Abstract
The objectives of this study were to isolate and identify garlic endophytes, and explore the characteristics of dominant strains. Garlic endophytes were studied through phenotypical characterization and comparative sequence analysis of 16S rDNA based on culture‐dependent approaches. Representative strains inferred from 16S rDNA sequencing were selected for further identification by gyrA and rpoB gene loci and phylogenetic analysis based on concateneted house‐keeping sequences. Seven kinds of Bacillus were found from garlic and black garlic, respectively. Further studies demonstrated that the total bacteria and endophytes showed a sharp decrease firstly, followed by a rapid rise, then maintained at a certain level, and finally slowed down during the black garlic processing. B. subtilis, B. methylotrophicus, and B. amyloliquefaciens were the dominant strains. The selected strains were capable of fermenting glucose, lactose, sucrose, and garlic polysaccharide to produce acid but no gas, with a strong ability of heat resistance. The results indicated that there were a certain number of garlic endophytes during the black garlic processing, and Bacillus was the dominant strains under the conventional culture‐dependent methods. This report provided useful information for the presence and type of garlic endophytes during the black garlic processing, which were of great significance to study the formation mechanism and quality improvement of black garlic in the future, as well as the security of garlic powder.
Keywords: black garlic; endophytes; isolation, identification, Bacillus
1. INTRODUCTION
Endophytes can be defined as those microbes that colonize the internal tissues of healthy plants, showing no obvious external sign of infection or negative effect on their host. There have been a hundred years of history on the research of endophytes, with endophytes found in almost every plant studied (Ryan, Germaine, Franks, Ryan, & Dowling, 2008). Plant endophytes which coexist with host plants for a long term can produce a series of the same bioactive secondary metabolites as the host plants, such as antitumor bioactive substances, with great potential for medical, agricultural, and industrial exploitation (Kim et al., 2007).
Garlic (Allium sativum L), a member of the family Alliaceae, enjoys the reputation of “antibiotics grown out of the land” (Raghu, Lu, & Sheen, 2012). Rahman (2007) reported that fructose‐containing carbohydrates were the main component of dry garlic, followed by sulfur compounds, proteins, fibers, and free amino acids. Garlic has a wide range of purposes on account of high nutritional value and unique flavor, regarded as one of the daily best healthy food, as demonstrated by some researchers (Ban et al., 2009; Benkeblia, 2004; Kim, 2016; Raghu et al., 2012). Black garlic, a novel garlic deep‐processed product, is obtained by maintaining fresh garlic at high temperature and controlled humidity for a period of time without any additional additives (Bae, Cho, Won, Lee, & Park, 2014; Toledano‐Medina, Pérez‐Aparicio, Moreno‐Rojas, & Merinas‐Amo, 2016). After processing, the functional components of black garlic increased significantly, such as reducing sugar, polyphenols, organic acids, and β‐carboline alkaloids, giving a more powerful efficacy than garlic (Lu, Li, Qiao, Qiu, & Liu, 2016). There are many reports about the bioactivity of black garlic on health, such as antioxidation (Lee et al., 2009), antiaging (Lee & Kim, 2010), hypoglycemic activity (Seo, Gweon, Lee, Kang, & Kim, 2009), antitumor (Dong et al., 2014), immunity enhancement (Wang et al., 2010), etc.
A few researchers declare that black garlic is a type of fermented products made by spontaneous fermentation of whole garlic bulbs (Kim et al., 2012; Lee et al., 2011; Sato, Kohno, & Niwano, 2006). However, little was known about existence and role played by microorganisms during the black garlic processing. Several studies had shown that there were a certain number of endophytes in garlic, which were mainly identified as bacteria and fungi. Shentu, Zhan, Ma, Yu, and Zhang (2014) had isolated an endophytic fungus strain 0248 from garlic, identified as Trichoderma brevicompactum based on morphological characteristics and the nucleotide sequences of ITS1‐5.8S‐ITS2 and tef1, with a marked inhibitory activity on Rhizoctonia solani and Botrytis cinerea due to trichodermin. The separation of endophytes from garlic and its bacteriostatic effect were conducted by Wei, Liu, Li, and Zhou (2013), indicating that endophytic bacteria occupied the majority of strains isolated from garlic, with a strong ability of acid production. Nevertheless, there are few reports on the changes in microorganisms during the black garlic processing, occurred high temperature and high humidity.
In general, 16S rDNA gene, which is highly conserved throughout prokaryotic organisms, is regarded as a framework for modern bacterial classification, but it has often proved to be insufficient and show limited variation for the discrimination of closely related taxa (Chun & Bae, 2000). Protein‐coding genes with higher mutation rates have been used for the differentiation and identification of closely related taxa in supplement to 16S rDNA. The gyrA gene (coding for DNA gyrase subunit A) and rpoB gene (encoding the RNA polymerase β‐subunit), have been used as markers for bacterial accurate identification and for phylogenetic study of B. subtilis and related taxa, as demonstrated by some researchers (Chun & Bae, 2000; Palmisano, Nakamura, Duncan, Istock, & Cohan, 2001).
In this study, the endophytes in garlic and black garlic samples were separated, characterized and identified based on conventional morphological approaches and molecular biological approaches. 16S rDNA sequencing was used for the first identification of isolates. Representatives of the different types based on 16S rDNA sequencing were selected for further identification by gyrA and rpoB sequencing and phylogenetic analysis based on these concateneted house‐keeping sequences. Then properties of dominant endophytes were explored to discover the strains used for the black garlic processing. The results provided a insight into the presence and type of garlic endophytes, which contributed to the further research of black garlic formation mechanism and quality improvement, and clarified the source of Bacillus in garlic powder for the security.
2. MATERIALS AND METHODS
2.1. Samples
Garlic (Allium sativum L.) was purchased from Laiwu (Shandong, China) without disease, insect injury and mechanical damage, and stored in cold storage at −2 ± 0.5°C. Black garlic was prepared from garlic in the laboratory.
2.2. Isolation and purification of endophytes from garlic and black garlic
Healthy white garlic and finished black garlic were chosen, with the outermost epidermis removed. The presterilization procedure was conducted in a clean aseptic bench as follows: initial sterilization with 75% alcohol for 10 min, soak with 0.3% NaClO for 20 min, immersion with 75% alcohol for 20 min, rinse with sterile water for 20 min twice, dry for 5 min. The last flushing sterile water was spread over a petri dish containing culture medium for the beef extract peptone agar medium (BPA), potato dextrose agar medium (PDA), and Gauze's medium no. 1 (GAU). The petri dishes were incubated at 37, 28, and 28°C for 36 hr, respectively. Subsequent experiments were performed after sterile surface was confirmed (Wei et al., 2013).
The samples were inoculated using the following two methods:
Under aseptic conditions, samples treated with surface disinfection, including inner epidermis, clove inside, clove outside, and clove root of garlic and black garlic, were cut into 0.5 × 0.5 cm tissue block, respectively, and placed on the surface of the medium containing BPA, PDA, and GAU medium, as suggested by Cui, Pan, Zhang, Zhao, and Wei (2008). The petri dishes were incubated at 37, 28, and 28°C for 2, 5, and 2 days, respectively.
25 g of garlic and black garlic samples undergoing surface disinfection were homogenized with 225 ml of sterile saline (0.9% NaCl, w/v). The mixture was stood for 15 min subsequently, which was submitted to serial 10‐fold dilutions in sterile saline to 10−1, 10−2, and 10−3 suspension. The 0.25 ml aliquots of diluent were spread on the surface of plates containing BPA, PDA, and GAU medium, then incubated at 37, 28, and 28°C for 2, 5, and 2 days, respectively.
After colony growth, the single colonies were picked up to three corresponding medium, and cultured at 37°C for 72 hr (Biscola et al., 2013; Wei et al., 2013). All described experiments were performed in triplicate.
2.3. Phenotypical characterization of endophytes from garlic and black garlic
The colonies with distinct characteristics, including morphology, size, and color, were purified using streak plate method, with an incubation at 37°C. The screening strains were transferred to a slant with solid nutrient agar medium for bacteria, or solid Gauze's medium no. 1 for actinomyces, stored at 4°C for further use (Shen, Fan, & Li, 1999). The colony characteristics visually analyzed on solid medium when cultivated to 24–48 hr. Then, the purified isolates were conducted gram staining and spore straining as described by Benson (2002), as observed under oil microscope.
The physiological and biochemical tests were conducted, respectively, as described by Buchanan & Gibbons (1984). According to results obtained, the taxonomic status of strain was acquired referring to “Bergey's Manual of Systematic Bacteriology” and “Common Bacteria Manual System Identification.”
2.4. Phylogenic analysis of endophytes from garlic and black garlic
Genomic DNA was extracted using TIANamp bacteria DNA kit (Qiangen, Beijing) from 3 ml of overnight culture inoculated with a single colony according to the manufacturer's instructions, used as template for amplification of the 16S rDNA fragment (Ahmadova et al., 2013).
Molecular identification was performed using 16S rDNA universal primers of bacteria 27F: (5′‐AGAGTTTGATCCTGGTCAGAACGAACGCT‐3′) and 1492R: (5′‐TACGGCTACCTTGTTACGACTTCACCCC‐3′) as described by Goto, Omura, Hara, and Sadaie (2000). The PCR system (50 μl) was composed of 2.0 μl of template DNA, 25.0 μl of 2× Taq Master Mix, 2.0 μl of 10 μmol/L forward and reverse primer each, and 19 μl of RNase‐Free Water. PCR amplification was performed under the following conditions: initial denaturation at 94°C for 3 min, 34 cycles of denaturation at 94°C for 30 s, primer annealing at 56°C for 30 s and DNA extension at 72°C for 90 s. A final extension step was added at 72°C for 10 min. The amplified products were analyzed on 1.0% (w/v) agarose gels in 5× Tris‐acetate EDTA buffer for 30 min at 100 V and made visible by UV transillumination. After the amplification products were purified, nucleotide sequencing was carried out by Sangon Biotech Co. Ltd. (Shanghai, China).
The sequences obtained were spliced and analyzed using software DNA MAN 5.0, and compared with those available in GenBank database by Basic Local Alignment Search Tool (BLAST). Then, type strains found in List of prokaryotic names with standing in nomenclature (LPSN) database and strains with high similarity were used to construct phylogenetic trees with MEGA 5.0 based on Neighbor‐Joining method (Chelo, Zé‐Zé, & Tenreiro, 2007). To determine the support for each clade, bootstrap analysis was performed with 1,000 replications.
2.5. Changes in total bacteria and endophytes during the black garlic processing
Fresh garlic with a simple treatment was sealed into the vacuum bag, processed in the heating oven at 80°C for 15 days as described by Zhang, Li, Lu, Liu, and Qiao (2015). Samples were immediately sealed and put back to the heating oven after daily aseptic sampling was conducted. The total number of bacteria in black garlic samples was determined by plate count agar medium (PCA) without any presterilization procedures referring to national standards of GB 4789.2–2010. The number and type of endophytes were detected using BPA medium.
The morphological characterization and 16S rDNA identification were carried as described above for garlic endophytes which were already isolated and purified. The housekeeping gene of gyrB gene locus was applied to confirm the strains unable to be distinguished based on 16S rDNA sequencing. PCR amplification was performed using gyrB universal primers UP‐1S: (5′‐GAAGTCATCATGACCGTTCTGCA‐3′) and UP‐2Sr: (5′‐AGCAGGGTACGGATGTGCGAGCC‐3′) designed according to Wang, Lee, Tai, and Kasai (2007). The PCR reaction mixture was 50 μl by reference to 16S rDNA identification. The amplification program was conducted according to La Duc, Satomi, Agata, and Venkateswaran (2004). PCR amplification products were sequenced by Sangon Biotech Co. Ltd. (Shanghai, China) after analyzed on 1% (w/v) agarose gels.
2.6. Partial sequencing of the gyrA and rpoB genes and multilocus sequence analysis
A set of primers, gyrA‐f (5′‐CAGTCAGGAAATGCGTACGTCCTT‐3′) and gyrA‐r (5′‐CAAGGTAATGCTCCAGGCATTGCT‐3′), corresponding to B. subtilis gyrA positions 43–1,070, was used to amplify gyrA gene. A primer pair, rpoB‐f (5′‐AGGTCAACTAGTTCAGTATGGAC‐3′) and rpoB‐r (5′‐AAGAACCGTAACCGGCAACTT‐3′), corresponding to nucleotides 6–585 of B. subtilis rpoB gene, was PCR amplified. The reaction mixture and PCR profile were consistent 16S rDNA sequencing, except that annealing temperature turned into 60°C for gyrA gene. The resultant amplicons purified were sequenced using the same primers by Sangon Biotech Co. Ltd. (Shanghai, China). The sequences of gyrA and ropB genes were aligned with reference strains using multiple‐alignment program, CLUSTALW 7.0.9. For multilocus sequence analysis, the selection of nucleotide substitution models was essential with jModelTest V2.1.4 for consensus sequences and three nucleotide fragments were combined for a congruency test using PAUP 4.0b10. Then phylogenetic inferences of the datasets were performed using the Bayesian Inference algorithm with MrBayes 3.1.2 based on best‐fitting model, following Ki, Zhang, and Qian (2009) and Weng, Chiou, Lin, and Yang (2009).
2.7. Effect of pH and temperature on the growth of selected strains
The effect of pH on the growth of dominant strain was determined by adjusting the pH of aliquots (30 ml) of beef extract peptone liquid medium from 3.0 up to 10.0 (with increments of one pH unit) with 1 M HCl or 1 M NaOH. After medium sterilization, the strains were incubated at 37°C for 24 hr. The OD value (λ = 600 nm) was measured every 2 hr, with blank medium as a control group, and observed the formation of bacteria membrane at the same time.
The strains preactivated for 12 hr were inoculated into flasks containing 30 ml of beef extract peptone liquid medium presterilized, which were cultivated in a thermostatic shaker incubator at 20, 30, 40, 50, and 60°C, respectively. With blank medium as a control group, the OD value (λ = 600 nm) was measured every 2 hr up to 24 hr of incubation, and observed the membrane produced at the liquid surface simultaneously.
3. RESULTS
3.1. Isolation and purification of endophytes from garlic and black garlic
As shown in Table 1, a certain number of strains were found in garlic and black garlic, whose morphological characteristics were gray wrinkled and white smooth. The number of colonies isolated from garlic was higher than that of the black garlic, indicating that some changes had taken place in microbial flora during the black garlic processing, with a slight decrease in the number. The strains grown on BPA medium were the most abundant among the three media, indicating that endophytic bacteria were dominant bacteria in garlic and black garlic. A small number of white smooth colonies were found on GAU medium, probably due to the fact that a few bacteria were grown for lack of bacteriostatic agent. After colony purification and preliminary screening, a total of 27 endophytes were found in the garlic (DS1–DS14) and black garlic (BS1–BS13). The morphological characterization and molecular biological identification were performed for screening strains.
Table 1.
The colony growth of garlic and black garlic samples
| Medium | Samples | Colony growth | Colony‐forming unit CFU/g |
|---|---|---|---|
| Potato dextrose agar medium | Garlic | Gray wrinkled colonies, White smooth colonies | 140 |
| Black garlic | Gray wrinkled colonies, White smooth colonies | 120 | |
| Beef extract peptone agar medium | Garlic | Gray wrinkled colonies, White smooth colonies | 240 |
| Black garlic | Gray wrinkled colonies, White smooth colonies | 160 | |
| Gauze's medium | Garlic | White smooth colonies | 40 |
| Black garlic | White smooth colonies | 20 |
3.2. Phenotypical characteristics of endophytes from garlic and black garlic
Morphological characteristics of the strains showed that colonies of 27 strains on solid medium incubated in aerobic conditions at 37°C for 48 hr were 2–4 mm in diameter, round, white to off‐white, smooth or rough and wrinkled, with typical characteristics of bacterial colonies. Gram staining and spore staining were conducted, indicating that 27 strains isolated from garlic and black garlic were gram‐positive Bacillus, with the thalli rod‐shaped or short rod‐shaped under a microscope.
Details on the physiological and biochemical characteristics of 27 isolates were shown in Table 2. All strains were able to produce protease, and most of the strains could produce extracellular amylase except DS11 and BS10 strains. All strains were capable of fermenting glucose to produce acid, and a large amount of organic acid could be produced by certain strains, which might affect the flavor of black garlic. Indole was not found for all strains. There were significant differences in the V‐P test and citrate utilization test. Based on the above results, 27 strains were identified preliminarily as Bacillus sp. according to “Bergey's Manual of Systematic Bacteriology.”
Table 2.
The physiological and biochemical properties of bacteria DS1–DS14 and BS1–BS13
| (a) Experiment | Result | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DS1 | DS2 | DS3 | DS4 | DS5 | DS6 | DS7 | DS8 | DS9 | DS10 | DS11 | DS12 | DS13 | DS14 | |
| Starch hydrolysis | + | + | + | + | + | + | + | + | + | + | − | + | + | + |
| Gelatin liquefaction | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Glucose fermentation | ||||||||||||||
| Acidogenic | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Aerogenic | − | − | − | + | − | − | − | − | − | + | − | − | − | − |
| Indole test | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Methyl test | − | + | − | − | − | − | − | − | + | − | − | + | − | + |
| V–P test | + | − | + | + | + | + | + | + | + | + | + | + | + | + |
| Citrate utilization | + | + | + | − | + | + | − | + | + | − | + | + | − | + |
| (b) Experiment | Result | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BS1 | BS2 | BS3 | BS4 | BS5 | BS6 | BS7 | BS8 | BS9 | BS10 | BS11 | BS12 | BS13 | |
| Starch hydrolysis | + | + | + | + | + | + | + | + | + | − | + | + | + |
| Gelatin liquefaction | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Glucose fermentation | |||||||||||||
| Acidogenic | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Aerogenic | − | − | − | + | − | − | − | − | + | − | − | − | − |
| Indole test | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Methyl red test | − | + | − | − | − | − | − | + | − | − | + | − | + |
| V–P test | − | − | + | + | + | + | + | + | + | + | + | + | + |
| Citrate utilization | − | + | + | − | + | + | − | + | − | + | + | − | + |
+, positive; −, negative reaction.
3.3. Phylogenetic analysis of endophytes from garlic and black garlic
Sequences of the 16S rRNA gene are generally used as a framework for bacterial classification. In general, the similarity between sequences more than 98% could be considered as the same species (Vaishampayan et al., 2009). PCR amplification of all strains was good, with a single band at around 1,500 bp. BLAST homology analysis showed a first match with similarity above 99% with Bacillus for all datasets. The Neighbor‐Joining tree constructed using MEGA 5.0 indicated that 27 isolates could be categorized into eight groups. Among them, six isolates, respectively, were found to be phylogenetically related to B. subtilis and B. methylotrophicus, with 99% similarity in their 16S rDNA sequences, making B. subtilis sp. and B. methylotrophicus sp. the most dominant strains.
Considering phenotypical characteristics and phylogenetic analysis, all strains were successfully identified at the species level as shown in Table 3. There were six similar strains in garlic and black garlic, B. aryabhattai, B. methylotrophicus, B. altitudinis, B. siamensis, B. pumilus, and B. subtilis, respectively, indicating that these strains might existed in the processing of black garlic. B. thuringiensis was found in garlic but disappeared in black garlic, indicating that this strain could not survive in the processing of black garlic for lack of high temperature tolerance. B. macroides found in black garlic disappeared in garlic, which should be due to the microbial contamination from the environment. The above results showed that a certain number of garlic endophytes were present in garlic and black garlic, which all belonged to Bacillus.
Table 3.
The phylogenic analysis results of the endophytes from garlic and black garlic
| Strains | Results |
|---|---|
| DS1, DS8 | Bacillus thuringiensis sp. |
| DS2, BS2 | Bacillus altitudinis sp. |
| DS3, DS5, DS6, BS3, BS5, BS6 | Bacillus methylotrophicus sp. |
| DS4, DS10, BS4, BS9 | Bacillus aryabhattai sp. |
| DS7, DS13, BS7, BS12 | Bacillus siamensis sp. |
| DS9, DS12, DS14, BS8, BS11, BS13 | Bacillus subtilis sp. |
| DS11, BS10 | Bacillus pumilus sp. |
| BS1 | Bacillus macroides sp. |
3.4. Changes in total bacteria and endophytes during the black garlic processing
As shown in Table 4, there was a certain number of garlic endophytes during the black garlic processing. Next, the number of colonies (631 ± 243 CFU/g) on PCA medium was greater than that (139 ± 54 CFU/g) of BPA medium, indicating that there were other types of strains present on the surface of garlic except for endophytes.
Table 4.
The quantitative changes in total bacteria and endophytes during the black garlic processing
| Sample no | Time (day) | Temperature (°C) | The count of endophytes (CFU/g) | The count of total bacteria (CFU/g) |
|---|---|---|---|---|
| A | 0 | 80 | 240 | 1,220 |
| B | 1 | 80 | 40 | 140 |
| C | 2 | 80 | 180 | 760 |
| D | 3 | 80 | 160 | 800 |
| E | 4 | 80 | 180 | 840 |
| F | 5 | 80 | 160 | 640 |
| G | 6 | 80 | 180 | 760 |
| H | 7 | 80 | 180 | 800 |
| I | 8 | 80 | 200 | 720 |
| J | 9 | 80 | 140 | 580 |
| K | 10 | 80 | 120 | 600 |
| L | 11 | 80 | 100 | 520 |
| M | 12 | 80 | 100 | 460 |
| N | 13 | 80 | 60 | 360 |
| O | 14 | 80 | 100 | 580 |
| P | 15 | 80 | 80 | 320 |
The number of total bacteria and endophytes dropped sharply from an initial value (day 0) of 1,220 and 240 CFU/g to the lowest value (day 1) of 140 and 40 CFU/g during the black garlic processing (Figure 1), probably due to the lack of tolerance of soil microorganisms to high temperatures and sulfides (Avato, Tursi, Vitali, Miccolis, & Candido, 2000; Kim, Kim, & Yook, 2015). After adapting to high temperatures and sulfides, the number of total bacteria and endophytes increased rapidly due to the redifferentiation of some spores, which remained stable until the eighth day. From the ninth day, the number gradually decreased, which might be due to the increase in total phenol and total acid content and the decrease in moisture content, as well as the depletion of nutrients on the garlic surface. Throughout the process, the number of total bacteria and endophytes detected in individual days was not consistent with the overall trend. The reason might be that there were individual differences between the samples, and the microbiological culture‐dependent methods had some limitations.
Figure 1.
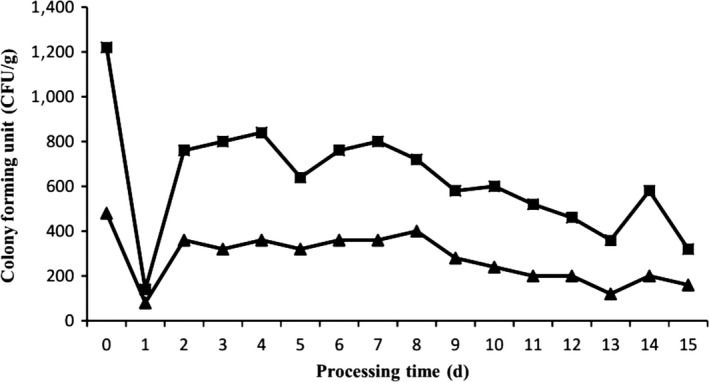
The number of total bacteria and endophytes during the black garlic processing. Fat contents: (■) total bacteria, (▲) endophytes.
3.5. Morphological characteristics of endophytes from the black garlic processing
Among the strains isolated and purified, a total of 78 endophytes were initially screened for further research, with eight different colony morphologies. Of these, most of the colonies were white, round, moist, smooth or off‐white, rough with irregular margins on BPA medium with the diameter from 2 to 4 mm after incubation (37°C, 24–48 hr), indicating that these forms of strains could withstand high temperature, significantly present in the black garlic processing. Microscopic analysis revealed that the majority of the isolates were endospore‐forming rods, so these isolates were expected to belong to Bacillus or related genera.
3.6. Identification of endophytes isolated from the black garlic processing
PCR amplification of all isolates was positive, with an average band length of 1,500 bp. After sequences were aligned with known sequences in the GenBank database, most strains could be identified successfully to the species level by the phylogenetic trees combined with colony morphological characteristics. However, several strains could not determine the specific species relationships due to the very high 16S rDNA/RNA gene affinity. The rate of molecular evolution inferred from gyrB gene sequences, a type II DNA topoisomerase, was faster than that inferred from 16S rDNA gene sequences, which could make up for the shortage of 16S rDNA gene (Wang et al., 2007). The Neighbor‐Joining tree revealed that 78 endophytes were found to be divided into 12 categories. Therefore, one strain from each category was selected for gyrB gene sequencing to ensure the accuracy of the identification results except for the above few strains that could not be identified by 16S rDNA sequencing.
Approximately 900 bp of the gyrB gene was successfully amplified for several strains according to gel electrophoresis. Trees derived from gyrB sequences based on GenBank database indicated that I6 belonged to B. amyloliquefaciens. Whereas the remaining strains belonged to B. subtilis. However, PCR amplification of the other strains failed despite adjusting the annealing temperature of 62, 65, 55, and 58°C. This might be due to the lack of primer specificity, which needed to redesign primers.
3.7. Multilocus sequence analysis
Since isolates identified as B. methylotrophicus, B. aryabhattai and members of the B. subtilis group on the basis of a first identification obtained with 16S rDNA sequencing were the main garlic endophytes and their identifications were insufficient, we applied gyrA and rpoB genes to discriminate these groups of isolates and obtain more reliable species affiliation. For each of these representative strains, approximate 950‐bp gyrA PCR product and 550‐bp rpoB PCR product were generated with species‐specific primer sets. The resultant partial gyrA and rpoB sequences were assembled and aligned manually using BLAST after sequencing, indicating that the gyrA and rpoB nucleotide sequences both showed much higher variations than the 16S rDNA sequences. The sequences were compared with those from Bacillus reference strains available from Agricultural Research Service Culture Collection Northern Regional Research Laboratory (NRRL) and Korean Collection for Type Cultures databases. Cluster analysis inferred from gyrA and rpoB nucleotide sequences revealed that the majority of identifications were consistent with 16S rDNA sequence analysis. Only very few isolates grouped with other Bacillus taxa unlike previous results. The optimal model of nucleotide substitution was GTR+I+G under the Akaike Information Criterion (AIC) principle by the calculation based on jModelTest V2.1.4 for consensus sequences. Phylogeny inferred from the concatenated housekeeping genes of 39 representative strains and reference strains using the Bayesian method revealed that 39 representative strains were distributed among seven clades of the Bayesian tree (Figure 2). Among the strains, the majority of isolates indentified based on 16S rDNA sequencing were clustered together, which belonged to known type strain, B. subtilis. In addition, the closely related taxa of B. subtilis group could be discriminated phylogenetically from each other, with a significant difference over 16S rDNA identification. For example, B. subtilis, B. sonorensis, and B. methylotrophicus were clearly differentiated. Most of strains were distinguishable clustering with the given reference strains, in accordance with the 16S rDNA identification. However, there were several inconsistencies between the multilocus sequence analysis and previously identified results. For example, K3 strain grouped with B. pumilus, whereas identified as B. aerophilus on the basis of 16S rDNA sequencing, which highlights the advantage of gyrA and rpoB sequence analysis to supplement 16S rRNA gene sequence analysis for efficient determination of closed related species. The results of consensus identification of garlic endophytes representatives based on the 16S rDNA, gyrA, gyrB, and rpoB genes were shown in Table 5, as well as a portion of the 16S rDNA alone identification.
Figure 2.
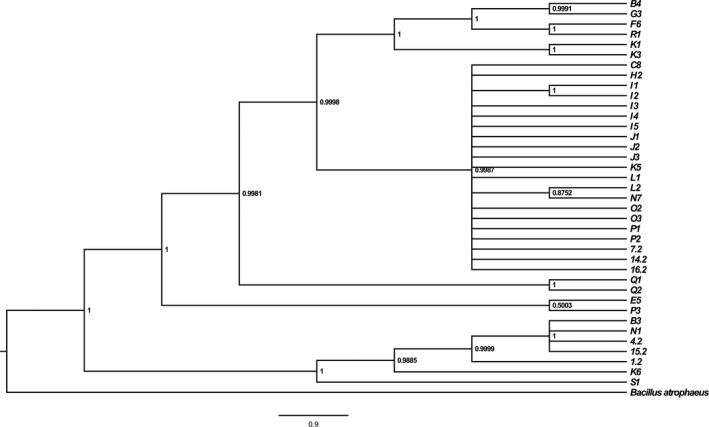
Bayesian trees inferred from the concatenated housekeeping genes (16S rDNA, gyrA, ropB), including 39 datasets determined in this study. These trees were constructed by the Bayesian method using MrBayes 3.1.2. B. atrophaeus was used as outgroup. The numbers at the nodes were posterior probabilities (PP).
Table 5.
Identification results of isolates from the black garlic processing
| Time | Code | Strains | Time | Code | Strains |
|---|---|---|---|---|---|
| Day 0 | A |
Staphylococcus epidermidis
Staphylococcus warneri Staphylococcus capitis Bacillus sonorensis Bacillus methylotrophicus Bacillus subtilis |
Day 8 | I |
Bacillus subtilis
Bacillus methylotrophicus Bacillus amyloliquefaciens |
| Day 1 | B |
Staphylococcus epidermidis
Staphylococcus warneri Bacillus methylotrophicus Bacillus amyloliquefaciens Bacillus megaterium Bacillus subtilis |
Day 9 | J |
Bacillus subtilis
Bacillus mojavensis |
| Day 2 | C |
Staphylococcus epidermidis
Bacillus subtilis |
Day 10 | K |
Bacillus pumilus
Bacillus altitudinis Bacillus subtilis Bacillus methylotrophicus |
| Day 3 | D | Staphylococcus epidermidis | Day 11 | L | Bacillus subtilis |
| Day 4 | E |
Staphylococcus epidermidis
Staphylococcus warneri Bacillus amyloliquefaciens |
Day 12 | M | Bacillus subtilis |
| Day 5 | F |
Staphylococcus epidermidis
Bacillus megaterium |
Day 13 | N |
Bacillus methylotrophicus
Bacillus amyloliquefaciens Bacillus subtilis |
| Day 6 | G |
Staphylococcus epidermidis
Bacillus subtilis |
Day 14 | O | Bacillus subtilis |
| Day 7 | H |
Staphylococcus epidermidis
Bacillus subtilis |
Day 15 | P |
Bacillus amyloliquefaciens
Bacillus subtilis |
The obtained results indicated that the majority of the isolates belonged to Bacillus and a minority of isolates were identified as species of nonendospore‐forming genus, Staphylococcus. Among them, Staphylococcus epidermidis was present in the first 7 days of processing, and there was no detection for the strain after the seventh day, which might be due to the reduction in moisture content and the increase in phenolic compounds with the processing of black garlic. In addition to individual days, B. subtilis existed throughout the black garlic processing, which was the most dominant strain under culture‐dependent approaches. This strain might have a important impact on the quality of black garlic. B. methylotrophicus and B. amyloliquefaciens were also present in the black garlic processing significantly, making them the second most dominant strains. They might also affect the quality of black garlic although less than B. subtilis. The remaining strains were present in individual days of the black garlic processing, indicating that they rarely existed in the garlic, which less affected the formation of black garlic.
3.8. Preliminary properties of selected strains from the black garlic processing
Four strains (F7, N1, N4, N7, respectively) of Bacillus were selected from the garlic endophytes obtained during the black garlic processing for subsequent experiments.
As shown in Table 6, four strains could produce gelatin hydrolase and extracellular amylase. In addition, all strains were capable of fermenting glucose, lactose, sucrose, and garlic polysaccharide, with a large amount of organic acid produced, which was of importance for the flavor of black garlic. There were significant differences in the V‐P test, litmus milk test, and citrate utilization test.
Table 6.
The physiological and biochemical properties of selected strains from the black garlic processing
| Experiment | Results | |||
|---|---|---|---|---|
| F7 | N1 | N4 | N7 | |
| Starch hydrolysis | + | + | + | + |
| Gelatin liquefaction | + | + | + | + |
| Glucose fermentation | ||||
| Acidogenic | + | + | + | + |
| Aerogenic | − | − | − | − |
| Lactose fermentation | ||||
| Acidogenic | + | + | + | + |
| Aerogenic | − | − | − | − |
| Sucrose fermentation | ||||
| Acidogenic | + | + | + | + |
| Aerogenic | − | − | − | − |
| Garlic polysaccharide fermentation | ||||
| Acidogenic | + | + | + | + |
| Aerogenic | − | − | − | − |
| Litmus milk test | − | + | + | + |
| Indole test | − | − | − | − |
| Methyl red test | + | + | + | + |
| V–P test | − | + | + | + |
| Citrate utilization | + | − | − | + |
+, positive; −, negative reaction.
Four strains of Bacillus were able to grow on the medium with an initial pH of 3–10 as shown in Table 7. Specifically, the optimum growth pH of F7 strain was 5, and it showed rapid growth in wide pH range from 5 to 7 for N1 strain, which was 5–8 for N4 and N7 strains. Besides, four strains were able to grow on the medium at different temperature from 20 to 60°C and optimum growth temperature was between 30 and 40°C. No growth was established at 70°C. Notably, N1, N4, and N7 strains had a general growth at 50°C. The above results indicated that these strains could tolerate high temperature and acidic conditions, which was of great significance for playing a role during the black garlic processing.
Table 7.
Growth of selected strains on different pH medium and different temperature
| (a) Strains | pH = 3 | pH = 4 | pH = 5 | pH = 6 | pH = 7 | pH = 8 | pH = 9 | pH = 10 |
|---|---|---|---|---|---|---|---|---|
| F7 | + | + | +++ | ++ | ++ | ++ | ++ | + |
| N1 | + | + | +++ | +++ | +++ | ++ | ++ | + |
| N4 | + | + | +++ | +++ | +++ | +++ | ++ | + |
| N7 | + | + | +++ | +++ | +++ | +++ | ++ | + |
| (b) Strains | 20°C | 30°C | 40°C | 50°C | 60°C | 70°C |
|---|---|---|---|---|---|---|
| F7 | ++ | +++ | +++ | + | + | − |
| N1 | ++ | +++ | +++ | ++ | + | − |
| N4 | ++ | +++ | +++ | ++ | + | − |
| N7 | ++ | +++ | +++ | ++ | + | − |
−, no growth; +, poor growth; ++, general growth; +++, better growth.
4. DISCUSSION
Black garlic was a newly processed food produced by maintaining fresh garlic at high temperature under controlled humidity condition for a long time (Liang et al., 2015), which was called fermented garlic by some researchers (Kim et al., 2012; Sato, Kohno, Hamano, & Niwano, 2006). However, there were few reports on the presence and role played by microbes during the black garlic processing. A series of studies were focused on the optimization of damp‐heat processing technology and the analysis of functional components. It was rarely related to the application of garlic endophytes as a fermenting agent to the black garlic processing (Ji et al., 2016). Therefore, the research on endophytes, which were used to accelerate the black garlic processing, enhance the black garlic flavor and functional substances and prolong the storage period, became a research hotspot.
Garlic polysaccharide, a fructans polysaccharides, was an important component of garlic as described by Wang, Huang, Zeng, and Wu (2004). During the black garlic processing, the fructans were decomposed gradually into small molecule carbohydrates, resulting in a significant increase in reducing sugar content and sweetness of black garlic (Zhang, Lei, et al., 2015). Bacillus, commonly found in soil, water sources and in association with plants, could withstand extreme environments and utilize a variety of carbon sources, enabling it to play a role in the processing of black garlic. It was reported that several strains, such as B. subtilis, B. aryabhattai, and B. coagulans had a strong capacity of acid production (Ramesh, Sharma, Sharma, Yadav, & Joshi, 2014; Wang, 2012), and the metabolites produced by certain Bacillus could form special flavor, such as alcohol aroma, sauce flavor and glutinous rice aroma (Cheng et al., 2014), which might have a significant impact on the flavor of black garlic. Meanwhile, some strains resembling B. subtilis and B. amyloliquefaciens could produce lipopeptide, peptide, and polyene substances, such as surfactin, iturin, and fengycin, which could inhibit the growth of pathogens (Kim et al., 2007; Raaijmakers, De Bruijn, Nybroe, & Ongena, 2010; Tan & Zou, 2001). Also, extracellular polysaccharides composed of mannose and glucose produced by several strains such as B. subtilis and B. amyloliquefaciens had a strong antioxidant activity (Yuan, Cai, Shan, Xu, & Wan, 2009). These active substances could significantly enhance the function of black garlic and extend the storage period, as well as the improvement of safety, which indicated that metabolic capabilities of Bacillus had important biotechnological applications. Accordingly, it was necessary to separate the endophytes in vitro and expand the culture for exploring the function of typical garlic endophytes due to their smaller number in garlic. Although there were many studies on Bacillus in recent years, our knowledge was incomplete and the studies remained in the initial stage. A better understanding of the identity and function of these garlic endophytes might provide information on the utilization of endophytes and the quality improvement of black garlic.
In summary, the report was the first to the isolate and identify garlic endophytes completely during the black garlic processing. The properties of dominant strains were investigated to obtain the target strains which could be applied to the black garlic processing. Our results provided theoretical evidence for the continued study of the formation mechanism of black garlic, and laid a foundation for the optimization of processing technology.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This project was supported by the National Natural Science Foundation of China (31371816), Special Fund for Agro‐scientific Research in the Public Interest of China (201303079) and Key R and D Program of Shandong Province (2016GNC113014).
Qiu Z, Lu X, Li N, Zhang M, Qiao X. Characterization of garlic endophytes isolated from the black garlic processing. MicrobiologyOpen. 2018;7:e547 https://doi.org/10.1002/mbo3.547
REFERENCES
- Ahmadova, A. , Todorov, S. D. , Choiset, Y. , Rabesona, H. , Zadi, T. M. , Kuliyev, A. , … Haertlé, T. (2013). Evaluation of antimicrobial activity, probiotic properties and safety of wild strain Enterococcus faecium AQ71 isolated from Azerbaijani. Motal cheese. Food Control, 30, 631–641. [Google Scholar]
- Avato, P. , Tursi, F. , Vitali, C. , Miccolis, V. , & Candido, V. (2000). Allylsulfide constituents of garlic volatile oil as antimicrobial agents. Phytomedicine, 7, 239–243. [DOI] [PubMed] [Google Scholar]
- Bae, S. E. , Cho, S. Y. , Won, Y. D. , Lee, S. H. , & Park, H. J. (2014). Changes in S‐allyl cysteine contents and physicochemical properties of black garlic during heat treatment. LWT‐Food Science and Technology, 55, 397–402. [Google Scholar]
- Ban, J. O. , Oh, J. H. , Kim, T. M. , Kim, D. J. , Jeong, H. S. , Han, S. B. , & Hong, J. T. (2009). Anti‐inflammatory and arthritic effects of thiacremonone, a novel sulfurcompound isolated from garlic via inhibition of NF‐κB. Arthritis Research & Therapy, 11, R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkeblia, N. (2004). Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). LWT‐Food Science and Technology, 37, 263–268. [Google Scholar]
- Benson, H. J. (2002). Microbiological applications: Laboratory manual in general microbiology. New York: McGraw‐Hill Higher Education. [Google Scholar]
- Biscola, V. , Todorov, S. D. , Capuano, V. S. C. , Abriouel, H. , Gálvez, A. , & Franco, B. D. G. M. (2013). Isolation and characterization of a nisin‐like bacteriocin produced by a Lactococcus lactis strain isolated from charqui, a Brazilian fermented, salted and dried meat product. Meat Science, 93, 607–613. [DOI] [PubMed] [Google Scholar]
- Buchanan, R. E. , & Gibbons, N. E. (1984). Bergey's manual of systematic bacteriology. Baltimore: William and Wilkens. [Google Scholar]
- Chelo, I. M. , Zé‐Zé, L. , & Tenreiro, R. (2007). Congruence of evolutionary relationships inside the Leuconostoc‐Oenococcus‐Weissella clade assessed by phylogenetic analysis of the 16S rRNA gene, dnaA, gyrB, rpoC and dnaK. International Journal of Systematic and Evolutionary Microbiology, 57, 276–286. [DOI] [PubMed] [Google Scholar]
- Cheng, W. , Wu, L. H. , Xu, Y. L. , Qin, J. Z. , Xie, G. P. , Wang, M. C. , & An, H. (2014). Research progress on brewing microbes in the production process of Luzhou‐flavour liquor. China Brew, 265, 1–4. [Google Scholar]
- Chun, J. , & Bae, K. S. (2000). Phylogenetic analysis of Bacillus subtilis and related taxa based on partial gyrA gene sequences. Antonie van Leeuwenhoek, 78, 123–127. [DOI] [PubMed] [Google Scholar]
- Cui, B. M. , Pan, Q. N. , Zhang, P. P. , Zhao, L. , & Wei, G. H. (2008). Isolation and identification of endogenetic bacteria and screening of their antagonistic bacteria in garlic. Acta Botanica Boreali‐Occidentalia Sinica, 11, 041. [Google Scholar]
- Dong, M. H. , Yang, G. Q. , Liu, H. C. , Liu, X. X. , Lin, S. X. , Sun, D. N. , & Wang, Y. S. (2014). Aged black garlic extract inhibits HT29 colon cancer cell growth via the PI3K/Akt signaling pathway. Biomedical Reports, 2, 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, K. , Omura, T. , Hara, Y. , & Sadaie, Y. (2000). Application of the partial 16S rDNA sequence as an index for rapid identification of species in the genus Bacillus. The Journal of General and Applied Microbiology, 46, 1–8. [DOI] [PubMed] [Google Scholar]
- Ji, Y. R. , Shi, J. , Wang, Y. M. , Liu, Y. F. , Dong, Y. , Yang, Q. L. , … Liu, Y. (2016). Separation and identification of endophytic bacteria strains and its increase effect in the production process of black garlic. Science and Technology of Food Industry, 1, 025. [Google Scholar]
- Ki, J. S. , Zhang, W. , & Qian, P. Y. (2009). Discovery of marine Bacillus species by 16S rRNA and rpoB comparisons and their usefulness for species identification. Journal of Microbiological Methods, 77, 48–57. [DOI] [PubMed] [Google Scholar]
- Kim, H. K. (2016). Garlic supplementation ameliorates UV‐induced photoaging in hairless mice by regulating antioxidative activity and MMPs expression. Molecules, 21, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. H. , Jung, E. Y. , Kang, D. H. , Chang, U. J. , Hong, Y. H. , & Suh, H. J. (2012). Physical stability, antioxidative properties, and photoprotective effects of a functionalized formulation containing black garlic extract. Journal of Photochemistry and Photobiology B: Biology, 117, 104–110. [DOI] [PubMed] [Google Scholar]
- Kim, S. Y. , Kim, J. Y. , Kim, S. H. , Bae, H. J. , Yi, H. , Yoon, S. H. , … Hong, S. Y. (2007). Surfactin from Bacillus subtilis displays anti‐proliferative effect via apoptosis induction, cell cycle arrest and survival signaling suppression. FEBS Letters, 581, 865–871. [DOI] [PubMed] [Google Scholar]
- Kim, D. , Kim, K. H. , & Yook, H. S. (2015). Analysis of active components of giant black garlic. Journal of the Korean Society of Food Science and Nutrition, 44, 1672–1681. [Google Scholar]
- La Duc, M. T. , Satomi, M. , Agata, N. , & Venkateswaran, K. (2004). gyrB as a phylogenetic discriminator for members of the Bacillus anthracis‐cereus‐thuringiensis group. Journal of Microbiological Methods, 56, 383–394. [DOI] [PubMed] [Google Scholar]
- Lee, E. N. , Choi, Y. W. , Kim, H. K. , Park, J. K. , Kim, H. J. , Kim, M. J. , … Yoon, S. (2011). Chloroform extract of aged black garlic attenuates TNF‐α‐induced ROS generation, VCAM–1 expression, NF‐κB activation and adhesiveness for monocytes in human umbilical vein endothelial cells. Phytotherapy Research, 25, 92–100. [DOI] [PubMed] [Google Scholar]
- Lee, Y. M. , Gweon, O. C. , Seo, Y. J. , Im, J. , Kang, M. J. , Kim, M. J. , & Kim, J. I. (2009). Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutrition Research and Practice, 3, 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. S. , & Kim, S. H. (2010). Safety evaluation of black garlic extract for development of cosmeceutical ingredients‐skin irritation and sensitization studies. Journal of the Korean Society of Food Science and Nutrition, 39, 1213–1219. [Google Scholar]
- Liang, T. F. , Wei, F. F. , Lu, Y. , Kodani, Y. , Nakada, M. , Miyakawa, T. , & Tanokura, M. (2015). Comprehensive NMR analysis of compositional changes of black garlic during thermal processing. Journal of Agricultural and Food Chemistry, 63, 683–691. [DOI] [PubMed] [Google Scholar]
- Lu, X. M. , Li, N. Y. , Qiao, X. G. , Qiu, Z. C. , & Liu, P. L. (2016). Composition analysis and antioxidant properties of black garlic extract. Journal of Food and Drug Analysis, 25, 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmisano, M. M. , Nakamura, L. K. , Duncan, K. E. , Istock, C. A. , & Cohan, F. M. (2001). Bacillus sonorensis sp. nov., a close relative of Bacillus licheniformis, isolated from soil in the Sonoran Desert, Arizona. International Journal of Systematic and Evolutionary Microbiology, 51, 1671–1679. [DOI] [PubMed] [Google Scholar]
- Raaijmakers, J. M. , De Bruijn, I. , Nybroe, O. , & Ongena, M. (2010). Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiology Reviews, 34, 1037–1062. [DOI] [PubMed] [Google Scholar]
- Raghu, R. , Lu, K. H. , & Sheen, L. Y. (2012). Recent research progress on garlic (大蒜 dà suàn) as a potential anticarcinogenic agent against major digestive cancers. Journal of Traditional and Complementary Medicine, 2, 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman, K. (2007). Effects of garlic on platelet biochemistry and physiology. Molecular Nutrition & Food Research, 51, 1335–1344. [DOI] [PubMed] [Google Scholar]
- Ramesh, A. , Sharma, S. K. , Sharma, M. P. , Yadav, N. , & Joshi, O. P. (2014). Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Applied Soil Ecology, 73, 87–96. [Google Scholar]
- Ryan, R. P. , Germaine, K. , Franks, A. , Ryan, D. J. , & Dowling, D. N. (2008). Bacterial endophytes: Recent developments and applications. FEMS Microbiol Letters, 278, 1–9. [DOI] [PubMed] [Google Scholar]
- Sato, E. , Kohno, M. , Hamano, H. , & Niwano, Y. (2006). Increased anti‐oxidative potency of garlic by spontaneous short‐term fermentation. Plant Foods for Human Nutrition, 61, 157–160. [DOI] [PubMed] [Google Scholar]
- Sato, E. , Kohno, M. , & Niwano, Y. (2006). Increased level of tetrahydro phytotherapy research‐β‐carboline derivatives in short‐term fermented garlic. Plant Foods for Human Nutrition, 61, 175–178. [DOI] [PubMed] [Google Scholar]
- Seo, Y. J. , Gweon, O. C. , Lee, Y. M. , Kang, M. J. , & Kim, J. I. (2009). Effect of garlic and aged black garlic on hyperglycemia and dyslipidemia in animal model of type 2 diabetes mellitus. Journal of Food Science and Nutrition, 14, 1–7. [Google Scholar]
- Shen, P. , Fan, X. R. , & Li, G. W. (1999). Microbiology experiment. Beijing: High Education. [Google Scholar]
- Shentu, X. P. , Zhan, X. H. , Ma, Z. , Yu, X. P. , & Zhang, C. X. (2014). Antifungal activity of metabolites of the endophytic fungus Trichoderma brevicompactum from garlic. Brazilian Journal of Microbiology, 45, 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, R. X. , & Zou, W. X. (2001). Endophytes: A rich source of functional metabolites. Natural Product Reports, 18, 448–459. [DOI] [PubMed] [Google Scholar]
- Toledano‐Medina, M. A. , Pérez‐Aparicio, J. , Moreno‐Rojas, R. , & Merinas‐Amo, T. (2016). Evolution of some physicochemical and antioxidant properties of black garlic whole bulbs and peeled cloves. Food Chemistry, 199, 135–139. [DOI] [PubMed] [Google Scholar]
- Vaishampayan, P. , Miyashita, M. , Ohnishi, A. , Satomi, M. , Rooney, A. , La Duc, M. T. , & Venkateswaran, K. (2009). Description of Rummeliibacillus stabekisii gen. nov., sp. nov. and reclassification of Bacillus pycnus Nakamura et al. 2002 as Rummeliibacillus pycnus comb. nov.. International Journal of Systematic and Evolutionary Microbiology, 59, 1094–1099. [DOI] [PubMed] [Google Scholar]
- Wang, X. (2012). Study on isolation, identification of lactic acid Bacillus and its preparation. Harbin, China: Northeast Agricultural University. [Google Scholar]
- Wang, D. N. , Feng, Y. H. , Yan, J. Z. , Wang, M. R. , Sasaki, J. I. , & Lu, C. L. (2010). Black garlic (Allium sativum) extracts enhance the immune system. Medical and Aromatic Plant Science Biotechnology, 4, 37–40. [Google Scholar]
- Wang, W. L. , Huang, X. S. , Zeng, L. S. , & Wu, J. Z. (2004). Advance on the polysaccharide of garlic (Allium sativum L.). Guangzhou Food Science and Technology, 20, 144–146. [Google Scholar]
- Wang, L. T. , Lee, F. L. , Tai, C. J. , & Kasai, H. (2007). Comparison of gyrB gene sequences, 16S rRNA gene sequences and DNA–DNA hybridization in the Bacillus subtilis group. International Journal of Systematic and Evolutionary Microbiology, 57, 1846–1850. [DOI] [PubMed] [Google Scholar]
- Wei, Z. Z. , Liu, M. Y. , Li, Z. , & Zhou, Y. J. (2013). Research on the separation and bacteriostasis of garlic endophytes. Heilongjiang Agricultural Sciences, 12, 003. [Google Scholar]
- Weng, F. Y. , Chiou, C. S. , Lin, P. H. P. , & Yang, S. S. (2009). Application of recA and rpoB sequence analysis on phylogeny and molecular identification of Geobacillus species. Journal of Applied Microbiology, 107, 452–464. [DOI] [PubMed] [Google Scholar]
- Yuan, J. F. , Cai, H. S. , Shan, X. Y. , Xu, C. X. , & Wan, H. G. (2009). Isolation and purification of exopolysaccharide from the fermentation broth of Bacillus sp. and its antioxidant effect. Microbiology China, 36, 1466–1470. [Google Scholar]
- Zhang, Z. S. , Lei, M. M. , Liu, R. , Gao, Y. F. , Xu, M. Y. , & Zhang, M. (2015). Evaluation of alliin, saccharide contents and antioxidant activities of black garlic during thermal processing. Journal of Food Biochemistry, 39, 39–47. [Google Scholar]
- Zhang, X. Y. , Li, N. Y. , Lu, X. M. , Liu, P. L. , & Qiao, X. G. (2015). Effects of temperature on the quality of black garlic. Journal of the Science of Food and Agriculture, 96, 2366–2372. [DOI] [PubMed] [Google Scholar]


