Abstract
Importance
There are conflicting results claiming that Alzheimer disease signature neurodegeneration may be more, less, or similarly advanced in individuals with β-amyloid peptide (Aβ)–negative (Aβ−) suspected non–Alzheimer disease pathophysiology (SNAP) than in Aβ-positive (Aβ+) counterparts.
Objective
To examine patterns of neurodegeneration in individuals with SNAP compared with their Aβ+ counterparts.
Design, Setting, and Participants
A longitudinal cohort study was conducted among individuals with mild cognitive impairment (MCI) and cognitively normal individuals receiving care at Alzheimer’s Disease Neuroimaging Initiative sites in the United States and Canada for a mean follow-up period of 30.5 months from August 1, 2005, to June 30, 2015. Several neurodegeneration biomarkers and longitudinal cognitive function were compared between patients with distinct SNAP (Aβ− and neurodegeneration-positive [Aβ−N+]) subtypes and their Aβ+N+ counterparts.
Main Outcomes and Measures
Participants were classified according to the results of their florbetapir F-18 (Aβ) positron emission tomography and their Alzheimer disease–associated neurodegeneration status (temporoparietal glucose metabolism determined by fluorodeoxyglucose F 18 [FDG]–labeled positron emission tomography and/or hippocampal volume [HV] determined by magnetic resonance imaging: participants with subthreshold HV values were regarded as exhibiting hippocampal volume atrophy [HV+], while subthreshold mean FDG values were considered as FDG hypometabolism [FDG+]).
Results
The study comprised 265 cognitively normal individuals (135 women and 130 men; mean [SD] age, 75.5 [6.7] years) and 522 patients with MCI (225 women and 297 men; mean [SD] age, 72.6 [7.8] years). A total of 469 individuals with MCI had data on neurodegeneration biomarkers; of these patients, 107 were Aβ−N+ (22.8%; 63 FDG+, 82 HV+, and 38 FDG+HV+) and 187 were Aβ+N+ (39.9%; 135 FDG+, 147 HV+, and 95 FDG+HV+ cases). A total of 209 cognitively normal participants had data on neurodegeneration biomarkers; of these, 52 were Aβ−N+ (24.9%; 30 FDG+, 33 HV+, and 11 FDG+HV+) and 37 were Aβ+N+ (17.7%; 22 FDG+, 26 HV+, and 11 FDG+HV+). Compared with their Aβ+ counterparts, all patients with MCI SNAP subtypes displayed better preservation of temporoparietal FDG metabolism (mean [SD] FDG: Aβ–N+, 1.25 [0.11] vs Aβ+N+, 1.19 [0.11]), less severe atrophy of the lateral temporal lobe, and lower mean (SD) cerebrospinal fluid levels of tau (59.2 [32.8] vs 111.3 [56.4]). In MCI with SNAP, sustained glucose metabolism and gray matter volume were associated with disproportionately low APOE ε4 (Aβ–N+, 18.7% vs Aβ+N+, 70.6%) and disproportionately high APOE ε2 (18.7% vs 4.8%) carrier prevalence. Slower cognitive decline and lower rates of progression to Alzheimer disease (Aβ–N+, 6.5% vs Aβ+N+, 32.6%) were also seen in patients with MCI with SNAP subtypes compared with their Aβ+ counterparts. In cognitively normal individuals, neurodegeneration biomarkers did not differ between Aβ−N+ and Aβ+N+ cases.
Conclusions and Relevance
In MCI with SNAP, low APOE ε4 and high APOE ε2 carrier prevalence may account for differences in neurodegeneration patterns between Aβ−N+ and Aβ+N+ cases independent from the neuroimaging biomarker modality used to define neurodegeneration associated with Alzheimer disease.
This longitudinal cohort study examines patterns of neurodegeneration patterns in individuals with β-amyloid peptide–negative suspected non-Alzheimer disease pathophysiology compared with their β-amyloid peptide–positive counterparts.
Key Points
Question
Do individuals with β-amyloid peptide–negative suspected non–Alzheimer disease (AD) pathophysiology exhibit patterns of AD-associated neurodegeneration comparable to those of their β-amyloid peptide–positive counterparts?
Findings
In this longitudinal cohort study, individuals with β-amyloid peptide–negative suspected non-AD pathophysiology displayed significantly less temporoparietal hypometabolism and temporal lobe atrophy, which was associated with the patients’ disproportionately low APOE ε4 and disproportionately high APOE ε2 carrier prevalence.
Meaning
In mild cognitive impairment with suspected non-AD pathophysiology, the patients’ genetic status seems to account for the extent of AD signature neurodegeneration independent from the neuroimaging biomarker modality used to define neurodegeneration associated with AD.
Introduction
Suspected non–Alzheimer disease pathophysiology (SNAP) is a biomarker construct that comprises approximately 23% of cognitively normal (CN) people older than 65 years and a similar proportion of those with mild cognitive impairment (MCI). The SNAP construct is based on the National Institute on Aging–Alzheimer Association criteria, which designates individuals as β-amyloid peptide positive (Aβ+) or negative (Aβ−) and as positive (N+) or negative (N−) for a neurodegeneration pattern characteristic of Alzheimer disease (AD). Neurodegeneration biomarkers associated with AD that are used to classify individuals according to the National Institute on Aging–Alzheimer Association criteria include hypometabolism in AD-specific regions measured with fluorodeoxyglucose F 18–labeled (FDG) positron emission tomography (PET), atrophy in AD-specific regions, such as the hippocampus, measured with structural magnetic resonance imaging, and cerebrospinal fluid (CSF) measures of total tau (t-tau) and phosphorylated tau at threonine 181 (p-tau181p). Individuals categorized as having SNAP are positive for AD-associated neurodegeneration biomarkers but negative for β-amyloid biomarkers (as measured using amyloid PET or CSF). They are often designated as Aβ−N+.
All studies investigating the SNAP concept have consistently demonstrated that, compared with Aβ+N+ individuals, those who are Aβ−N+ possess significantly lower frequencies of apolipoprotein E (APOE) ε4 (OMIM 107741.0016) carriers. In CN individuals, as well as those with MCI and AD, APOE ε4 carrier status has been associated with neurodegeneration in the AD signature regions: inferior temporal, lateral parietal, and posterior cingulated and precuneus regions. This association suggests that, because of the relatively low prevalence of APOE ε4, individuals with SNAP might have less advanced AD signature neurodegeneration than do Aβ+N+ individuals, who possess a relatively high prevalence of APOE ε4 carriers. There are, however, conflicting results claiming that neurodegeneration may be more, less, or similarly advanced in Aβ−N+ individuals than in Aβ+N+ individuals. Frequency of APOE ε2 (OMIM 107741.0001) positivity is presumably associated with lower cerebral Aβ retention, and its link with cerebral neurodegeneration has so far not been examined in SNAP, to our knowledge.
We investigated the extent of changes of whole-brain glucose metabolism, gray matter volume, and concentrations of t-tau and p-tau181p in CSF to capture differences in the severity of neurodegeneration between various Aβ−N+ subgroups and their Aβ+ counterparts. Results were associated with the individuals’ APOE ε2 and APOE ε4 carrier status, focusing on the question of whether the genetics of those who are Aβ−N+ may drive their patterns of neurodegeneration. All analyses were performed in CN individuals, as well as those with early MCI and late MCI, who were receiving care at Alzheimer’s Disease Neuroimaging Initiative (ADNI) sites.
Methods
Participants
We included 265 CN individuals (mean [SD] age, 75.5 [6.7] years; 49.1% male), as well as 302 patients with early MCI and 220 with late MCI who were enrolled in ADNI GO or ADNI2. Full methodological information on participants, image acquisition, PET preprocessing, and CSF and data analysis are provided in the eAppendix in the Supplement. Results of APOE testing were dichotomized into APOE ε2 or APOE ε4 allele carrier (APOE ε2+ or APOE ε4+) or noncarrier (APOE ε2− or APOE ε4−) status. The florbetapir PET examination was considered as a baseline, and during a mean (SD) observation period of 30.5 (11.4) months from August 1, 2005, to June 30, 2015, cognitive function was assessed annually using the Alzheimer Disease Assessment Scale–Cognitive Subscale and the Rey Auditory Verbal Learning Test. Progression to probable AD was diagnosed at each center according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association criteria. All participants gave their written informed consent as approved by the institutional review board of each participating institution.
Florbetapir F-18–Labeled and FDG PET and Structural Magnetic Resonance Imaging
Florbetapir standardized uptake value ratios (SUVRs) were created from a volume-weighted mean of the mean florbetapir uptake from the gray matter of the lateral and medial frontal, anterior, and posterior cingulate; lateral parietal; and lateral temporal regions normalized to the cerebellar reference region (white and gray matter). Mean FDG, generated as a composite region-of-interest (ROI) measure from the mean of predefined meta-ROIs (right and left angular gyri, bilateral posterior cingulate, and right and left inferior temporal gyri), and voxelwise, spatially normalized FDG-PET results were intensity normalized using a pons and vermis reference region. Hippocampal volume (HV) estimated from 3-dimensional magnetization-prepared rapid acquisition and multiple gradient-echoes (MPRAGE) images with 3 Tesla magnetic field strength using FreeSurfer (Laboratory for Computational Neuroimaging at the Athinoula A. Martinos Center for Biomedical Imaging) was summed across hemispheres and adjusted by total intracranial volume. White matter hyperintensity volumes as a percentage of intracranial volume were calculated using fluid-attenuated inversion recovery and MPRAGE images as described previously. For voxel-based morphometry (VBM), gray matter MPRAGE images were warped to a study-specific mean template, spatially normalized to Montreal Neurological Institute (MNI) coordinate space, and smoothed with a 12-mm full-width at half maximum gaussian kernel.
Cerebrospinal Fluid
At baseline, 170 of 209 CN individuals with neurodegeneration biomarker data (81.3%) had CSF Aβ1-12 and p-tau181p, and 167 had t-tau available; in those with MCI, 431 of 469 patients with neurodegeneration biomarker data (91.9%) had Aβ1-42 and p-tau181p, and 414 had t-tau. Cerebrospinal fluid data were not used to classify participants but rather to assess tau as a marker of neurodegeneration.
Image Biomarker Cutoffs
Baseline florbetapir F-18 SUVR, mean FDG, and HV were the biomarkers of interest to classify CN individuals and patients with MCI as Aβ− or Aβ+ and as N− or N+. The threshold for a positive florbetapir F-18 SUVR was 1.11. Our neurodegeneration biomarkers of interest (mean FDG and HV) were classified as abnormal when their values were equal to or below the 90th percentile values of an ADNI AD cohort (n = 194; mean [SD] age, 75.1 [7.9] years; mean [SD] education, 15.9 [2.7] years; 115 male [59.3%]). Resulting cutoffs were 1.25 for mean FDG and 4.65 × 10−3 for the normalized HV units. Participants were classified as N+ if 1 or both biomarkers were abnormal; in additional analyses, they were further described as FDG+ if glucose metabolism was abnormal and as HV+ if HV was abnormal.
Statistical Analysis
Differences between (1) Aβ−N+, Aβ−FDG+, and Aβ−HV+ individuals and their Aβ+ counterparts, and (2) within the Aβ−N+ group between those who were APOE ε2− and those who were APOE ε2+ or between those who were APOE ε4− and those who were APOE ε4+ on 15 variables of interest were assessed using general linear models or logistic regression analysis adjusted for age, sex, and education. For the comparison of Aβ−N+APOE ε2− and Aβ−N+APOE ε2+ (or Aβ−N+APOE ε4− and Aβ−N+APOE ε4+) individuals, models were further adjusted for APOE ε4 (or APOE ε2) status. Bonferroni-corrected P ≤ .05/15 = 0.003 was deemed statistically significant.
To examine the effects of Aβ and APOE genotype, whole-brain voxelwise FDG analysis and whole-brain VBM were conducted, contrasting Aβ−N+, Aβ−FDG+, and Aβ−HV+ individuals with their Aβ+ counterparts, and within the Aβ−N+ group of individuals, contrasting APOE ε2− against APOE ε2+ individuals and APOE ε4− against APOE ε4+ individuals, using 2-sample t tests adjusted for age, sex, education, and intracranial volume (global normalization, for VBM only). Models were further adjusted for florbetapir SUVR (for contrasting APOE ε2 and APOE ε4 genotypes) or for APOE ε4 or APOE ε2 status (for contrasting the APOE ε2 or the APOE ε4 genotype). Clusters reported were corrected for multiple dependent comparisons at cluster-level P < .05 (voxelwise thresholding at P < .001 uncorrected, extent threshold k = 260 voxels).
Mixed-effects linear models adjusted for age, sex, and education (each including a random intercept) were conducted with group (main effect), time in years (main effect), and group × time in years (interaction effect) on the longitudinal Alzheimer Disease Assessment Scale–Cognitive Subscale or Auditory Verbal Learning Test. Group was included as a set of pairwise dummy variables (eAppendix in the Supplement). Bonferroni-corrected P ≤ .05/6 = 0.008 or P ≤ .05/4 = 0.01 (for the comparison of APOE genotypes) was deemed statistically significant. Statistical analysis was conducted using SPSS version 23.0 (SPSS Inc) and SPM12 in MATLAB R2015b (The MathWorks Inc).
Results
Patients With MCI
In all, 522 patients with MCI participated in the study. Of these, 225 were women and 297 were men (mean [SD] age, 72.6 [7.8] years). Neurodegeneration biomarker data were missing for 53 patients with MCI. The remaining 469 patients were classified as follows: 103 were Aβ−N− (22.0%), 72 were Aβ+N− (15.4%), 107 were Aβ−N+ (22.8%), and 187 were Aβ+N+ (39.9%). Of the Aβ−N+ group, 63 (58.9%) were FDG+, 82 (76.6%) were HV+, and 38 (35.5%) were FDG+HV+. Of the Aβ+N+ group, 135 (72.2%) were FDG+, 147 (78.6%) were HV+, and 95 (50.8%) were FDG+HV+.
Compared with their Aβ+ counterparts, individuals who were Aβ−N+, Aβ−FDG+, and Aβ−HV+ comprised more APOE ε2 carriers (Aβ−N+, 20 [18.7%]; Aβ+N+, 9 [4.8%]; Aβ−FDG+, 13 [20.6%]; Aβ+FDG+, 7 [5.2%]; Aβ−HV+, 16 [19.5%]; and Aβ+HV+, 6 [4.1%]) but fewer APOE ε4 carriers (Aβ−N+, 20 [18.7%]; Aβ+N+, 132 [70.6%]; Aβ−FDG+, 16 [25.4%]; Aβ+FDG+, 101 [74.8%]; Aβ−HV+, 12 [14.6%]; and Aβ+HV+, 103 [70.1%] and had higher mean (SD) FDG meta-ROIs (Aβ−N+, 1.25 [0.11]; Aβ+N+, 1.19 [0.11]; Aβ−FDG+, 1.18 [0.06]; Aβ+FDG+, 1.14 [0.08]; Aβ−HV+, 1.27 [0.08]; and Aβ+HV+, 1.20 [0.07]), lower mean (SD) CSF t-tau (Aβ−N+, 59.2 [32.8] pg/mL; Aβ+N+, 111.3 [56.4] pg/mL; Aβ−FDG+, 62.2 [37.2] pg/mL; Aβ+FDG+, 115.7 [58.3] pg/mL; Aβ−HV+, 58.3 [31.2] pg/mL; and Aβ+HV+, 111.3 [57.2] pg/mL) and p-tau181p levels (Aβ−N+, 25.0 [11.8] pg/mL; Aβ+N+, 53.9 [24.7] pg/mL; Aβ−FDG+, 26.1 [13.0] pg/mL; Aβ+FDG+, 56.7 [26.5] pg/mL; Aβ−HV+, 24.6 [10.9] pg/mL; and Aβ+HV+, 53.4 [23.5] pg/mL), and less cognitive impairment (Table).
Table. Baseline Variables in Aβ−N+ Subtypes of MCI Compared With Their Aβ+ Counterpartsa.
| Characteristic | Aβ−N+ (n = 107) |
Aβ+N+ (n = 187) |
P Value | Aβ−FDG+ (n = 63) |
Aβ+FDG+ (n = 135) |
P Value | Aβ−HV+ (n = 82) |
Aβ+HV+ (n = 147) |
P Value |
|---|---|---|---|---|---|---|---|---|---|
| Age at baseline florbetapir F-18 scan, mean (SD), y |
73.5 (7.6) | 74.8 (6.4) | .13 | 73.7 (8.0) | 74.3 (6.7) | .59 | 74.6 (7.6) | 75.3 (5.9) | .42 |
| Male sex, No. (%) | 66 (61.7) | 115 (61.5) | .97 | 40 (63.5) | 82 (60.7) | .69 | 49 (59.8) | 95 (64.6) | .52 |
| Education, mean (SD), y |
16.2 (2.6) | 16.1 (2.8) | .86 | 16 (2.6) | 16.2 (2.9) | .61 | 16.3 (2.6) | 16.3 (2.7) | .88 |
| Carriers, No. (%) | |||||||||
| APOE ε2 | 20 (18.7) | 9 (4.8) | <.001 | 13 (20.6) | 7 (5.2) | .002 | 16 (19.5) | 6 (4.1) | <.001 |
| APOE ε4 | 20 (18.7) | 132 (70.6) | <.001 | 16 (25.4) | 101 (74.8) | <.001 | 12 (14.6) | 103 (70.1) | <.001 |
| FDG, mean (SD), meta-ROIs |
1.25 (0.11) | 1.19 (0.11) | <.001 | 1.18 (0.06) | 1.14 (0.08) | .001 | 1.27 (0.08) | 1.20 (0.07) | <.001 |
| HV/ICV, mean (SD) | 4.4 × 10−3
(0.7 × 10−3) |
4.2 × 10−3
(0.6 × 10−3) |
.39 | 4.5 × 10−3
(0.9 × 10−3) |
4.3 × 10−3
(0.7 × 10−3) |
.22 | 4.1 × 10−3
(0.5 × 10−3) |
4.0 × 10−3
(0.5 × 10−3) |
.77 |
| CSF, mean (SD), pg/mL |
|||||||||
| t-tau | 59.2 (32.8) | 111.3 (56.4) | <.001 | 62.2 (37.2) | 115.7 (58.3) | <.001 | 58.3 (31.2) | 111.3 (57.2) | <.001 |
| p-tau181p | 25.0 (11.8) | 53.9 (24.7) | <.001 | 26.1 (13.0) | 56.7 (26.5) | <.001 | 24.6 (10.9) | 53.4 (23.5) | <.001 |
| WMH/ICV, mean (SD) |
5.6 × 10−3
(6 × 10−3) |
6.5 × 10−3
(7.3 × 10−3) |
.69 | 5.5 × 10−3
(6 × 10−3) |
6.8 × 10−3
(7.5 × 10−3) |
.23 | 6.1 × 10−3
(6.2 × 10−3) |
6.5 × 10−3
(7.5 × 10−3) |
.96 |
| Arterial hypertension, No. (%) |
57 (53.3) | 100 (53.5) | .76 | 36 (57.1) | 73 (54.1) | .71 | 45 (54.9) | 76 (51.7) | .49 |
| Alzheimer disease, No. (%) |
|||||||||
| Family history | 33 (30.8) | 56 (29.9) | .97 | 22 (34.9) | 40 (29.6) | .36 | 26 (31.7) | 44 (29.9) | .87 |
| Progression | 7 (6.5) | 61 (32.6) | <.001 | 6 (9.5) | 49 (36.3) | .001 | 6 (7.3) | 53 (36.1) | <.001 |
| Baseline score, mean (SD) |
|||||||||
| ADAS-Cog | 8.4 (3.9) | 11.2 (4.7) | <.001 | 9.1 (4.2) | 11.7 (4.8) | <.001 | 8.8 (3.9) | 11.6 (4.7) | <.001 |
| RAVLT | 38.6 (10.6) | 32.6 (9.6) | <.001 | 38.1 (10.4) | 32.1 (9.9) | <.001 | 37.9 (10.1) | 31.5 (8.5) | <.001 |
| Group × time on ADAS-Cog, mean (SD)b |
1.9 (0.2) | <.001 | 1.8 (0.6) | .003 | 2.1 (0.4) | <.001 | |||
| Group × time on RAVLT, mean (SD)b |
−1.9 (0.4) | <.001 | −2.2 (0.5) | <.001 | −2.0 (0.4) | <.001 | |||
Abbreviations: Aβ, β-amyloid; ADAS-cog, Alzheimer Disease Assessment Scale–Cognitive Subscale; CSF, cerebrospinal fluid; FDG, fluorodeoxyglucose F 18; HV, hippocampal volume; ICV, intracranial volume; MCI, mild cognitive impairment; N, neurodegeneration; p-tau181p, tau phosphorylated at threonine 181; RAVLT, Rey Auditory Verbal Learning Test; ROIs, regions of interest; t-tau, total tau; WMH, white matter hyperintensity.
Bonferroni-adjusted P values are significant at .003 or less (for cross-sectional analysis) or at .01 or less (for longitudinal analysis). Statistical models were adjusted for age, sex, and education.
For the interaction effects, estimates are provided.
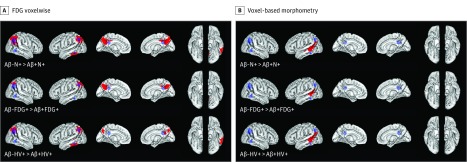
In the voxelwise FDG analysis, Aβ−N+ individuals displayed better preserved parietal and temporal glucose metabolism overlapping with the FDG meta-ROIs compared with Aβ+N+ individuals (Figure 1). Significant cluster peak voxels were found in the bilateral precuneus and the left inferior temporal gyrus of Aβ−N+ individuals (Figure 1A and eTable 1 in the Supplement). In the VBM analysis, Aβ−N+ individuals had higher temporal gray matter volume than did Aβ+N+ individuals (Figure 1B), with significant cluster peak voxels found in the left middle temporal gyrus (eTable 1 in the Supplement). Compared with FDG− and HV− individuals, FDG+ and HV+ individuals displayed similar neurodegeneration patterns (Figure 1 and eTable 1 in the Supplement).
Figure 1. Patterns of Neurodegeneration in Individuals With β-Amyloid Peptide–Negative (Aβ−) and Neurodegeneration-Positive (N+) Subtypes of Mild Cognitive Impairment Compared With Their Aβ+ Counterparts.
A, Brain surface images demonstrating the results of 3 contrasts in the whole-brain fluorodeoxyglucose F 18–labeled (FDG) voxelwise analysis. B, Brain surface images demonstrating the results of 3 contrasts in voxel-based morphometry. Red indicates clusters that met the significant cluster-level threshold of P < .05 corrected (voxelwise threshold P < .001 uncorrected, k = 260 voxels; see eTable 1 in the Supplement for peak voxel cluster region demonstration). The blue regions of interest (ROIs) represent prespecified meta-ROIs (bilateral inferior temporal gyrus, bilateral angular gyrus, and bilateral posterior-cingulate precuneus region) used to create mean FDG as a composite measure. There were no regions in which Aβ−N+ individuals showed lower FDG metabolism and lower gray matter volume than did Aβ+N+ individuals (reverse contrasts in eFigure 1 in the Supplement). HV indicates hippocampal volume.
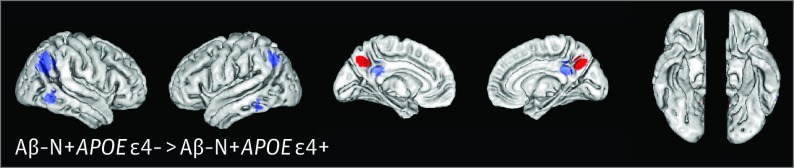
Within only the Aβ−N+ individuals, APOE ε4− compared with APOE ε4+ cases revealed better sustained parietal glucose metabolism close to the FDG meta-ROIs (Figure 2), with significant cluster peak voxels found in the bilateral precuneus (eTable 1 in the Supplement), but VBM results did not differ significantly. On a voxelwise level (for FDG and VBM), no differences were found when comparing APOE ε2− and APOE ε2+ cases.
Figure 2. Patterns of Glucose Metabolism in β-Amyloid Peptide–Negative (Aβ−) and Neurodegeneration-Positive (N+) Noncarriers of APOE ε4 With Mild Cognitive Impairment Noncarriers Compared With Aβ−N+ Carriers of APOE ε4.
Brain surface images demonstrate the results of whole-brain fluorodeoxyglucose F 18–labeled (FDG) voxelwise analysis in Aβ−N+ noncarriers of APOE ε4 contrasted against Aβ−N+ carriers of APOE ε4. Red indicates clusters that met the significant cluster-level threshold of P < .05 corrected (voxelwise threshold P < .001 uncorrected, k = 260 voxels; eTable 1 in the Supplement). The blue regions of interest (ROIs) represent prespecified meta-ROIs. There were no regions in which APOE ε4− individuals showed lower glucose metabolism than did APOE ε4+ individuals (reverse contrasts in eFigure 2 in the Supplement).
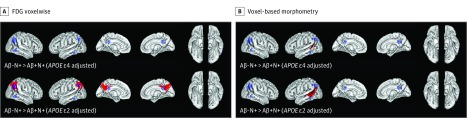
After including APOE ε4 status as an additional covariate in the voxelwise contrasts of Aβ−N+ vs Aβ+N+ individuals, we found that temporoparietal glucose metabolism was no longer preserved and that better sustained left middle temporal gray matter volume became remarkably smaller in the Aβ−N+ individuals (from 3209 to 890 voxels) (Figure 3). After including APOE ε2 status as an additional variable into the voxelwise FDG contrast, we found that group differences between Aβ−N+ and Aβ+N+ individuals remained significant in parietal, but not in lateral, temporal regions (Figure 1A and Figure 3A). However, for VBM, adjustment for APOE ε2 status did not affect significant group differences in the left middle temporal gyrus between Aβ−N+ and Aβ+N+ individuals (Figure 3B). Model adjustments for APOE ε2 or APOE ε4 status did not change the significant group differences in CSF t-tau and p-tau181p levels between Aβ−N+ and Aβ+N+ individuals.
Figure 3. APOE-Dependent Alzheimer Disease (AD) Signature Patterns of Neurodegeneration in Individuals With β-Amyloid Peptide–Negative (Aβ−) and Neurodegeneration-Positive (N+) Mild Cognitive Impairment (MCI).
A, Brain surface images demonstrating the results of whole-brain fluorodeoxyglucose F 18–labeled (FDG) voxelwise analysis. After adjustment for APOE ε4 carrier status, the glucose metabolism in the temporoparietal regions in Aβ−N+ vs Aβ+N+ patients with MCI was no longer preserved. After adjustment for APOE ε2 carrier status, sustained glucose metabolism in parietal but not in temporal AD signature regions remained significant in Aβ−N+ vs Aβ+N+ patients with MCI, as indicated by the red cluster peak voxels (cluster-level threshold P < .05 corrected; voxelwise threshold P < .001 uncorrected, k = 260 voxels). B, Brain surface images demonstrating the results of voxel-based morphometry. Significant cluster peak voxels (cluster-level threshold P < .05 corrected; voxelwise threshold P < .001 uncorrected, k = 260 voxels) indicating less gray matter volume atrophy in the left middle temporal gyrus in Aβ−N+ vs Aβ+N+ patients with MCI remained after additional adjustment for APOE ε4 or APOE ε2 carrier status. The extent of significant better preserved gray matter volume, however, decreased after APOE ε4 adjustment. The blue regions of interest (ROIs) represent prespecified meta-ROIs. There were no regions in which Aβ−N+ individuals showed lower FDG metabolism and lower gray matter volumes than did Aβ+N+ individuals after inclusion of APOE ε4 or APOE ε2 carrier status as additional variables (reverse contrasts in eFigure 3 in the Supplement).
Progression rates of AD were significantly lower in Aβ−N+, Aβ−FDG+, and Aβ−HV+ patients with MCI compared with their Aβ+ counterparts. In addition, Aβ−N+, Aβ−FDG+, and Aβ−HV+ individuals declined at an annual rate of 1.8 to 2.2 points slower than their Aβ+ counterparts (for the Alzheimer Disease Assessment Scale–Cognitive Subscale and Rey Auditory Verbal Learning Test) (Table).
Cognitively Normal Individuals
Neurodegeneration biomarker data were missing for 56 CN individuals. The remaining 209 CN individuals were classified as follows: 92 (44.0%) were Aβ−N−, 28 (13.4%) were Aβ+N−, 52 (24.9%) were Aβ−N+, and 37 (17.7%) were Aβ+N+. Of the Aβ−N+ group, 30 (57.7%) were FDG+, 33 (63.5%) were HV+, and 11 (21.2%) were FDG+HV+. Of the Aβ+N+ group, 22 (59.5%) were FDG+, 26 (70.3%) were HV+, and 11 (29.7%) were FDG+HV+ (eTable 2 in the Supplement).
Compared with Aβ+N+ individuals, Aβ−N+ individuals comprised fewer APOE ε4 carriers; there were no differences between Aβ−N+, Aβ−FDG+, and Aβ−HV+ and their Aβ+ counterparts or Aβ−N+APOE ε2− and Aβ−N+APOE ε2+ or Aβ−N+APOE ε4− and Aβ−N+APOE ε4+ individuals on any further variables (eTable 2 in the Supplement) and on voxelwise contrasts.
Discussion
Compared with Aβ+N+ patients with MCI, the SNAP MCI group had a lower proportion of APOE ε4 carriers but a greater proportion of APOE ε2 carriers and less severe abnormalities on neurodegeneration biomarkers associated with AD, such as glucose metabolism, brain volume, and CSF levels of p-tau181p or t-tau. The findings did not depend on the imaging biomarker modality used to define AD-specific patterns of neurodegeneration and were similarly detectable in those classified by glucose metabolism and HV. Better preserved glucose metabolism and gray matter volume were at least partly associated with the disproportionately low APOE ε4 and with the disproportionately high APOE ε2 carrier status in the group of Aβ−N+ patients with MCI. Less severe neurodegeneration may account for slower cognitive decline and lower rates of progression of AD in Aβ−N+ individuals than in Aβ+N+ patients with MCI. In CN participants, the severity of AD-associated neurodegeneration did not differ between Aβ−N+ and Aβ+N+ individuals.
These data replicate and complement previous findings in ADNI patients with MCI, demonstrating better preserved temporoparietal glucose metabolism in Aβ−N+ than in Aβ+N+ individuals using an ROI-based approach without statistical adjustment for APOE ε4 or APOE ε2 status. Compared with Aβ+N+ patients with MCI, however, participants with SNAP displayed not only glucose metabolism differences but also less severe neurodegeneration associated with AD using distinct biomarkers, such as lateral temporal gray matter atrophy or increased CSF levels of t-tau and p-tau181p (with the latter finding also having been reported in a previous study). Severity of HV atrophy was an exception, as it did not differ between Aβ−N+ and Aβ+N+ patients with MCI when using either ROI or voxelwise approaches. In patients with SNAP, better preserved temporoparietal metabolism and a higher volume of lateral temporal lobe gray matter in the presence of more severe HV atrophy may indicate decelerated neurodegeneration (tau) spread outside the medial temporal lobe in the absence of β-amyloid. Lower CSF levels of tau and better sustained glucose metabolism in patients with SNAP support the commonalities between those biomarkers. A recent imaging study in various AD phenotypes using 18F-AV-1451 as a PET ligand to detect tau in vivo confirms substantial overlap between greater tau tracer retention and reduced cortical glucose metabolism. Presumably, in Aβ−N+ compared with Aβ+N+ patients with MCI, lower levels of CSF tau in the presence of comparable HV loss may denote SNAP as a non-AD state. As has been demonstrated in exemplary autopsy cases, medial temporal lobe atrophy in patients with SNAP could be associated with hippocampal sclerosis, TDP43 pathologic conditions, or argyrophilic grain disease. This finding overall suggests a nonspecificity of neurodegeneration biomarkers, which could indicate a slightly different mix of non-AD conditions, such as cumulative ischemia, developmental factors, corticobasal degeneration, or primary progressive aphasia, especially in case of β-amyloid negativity. We found that patients with MCI and SNAP were not more likely to have vascular risk factors or white matter hyperintensity than were Aβ+N+ patients with MCI (which is also a replication of a previous finding in ADNI), making an association between neurodegeneration from SNAP and cerebrovascular disease unlikely.
On a voxelwise level, patterns of less severe neurodegeneration were comparable between subtypes of MCI with SNAP, whether they were selected through FDG meta-ROI hypometabolism or HV atrophy or whether they revealed an overlap on the 2 biomarker abnormalities. In other words, compared with their Aβ+ counterparts, Aβ−FDG+ individuals displayed the same patterns of gray matter volume differences as did Aβ−HV+ patients with SNAP, who in turn showed comparable glucose metabolism patterns as Aβ−FDG+ patients with MCI. Also, when contrasted with their Aβ+ counterparts, Aβ−FDG+ and Aβ−HV+ individuals had similar results with regard to demographics, genetics, cognitive function, and CSF tau concentrations. These data support the results of recent analyses demonstrating that the use of different measures of neurodegeneration (in our study, FDG meta-ROI hypometabolism and HV atrophy) to classify individuals as N+ provides quite similar information about those cases.
Compared with their Aβ+ counterparts, patients with MCI and SNAP showed fewer APOE ε4 but higher APOE ε2 carrier frequencies. Although APOE ε4 positivity is linked to decreased β-amyloid clearance and amyloid fibril formation, APOE ε2 carrier status is associated with higher rates of Aβ clearance. The APOE isoforms are, however, also associated with cognitive changes, such that APOE ε4 carriers show cognitive disturbances while APOE ε2 carriers reveal less cognitive decline. The constellation of differing frequencies of APOE isoforms in Aβ−N+ patients with MCI seems thus to substantially account for the β-amyloid negativity in patients with SNAP. Moreover, Aβ− and APOE ε4 negativity in the presence of APOE ε2 could be a powerful combination contributing to the deceleration of longitudinal cognitive decline in MCI with SNAP.
Several studies claim that there is an interaction between APOE ε4 and Aβ load on AD signature neurodegeneration. There is, however, additional evidence that APOE ε4 positivity itself is associated with differences in glucose metabolism and gray matter volume in AD signature regions, independent from cortical Aβ load. Indeed, APOE ε4 carrier status has directly been linked to neuronal degeneration; to impairment of axonal transport mechanisms, neuronal plasticity, and synaptogenesis; and to increased phosphorylation of tau. Those mechanisms seem to underlie biomarker abnormalities found in APOE ε4 carriers. Our data conversely demonstrate that APOE ε4 noncarrier status is associated with better preserved glucose metabolism and less gray matter atrophy in AD signature regions. Nevertheless, in MCI with SNAP, the link between APOE ε4 negativity and less severe neurodegeneration associated with AD is probably also associated with the patients’ β-amyloid negativity. In other words, less severe AD-signature neurodegeneration in MCI with SNAP most likely results from both independent and related effects of low APOE ε4 carrier frequencies and Aβ negativity.
Despite the general notion of associations between APOE ε2 positivity, reduced β-amyloid pathologic findings, and slower cognitive deterioration, there are still controversies about linking APOE ε2 carrier status and neurodegeneration associated with AD. In MCI with SNAP, the high frequencies of APOE ε2 carriers also seem to contribute to better sustained AD signature glucose metabolism, although these effects are less prominent than those of the APOE ε4 allele. Our findings contradict those of recent animal studies, which did not detect associations between APOE ε2 positivity and alterations of neurodegeneration markers. The association between APOE ε2 and glucose metabolism has to be considered in light of the amyloid negativity of the patients with MCI and SNAP, which itself is associated with APOE ε2 positivity and thus probably mediates preserved AD signature FDG metabolism in the APOE ε2 carriers.
Besides varying frequencies of APOE ε4 positivity, we did not capture any significant differences between CN Aβ−N+ and Aβ+N+ ADNI individuals on severity of neurodegeneration associated with AD, vascular risk factors, or white matter hyperintensity burden, which replicates previous findings of the Mayo Clinic Study of Aging cohort comparing CN participants with SNAP and their Aβ+ counterparts. When considering control participants from other cohorts, such as the Harvard Aging Brain Study or the Australian Imaging, Biomarker and Lifestyle study, CN Aβ+N+ vs CN Aβ−N+ individuals displayed faster cognitive decline and greater rates of progression of MCI and AD. Both studies comprised larger numbers of up to 573 participants observed for up to 8 years, which may account for the discrepancies.
Limitations
Our study has some limitations. It is possible that the amyloid status of patients with SNAP is a false-negative misclassification. This could be the case for participants revealing a constellation of Aβ negativity on results of PET but Aβ1-42 positivity in CSF, and vice versa, or for those turning β-amyloid positive during the time comprising participants displaying subthreshold Aβ levels. Second, our frequencies of Aβ+ APOE ε2 and Aβ− APOE ε4 carriers were low (especially in FDG+ and HV+ CN individuals), which limited the performance of further voxelwise contrasts between Aβ−FDG+APOE ε4− and Aβ−FDG+APOE ε4+ individuals. Those low frequencies may further have hindered the detection of significant voxelwise differences between APOE ε2+ and APOE ε2− patients with MCI and SNAP, especially as we applied a more conservative significance threshold.
Conclusions
Suspected non-Alzheimer disease pathophysiology is a biomarker-based concept commonly found in CN individuals and in patients with MCI. The increasing use of biomarkers to classify individuals according to their β-amyloid and neurodegeneration status will entail more frequent detection of Aβ−N+ individuals. There is thus a need to integrate patients with SNAP into a clinical and scientific context, especially in association with their Aβ+ counterparts. In this context, we provide pathophysiological insights to help researchers better understand the SNAP biomarker construct. These results indicate the importance of the genetic background of the individuals and the less severe neurodegenerative process and cognitive decline associated with SNAP.
eAppendix. Supplemental Material and Methods
eTable 1. Cluster Peak Voxels in MCI Aβ-N+ Individuals and in MCI Aβ-N+APOEε4 Noncarriers
eTable 2. Baseline Variables in Cognitively Normal Aβ-N+ Subtypes Compared With Their Aβ Positive Counterparts
eFigure 1. Patterns of Neurodegeneration in Individuals With β-Amyloid Peptide–Positive (Aβ+) and Neurodegeneration-Positive (N+) Subtypes of Mild Cognitive Impairment Compared With Their Aβ- Counterparts
eFigure 2. Patterns of Glucose Metabolism in β-Amyloid Peptide–Negative (Aβ−) and Neurodegeneration-Positive (N+) Carriers of APOE ε4 With Mild Cognitive Impairment Noncarriers Compared With Aβ−N+ Noncarriers of APOE ε4
eFigure 3. APOE-Dependent Alzheimer Disease (AD) Signature Patterns of Neurodegeneration in Individuals With β-Amyloid Peptide–Negative (Aβ−) and Neurodegeneration-Positive (N+) Mild Cognitive Impairment (MCI)
eReferences.
References
- 1.Jack CR Jr, Knopman DS, Chételat G, et al. Suspected non-Alzheimer disease pathophysiology—concept and controversy. Nat Rev Neurol. 2016;12(2):117-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. [DOI] [PubMed] [Google Scholar]
- 3.Alcolea D, Martínez-Lage P, Sánchez-Juan P, et al. Amyloid precursor protein metabolism and inflammation markers in preclinical Alzheimer disease. Neurology. 2015;85(7):626-633. [DOI] [PubMed] [Google Scholar]
- 4.Caroli A, Prestia A, Galluzzi S, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Mild cognitive impairment with suspected nonamyloid pathology (SNAP): prediction of progression. Neurology. 2015;84(5):508-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack CR Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging–Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71(6):765-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack CR Jr, Wiste HJ, Weigand SD, et al. Age-specific population frequencies of cerebral β-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: a cross-sectional study. Lancet Neurol. 2014;13(10):997-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knopman DS, Jack CR Jr, Wiste HJ, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78(20):1576-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knopman DS, Jack CR Jr, Wiste HJ, et al. Brain injury biomarkers are not dependent on β-amyloid in normal elderly. Ann Neurol. 2013;73(4):472-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mormino EC, Betensky RA, Hedden T, et al. Synergistic effect of β-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014;71(11):1379-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12(10):957-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vos SJ, Verhey F, Frölich L, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain. 2015;138(pt 5):1327-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drzezga A, Riemenschneider M, Strassner B, et al. Cerebral glucose metabolism in patients with AD and different APOE genotypes. Neurology. 2005;64(1):102-107. [DOI] [PubMed] [Google Scholar]
- 13.Knopman DS, Jack CR Jr, Wiste HJ, et al. 18F-fluorodeoxyglucose positron emission tomography, aging, and apolipoprotein E genotype in cognitively normal persons. Neurobiol Aging. 2014;35(9):2096-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wisse LE, Butala N, Das SR, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Suspected non-AD pathology in mild cognitive impairment. Neurobiol Aging. 2015;36(12):3152-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serrano-Pozo A, Qian J, Monsell SE, Betensky RA, Hyman BT. APOEε2 is associated with milder clinical and pathological Alzheimer disease. Ann Neurol. 2015;77(6):917-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doraiswamy PM, Bieber F, Kaiser L, Krishnan KR, Reuning-Scherer J, Gulanski B. The Alzheimer’s Disease Assessment Scale: patterns and predictors of baseline cognitive performance in multicenter Alzheimer’s disease trials. Neurology. 1997;48(6):1511-1517. [DOI] [PubMed] [Google Scholar]
- 17.Rey A. L’Examen Clinique en Psychologie. Paris, France: Presses Universitaires de France; 1964. [Google Scholar]
- 18.Landau SM, Harvey D, Madison CM, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32(7):1207-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz C, Fletcher E, DeCarli C, Carmichael O. Fully-automated white matter hyperintensity detection with anatomical prior knowledge and without FLAIR. Inf Process Med Imaging. 2009;21:239-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landau SM, Lu M, Joshi AD, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann Neurol. 2013;74(6):826-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schöll M, Lockhart SN, Schonhaut DR, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89(5):971-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrie EC, Cross DJ, Galasko D, et al. Preclinical evidence of Alzheimer changes: convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol. 2009;66(5):632-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(pt 5):1551-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landau SM, Horng A, Fero A, Jagust WJ; Alzheimer’s Disease Neuroimaging Initiative . Amyloid negativity in patients with clinically diagnosed Alzheimer disease and MCI. Neurology. 2016;86(15):1377-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128(6):755-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Josephs KA, Duffy JR, Fossett TR, et al. Fluorodeoxyglucose F18 positron emission tomography in progressive apraxia of speech and primary progressive aphasia variants. Arch Neurol. 2010;67(5):596-605. [DOI] [PubMed] [Google Scholar]
- 28.Kim GH, Lee JH, Seo SW, et al. Hippocampal volume and shape in pure subcortical vascular dementia. Neurobiol Aging. 2015;36(1):485-491. [DOI] [PubMed] [Google Scholar]
- 29.Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: childhood socioeconomic status influences adult hippocampal size. Ann Neurol. 2012;71(5):653-660. [DOI] [PubMed] [Google Scholar]
- 30.Jack CR Jr, Wiste HJ, Weigand SD, et al. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain. 2015;138(pt 12):3747-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y, Mahley RW. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer's diseases. Neurobiol Dis. 2014;72(pt A):3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanekiyo T, Xu H, Bu G. ApoE and Aβ in Alzheimer’s disease: accidental encounters or partners? Neuron. 2014;81(4):740-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunz L, Schröder TN, Lee H, et al. Reduced grid-cell–like representations in adults at genetic risk for Alzheimer’s disease. Science. 2015;350(6259):430-433. [DOI] [PubMed] [Google Scholar]
- 34.Salvato G. Does apolipoprotein E genotype influence cognition in middle-aged individuals? Curr Opin Neurol. 2015;28(6):612-617. [DOI] [PubMed] [Google Scholar]
- 35.Shinohara M, Kanekiyo T, Yang L, et al. APOE2 eases cognitive decline during aging: clinical and preclinical evaluations [published online March 2, 2016]. Ann Neurol. 2016. doi: 10.1002/ana.24628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carbonell F, Charil A, Zijdenbos AP, Evans AC, Bedell BJ. β-Amyloid is associated with aberrant metabolic connectivity in subjects with mild cognitive impairment. J Cereb Blood Flow Metab. 2014;34(7):1169-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiang GC, Insel PS, Tosun D, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Impact of apolipoprotein E4–cerebrospinal fluid β-amyloid interaction on hippocampal volume loss over 1 year in mild cognitive impairment. Alzheimers Dement. 2011;7(5):514-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowe VJ, Weigand SD, Senjem ML, et al. Association of hypometabolism and amyloid levels in aging, normal subjects. Neurology. 2014;82(22):1959-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arenaza-Urquijo EM, Gonneaud J, Fouquet M, et al. Interaction between years of education and APOE ε4 status on frontal and temporal metabolism. Neurology. 2015;85(16):1392-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jagust WJ, Landau SM; Alzheimer’s Disease Neuroimaging Initiative . Apolipoprotein E, not fibrillar β-amyloid, reduces cerebral glucose metabolism in normal aging. J Neurosci. 2012;32(50):18227-18233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nosheny RL, Insel PS, Truran D, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Variables associated with hippocampal atrophy rate in normal aging and mild cognitive impairment. Neurobiol Aging. 2015;36(1):273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen RC, Wiste HJ, Weigand SD, et al. Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA Neurol. 2016;73(1):85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burnham SC, Bourgeat P, Doré V, et al. ; AIBL Research Group . Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer’s disease pathophysiology (SNAP) or Alzheimer’s disease pathology: a longitudinal study. Lancet Neurol. 2016;15(10):1044-1053. [DOI] [PubMed] [Google Scholar]
- 44.Mormino EC, Papp KV, Rentz DM, et al. Heterogeneity in suspected non–Alzheimer disease pathophysiology among clinically normal older individuals. JAMA Neurol. 2016;73(10):1185-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon BA, Blazey T, Su Y, et al. Longitudinal β-amyloid deposition and hippocampal volume in preclinical Alzheimer disease and suspected non–Alzheimer disease pathophysiology. JAMA Neurol. 2016;73(10):1192-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vos SJ, Gordon BA, Su Y, et al. NIA-AA staging of preclinical Alzheimer disease: discordance and concordance of CSF and imaging biomarkers. Neurobiol Aging. 2016;44:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplemental Material and Methods
eTable 1. Cluster Peak Voxels in MCI Aβ-N+ Individuals and in MCI Aβ-N+APOEε4 Noncarriers
eTable 2. Baseline Variables in Cognitively Normal Aβ-N+ Subtypes Compared With Their Aβ Positive Counterparts
eFigure 1. Patterns of Neurodegeneration in Individuals With β-Amyloid Peptide–Positive (Aβ+) and Neurodegeneration-Positive (N+) Subtypes of Mild Cognitive Impairment Compared With Their Aβ- Counterparts
eFigure 2. Patterns of Glucose Metabolism in β-Amyloid Peptide–Negative (Aβ−) and Neurodegeneration-Positive (N+) Carriers of APOE ε4 With Mild Cognitive Impairment Noncarriers Compared With Aβ−N+ Noncarriers of APOE ε4
eFigure 3. APOE-Dependent Alzheimer Disease (AD) Signature Patterns of Neurodegeneration in Individuals With β-Amyloid Peptide–Negative (Aβ−) and Neurodegeneration-Positive (N+) Mild Cognitive Impairment (MCI)
eReferences.