Abstract
Sarcoidosis is a multisystem disease with tremendous heterogeneity in disease manifestations, severity, and clinical course that varies among different ethnic and racial groups. To better understand this disease and to improve the outcomes of patients, a National Heart, Lung, and Blood Institute workshop was convened to assess the current state of knowledge, gaps, and research needs across the clinical, genetic, environmental, and immunologic arenas. We also explored to what extent the interplay of the genetic, environmental, and immunologic factors could explain the different phenotypes and outcomes of patients with sarcoidosis, including the chronic phenotypes that have the greatest healthcare burden. The potential use of current genetic, epigenetic, and immunologic tools along with study approaches that integrate environmental exposures and precise clinical phenotyping were also explored. Finally, we made expert panel–based consensus recommendations for research approaches and priorities to improve our understanding of the effect of these factors on the health outcomes in sarcoidosis.
Keywords: genetics, environment, immunology, granuloma, phenotype
The pathobiologic mechanisms underlying the clinical phenotypic heterogeneity of sarcoidosis are poorly understood (1). This is due to an incomplete understanding of disease etiology as well as a lack of understanding as to how variability in genetic, environmental, epigenetic, and immunologic factors may result in widely different clinical manifestations, outcomes, and responses to therapy in sarcoidosis. Thus, although there has been considerable progress in understanding common biologic mechanisms in sarcoidosis, the determinants of disease heterogeneity remain poorly understood. A better understanding of the biologic underpinnings of the clinical heterogeneity of sarcoidosis is needed for personalized medicine approaches to improve the health and outcomes of patients with sarcoidosis, particularly those who suffer from the most severe manifestations.
Here, we summarize the current state of knowledge of the genetic, environmental, and immunologic basis of sarcoidosis as well as knowledge gaps in these areas at the time of the National Heart, Lung, and Blood Institute (NHLBI) workshop. Furthermore, focusing on these issues within the context of disease heterogeneity and health disparities faced by patients with sarcoidosis, we make recommendations for future research priorities aimed to better understand and reduce health disparities in sarcoidosis.
Genetics of Sarcoidosis
Current State of Knowledge
Before genome-wide association studies (GWAS), significant associations between sarcoidosis and several loci of the human leukocyte antigen gene (HLA) were established (2–6) (see Tables E1 and E2 in the online supplement). In 2008, a GWAS in a German population found association to annexin A11 (ANXA11) (7), which has since been replicated in multiple studies and populations (8–11). A fine-mapping study of ANXA11 found two additional ANXA11 sarcoidosis-associated variants only in African American individuals (AA) (11). A recent GWAS of sarcoidosis conducted in AA reported a NOTCH4 single-nucleotide polymorphism (SNP) reaching genome-wide significance (12). A genome-wide study comparing sarcoidosis by ancestry implicated the X-linked inhibitor of apoptosis (XIAP) associated factor 1 gene (XAF1) on chromosome 17p13.1 in AA with sarcoidosis (13). In sarcoidosis granulomas, XAF1 expression was absent, but there was high expression of the XAF1 downstream target, XIAP, suggesting the XIAP/XAF1 apoptosis pathway may play a role in the maintenance of sarcoidosis granulomas (13).
Although sarcoidosis is most strongly associated with the HLA region on chromosome 6, with HLA-DRB1*11:01 increasing risk in both AA and white individuals, other DRB1 alleles, such as 12:01 or 15:03, and 15:01 or 04:01, have race-specific associations in AA and white individuals, respectively (4). In Europeans, DRB1*03:01 has a strong association with increased disease risk but also with disease resolution (14). In AA, 03:01 was protective against disease risk, whereas 03:02 was associated with disease risk and resolution (9). A scan of SNPs on the Immunochip (15) in a German cohort implicated genes within the IL23/T-helper (Th)17-signaling pathway, but these effects were not replicated in an AA cohort (16). A study of a European cohort identified a truncating splice site mutation of the butyrophilin-like 2 gene (SNP rs2076530), which is predicted to alter the structure and function of the related butyrophilin-like 2 protein, a member of the B7 cell receptor family that normally suppresses activation of T cells by antigen-presenting cells (17). These findings were confirmed in other studies (16), and in AA, although with some differences in genetic variants and strength of association (12). These findings suggest that the etiology of sarcoidosis may differ by ancestry, and thus there be more than one pathway to disease.
The genetics of organ-specific manifestations of sarcoidosis have been sparsely studied, and the quality of these data is limited by small sample size (e.g., relative to aforementioned GWAS studies) and lack of genome-wide significance, but some evidence suggests that HLA subtypes may be linked with extrapulmonary involvement. For instance, HLA-DRB1*04/*15 have been associated with extrapulmonary involvement (18), HLA-DRB1*03:01 with Löfgren syndrome (14), HLA-DQB1*06:01 with cardiac sarcoidosis (19), and HLA-DRB1*04 with uveitis (20). More recently, evidence of genes in the nucleotide-binding oligomerization domain-containing protein 2 pathway, transforming growth factor–β activated kinase 1/mitogen-activated protein kinase kinase kinase 7–binding protein (TAB)-2 in European Americans and TAB2, mitogen-activated protein kinase 13, and TAB1 in AA, were associated with skin and bone/joint involvement in sarcoidosis (21), whereas a SNP in a zinc finger gene, ZNF592, was found associated with neurosarcoidosis in AA and European Americans (22). Further refinement of sarcoidosis phenotypes may lead to additional novel genetic associations that elucidate mechanisms behind extrathoracic manifestation of disease.
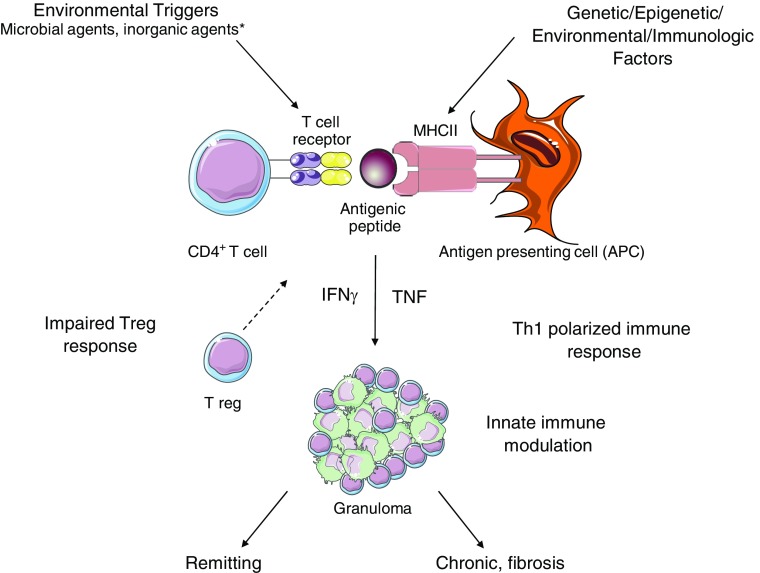
Because both heredity and environment play important roles in sarcoidosis etiology, investigating how genes and environment interact in disease pathogenesis is key (Figure 1). Environmental interactions have been found in sarcoidosis between HLA DRB1*11:01 and insecticide exposure at work and exposure to mold and musty odors, and between DRB1*15:01 and insecticide exposure at work (23). More recently, interactions between insecticide exposure and the fucosyltransferase 9 gene on chromosome 6q16.1 was found (24). Although several studies have found significant increased risk for sarcoidosis among siblings of affected cases, and a recent twin study estimated heritability of sarcoidosis at 66%, only 33% of disease heritability is accounted for by common genetic variants, and 27% of that remains after accounting for the known HLA associations (25–27). This suggests that further investigation involving multiple exposures genome-wide data and sophisticated statistical modeling are needed to discover additional gene–environment interactions that might account for other sources of sarcoidosis heritability and help better understand overall disease susceptibility.
Figure 1.
Schematic of the current state of the genetic, immunological, and environmental basis of sarcoidosis. Environmental triggers, mostly microbial in origin, interact with genetic, epigenetic, environmental, and immunologic host factors resulting in a hyperpolarized T-helper (Th)1 response to pathogenic tissue antigens and epithelioid granuloma formation. The local Th1 immune responses are associated with impaired regulatory T-cell function and innate immune stimulation (e.g., Toll-like receptor 2, serum amyloid A). The determinants of the different clinical phenotypes and outcomes remain uncertain. *There is a lack of consensus on whether inorganic agents can trigger multisystem sarcoidosis. MHC = major histocompatibility complex; TNF = tumor necrosis factor.
Gaps in Knowledge and Resources for Future Work
There are significant genetic data resources available (dbGaP) (28), including data from a study using the Illumina HumanOmni1-Quad array for ∼1.1 million SNPs in an AA cohort of 1,273 cases and 1,465 control subjects and a white cohort of 442 cases and 339 control subjects. Other data are being generated from ongoing studies, including GWAS data from a larger white cohort and exome and targeted sequencing as well as whole-genome sequencing that will be available as a resource. Although the list of completed and ongoing genetics projects is extensive, the greatest potential gain to future sarcoidosis genetics studies may be through the integration of genetics and omics data, as per the article by Crouser and colleagues (29); to move genetics research beyond associations to functional characterization will require a multidimensional examination of the clinical and molecular data (Table 1). More definitive studies will be required to verify these associations, including evidence for replication, which is a challenge given that sarcoidosis is a rare disease with limited numbers of individuals affected.
Table 1.
Knowledge gaps in the pathogenesis of sarcoidosis
| Genetic knowledge gaps |
| Understanding of the relationships between genotype and clinical phenotype is limited. |
| Understanding of genetic factors influencing immunologic variability is limited. |
| The role played by epigenetic factors (noncoding RNA, methylation, histone acetylation) in sarcoidosis is limited. |
| Knowledge gaps relating to environmental factors |
| The role of microbial and nonmicrobial antigens in the pathogenesis of sarcoidosis has not been firmly established. |
| Environmental factors that modify sarcoidosis disease course remain unknown. |
| The role played by the microbiome (lung, gastrointestinal tract) remains to be determined. |
| Knowledge gaps of the immunology of sarcoidosis |
| The role of T-cell subsets remains controversial. |
| The role of macrophage polarization in sarcoidosis granuloma formation is unclear. |
| The role of B cells remains largely unexplored. |
| The complex interaction among these cell lines during granuloma formation is difficult to model, and, as such, is largely unknown. |
Environmental Basis for Sarcoidosis
Current State of Knowledge
Environmental exposures play a putative role in sarcoidosis pathogenesis by directly triggering granulomatous inflammation and by indirectly inducing epigenetic and immunologic changes that alter the risk of sarcoidosis (Figure 1). Investigators in ACCESS (A Case Control Etiologic Study of Sarcoidosis) observed positive associations between sarcoidosis risk and certain occupations, such as agricultural employment, exposure to insecticides, and mold/mildew work environments, with modest increased risks (odds ratios, ∼1.5) (30). Occupational exposures have been associated with sarcoidosis in other cohort studies, including healthcare workers and firefighters. Despite these studies, the relevant environmental antigens remain uncertain. Furthermore, it is unclear if these exposures reflect direct environmental triggers or indirect influence impacting host response readiness. Differences in exposures related to sex and ancestry should be examined in future studies.
Inorganic and complex environmental airborne exposures have been associated with sarcoidosis-like granulomatous pneumonitis, with chronic beryllium disease being a well-studied example (31). Another example involves the “sarcoidosis-like” pulmonary disease experienced by first-response rescue workers from the World Trade Center disaster (32). In the absence of multisystem granulomatous inflammation, it is debated whether these diseases should be grouped under the sarcoidosis domain or remain an independent etiologically defined pulmonary disease (33). Furthermore, other inorganic exposures have been implicated as triggers for a systemic sarcoidosis-like response, including silica (34).
Multiple lines of evidence support a potential microbial etiology of sarcoidosis, particularly involving mycobacteria and/or propionibacterial organisms (35). Mycobacterial nucleic acids and proteins have been isolated from sarcoidosis tissue specimens (36–39); meta-analysis of studies revealed a positive association with pathogenic mycobacteria in sarcoidosis tissues, some genetically distinct from Mycobacterium tuberculosis (37). Multiple studies document sarcoidosis B-cell and Th1 immune responses to specific mycobacterial proteins compared with controls, suggesting a potential antigenic role. Candidate pathogenic antigens from mycobacterial organisms include mycobacterial catalase-peroxidase G, early secreted antigenic target of 6 kD, superoxide dismutase, and heat shock proteins (40–43). Evaluation of transcriptomic signatures in peripheral blood of patients with of sarcoidosis and tuberculosis infection reveal extensive overlap, potentially supporting a mycobacterial link to sarcoidosis etiology (41, 44–46).
Propionibacterium acnes nucleic acids and proteins have been identified in sarcoidosis tissues but also in many controls (38, 47, 48). T- and B-cell immune responses to P. acnes proteins have also been seen in both sarcoidosis and control groups (49, 50). A recent meta-analysis involving nine sarcoidosis case–control studies of P. acnes revealed significantly elevated sarcoidosis risk (odds ratio, 19.58; 95% confidence interval, 13.06–29.36) (51). However, one recent study of the microbiome of the upper and lower airway of subjects with idiopathic interstitial pneumonia, sarcoidosis, Pneumocystis jiroveci pneumonia, and healthy control subjects found no significant differences in airway microbiota composition between the different groups, highlighting the challenges of defining microbes in pathogenic disease processes (52).
Gaps in Knowledge and Lack of Consensus
Despite the link to microbes in sarcoidosis, there is no consensus on the role microbes play in disease etiology (Table 2). Some hypothesize that chronic sarcoidosis is caused by active, replicating mycobacterial, propionibacterial, or other microbial organisms (44). Others suggest the inability to identify microorganisms by histologic staining or culture at any point in the disease, despite years of immunosuppressive or anti–tumor necrosis factor (TNF) therapies as strong arguments against a role for actively replicating microbial agents in sarcoidosis (53). In support of the former, investigators point to the fact that current culture and staining methods identify less than 2% of current microbial communities present within the human biological specimens (54). For the latter, investigators point to the fact that the basic clinical course of chronic sarcoidosis (i.e., slowly progressive disease when untreated, responses to chronic immunosuppressive therapies without evidence of recurrent or relapsing infection) is unlike known active infectious diseases. An alternative hypothesis posits that chronic sarcoidosis is the result of aberrant misfolding, aggregation, and progressive accumulation of serum amyloid A (SAA) within granulomas in a progressive amyloid-like manner, with released SAA fragments promoting persistent and enhanced Th1 responses to local tissue antigens (53, 55).
Table 2.
Summary recommendations for future research
| Research Question to be Addressed | Recommended Scientific Approach |
|---|---|
| What is (are) the environmental exposure(s) causing sarcoidosis and influencing diverse clinical phenotypes? | Studies to identify environmental exposures that are associated with extreme disease phenotypes |
| What are the immune mechanisms, including incompletely understood local innate and adaptive immune responses, that could be targeted to more effectively treat sarcoidosis? | Support efforts to develop relevant animal and in vitro disease models |
| Support hypothesis-driven research to identify molecular mechanisms and potential therapeutic targets | |
| What is the genetic basis of severe sarcoidosis phenotypes? | Leverage high-throughput, low-cost genome-wide technology |
| Genome-wide association studies | |
| Gene sequencing | |
| Epigenetics | |
| How does the host microbiome influence the risk for sarcoidosis or severe sarcoidosis phenotypes? | Support studies to comprehensively evaluate the microbiome of the lungs and gastrointestinal tract and correlate with clinical and immunological sarcoidosis phenotypes |
| What are the molecular pathways and biomarkers that contribute to chronic multisystem sarcoidosis, and how can this information contribute to improved care? | Conduct longitudinal studies to assess various candidate biomarkers that would serve to assist in establishing the diagnosis, prognosis, and response to treatment |
| How do we account for the complex interactions of multiple environmental and host factors as they relate to the phenotypic variability of sarcoidosis? | Bioinformatic analysis of comprehensive data sets derived from large patient cohorts followed longitudinally |
These controversies highlight the many unknown immune factors that play a role in the chronic disease pathogenesis (Table 1). It is unknown whether specific environmental antigens are important in determining clinical phenotype or outcome or if the same antigens cause multiple/any clinical phenotypes. It further remains unclear if microbes play a role in chronic disease pathogenesis indirectly by altering host immunity and its response to certain environmental antigens or as a source of antigens. It is reasonable to speculate that microbes could be critical to chronic pathology if trapped in preexisting granulomas, which are known to be “sticky,” as shown for mycobacterial or prion diseases (56). Importantly, it remains unknown whether environmental exposures linked to sarcoidosis result in distinct epigenetic signatures. If so, determining the epigenetic profiles that associate with different disease outcomes may provide biomarkers that could predict disease risk, clinical course, or treatment responses and guide treatment (e.g., avoidance of specific exposures).
Immunology of Sarcoidosis
Current State of Knowledge
The pathologic hallmark of sarcoidosis is epithelioid noncaseating granulomas associated with infiltration of CD4+ T cells in affected organs. Scattered macrophages, giant cells, CD8+ T cells, and B cells may be seen within or around granulomas with rare neutrophils and eosinophils. CD4+ T-cell and B-cell lymphopenia are common in peripheral blood. The CD4+ T cells in lung tissue, bronchoalveolar lavage (BAL), and blood are polarized to a Th1 effector phenotype, expressing IFN-γ, TNF-α, and often IL-2 (Figure 1). This polarized response is seen throughout the disease course, without evidence of transition to a Th2 response producing IL-4 or IL-5. Clinical evidence that sarcoidosis is a Th1-driven disorder includes the association with Th1-promoting therapies such as IFN-α (57). Th1-associated gene-expression signatures have been found in several transcriptomic studies, with association with clinical decline in sarcoidosis, supporting a primary role for Th1 responses in disease pathogenesis and clinical outcome (58, 59). Th17-polarized effector T cells have also been detected in sarcoidosis-affected tissues, BAL, and blood, but of lower frequency than Th1 cells (60–63). Although the role of Th17 cells in sarcoidosis etiology remains unclear, studies suggest Th17 effector responses, including IFN-γ–producing Th17.1 cells (64), may influence disease severity and clinical course (61).
T cells in the blood and BAL of patients with sarcoidosis have impaired induction of nuclear factor-κB and cytokine expression (65). This T-cell dysfunction is associated with higher expression of programmed cell death 1 protein, a coinhibitory receptor, which in turn correlates with clinical disease activity (66). Similar findings have been seen in antigen-specific CD4+ T cells from blood and BAL of subjects with chronic beryllium disease (67). These findings suggest an upregulation of coinhibitory receptors on CD4+ T cells, likely from persistent antigen exposure.
Regulatory T cells (Treg) have been investigated in sarcoidosis. Several studies associate active sarcoidosis with diminished Treg suppressor function (68, 69). Other studies demonstrate that pharmacological interventions can boost Treg functional capacity and restore the Treg functional deficits observed in patients with sarcoidosis with active disease (70, 71). Additional studies are necessary to delineate whether the defective Treg responses are primary to disease pathogenesis or a secondary result of persistent antigenic stimulation of CD4 + T cell (72–74).
CD4+ T cells in the BAL express biased T-cell receptor Vα and Vβ genes, consistent with oligoclonal expansions of antigen-experienced T cells. The best studied example involves the expansion of CD4+ T cells expressing the T-cell receptor Vα2.3 (AV2S3) gene in the BAL of patients with sarcoidosis expressing HLA-DRB1*03:01 (14, 75). In Löfgren syndrome, an association between multifunctional T-cell cytokine responses to a candidate pathogenic antigen mycobacterial catalase-peroxidase G and T cells expressing Vα2.3 was observed (76). In some patients, adaptive responses to candidate pathogenic antigens are detectable years after diagnosis (40). T-cell reactivity to multiple mycobacterial proteins in sarcoidosis has been reported, as discussed above (40–43).
Although most studies in sarcoidosis have focused on adaptive immunity, granuloma formation can occur in the absence of an adaptive immunity (77). The innate Toll-like receptors (TLRs) have been implicated in sarcoidosis pathobiology (76, 77). Immune response to ligands of the TLR-2/TLR-1 heterodimer was seen in the presence of reduced responses to TLR-2/TLR-6 ligands in sarcoidosis peripheral blood cells (78). SAA, an innate ligand, has been found to selectively accumulate within sarcoidosis granulomas (55) and induce Th1 cytokine responses (e.g., TNF, IL-18) in sarcoidosis BAL cells. It also accumulated in an experimental model of granulomatous lung inflammation, mediated in part through TLR-2. SAA has multiple innate receptors that may influence outcomes in sarcoidosis by promoting Th1 responses, contributing to alternatively activated macrophage differentiation and Th17 responses in vitro (79, 80). Several studies have identified SAA in BAL fluid and blood as a biomarker for active sarcoidosis correlating with stage of disease, supporting its role in chronic disease (55, 81–83).
A major conceptual challenge in sarcoidosis is to understand how pulmonary fibrosis occurs in an environment dominated by the expression of Th1 cytokines, such as IFN-γ, which inhibit collagen synthesis. Recent studies have identified alternatively activated macrophage subpopulations in fibrotic sarcoidosis associated with expression of C-C motif chemokine ligand 18, a chemokine that is linked to fibrosis in other lung diseases (84). However, the phenotype of these macrophages is not typical for M2 macrophages that develop in a classical Th2 environment. Another study reported that biopsies of sarcoidosis myositis contained alternatively activated M2 macrophages expressing C-C motif chemokine ligand 18 that were histologically localized to areas of myofibrosis (85).
Gaps in Knowledge and Resources for Future Work
A major shortcoming in the study of sarcoidosis is the lack of known antigens driving disease initiation and persistence in those subjects with chronic disease. In the absence of a known antigenic driver(s) of disease, a second critical gap in sarcoidosis research is the lack of an adequate animal or in vitro model. These two critical gaps go hand in hand, because an adequate model that replicates the human disease is unlikely to be developed in the absence of a solid understanding of the antigens that drive disease onset and persistence.
There are a number of gaps in our knowledge of innate and adaptive immunity in sarcoidosis. For example, the role of macrophages, and particularly macrophage polarization, in the transition from inflammatory to fibrotic forms of sarcoidosis remains to be determined. Similarly, although hypergammaglobulinemia is present in many patients (86), and low titers of autoantibodies have also been observed (87), the role of B cells and autoantibodies in the pathogenesis of sarcoidosis remains uncertain. The lack of understanding of the roles of Treg cells, B cells, and Th17 cells in disease pathogenesis will likely be filled once the critical first two gaps noted above are addressed. The last major gap is the role of innate immunity in driving granuloma formation and persistence, as well as the role of SAA, in orchestrating chronic granuloma formation in humans.
Summary of Knowledge Gaps and Critical Questions
A number of critical questions regarding the immunopathogenesis of sarcoidosis remain (Table 1) that help dictate research priorities. Identification of environmental and host antigens that cause sarcoidosis remains a high-risk, high-reward endeavor. This risk includes the possibility that the disease-relevant antigens may change from disease initiation through different clinical phenotypes and outcomes. However, the potential impact of such discovery remains high when considering strategies for new treatments, cure, and ultimately disease prevention.
The lack of an experimental model of sarcoidosis hampers progress in our understanding and discovery of new therapies for this human disease. Although aspects of pathogenic mechanisms may be explored in newer animal models of granulomatous diseases (88, 89), the relevance to human sarcoidosis needs to be validated. Other approaches include modeling aspects of sarcoidosis granuloma formation in vitro using a limited number of cells and cell types (90). These approaches are a lower-risk investment but are unlikely to capture the full complexity of granulomatous inflammation in sarcoidosis. However, the information gleaned from these lower-cost approaches can be used to assist in the development of a representative animal model that recapitulates features of chronic sarcoidosis. Such a model would allow preclinical testing of novel therapies for treatment or cure. Given these limitations, human-based studies on the pathogenic mechanisms that may be important in sarcoidosis remain critical to further understanding of this disease, and human studies are the gold standard for the validation of new models.
A critical aspect for a study with a goal of understanding the biologic underpinnings of chronic sarcoidosis and its most impactful phenotypes is sufficient follow-up to objectively determine clinical course and outcome. Integration of biologic data with clinical phenotyping, environmental assessment, and consideration of clinical course is critical to examine the genomic/genetic/epigenetic/immunologic/environmental interactions that likely dictate chronic disease and severe phenotypes. These studies more often lead to hypothesis-generating data and will need to be validated by mechanistic studies.
In summary, the authors propose recommendations that include a renewed emphasis on hypothesis testing of recent discoveries related to sarcoidosis pathobiology as well as exploiting the rapid advancements in technology for genetic, immunologic, and molecular phenotyping of patients with sarcoidosis with defined clinical manifestations and clinical course (Table 2).
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank their patients; study participants who have enrolled in sarcoidosis-related research studies; the many organizations that have supported the sarcoidosis community and its research mission; Drs. Jerry Eu, George Mensah, Lora Reineck, and Antonello Punturieri, and the National Heart, Lung, and Blood Institute; and other Institutes/Centers of the National Institutes of Health for the support of this workshop. They also thank the remainder of the workshop participants for thoughtful discussions that led to the development of these recommendations and Megan Marchant for assistance with editing and formatting this manuscript.
Footnotes
Supported by National Institutes of Health Grants HL112708 (D.R.M.), HL113326 (C.G.M.), HL114587 (N.Y.H.), HL112695 (N.Y.H.), HL112694 (W.D.), HL127301 (W.D.), and HL136137 (A.P.F).
Author Contributions: All authors participated in concept, design, drafting of the manuscript, and critical review of the manuscript. All authors read and approved the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Bogunia-Kubik K, Tomeczko J, Suchnicki K, Lange A. HLA-DRB1*03, DRB1*11 or DRB1*12 and their respective DRB3 specificities in clinical variants of sarcoidosis. Tissue Antigens. 2001;57:87–90. doi: 10.1034/j.1399-0039.2001.057001087.x. [DOI] [PubMed] [Google Scholar]
- 3.Foley PJ, McGrath DS, Puscinska E, Petrek M, Kolek V, Drabek J, et al. Human leukocyte antigen-DRB1 position 11 residues are a common protective marker for sarcoidosis. Am J Respir Cell Mol Biol. 2001;25:272–277. doi: 10.1165/ajrcmb.25.3.4261. [DOI] [PubMed] [Google Scholar]
- 4.Rossman MD, Thompson B, Frederick M, Maliarik M, Iannuzzi MC, Rybicki BA, et al. ACCESS Group. HLA-DRB1*1101: a significant risk factor for sarcoidosis in blacks and whites. Am J Hum Genet. 2003;73:720–735. doi: 10.1086/378097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma SK, Balamurugan A, Pandey RM, Saha PK, Mehra NK. Human leukocyte antigen-DR alleles influence the clinical course of pulmonary sarcoidosis in Asian Indians. Am J Respir Cell Mol Biol. 2003;29:225–231. doi: 10.1165/rcmb.2003-0007OC. [DOI] [PubMed] [Google Scholar]
- 6.Swider C, Schnittger L, Bogunia-Kubik K, Gerdes J, Flad H, Lange A, et al. TNF-alpha and HLA-DR genotyping as potential prognostic markers in pulmonary sarcoidosis. Eur Cytokine Netw. 1999;10:143–146. [PubMed] [Google Scholar]
- 7.Hofmann S, Franke A, Fischer A, Jacobs G, Nothnagel M, Gaede KI, et al. Genome-wide association study identifies ANXA11 as a new susceptibility locus for sarcoidosis. Nat Genet. 2008;40:1103–1106. doi: 10.1038/ng.198. [DOI] [PubMed] [Google Scholar]
- 8.Feng X, Zang S, Yang Y, Zhao S, Li Y, Gao X, et al. Annexin A11 (ANXA11) gene polymorphisms are associated with sarcoidosis in a Han Chinese population: a case-control study. BMJ Open. 2014;4:e004466. doi: 10.1136/bmjopen-2013-004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin AM, Iannuzzi MC, Montgomery CG, Trudeau S, Datta I, McKeigue P, et al. Association of ANXA11 genetic variation with sarcoidosis in African Americans and European Americans. Genes Immun. 2013;14:13–18. doi: 10.1038/gene.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morais A, Lima B, Peixoto M, Melo N, Alves H, Marques JA, et al. Annexin A11 gene polymorphism (R230C variant) and sarcoidosis in a Portuguese population. Tissue Antigens. 2013;82:186–191. doi: 10.1111/tan.12188. [DOI] [PubMed] [Google Scholar]
- 11.Mrazek F, Stahelova A, Kriegova E, Fillerova R, Zurkova M, Kolek V, et al. Functional variant ANXA11 R230C: true marker of protection and candidate disease modifier in sarcoidosis. Genes Immun. 2011;12:490–494. doi: 10.1038/gene.2011.27. [DOI] [PubMed] [Google Scholar]
- 12.Adrianto I, Lin CP, Hale JJ, Levin AM, Datta I, Parker R, et al. Genome-wide association study of African and European Americans implicates multiple shared and ethnic specific loci in sarcoidosis susceptibility. Plos One. 2012;7:e43907. doi: 10.1371/journal.pone.0043907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin AM, Iannuzzi MC, Montgomery CG, Trudeau S, Datta I, Adrianto I, et al. Admixture fine-mapping in African Americans implicates XAF1 as a possible sarcoidosis risk gene. Plos One. 2014;9:e92646. doi: 10.1371/journal.pone.0092646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grunewald J, Eklund A. Löfgren’s syndrome: human leukocyte antigen strongly influences the disease course. Am J Respir Crit Care Med. 2009;179:307–312. doi: 10.1164/rccm.200807-1082OC. [DOI] [PubMed] [Google Scholar]
- 15.Cortes A, Brown MA. Promise and pitfalls of the Immunochip. Arthritis Res Ther. 2011;13:101. doi: 10.1186/ar3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer A, Ellinghaus D, Nutsua M, Hofmann S, Montgomery CG, Iannuzzi MC, et al. GenPhenReSa Consortium. Identification of immune-relevant factors conferring sarcoidosis genetic risk. Am J Respir Crit Care Med. 2015;192:727–736. doi: 10.1164/rccm.201503-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valentonyte R, Hampe J, Huse K, Rosenstiel P, Albrecht M, Stenzel A, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357–364. doi: 10.1038/ng1519. [DOI] [PubMed] [Google Scholar]
- 18.Darlington P, Gabrielsen A, Sörensson P, Tallstedt L, Padyukov L, Eklund A, et al. HLA-alleles associated with increased risk for extra-pulmonary involvement in sarcoidosis. Tissue Antigens. 2014;83:267–272. doi: 10.1111/tan.12326. [DOI] [PubMed] [Google Scholar]
- 19.Naruse TK, Matsuzawa Y, Ota M, Katsuyama Y, Matsumori A, Hara M, et al. HLA-DQB1*0601 is primarily associated with the susceptibility to cardiac sarcoidosis. Tissue Antigens. 2000;56:52–57. doi: 10.1034/j.1399-0039.2000.560107.x. [DOI] [PubMed] [Google Scholar]
- 20.Darlington P, Tallstedt L, Padyukov L, Kockum I, Cederlund K, Eklund A, et al. HLA-DRB1* alleles and symptoms associated with Heerfordt’s syndrome in sarcoidosis. Eur Respir J. 2011;38:1151–1157. doi: 10.1183/09031936.00025011. [DOI] [PubMed] [Google Scholar]
- 21.Bello GA, Adrianto I, Dumancas GG, Levin AM, Iannuzzi MC, Rybicki BA, et al. Role of NOD2 pathway genes in sarcoidosis cases with clinical characteristics of Blau syndrome. Am J Respir Crit Care Med. 2015;192:1133–1135. doi: 10.1164/rccm.201507-1344LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lareau CA, Adrianto I, Levin AM, Iannuzzi MC, Rybicki BA, Montgomery CG. Fine mapping of chromosome 15q25 implicates ZNF592 in neurosarcoidosis patients. Ann Clin Transl Neurol. 2015;2:972–977. doi: 10.1002/acn3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossman MD, Thompson B, Frederick M, Iannuzzi MC, Rybicki BA, Pander JP, et al. ACCESS Group. HLA and environmental interactions in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2008;25:125–132. [PubMed] [Google Scholar]
- 24.Li J, Yang J, Levin AM, Montgomery CG, Datta I, Trudeau S, et al. Efficient generalized least squares method for mixed population and family-based samples in genome-wide association studies. Genet Epidemiol. 2014;38:430–438. doi: 10.1002/gepi.21811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rybicki BA, Iannuzzi MC, Frederick MM, Thompson BW, Rossman MD, Bresnitz EA, et al. ACCESS Research Group. Familial aggregation of sarcoidosis: a case-control etiologic study of sarcoidosis (ACCESS) Am J Respir Crit Care Med. 2001;164:2085–2091. doi: 10.1164/ajrccm.164.11.2106001. [DOI] [PubMed] [Google Scholar]
- 26.Rybicki BA, Kirkey KL, Major M, Maliarik MJ, Popovich J, Jr, Chase GA, et al. Familial risk ratio of sarcoidosis in African-American sibs and parents. Am J Epidemiol. 2001;153:188–193. doi: 10.1093/aje/153.2.188. [DOI] [PubMed] [Google Scholar]
- 27.Sverrild A, Backer V, Kyvik KO, Kaprio J, Milman N, Svendsen CB, et al. Heredity in sarcoidosis: a registry-based twin study. Thorax. 2008;63:894–896. doi: 10.1136/thx.2007.094060. [DOI] [PubMed] [Google Scholar]
- 28.National Center for Biotechnology Information dbGaP: database of Genotypes and Phenotypes [accessed 2015 Sep 1]. Available from: https://www.ncbi.nlm.nih.gov/gap.
- 29.Crouser ED, Fingerlin TE, Yang, Maier LA, Nana-Sinkam P, Collman RG, et al. Application of ‘omics’ and systems biology to sarcoidosis research: NHLBI Workshop to Better Understand Disease Variability and Improve Outcomes in Sarcoidosis. Ann Am Thorac Soc. doi: 10.1513/AnnalsATS.201707-567OT. [online ahead of print] 20 Oct 2017; DOI: doi: 10.1513/AnnalsATS.201707-567OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman LS, Rose CS, Bresnitz EA, Rossman MD, Barnard J, Frederick M, et al. ACCESS Research Group. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 31.Mayer AS, Hamzeh N, Maier LA. Sarcoidosis and chronic beryllium disease: similarities and differences. Semin Respir Crit Care Med. 2014;35:316–329. doi: 10.1055/s-0034-1377059. [DOI] [PubMed] [Google Scholar]
- 32.Izbicki G, Chavko R, Banauch GI, Weiden MD, Berger KI, Aldrich TK, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414–1423. doi: 10.1378/chest.06-2114. [DOI] [PubMed] [Google Scholar]
- 33.Judson MA, Baughman RP. How many organs need to be involved to diagnose sarcoidosis? An unanswered question that, hopefully, will become irrelevant. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:6–7. [PubMed] [Google Scholar]
- 34.Rafnsson V, Ingimarsson O, Hjalmarsson I, Gunnarsdottir H. Association between exposure to crystalline silica and risk of sarcoidosis. Occup Environ Med. 1998;55:657–660. doi: 10.1136/oem.55.10.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen ES, Moller DR. Etiologies of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:6–18. doi: 10.1007/s12016-015-8481-z. [DOI] [PubMed] [Google Scholar]
- 36.Drake WP, Pei Z, Pride DT, Collins RD, Cover TL, Blaser MJ. Molecular analysis of sarcoidosis tissues for mycobacterium species DNA. Emerg Infect Dis. 2002;8:1334–1341. doi: 10.3201/eid0811.020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta D, Agarwal R, Aggarwal AN, Jindal SK. Molecular evidence for the role of mycobacteria in sarcoidosis: a meta-analysis. Eur Respir J. 2007;30:508–516. doi: 10.1183/09031936.00002607. [DOI] [PubMed] [Google Scholar]
- 38.Oswald-Richter KA, Beachboard DC, Seeley EH, Abraham S, Shepherd BE, Jenkins CA, et al. Dual analysis for mycobacteria and propionibacteria in sarcoidosis BAL. J Clin Immunol. 2012;32:1129–1140. doi: 10.1007/s10875-012-9700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song Z, Marzilli L, Greenlee BM, Chen ES, Silver RF, Askin FB, et al. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med. 2005;201:755–767. doi: 10.1084/jem.20040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen ES, Wahlström J, Song Z, Willett MH, Wikén M, Yung RC, et al. T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol. 2008;181:8784–8796. doi: 10.4049/jimmunol.181.12.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubaniewicz A, Trzonkowski P, Dubaniewicz-Wybieralska M, Dubaniewicz A, Singh M, Myśliwski A. Mycobacterial heat shock protein-induced blood T lymphocytes subsets and cytokine pattern: comparison of sarcoidosis with tuberculosis and healthy controls. Respirology. 2007;12:346–354. doi: 10.1111/j.1440-1843.2007.01076.x. [DOI] [PubMed] [Google Scholar]
- 42.Oswald-Richter K, Sato H, Hajizadeh R, Shepherd BE, Sidney J, Sette A, et al. Mycobacterial ESAT-6 and katG are recognized by sarcoidosis CD4+ T cells when presented by the American sarcoidosis susceptibility allele, DRB1*1101. J Clin Immunol. 2010;30:157–166. doi: 10.1007/s10875-009-9311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oswald-Richter KA, Beachboard DC, Zhan X, Gaskill CF, Abraham S, Jenkins C, et al. Multiple mycobacterial antigens are targets of the adaptive immune response in pulmonary sarcoidosis. Respir Res. 2010;11:161. doi: 10.1186/1465-9921-11-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koth LL, Solberg OD, Peng JC, Bhakta NR, Nguyen CP, Woodruff PG. Sarcoidosis blood transcriptome reflects lung inflammation and overlaps with tuberculosis. Am J Respir Crit Care Med. 2011;184:1153–1163. doi: 10.1164/rccm.201106-1143OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maertzdorf J, Weiner J, III, Mollenkopf HJ, Bauer T, Prasse A, Müller-Quernheim J, et al. TBornotTB Network. Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc Natl Acad Sci USA. 2012;109:7853–7858. doi: 10.1073/pnas.1121072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thillai M, Eberhardt C, Lewin AM, Potiphar L, Hingley-Wilson S, Sridhar S, et al. Sarcoidosis and tuberculosis cytokine profiles: indistinguishable in bronchoalveolar lavage but different in blood. Plos One. 2012;7:e38083. doi: 10.1371/journal.pone.0038083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eishi Y, Suga M, Ishige I, Kobayashi D, Yamada T, Takemura T, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198–204. doi: 10.1128/JCM.40.1.198-204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishige I, Eishi Y, Takemura T, Kobayashi I, Nakata K, Tanaka I, et al. Propionibacterium acnes is the most common bacterium commensal in peripheral lung tissue and mediastinal lymph nodes from subjects without sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22:33–42. [PubMed] [Google Scholar]
- 49.Ebe Y, Ikushima S, Yamaguchi T, Kohno K, Azuma A, Sato K, et al. Proliferative response of peripheral blood mononuclear cells and levels of antibody to recombinant protein from Propionibacterium acnes DNA expression library in Japanese patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:256–265. [PubMed] [Google Scholar]
- 50.Schupp JC, Tchaptchet S, Lützen N, Engelhard P, Müller-Quernheim J, Freudenberg MA, et al. Immune response to Propionibacterium acnes in patients with sarcoidosis—in vivo and in vitro. BMC Pulm Med. 2015;15:75. doi: 10.1186/s12890-015-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Hu Y, Li H. Role of Propionibacterium acnes in sarcoidosis: a meta-analysis. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:262–267. [PubMed] [Google Scholar]
- 52.Garzoni C, Brugger SD, Qi W, Wasmer S, Cusini A, Dumont P, et al. Microbial communities in the respiratory tract of patients with interstitial lung disease. Thorax. 2013;68:1150–1156. doi: 10.1136/thoraxjnl-2012-202917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen ES, Moller DR. Sarcoidosis: scientific progress and clinical challenges. Nat Rev Rheumatol. 2011;7:457–467. doi: 10.1038/nrrheum.2011.93. [DOI] [PubMed] [Google Scholar]
- 54.Whitley R. The new age of molecular diagnostics for microbial agents. N Engl J Med. 2008;358:988–989. doi: 10.1056/NEJMp0708085. [DOI] [PubMed] [Google Scholar]
- 55.Chen ES, Song Z, Willett MH, Heine S, Yung RC, Liu MC, et al. Serum amyloid A regulates granulomatous inflammation in sarcoidosis through Toll-like receptor-2. Am J Respir Crit Care Med. 2010;181:360–373. doi: 10.1164/rccm.200905-0696OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cosma CL, Humbert O, Ramakrishnan L. Superinfecting mycobacteria home to established tuberculous granulomas. Nat Immunol. 2004;5:828–835. doi: 10.1038/ni1091. [DOI] [PubMed] [Google Scholar]
- 57.Ramos-Casals M, Mañá J, Nardi N, Brito-Zerón P, Xaubet A, Sánchez-Tapias JM, et al. HISPAMEC Study Group. Sarcoidosis in patients with chronic hepatitis C virus infection: analysis of 68 cases. Medicine (Baltimore) 2005;84:69–80. doi: 10.1097/01.md.0000157577.69729.e6. [DOI] [PubMed] [Google Scholar]
- 58.Su R, Li MM, Bhakta NR, Solberg OD, Darnell EP, Ramstein J, et al. Longitudinal analysis of sarcoidosis blood transcriptomic signatures and disease outcomes. Eur Respir J. 2014;44:985–993. doi: 10.1183/09031936.00039714. [DOI] [PubMed] [Google Scholar]
- 59.Zhou T, Zhang W, Sweiss NJ, Chen ES, Moller DR, Knox KS, et al. Peripheral blood gene expression as a novel genomic biomarker in complicated sarcoidosis. PLoS One. 2012;7:e44818. doi: 10.1371/journal.pone.0044818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Facco M, Cabrelle A, Teramo A, Olivieri V, Gnoato M, Teolato S, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax. 2011;66:144–150. doi: 10.1136/thx.2010.140319. [DOI] [PubMed] [Google Scholar]
- 61.Ostadkarampour M, Eklund A, Moller D, Glader P, Olgart Höglund C, Lindén A, et al. Higher levels of interleukin IL-17 and antigen-specific IL-17 responses in pulmonary sarcoidosis patients with Löfgren’s syndrome. Clin Exp Immunol. 2014;178:342–352. doi: 10.1111/cei.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richmond BW, Ploetze K, Isom J, Chambers-Harris I, Braun NA, Taylor T, et al. Sarcoidosis Th17 cells are ESAT-6 antigen specific but demonstrate reduced IFN-γ expression. J Clin Immunol. 2013;33:446–455. doi: 10.1007/s10875-012-9817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ten Berge B, Paats MS, Bergen IM, van den Blink B, Hoogsteden HC, Lambrecht BN, et al. Increased IL-17A expression in granulomas and in circulating memory T cells in sarcoidosis. Rheumatology (Oxford) 2012;51:37–46. doi: 10.1093/rheumatology/ker316. [DOI] [PubMed] [Google Scholar]
- 64.Ramstein J, Broos CE, Simpson LJ, Ansel KM, Sun SA, Ho ME, et al. IFN-γ-producing T-helper 17.1 cells are increased in sarcoidosis and are more prevalent than T-helper type 1 cells. Am J Respir Crit Care Med. 2016;193:1281–1291. doi: 10.1164/rccm.201507-1499OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee DU, Agarwal S, Rao A. Th2 lineage commitment and efficient IL-4 production involves extended demethylation of the IL-4 gene. Immunity. 2002;16:649–660. doi: 10.1016/s1074-7613(02)00314-x. [DOI] [PubMed] [Google Scholar]
- 66.Braun NA, Celada LJ, Herazo-Maya JD, Abraham S, Shaginurova G, Sevin CM, et al. Blockade of the programmed death-1 pathway restores sarcoidosis CD4(+) T-cell proliferative capacity. Am J Respir Crit Care Med. 2014;190:560–571. doi: 10.1164/rccm.201401-0188OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palmer BE, Mack DG, Martin AK, Gillespie M, Mroz MM, Maier LA, et al. Up-regulation of programmed death-1 expression on beryllium-specific CD4+ T cells in chronic beryllium disease. J Immunol. 2008;180:2704–2712. doi: 10.4049/jimmunol.180.4.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taflin C, Miyara M, Nochy D, Valeyre D, Naccache J-M, Altare F, Salek-Peyron P, Badoul C, Bruneval P, Haroche P, et al. FoxP3+ regulatory T cells suppress early stages of granuloma formation but have little impact on sarcoidosis lesions. Am J Pathol. 2009;174:497–508. doi: 10.2353/ajpath.2009.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Idali F, Wahlström J, Müller-Suur C, Eklund A, Grunewald J. Analysis of regulatory T cell associated forkhead box P3 expression in the lungs of patients with sarcoidosis. Clin Exp Immunol. 2008;152:127–137. doi: 10.1111/j.1365-2249.2008.03609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prasse A, Zissel G, Lützen N, Schupp J, Schmiedlin R, Gonzalez-Rey E, Rensing-Ehl A, Bacher G, Cavalli V, Bevec D, et al. Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am J Respir Crit Care Med. 2010;182:540–548. doi: 10.1164/rccm.200909-1451OC. [DOI] [PubMed] [Google Scholar]
- 71.Julian MW, Shao G, Schlesinger LS, Huang Q, Cosmar DG, Bhatt NY, Culver DA, Baughman RP, Wood KL, Crouser ED. Nicotine treatment improves Toll-like receptor 2 and Toll-like receptor 9 responsiveness in active pulmonary sarcoidosis. Chest. 2013;143:461–470. doi: 10.1378/chest.12-0383. [DOI] [PubMed] [Google Scholar]
- 72.Coleman CA, Muller-Trutwin MC, Apetrei C, Pandrea I. T regulatory cells: aid or hindrance in the clearance of disease? J Cell Mol Med. 2007;11:1291–1325. doi: 10.1111/j.1582-4934.2007.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang H, Lu Z, Jiang C, Liu J, Wang Y, Xu Z. Imbalance between Th17 and regulatory T-Cells in sarcoidosis. Int J Mol Sci. 2013;14:21463–21473. doi: 10.3390/ijms141121463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tondell A, Moen T, Borset M, Salvesen O, Ro AD, Sue-Chu M. Bronchoalveolar lavage fluid IFN-γ+ Th17 cells and regulatory T cells in pulmonary sarcoidosis. Mediators Inflamm. 2014;2014:438070. doi: 10.1155/2014/438070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grunewald J, Janson CH, Eklund A, Ohrn M, Olerup O, Persson U, et al. Restricted V alpha 2.3 gene usage by CD4+ T lymphocytes in bronchoalveolar lavage fluid from sarcoidosis patients correlates with HLA-DR3. Eur J Immunol. 1992;22:129–135. doi: 10.1002/eji.1830220120. [DOI] [PubMed] [Google Scholar]
- 76.Wikén M, Ostadkarampour M, Eklund A, Willett M, Chen E, Moller D, et al. Antigen-specific multifunctional T-cells in sarcoidosis patients with Lofgren’s syndrome. Eur Respir J. 2012;40:110–121. doi: 10.1183/09031936.00166110. [DOI] [PubMed] [Google Scholar]
- 77.Pagán AJ, Ramakrishnan L. Immunity and immunopathology in the tuberculous granuloma. Cold Spring Harb Perspect Med. 2014;5:a018499. doi: 10.1101/cshperspect.a018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gabrilovich MI, Walrath J, van Lunteren J, Nethery D, Seifu M, Kern JA, et al. Disordered Toll-like receptor 2 responses in the pathogenesis of pulmonary sarcoidosis. Clin Exp Immunol. 2013;173:512–522. doi: 10.1111/cei.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Migita K, Koga T, Torigoshi T, Motokawa S, Maeda Y, Jiuchi Y, et al. Induction of interleukin-23 p19 by serum amyloid A (SAA) in rheumatoid synoviocytes. Clin Exp Immunol. 2010;162:244–250. doi: 10.1111/j.1365-2249.2010.04242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun L, Zhou H, Zhu Z, Yan Q, Wang L, Liang Q, et al. Ex vivo and in vitro effect of serum amyloid a in the induction of macrophage M2 markers and efferocytosis of apoptotic neutrophils. J Immunol. 2015;194:4891–4900. doi: 10.4049/jimmunol.1402164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bargagli E, Magi B, Olivieri C, Bianchi N, Landi C, Rottoli P. Analysis of serum amyloid A in sarcoidosis patients. Respir Med. 2011;105:775–780. doi: 10.1016/j.rmed.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 82.De Vries J, Rothkrantz-Kos S, van Dieijen-Visser MP, Drent M. The relationship between fatigue and clinical parameters in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:127–136. [PubMed] [Google Scholar]
- 83.Rubinstein I, Knecht A, de Beer FC, Baum GL, Pras M. Serum amyloid-A protein concentrations in sarcoidosis. Isr J Med Sci. 1989;25:461–462. [PubMed] [Google Scholar]
- 84.Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, Goldmann T, et al. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173:781–792. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- 85.Prokop S, Heppner FL, Goebel HH, Stenzel W. M2 polarized macrophages and giant cells contribute to myofibrosis in neuromuscular sarcoidosis. Am J Pathol. 2011;178:1279–1286. doi: 10.1016/j.ajpath.2010.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Daniele RP, McMillan LJ, Dauber JH, Rossman MD. Immune complexes in sarcoidosis: a correlation with activity and duration of disease. Chest. 1978;74:261–264. doi: 10.1378/chest.74.3.261. [DOI] [PubMed] [Google Scholar]
- 87.Nakamura H, Genma R, Mikami T, Kitahara A, Natsume H, Andoh S, et al. High incidence of positive autoantibodies against thyroid peroxidase and thyroglobulin in patients with sarcoidosis. Clin Endocrinol (Oxf) 1997;46:467–472. doi: 10.1046/j.1365-2265.1997.1630976.x. [DOI] [PubMed] [Google Scholar]
- 88.Falta MT, Tinega AN, Mack DG, Bowerman NA, Crawford F, Kappler JW, et al. Metal-specific CD4+ T-cell responses induced by beryllium exposure in HLA-DP2 transgenic mice. Mucosal Immunol. 2016;9:218–228. doi: 10.1038/mi.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harding JS, Schreiber HA, Sandor M. Granuloma transplantation: an approach to study mycobacterium-host interactions. Front Microbiol. 2011;2:245. doi: 10.3389/fmicb.2011.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hao W, Crouser ED, Friedman A. Mathematical model of sarcoidosis. Proc Natl Acad Sci USA. 2014;111:16065–16070. doi: 10.1073/pnas.1417789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.