andreoni L, et al. . Neurofilament ELISA validation. J Immunol Methods. 2010;352(1-2):23-31. [DOI] [PubMed] [Google Scholar]
- 41.Robin X, Turck N, Hainard A, et al. . pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elamin M, Bede P, Byrne S, et al. . Cognitive changes predict functional decline in ALS: a population-based longitudinal study. Neurology. 2013;80(17):1590-1597. [DOI] [PubMed] [Google Scholar]
- 43.Menke RA, Gray E, Lu CH, et al. . CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann Clin Transl Neurol. 2015;2(7):748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lillo P, Mioshi E, Burrell JR, Kiernan MC, Hodges JR, Hornberger M. Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS One. 2012;7(8):e43993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burrell JR, Kiernan MC, Vucic S, Hodges JR. Motor neuron dysfunction in frontotemporal dementia. Brain. 2011;134(pt 9):2582-2594. [DOI] [PubMed] [Google Scholar]
- 46.Cerami C, Marcone A, Crespi C, et al. . Motor neuron dysfunctions in the frontotemporal lobar degeneration spectrum: a clinical and neurophysiological study. J Neurol Sci. 2015;351(1-2):72-77. [DOI] [PubMed] [Google Scholar]
- 47.Chiò A, Calvo A, Moglia C, Mazzini L, Mora G; PARALS study group . Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry. 2011;82(7):740-746. [DOI] [PubMed] [Google Scholar]
- 48.Turner MR, Gray E. Are neurofilaments heading for the ALS clinic? J Neurol Neurosurg Psychiatry. 2016;87(1):3-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Natural Logarithm Transformation of CSF NFL Concentration With Subsequent Normal Distribution of Data
eFigure 2. ROC Curves of NFL Concentrations for Discrimination Between ALS and Controls/FTD/MN, Between ALS and Controls, and Between ALS and MN/FTD
eFigure 3. Effect of Time From Onset to Lumbar Puncture on the Concentration of NFL in the ALS Group
eFigure 4. Effect of Disease Progression Rate From Onset to Lumbar Puncture (∆FS) on NFL Concentration in the ALS Group
eFigure 5. ALSFRS-R (A-B-C-D) and MITOS (E-F-G-H) Change Relative to Time (Months From Lumbar Puncture) by Subtypes
eTable. Characteristics of the ALS Group