Abstract
Objectives
MEDI4893 is a novel, long‐acting human monoclonal antibody targeting Staphylococcus aureus (SA) alpha toxin (AT). This report presents the results of the exploratory analyses from a randomised phase 1 dose‐escalation study in healthy human subjects receiving single intravenous MEDI4893 doses or placebo.
Methods
Anti‐AT antibodies and AT expression were measured as described previously. Nasal swabs were analysed by culture and PCR. Data were summarised by treatment groups and visits by using SAS System Version 9.3.
Results
Subjects receiving 2250 or 5000 mg of MEDI4893 had the highest serum anti‐AT neutralising antibody (NAb) levels: approximately 180‐ to 240‐, 70‐ to 100‐ and sevenfold to 10‐fold higher than respective baseline levels at peak, 30 and 360 days, respectively. In these subjects, levels of serum anti‐AT NAbs were >3.2 International Units (IU) mL−1 for at least 211 days. In the upper respiratory tract, anti‐AT NAb levels increased with MEDI4893 dose. No apparent effect of MEDI4893 on SA nasal colonisation, hla gene sequence or AT expression was observed. Five AT variants were detected, their lytic activity was fully neutralised by MEDI4893.
Discussion
Our results indicate that (1) MEDI4893 administration at 2250 and 5000 mg would provide effective immunoprophylaxis against systemic SA disease; (2) MEDI4983 distributes to the upper respiratory tract and retains neutralising activity against AT; and (3) potential for emergence of MEDI4893 resistance is low.
Conclusion
Intravenous administration of MEDI4893 maintained levels of anti‐AT NAbs in serum and nasal mucosa that may provide effective immunoprophylaxis against SA disease and support continued clinical development of MEDI4893.
Keywords: alpha toxin, immunoprophylaxis, MEDI4893, Staphylococcus aureus
Introduction
Staphylococcus aureus is a major bacterial pathogen that causes various infections ranging from mild skin and soft‐tissue infections to serious invasive diseases such as endocarditis, osteomyelitis and necrotising pneumonia.1, 2, 3 Bacterial pneumonia is the second leading type of nosocomial infections and is the leading cause of death from nosocomial infection in the United States.4 Approximately one‐quarter of mechanically ventilated patients in intensive care units develop S. aureus pneumonia; half of these cases are caused by methicillin‐resistant S. aureus (MRSA).5
During infection, S. aureus releases a variety of different toxins. Alpha toxin (AT; also known as alpha haemolysin) is encoded by the hla gene and is a key virulence factor that contributes to S. aureus pathogenesis by causing tissue invasion and necrosis, promoting immune evasion and altering bacterial killing in macrophages.6, 7, 8, 9, 10, 11 Staphylococcus aureus strains deficient in AT expression are less virulent in animal models of dermonecrosis, pneumonia, sepsis, endocarditis and mastitis.11, 12, 13, 14, 15, 16, 17 Targeted neutralisation of AT could therefore prevent or limit S. aureus disease. This hypothesis is supported by studies that demonstrated a reduction in S. aureus disease severity in murine infection models after active or passive immunisation directed against AT.8, 11, 14, 18, 19, 20, 21 In addition, high levels of anti‐AT antibodies in patients with bacteremia, sepsis and endocarditis have been associated with improved outcomes.22, 23, 24
The emergence and spread of antibiotic‐resistant S. aureus strains complicate the management of S. aureus infections and highlight the need to consider novel approaches such as immunoprophylaxis. As AT plays an important role in S. aureus pathogenesis, pre‐emptive targeting of AT by a specific monoclonal antibody may prevent S. aureus disease in at‐risk populations, such as mechanically ventilated patients colonised with S. aureus in the lower respiratory tract. MEDI4893 is a potent and long‐acting human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that neutralises S. aureus AT.25 In animal models, MEDI4893 has been shown to prevent lethal S. aureus pneumonia, reduce the pathology associated with infection and accelerate pulmonary bacterial clearance even in the absence of concurrent antimicrobial therapy.26
Results from a phase 1, first‐in‐human, randomised, double‐blind, placebo‐controlled, dose‐escalation study demonstrated the safety and tolerability of MEDI4893. Pharmacokinetic analyses from this study demonstrated approximately dose‐dependent increases in MEDI4893 levels in serum and nasal wash after a single intravenous (IV) administration and a serum half‐life of 80–112 days.27 This report extends the findings of the phase 1 study with results of exploratory analyses evaluating the levels of anti‐AT antibody in serum and nasal wash samples and the effect of MEDI4893 administration on S. aureus colonisation status, bacterial load, AT expression, and hla gene and AT protein sequence.
Results
Levels of anti‐AT IgG and neutralising antibodies in serum
Serum anti‐AT IgG and anti‐AT neutralising antibodies were detected in all treatment groups prior to infusion, and their baseline levels were similar across all treatment groups. No significant changes in the baseline anti‐AT IgG and neutralising antibody levels were observed in the placebo group during the entire 360‐day follow‐up period, which suggests that AT‐specific humoural immunity remains stable in healthy individuals for a prolonged time.
Upon administration of MEDI4893, serum anti‐AT IgG and neutralising antibody levels increased; the highest levels were observed at the end of infusion. The magnitude of increase from the baseline to the peak levels correlated with MEDI4893 dose (Figure 1; Table 1). Thereafter, the concentrations declined in a biexponential manner through 360 days after dosing. In MEDI4893 cohorts 3 (2250 mg) and 4 (5000 mg), the serum anti‐AT neutralising antibody levels were approximately 70‐ to 100‐ and sevenfold to 10‐fold higher than baseline levels on days 31 and 361, respectively. In subjects that received either 2250 or 5000 mg of MEDI4893, the levels of serum AT neutralising antibodies exceeded the threshold of 3.2 IU mL−1 for at least 211 days after MEDI4893 administration.
Figure 1.
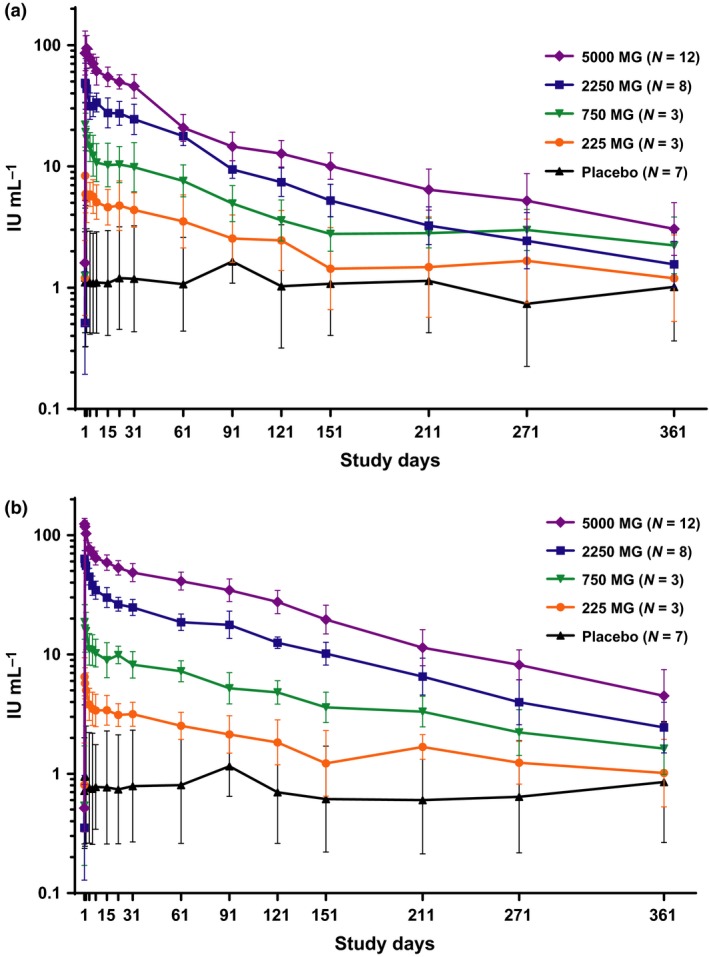
Anti‐alpha toxin IgG and neutralizing antibody levels in serum. Each sample was tested in duplicate. Anti‐AT IgG (a) and neutralising antibody (b) in IU per millilitre of serum samples were summarised in mean and standard deviation in log scale for each treatment group at each visit time point.
Table 1.
Geometric mean fold increase in serum anti‐alpha toxin neutralising levels over baseline
| Days | Placebo (n = 7) | 225 mg (n = 3) | 750 mg (n = 3) | 2250 mg (n = 8) | 5000 mg (n = 12) |
|---|---|---|---|---|---|
| 1 (pre‐infusion) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1 (end of infusion) | 1.3 | 8.1 | 34.7 | 179.4 | 240.5 |
| 1 (8 h postinfusion) | 1.3 | 7.1 | 30.6 | 164.3 | 230.9 |
| 2 | 1.1 | 6.2 | 29.1 | 156.7 | 193.9 |
| 4 | 1.1 | 4.7 | 20.5 | 127.2 | 147.7 |
| 6 | 1.0 | 4.3 | 20.1 | 106.9 | 138.1 |
| 8 | 1.1 | 4.2 | 19.0 | 97.9 | 126.6 |
| 15 | 1.1 | 4.2 | 16.6 | 85.0 | 115.7 |
| 22 | 1.0 | 3.9 | 18.3 | 67.4 | 104.8 |
| 31 | 1.1 | 3.9 | 15.2 | 70.1 | 96.4 |
| 61 | 1.1 | 3.1 | 13.4 | 52.7 | 81.1 |
| 91 | 1.1 | 2.6 | 9.6 | 50.2 | 69.5 |
| 121 | 1.0 | 2.3 | 8.9 | 35.5 | 54.5 |
| 151 | 0.9 | 2.2 | 6.7 | 28.8 | 40.9 |
| 211 | 0.9 | 2.1 | 6.1 | 18.5 | 21.0 |
| 271 | 1.0 | 1.5 | 4.1 | 11.3 | 16.5 |
| 361 | 1.3 | 1.3 | 3.0 | 6.9 | 9.7 |
Levels of anti‐AT IgG and neutralising antibodies in the upper respiratory tract
Anti‐AT IgG and anti‐AT neutralising antibody levels in the upper respiratory tract were measured on days 1 (MEDI4893 predose), 8 and 31. These antibody levels were MEDI4893 dose‐dependent and reached a plateau by day 8 (Figure 2). Compared with the baseline levels, the anti‐AT neutralising antibody levels in the nasal wash samples were approximately eightfold to ninefold and 30‐fold higher in MEDI4893 cohorts 3 (2250 mg) and 4 (5000 mg), respectively (Table 2).
Figure 2.
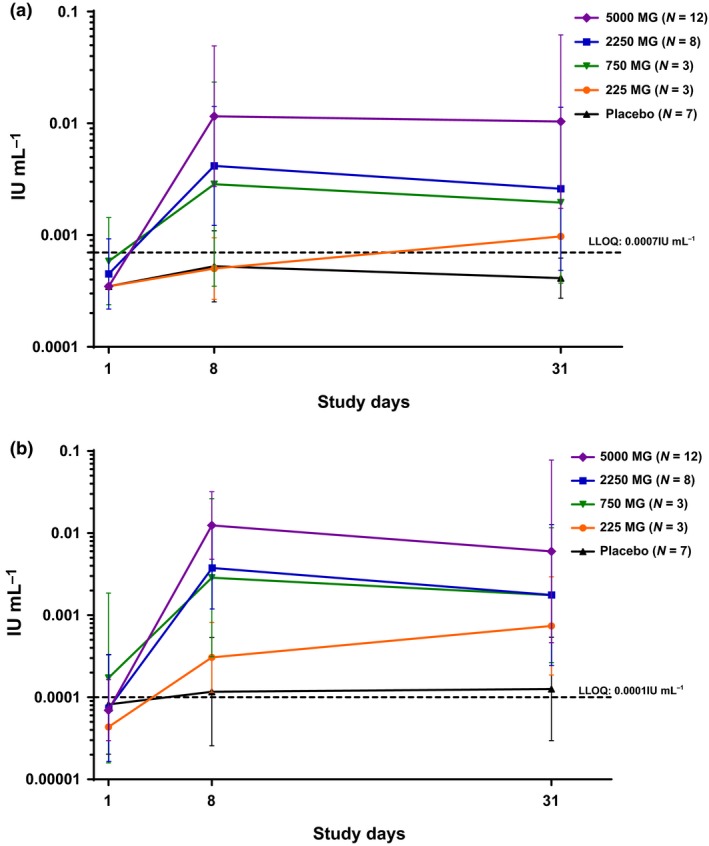
Anti‐alpha toxin IgG and neutralising antibody levels in the upper respiratory tract. Each sample was tested in duplicate. Anti‐AT IgG (a) and neutralising antibody (b) in IU per millilitre of nasal wash samples were summarised in mean and standard deviation in log scale for each treatment group at each visit time point. The dashed line represents the LLOQ of the assay, and LLOQ/2 was used for measurements below the LLOQ.
Table 2.
Geometric mean fold increase in nasal anti‐alpha toxin neutralising levels over baseline
| Days | Placebo (n = 7) | 225 mg (n = 3) | 750 mg (n = 3) | 2250 mg (n = 8) | 5000 mg (n = 12) |
|---|---|---|---|---|---|
| 8 | 1.2 | 1.4 | 4.9 | 9.3 | 33.3 |
| 31 | 1.2 | 2.8 | 3.4 | 7.5 | 29.8 |
Detection of Staphylococcus aureus nasal colonisation by PCR and culture
Both polymerase chain reaction (PCR) analysis and bacterial cultures were used to detect methicillin‐susceptible S. aureus (MSSA) and MRSA nasal colonisation in the different treatment groups. PCR detection was in agreement (96.3%) with the standard microbiological culture results. Approximately one‐third of the subjects in the MEDI4893 total group were colonised by MSSA at all time points evaluated (range, 26.9–37.5% as measured by PCR and 29.2–38.5% as measured by culture), whereas fewer subjects in the placebo group were colonised by MSSA (range, 0–16.7% as measured by both PCR and culture). No subjects were positive for MRSA colonisation in either assay. No significant differences in the basal levels of anti‐AT IgG and neutralising antibodies were observed between S. aureus‐colonised and noncolonised subjects (P > 0.05).
The changes in PCR cycle threshold (Ct) values and culture scores relative to baseline (pre‐infusion, day 1) were monitored over the course of 360 days (Figure 3). The results showed no consistent dose‐dependent MEDI4893‐mediated effect on nasal S. aureus colonisation.
Figure 3.
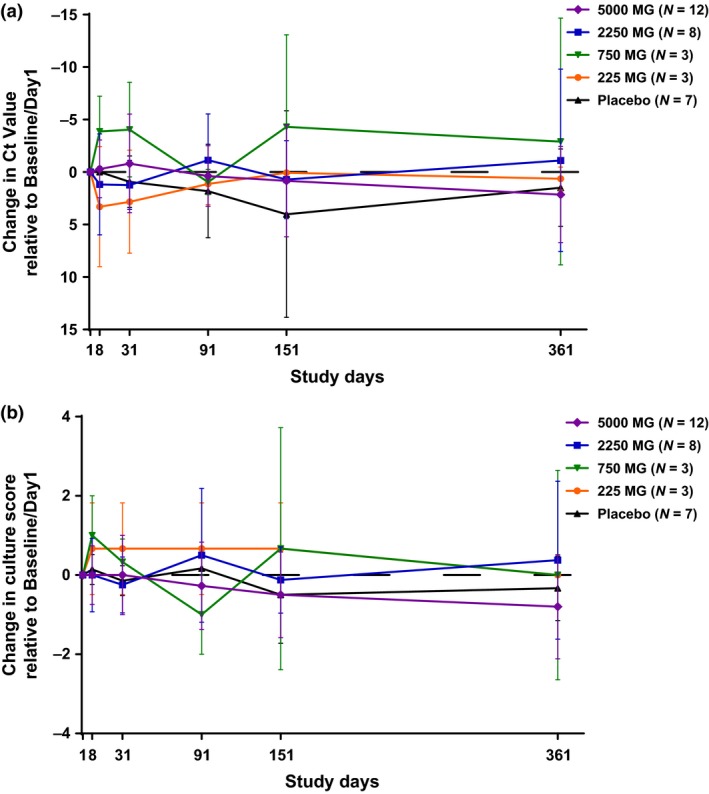
Changes in nasal Staphylococcus aureus colonisation relative to baseline in subject groups. Two nasal swabs, one from each nostril, were collected per study subject. One swab was used for S. aureus identification and enumeration by culture, another swab was used for Cepheid Xpert SA Nasal Complete PCR assay. Changes from the baseline measurement of S. aureus colonisation in PCR Ct values (a) and culture scores (b) of nasal swab samples were summarised in mean and standard deviation for each treatment group at each visit time point. The dashed line at 0 represents no change from baseline.
AT gene and protein sequence in Staphylococcus aureus nasal isolates
Alpha toxin gene was detected in 54 of 56 nasal isolates by PCR amplification. Based on the amino acid sequences, five AT subtypes were identified in S. aureus isolates obtained from 14 subjects enrolled in this study (Table 3). AT protein sequence was identical to the USA300 reference sequence (subtype 1) in 37% of isolates. Amino acid substitutions were detected at positions 78, 113, 135, 155, 234, 265 and 301 in subtypes 4, 11, 45 and 58. None of the substitutions were detected in the MEDI4893 binding region that encompasses amino acid residues 203 through 226 and 287 through 297, which is consistent with the reported data that this region is highly conserved among a diverse collection of S. aureus clinical isolates.28
Table 3.
Alpha toxin amino acid sequence subtypes from Staphylococcus aureus nasal isolates
| AT subtypea | Isolatesb (n = 54) | Subjectc (n = 14) | Amino acid position | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Count | % | Count | % | 78 | 113 | 135 | 234 | 301 | |
| USA300 (reference) | N/A | N/A | N/A | N/A | L | Q | T | D | I |
| 1 | 20 | 37 | 7 | 50 | L | Q | T | D | I |
| 4 | 7 | 13 | 2 | 14 | L | Q | T | D | T |
| 58 | 6 | 11 | 1 | 7 | L | Q | A | D | I |
| 11 | 16 | 30 | 5 | 36 | L | Q | T | E | T |
| 45 | 5 | 9 | 1 | 7 | I | B | T | D | I |
N/A, not applicable.
Subtypes 1, 4, 11 and 45 correspond to AT subtypes as defined previously.28 Subtype 58 is novel and extends the previously reported list of AT subtypes.28 Highlighted in bold are amino acid substitutions in subtypes 4, 11, 45 and 58 as compared to USA300 reference sequence (subtype 1). No amino acid changes are recorded downstream of stop codon at position 113 of subtype 45.
Two MSSA‐positive isolates did not yield AT‐specific PCR product; thus, AT sybtypes were not determined for those isolates.
Two subjects with isolates from two AT subtypes (subject 1097401023: AT subtypes 1 and 4; subject 1097401024: AT subtypes 1 and 11).
AT protein expression in Staphylococcus aureus nasal isolates
No major changes in AT protein expression levels were observed among S. aureus isolates through day 361 across all treatment groups (Figure 4).
Figure 4.
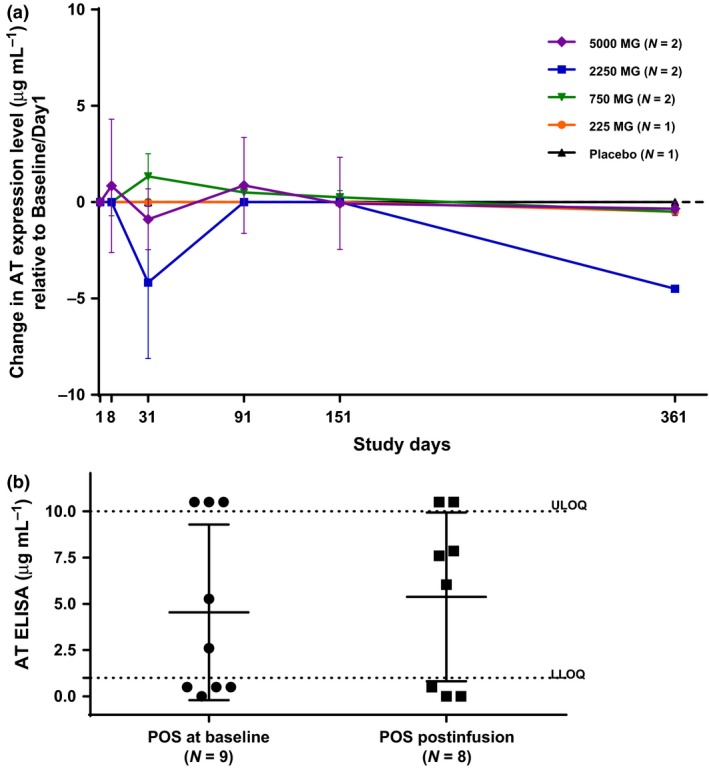
Alpha toxin protein expression in Staphylococcus aureus nasal isolates. In vitro AT expression was measured in the supernatants of overnight S. aureus cultures grown in TSB. Each sample was tested in duplicate. (a) For subjects who were S. aureus positive at baseline, changes of AT expression levels from the baseline were summarised in mean and standard deviation for each treatment group at each visit time point. The dashed line at 0 represents no change. (b) For subjects who were S. aureus negative at baseline but tested positive at postinfusion visits, the AT expression levels were compared with isolates from baseline. The dashed lines are the LLOQ (1 μg mL−1) and the upper limit of quantitation (ULOQ) (10 μg mL−1), where 10.5, 0.5 and 0 μg mL−1 were used for measurements greater than the ULOQ, positive (POS), or below the LLOQ, and negative, respectively.
Discussion
This randomised, dose‐escalation phase 1 study of MEDI4893 in healthy subjects evaluated AT‐specific IgG and neutralising antibody levels in serum and nasal wash samples after a single IV infusion, as well as the impact of MEDI4893 on S. aureus nasal colonisation, strain AT genotype and protein expression. Our results indicate that after MEDI4893 administration, anti‐AT neutralising antibody levels in serum increased by up to 240.5‐fold (MEDI4893 5000 mg cohort) compared with baseline and were maintained above the baseline levels for at least 121 days in all MEDI4893 cohorts. Previous studies have suggested that anti‐AT antibody levels of ≥ 3.2 IU mL−1 correlate with protection against S. aureus infections and represent ≥ 98th percentile value in a healthy human population.24 All subjects dosed with MEDI4893 had anti‐AT neutralising antibody levels above 3.2 IU mL−1 for at least 30 days after dosing. The neutralising antibody levels in subjects dosed with 2250 and 5000 mg were above 3.2 IU mL−1 for at least 211 days, suggesting that MEDI4893 administration at these doses would provide effective immunoprophylaxis against systemic S. aureus disease.
After a single IV administration of MEDI4893 in healthy subjects, anti‐AT neutralising antibody levels in the upper respiratory tract increased and reached a steady‐state equilibrium between serum and nasal mucosa. This indicates that MEDI4983 distributes to the upper respiratory tract and retains neutralising activity against AT. As expression of hla gene increases steadily upon transition from nasal colonisation to bacteremia and heart lesions in animal models,29 and elevated haemolytic activity by S. aureus strains is one of the risk factors that predict progression to ventilator‐associated pneumonia,30 sequestration of AT by MEDI4893 at the site of colonisation (e.g. nares) is expected to contribute to the prevention of infection and dissemination of S. aureus to the rest of the body.
Although increased AT expression accompanies the transition of S. aureus from a commensal to a pathogenic bacterium, hla expression in the nares is low, and deletion of the hla gene does not affect S. aureus nasal colony counts.29, 31 Exposure to MEDI4893 over the course of 360 days had no significant effect on nasal colonisation and caused no major changes in in vivo AT expression by S. aureus isolates. These results are consistent with published data showing that AT does not play a significant role in maintaining S. aureus nasal colonisation.
Because MEDI4893 targets the secreted toxin (AT) and not the pathogen (S. aureus) itself, any emergence of resistance would be likely to manifest as amino acid substitutions in AT that would compromise binding and neutralisation by MEDI4893. In the present study, five different AT subtypes were observed in S. aureus isolates, none of which possessed AT variants with amino acid substitutions in MEDI4893 binding region despite prolonged exposure of S. aureus strains to MEDI4893 due to its extended half‐life. However, it is possible that amino acid substitutions outside of the defined MEDI4893 binding region may change the conformation of the toxin and affect the MEDI4893–AT interaction as a consequence. Four of the S. aureus subtypes (subtypes 1, 4, 11 and 45) in this study were also detected in a recent global surveillance study of a large collection (n = 994) of S. aureus isolates; those AT variants possessed lytic activity that was fully neutralised by MEDI4893.28 Subtype 58 detected in this study is novel and, upon testing, demonstrated lytic activity that was fully neutralised by MEDI4893. The aforementioned global surveillance study showed that only 19 of 994 isolates (1.8%) harboured AT with substitutions in MEDI4893 binding region, of which only a single AT variant (G218V) had lytic activity not neutralised by MEDI4893. Because lytic activity from that particular isolate (subtype 10) was neutralised by polyclonal IgG directed against γ‐haemolysin and not neutralised by a polyclonal anti‐AT IgG, the lytic activity from subtype 10 was probably derived from γ‐haemolysin.28 Thus, the MEDI4893 molecular target is well conserved, and the potential for emergence of MEDI4893 resistance appears to be low.
Limitations of this study include a small subject sample size and lack of geographical diversity arising from recruitment at a single site in the United States. A larger and more geographically diverse phase 2 study is currently underway in Europe to evaluate the safety and efficacy of MEDI4893 in reducing the incidence of S. aureus pneumonia in mechanically ventilated patients. Data from this phase 2 study will extend the findings reported here, and we will continue to evaluate the impact of MEDI4893 on both the S. aureus pathogen and the human immune system.
Methods
Study design
This first‐in‐human, double‐blind, randomised, placebo‐controlled, dose‐escalation, phase 1 study was conducted at a single‐study centre in the United States (www.clinicaltrials.gov: NCT01769417). Thirty‐three healthy subjects were enrolled and randomly assigned to receive either MEDI4893, at 225 mg (n = 3); 750 mg (n = 3); 2250 mg (n = 8), or 5000 mg (n = 12), or placebo (n = 7). The subjects were followed for 360 days after the dosing.
Written informed consent was obtained from subjects before study procedures began. The institutional review board approved the study protocol and informed consent documents. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and the International Council for Harmonisation guidelines on Good Clinical Practice.
Characterisation of Staphylococcus aureus isolates
Nasal swabs, one from each nostril, were collected before nasal wash collection and transported in Universal Transport Medium tubes to International Health Management Associates, Inc, within 2 days of collection. Upon receipt, one nasal swab was used to inoculate a blood agar plate (tryptic soy agar with 5% sheep blood; Remel Microbiology Products, Lenexa, KS, USA) and streaked into four quadrants for S. aureus identification and enumeration.32 Plates were incubated at 37°C in an atmosphere of 5% CO2 for 18–24 h. Colonies with a staphylococcal morphology (smooth, butyrous colonies with a low convex edge and yellow to off‐white colour) were identified to the species level by matrix‐assisted laser desorption–ionisation time‐of‐flight mass spectrometry and evaluated for production of coagulase (BactiStaph Latex Agglutination Test Kit; Thermo Scientific, Waltham, MA, USA) and catalase (Gibson Biosciences, Lexington, KY, USA). In addition, isolates were screened for susceptibility to methicillin using the cefoxitin disc method (cefoxitin, 30 μg; BBL Sensi‐Disc; BD, Franklin Lakes, NJ, USA) according to Clinical Laboratory and Standards Institute guidelines.33 DNA was extracted from isolates identified as S. aureus for PCR amplification and sequencing of the hla gene as previously described.34 The AT protein sequences were obtained by translation of the consensus DNA sequences. Amino acid substitutions were identified using USA300 FPR3757 as a reference sequence.35 AT subtypes were assigned, and a novel AT variant (subtype 58) was tested for lytic activity and neutralisation by MEDI4893 as described previously.28
The second nasal swab was tested using the Xpert SA Nasal Complete PCR assay (Cepheid, Sunnyvale, CA, USA) according to the manufacturer's protocol.
In vitro AT expression was determined in the supernatants obtained from S. aureus cultures grown overnight in tryptic soy broth, as described previously.34 Purified AT was used as a standard (0.01–10 ng mL−1). An overnight culture supernatant prepared from a Δhla strain and diluted 1:100 000 into tryptic soy broth was used as a negative control (blank) for AT detection and also as the diluent for purified control AT protein used for quality control and protein quantitation. The sample was positive if the optical density readout was at least three standard deviations above the negative controls. The quantification range for a positive sample was between 1 and 10 μg mL−1.
Anti‐AT IgG and neutralising antibody levels in human serum and upper respiratory tract (nasal wash)
Blood samples were collected on days 1, 2, 4, 6, 8, 15, 22, 31, 61, 91, 121, 151, 211, 271 and 361. Quantification of anti‐AT IgG and neutralising antibodies in serum samples was performed as described previously using an enzyme‐linked immunosorbent assay (ELISA) and a red blood cell‐based AT neutralisation assay, respectively.34 Nasal wash samples were collected on the same dates as blood samples, except for day 6. Bioanalytical methods to quantify anti‐AT IgG and neutralising antibodies in human serum samples were adapted and modified to quantify anti‐AT IgG and neutralising antibodies in the nasal wash samples.
The standard and quality control samples used in these assays were calibrated to the National Institute for Biological Standards and Control reference standard,36 and antibody levels for samples were reported in IU per millilitre. The lower limit of quantitation (LLOQ) for the ELISA assay was 0.0001 IU mL−1. LLOQ for the AT neutralisation assay was 0.0007 IU mL−1.
Statistical methods
The changes from baseline screening of S. aureus colonisation in PCR cycle threshold values and culture scores and the changes in AT expression level for S. aureus‐positive subjects at screening were summarised in descriptive statistics of mean and standard deviation in log scale by treatment group and visits. The AT expression levels for S. aureus isolates from subjects who were negative at baseline but tested positive during postinfusion visits were compared with isolates from screening, and a two‐sample t‐test was used for comparison.
Anti‐AT neutralising antibodies and IgG levels in serum and upper respiratory tract, in IU per millilitre and fold change from baseline, were summarised by using the geometric mean by study day and by treatment group.
SAS System Version 9.3 was used for summary statistics, and Prism 6.05 was used to generate figures.
Acknowledgments
We thank Monica Chiaramonte, Jeffery Brubaker, Bret Sellman and Michael McCarthy for critical review and help with the manuscript preparation.
This study was sponsored by MedImmune, the global biologics R&D arm of AstraZeneca. Medical writing support was provided by Monica Chiaramonte of MedImmune.
Disclosures
This study was supported by MedImmune, the global biologics R&D arm of AstraZeneca. All authors are or were employees of MedImmune and own or owned stock and/or stock interests in AstraZeneca while engaged in the research described in this manuscript.
References
- 1. Bamberger DM, Boyd SE. Management of Staphylococcus aureus infections. Am Fam Physician 2005; 72: 2474–2481. [PubMed] [Google Scholar]
- 2. Klevens RM, Morrison MA, Nadle J et al Invasive methicillin‐resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298: 1763–1771. [DOI] [PubMed] [Google Scholar]
- 3. Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998; 339: 520–532. [DOI] [PubMed] [Google Scholar]
- 4. Spellberg B, Talbot G. Recommended design features of future clinical trials of antibacterial agents for hospital‐acquired bacterial pneumonia and ventilator‐associated bacterial pneumonia. Clin Infect Dis 2010; 51(Suppl 1): S150–S170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Esperatti M, Ferrer M, Theessen A et al Nosocomial pneumonia in the intensive care unit acquired by mechanically ventilated versus nonventilated patients. Am J Respir Crit Care Med 2010; 182: 1533–1539. [DOI] [PubMed] [Google Scholar]
- 6. Bhakdi S, Tranum‐Jensen J. Alpha‐toxin of Staphylococcus aureus . Microbiol Rev 1991; 55: 733–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen TS, Hilliard JJ, Jones‐Nelson O et al Staphylococcus aureus alpha toxin potentiates opportunistic bacterial lung infections. Sci Transl Med 2016; 8: 329ra331. [DOI] [PubMed] [Google Scholar]
- 8. Tkaczyk C, Hua L, Varkey R et al Identification of anti‐alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin Vaccine Immunol 2012; 19: 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walev I, Martin E, Jonas D et al Staphylococcal alpha‐toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect Immun 1993; 61: 4972–4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilke GA, Wardenburg JB. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus α‐hemolysin–mediated cellular injury. Proc Natl Acad Sci USA 2010; 107: 13473–13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ragle BE, Bubeck WJ. Anti‐alpha‐hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun 2009; 77: 2712–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bayer AS, Ramos MD, Menzies BE, Yeaman MR, Shen AJ, Cheung AL. Hyperproduction of alpha‐toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infect Immun 1997; 65: 4652–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bramley AJ, Patel AH, O'Reilly M, Foster R, Foster TJ. Roles of alpha‐toxin and beta‐toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect Immun 1989; 57: 2489–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bubeck Wardenburg J, Palazzolo‐Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton‐Valentine leukocidin is not a virulence determinant in murine models of community‐associated methicillin‐resistant Staphylococcus aureus disease. J Infect Dis 2008; 198: 1166–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kernodle DS, Voladri RK, Menzies BE, Hager CC, Edwards KM. Expression of an antisense hla fragment in Staphylococcus aureus reduces alpha‐toxin production in vitro and attenuates lethal activity in a murine model. Infect Immun 1997; 65: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kobayashi SD, Malachowa N, Whitney AR et al Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis 2011; 204: 937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wardenburg JB, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun 2007; 75: 1040–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adhikari RP, Karauzum H, Sarwar J et al Novel structurally designed vaccine for S. aureus alpha‐hemolysin: protection against bacteremia and pneumonia. PLoS One 2012; 7: e38567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 2008; 205: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kennedy AD, Bubeck Wardenburg J, Gardner DJ et al Targeting of alpha‐hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 2010; 202: 1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Menzies BE, Kernodle DS. Passive immunization with antiserum to a nontoxic alpha‐toxin mutant from Staphylococcus aureus is protective in a murine model. Infect Immun 1996; 64: 1839–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adhikari RP, Ajao AO, Aman MJ et al Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis 2012; 206: 915–923. [DOI] [PubMed] [Google Scholar]
- 23. Jacobsson G, Colque‐Navarro P, Gustafsson E, Andersson R, Mollby R. Antibody responses in patients with invasive Staphylococcus aureus infections. Eur J Clin Microbiol Infect Dis 2010; 29: 715–725. [DOI] [PubMed] [Google Scholar]
- 24. Ruotsalainen E, Karden‐Lilja M, Kuusela P et al Methicillin‐sensitive Staphylococcus aureus bacteraemia and endocarditis among injection drug users and nonaddicts: host factors, microbiological and serological characteristics. J Infect 2008; 56: 249–256. [DOI] [PubMed] [Google Scholar]
- 25. Oganesyan V, Peng L, Damschroder MM et al Mechanisms of neutralization of a human anti‐α‐toxin antibody. J Biol Chem 2014; 289: 29874–29880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hua L, Hilliard JJ, Shi Y et al Assessment of an anti‐alpha‐toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus‐induced pneumonia. Antimicrob Agents Chemother 2014; 58: 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu X, Robbie GJ, Wu Y et al Safety, tolerability, and pharmacokinetics of MEDI4893, an investigational, extended half‐life, anti‐Staphylococcus aureus alpha‐toxin human monoclonal antibody, in healthy adults. Antimicrob Agents Chemother 2016; 61: pii: e01020‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tabor DE, Yu L, Mok H et al Staphylococcus aureus alpha‐toxin is conserved among diverse hospital respiratory isolates collected from a global surveillance study and is neutralized by monoclonal antibody MEDI4893. Antimicrob Agents Chemother 2016; 60: 5312–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jenkins A, Diep BA, Mai TT et al Differential expression and roles of Staphylococcus aureus virulence determinants during colonization and disease. MBio 2015; 6: e02272‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stulik L, Malafa S, Hudcova J et al Alpha‐hemolysin activity of methicillin‐susceptible Staphylococcus aureus predicts ventilator‐associated pneumonia. Am J Respir Crit Care Med 2014; 190: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 31. Burian M, Wolz C, Goerke C. Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans. PLoS One 2010; 5: e10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garcia LS. Clinical Microbiology Procedures Handbook. American Society for Microbiology Press: Washington, DC, 2010. [Google Scholar]
- 33. Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing, 25th informational supplement. CLSI document M100‐S25. CLSI: Wayne, PA, 2015. [Google Scholar]
- 34. Sharma‐Kuinkel BK, Wu Y, Tabor DE et al Characterization of alpha‐toxin hla gene variants, alpha‐toxin expression levels, and levels of antibody to alpha‐toxin in hemodialysis and postsurgical patients with Staphylococcus aureus bacteremia. J Clin Microbiol 2015; 53: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Diep BA, Gill SR, Chang RF et al Complete genome sequence of USA300, an epidemic clone of community‐acquired meticillin‐resistant Staphylococcus aureus . Lancet 2006; 367: 731–739. [DOI] [PubMed] [Google Scholar]
- 36. National Institute for Biological Standards and Control . WHO International Standard: The 3rd International Standard for Staphylococcus Alpha Antitoxin, Equine, Version 5.0. NIBSC: Potters Bar, Hertfordshire, UK, 2010. Available from http://www.nibsc.org/documents/ifu/STA.pdf. Retrieved January 16, 2018. [Google Scholar]


