Abstract
The Long-Term Oxygen Treatment Trial demonstrated that long-term supplemental oxygen did not reduce time to hospital admission or death for patients who have stable chronic obstructive pulmonary disease and resting and/or exercise-induced moderate oxyhemoglobin desaturation, nor did it provide benefit for any other outcome measured in the trial. Nine months after initiation of patient screening, after randomization of 34 patients to treatment, a trial design amendment broadened the eligible population, expanded the primary outcome, and reduced the goal sample size. Within a few years, the protocol underwent minor modifications, and a second trial design amendment lowered the required sample size because of lower than expected treatment group crossover rates. After 5.5 years of recruitment, the trial met its amended sample size goal, and 1 year later, it achieved its follow-up goal. The process of publishing the trial results brought renewed scrutiny of the study design and the amendments. This article expands on the previously published design and methods information, provides the rationale for the amendments, and gives insight into the investigators’ decisions about trial conduct. The story of the Long-Term Oxygen Treatment Trial may assist investigators in future trials, especially those that seek to assess the efficacy and safety of long-term oxygen therapy.
Clinical trial registered with clinicaltrials.gov (NCT00692198).
Keywords: chronic obstructive pulmonary disease, hypoxemia, oxygen, randomized controlled trial, survival
Chronic obstructive pulmonary disease (COPD) has become the third leading cause of death in the United States (1, 2). Two previous landmark studies demonstrated that long-term oxygen therapy (LTOT) decreases mortality among patients who have COPD and severe resting hypoxemia (i.e., PaO2 ≤ 55 mm Hg on two occasions) (3, 4). Only two published randomized clinical trials have tested LTOT in patients who have less severe (i.e., “moderate”) resting hypoxemia associated with COPD, but these studies were underpowered for the mortality outcome and did not detect a survival advantage (5, 6). These four studies had a combined total enrollment of 501 participants. Thus, the evidence for LTOT efficacy to reduce mortality has rested on positive results from two small trials in patients who had severe resting hypoxemia. The evidence gaps regarding the benefits and harms of LTOT in patients with stable COPD who have resting and/or exercise-induced moderate desaturation provided the rationale for the Long-Term Oxygen Treatment Trial (LOTT) (7).
The LOTT did not find a difference between LTOT and no-LTOT groups in time to hospital admission or death for patients who had stable COPD and resting and/or exercise-induced moderate desaturation, nor did it detect benefits for any other outcome measured in the trial (7). Publication of the LOTT primary outcome results brought renewed scrutiny of its study design and its transformation from a trial of 1) 24-hour oxygen therapy versus no supplemental oxygen for COPD with moderate resting oxyhemoglobin desaturation to a trial of 2) supplemental oxygen versus no supplemental oxygen for COPD with moderate resting or moderate exercise oxyhemoglobin desaturation. As well as broadening its eligible population, the amended study design expanded the LOTT’s primary outcome from death to the composite event of death or first hospitalization, whichever occurred first. These design amendments allowed the trial to meet its sample size and follow-up duration goals in a feasible time frame, but they complicated the interpretation of the study results.
Investigators studying the efficacy and safety of home oxygen therapy in other populations and for other indications may face some of the same challenges confronted by the LOTT investigators. These investigators and others who study widely used treatments in the community that lack evidence of efficacy may benefit from review of the LOTT design, methodology, and history. This article expands on the information published in the LOTT primary outcome article (7), two other LOTT-related articles (8, 9), one review article (10), and one abstract (11), uniquely provides the “story” behind the development and revision of the LOTT protocol, and shares some of the lessons learned from the LOTT experience.
Background and Scientific Premise
In the 1980s, the Nocturnal Oxygen Therapy Trial (NOTT) and Medical Research Council (MRC) randomized clinical trials demonstrated that LTOT decreases mortality among patients who have COPD and severe resting hypoxemia (3, 4). Those oxygen trial data translated into clinical practice, where Medicare coverage policies presumed that those meeting NOTT eligibility criteria would benefit from LTOT whereas those not meeting NOTT eligibility criteria would not benefit. Significant differences may exist between the patients selected for the NOTT trial and those eligible for LTOT under current Medicare regulations, so the NOTT results might lack generalizability.
After completion of the NOTT, scientists raised concerns about clinically important adverse effects of LTOT. Oxidative stress may contribute to COPD progression through molecular pathways believed to be involved in its pathogenesis (12). Individuals who have impaired upregulation of oxidant defense mechanisms or those who sporadically use oxygen may have an increased risk for toxic effects of supplemental oxygen.
Medicare covers the costs of supplemental oxygen treatment for the indication of resting, exercise, or nocturnal arterial oxyhemoglobin desaturation below 89% (or severe resting hypoxemia [i.e., PaO2 ≤ 55 mm Hg]), with some exceptions (e.g., pulmonary hypertension, polycythemia) for those with a saturation of 89% or a PaO2 of 56–59 mm Hg, and some qualifications for exercise and nocturnal desaturation. However, the evidence base for LTOT mainly came from studies of patients with COPD who had resting oxyhemoglobin desaturation (3, 4). Supplemental oxygen became widely available after the NOTT, resulting in approximately 5% of Medicare part B beneficiaries receiving home oxygen annually (13) at a high cost to Medicare (14). Reliable estimates of the number of prescriptions written for supplemental oxygen for the indication of exercise-induced desaturation are not readily available, and many patients who are prescribed supplemental oxygen may not have severe resting hypoxemia (15).
In May 2004, a working group convened by the National Heart, Lung, and Blood Institute (NHLBI, National Institutes of Health), the Centers for Medicare and Medicaid Services (CMS), and the Agency for Healthcare Research and Quality reviewed the current state of knowledge of the efficacy, the research needs, and the technical issues related to chronic supplemental oxygen therapy for COPD that might influence the feasibility and design of clinical trials of this treatment. The working group recommended the conduct of four high-priority trials that aim to answer crucial questions in the treatment of patients with COPD: Study 1, oxygen supplementation during ambulation (very high priority); Study 2, continuous oxygen supplementation in patients with moderate hypoxemia (very high priority); Study 3, nocturnal oxygen treatment of desaturation during sleep (high priority); and Study 4, detailed and individualized prescriptions for long-term oxygen supplementation (high priority) (16). The LOTT arose from this background.
Original Study Design and Subsequent Protocol Amendments
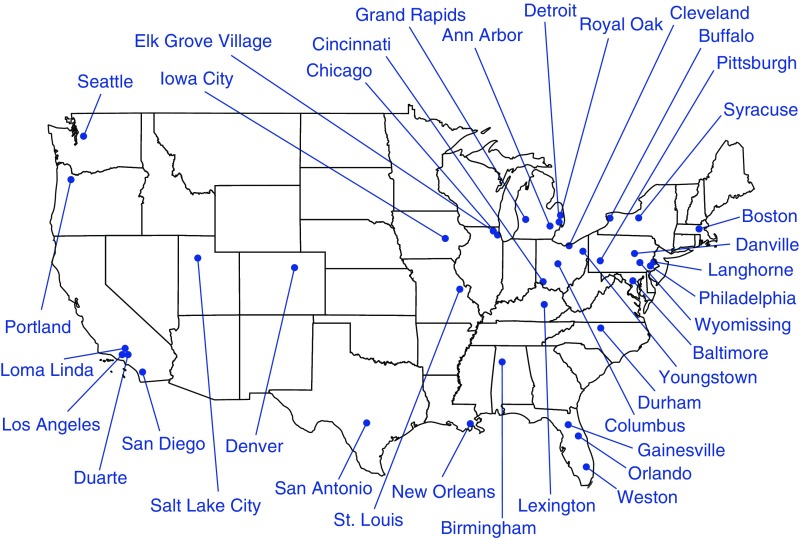
In November 2005, the NHLBI released solicitations requesting proposals for regional clinical centers and a data coordinating center (DCC) for a trial of round-the-clock oxygen therapy in people who have COPD associated with resting hypoxemia of moderate severity, increased breathlessness, and increased risk of mortality. In March 2006, the NHLBI and CMS announced their intention to work together to conduct such a trial. After peer review of the proposals, the NHLBI awarded contracts to 14 regional clinical centers and one DCC in October 2006 (Figure 1), and the investigators began the work of design and conduct of the trial. At the first Steering Committee meeting, a working group was nominated to develop eligibility criteria for the LOTT. Working from the parameters specified in the NHLBI request for proposals and from the LOTT proposals that were awarded contracts, the Steering Committee members tracked types of patients seen in their clinics, reviewed published literature, and solicited community partners, all in the pursuit of developing viable and relevant eligibility criteria. During multiple in-person meetings and many teleconferences, the Steering Committee debated each proposed criterion at the general level (e.g., smoking status [current and/or previous]) and at the specific level (e.g., threshold for resting hypoxemia, allowable FEV1 range). The committee spent a significant amount of time debating the use of oxyhemoglobin saturation by pulse oximetry (SpO2) versus use of PaO2 by arterial blood gas to determine eligibility. The protocol, manuals, forms, training, and other study preparatory activities were completed in 2008.
Figure 1.
Geographic distribution of Long-Term Oxygen Treatment Trial (LOTT) clinics. Forty-seven LOTT clinics in 37 locations screened participants for the LOTT; 42 clinics randomized at least one participant. The map figure may be viewed on the public portion of the LOTT website at www.lottsite.org.
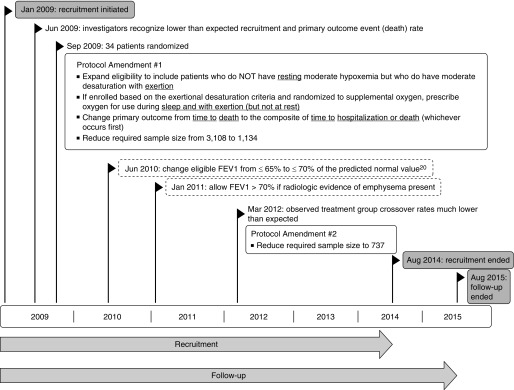
In January 2009, the LOTT initiated recruitment (Figure 2). Participants were randomly assigned to 24-hour supplemental oxygen or no supplemental oxygen. Within a few months, it became evident that fewer potential study participants than expected met the trial eligibility criteria and that investigators underestimated the difficulty in successfully recruiting for the LOTT. Many who met the study criteria were already prescribed oxygen, and a greater number than expected were unwilling to stop its use. Investigators found that the eligible patient group had a higher than expected nonvalidated oxygen prescription rate. In addition, many nonusers of supplemental oxygen were unwilling to commit to 24-hour oxygen use for their expected 4.5-year participation the trial. As investigators presented the trial to the medical community and searched for potential recruits, the need for evidence-based treatment recommendations for the numerous patients who had normal oxyhemoglobin saturation or moderate desaturation at rest but had oxyhemoglobin desaturation during exercise became more evident. In June 2009, the investigators recognized that poor recruitment might lead to trial closure. To address the recruitment concerns, the investigators felt that the trial design had to change to become more acceptable to patients and physicians. The revised trial had to have clinical relevance, and hence appeal, for the entire COPD community, for it to succeed.
Figure 2.
Long-Term Oxygen Treatment Trial protocol and study conduct timeline. Shaded objects refer to recruitment and follow-up timing. Nonshaded boxes that have a boldface outline show study protocol amendments. Dashed nonshaded boxes show additional protocol changes.
During the first year of enrollment, it also became clear that the trial would not have sufficiently high event rates for the primary outcome of survival time. Thus, the LOTT lacked feasibility in terms of successfully conducting the trial because it would require a larger sample size in a limited time frame. Because patients hospitalized with COPD have a significantly increased risk for poor outcomes (e.g., death) and supplemental oxygen could reduce hospitalization rates (e.g., fewer COPD exacerbations, fewer cardiovascular events), the investigators felt that adding hospitalization to the primary outcome had face validity and would significantly improve study feasibility.
In September 2009, after randomization of 34 patients, the LOTT investigators proposed, and the Data and Safety Monitoring Board (DSMB) approved, protocol amendment #1 (Figure 2) that expanded the LOTT eligibility criteria to allow enrollment of participants who had an adequate oxyhemoglobin saturation at rest associated with moderate oxyhemoglobin desaturation during exercise. The amended protocol also changed the primary outcome from time to death from any cause to a composite outcome of time to hospitalization for any cause or death from any cause, whichever occurred first. As under the original design, participants were randomized to supplemental oxygen or no supplemental oxygen at a 1:1 ratio; for those randomized to supplemental oxygen, the prescription was determined according to their type of desaturation. Treatment group assignment was not masked.
The study design changes of LOTT protocol amendment #1 reduced the required sample size from 3,108 to 1,134 participants. Of the participants who had adequate oxyhemoglobin saturation at rest associated with moderate exercise-induced desaturation, those randomized to supplemental oxygen were prescribed supplemental oxygen for use only during exercise and sleep. Of the participants who had moderate resting desaturation, those randomized to supplemental oxygen were prescribed 24-hour supplemental oxygen. The 34 participants randomized to treatment under the original design (all with resting hypoxemia) continued their treatment and participation seamlessly because they were part of the latter randomized group. The protocol amendment #1 changes had broadened the pool of eligible patients, expanded the primary outcome (more events), kept original recruits on their original treatment, kept original recruits as a relevant part of the transformed trial, and broadened the investigative focus and relevance for patients and health care professionals.
To assist with enrollment of patients who met the spirit of the trial, the Steering Committee implemented (June 2010 and January 2011) a few minor protocol modifications that broadened eligibility based on spirometric test results (Figure 2 and Table 1). However, the least restrictive spirometric eligibility criteria also required radiologic evidence of emphysema.
Table 1.
Long-Term Oxygen Treatment Trial participant eligibility criteria
| Inclusion (All Must Be Met) |
| • COPD-dominated lung disease |
| • Age at least 40 yr |
| • At least 10–pack-year cigarette-smoking history |
| • Modified Medical Research Council dyspnea score ≥ 1 (short of breath when hurrying on the level or when walking up a slight hill) (21, 22) |
| • Post-bronchodilator FEV1/FVC < 0.70 |
| • Post-bronchodilator FEV11) ≤70% of the predicted normal value (20) or 2) >70% of the predicted normal value (20) if study physician determines that there is radiologic evidence of emphysema |
| • Resting SpO2 89–93% (moderate resting hypoxemia) or resting SpO2 ≥ 94% and desaturation during exercise defined as SpO2 < 90% for at least 10 consecutive s during the 6-min walk test (normal resting saturation but moderate hypoxemia with exercise) |
| • Medicare Part A and Part B beneficiary, insurance willing to pay costs of treatment and study procedures and visits, or willing to self-pay costs |
| • Approval by study physician for randomization to either treatment group |
| • No exacerbation requiring antibiotics or new/increased dose of systemic corticosteroids in the 30 d before screening |
| • Not less than 30 d postdischarge from an acute care hospital (for COPD or other condition) before screening |
| • If participant regularly uses supplemental oxygen before screening, all of the following must be met before randomization: |
| - Participant agrees to stop using supplemental oxygen if randomized to no supplemental oxygen |
| - Participant’s physician agrees in writing to rescind order for supplemental oxygen if participant is randomized to no supplemental oxygen |
| - Participant must not use supplemental oxygen for the four calendar days before randomization and must report that he/she had no problems doing without the oxygen |
| • Signature of written contract agreeing not to smoke while using supplemental oxygen |
| Exclusion (None May Be Met) |
| • COPD exacerbation requiring antibiotics, new or increased dose of systemic corticosteroids, or oxygen treatment after screening starts and before randomization (chronic use of corticosteroids while health is stable is not exclusionary) |
| • New prescription of supplemental oxygen after screening starts and before randomization |
| • Thoracic surgery or other procedure in the 6 mo before evaluation likely to cause instability of pulmonary status |
| • Non-COPD lung disease that would affect oxygenation or survival |
| • Epworth Sleepiness Scale (23) score greater than 15 |
| • SpO2 below 80% for at least 1 consecutive minute during the 6-min walk |
| • Disease or condition expected to cause death or inability to perform procedures for the trial or inability to comply with therapy within 6 mo of randomization, as judged by study physician |
| • Participation in another intervention study |
In March 2012, it became evident to the LOTT DSMB that the observed treatment group crossover rates were much lower than the rates assumed for design of the trial. As a result, the DSMB approved protocol amendment #2 that reduced the final sample size to 737 participants (Figure 2).
Final Study Design
Two protocol amendments, and a few protocol modifications, led to the final LOTT study design; a multicenter, randomized clinical trial that tested whether supplemental oxygen therapy versus no supplemental oxygen therapy decreases time to the composite event (Table 2), all-cause hospital admission or all-cause mortality, whichever occurs first, among individuals with COPD who have moderate oxyhemoglobin desaturation at rest or normal oxyhemoglobin saturation at rest associated with moderate oxyhemoglobin desaturation during exercise.
Table 2.
Long-Term Oxygen Treatment Trial hypotheses
| For patients who have COPD and 1) moderate resting desaturation or 2) normal resting saturation and moderate desaturation during exercise, treatment with supplemental oxygen, in comparison with no treatment with supplemental oxygen, will lead to: |
| Primary |
| Ha 1: Increased time to all-cause mortality or all-cause hospitalization |
| Secondary |
| Ha 2: Increased time to all-cause mortality |
| Ha 3: Increased time to all-cause hospitalization |
| Ha 4: Improved disease-specific quality of life |
| Ha 5: Improved preference-weighted health-related quality of life |
| Ha 6: Decreased disease impact (e.g., reduced dyspnea, longer 6-min walk distance, reduced COPD exacerbation rate) |
| Ha 7: Improved quality-adjusted survival |
| Ha 8: Lower health care utilization |
| Ha 9: Better maintenance of nutritional status (e.g., body mass index) |
| Ha 10: Improved general quality of life |
| Ha 11: Better sleep quality |
| Ha 12: Less depression and less anxiety |
| Ha 13: Delayed onset of severe hypoxemia (defined as room air SpO2 88% or less) |
| Ha 14: Lower risk of cardiovascular disease outcomes (e.g., acute coronary syndrome, chronic heart failure exacerbation, mortality secondary to these outcomes) |
| Ha 15: Longer survival and better outcomes in those with greater adherence to supplemental oxygen use in comparison with those with lesser adherence |
| Exploratory |
| Analyses will be performed to test the consistency of treatment effects across subgroups defined by baseline demographic and clinical characteristics. Subgroups to be examined include but are not limited to those defined by age, race/ethnicity, sex, oxyhemoglobin saturation during exercise, lung function (e.g., FEV1), and smoking status |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; SpO2 = oxyhemoglobin saturation by pulse oximetry.
Bold type items added as protocol amendment #1 (see Figure 2).
The LOTT completed recruitment in August 2014 and ended participant follow-up in August 2015 (Figure 2). Each participant had a minimum possible follow-up of 1 year and a maximum possible follow-up of 6.5 years; follow-up duration depended on the date of enrollment, survival, and willingness to continue with the trial.
The LOTT is registered with ClinicalTrials.gov (17). The design issues reported herein meet the requirements of the CONSORT (Consolidated Standards of Reporting Trials) checklist (18).
Study Entities and Collaborations
The collaboration between the NHLBI and CMS supported the LOTT. The NHLBI had responsibility for administration of the trial and the sites conducting the trial. Medicare covered the costs of the treatment and clinical procedures for their beneficiaries under the National Coverage Determination effective March 2006 that extended coverage for home oxygen use to Medicare beneficiaries participating in trials approved by the CMS and sponsored by the NHLBI (19). The trial enrolled Medicare beneficiaries, those who had non-Medicare insurance that covered the costs of the treatment and clinical procedures for the trial, and those willing to pay out of pocket for those costs. Medicare-approved suppliers provided the oxygen equipment. The clinical sites, DCC, NHLBI, and CMS conducted the trial.
Setting
To assist with a broad recruitment effort, the NHLBI structured the LOTT clinical sites to consist of regional clinical centers and additional satellite sites (Figure 1). Each regional clinical center director decided whether to recruit satellite sites to assist with meeting the regional clinical center’s recruitment goal. Although the regional clinical centers were all academic medical centers, the satellite sites included a mixture of academic medical centers, Veterans Affairs medical centers, and community-based private practices. Eleven of the 14 regional clinical centers recruited at least one satellite site; satellite sites contributed 54% of the LOTT study population.
Patients and Eligibility
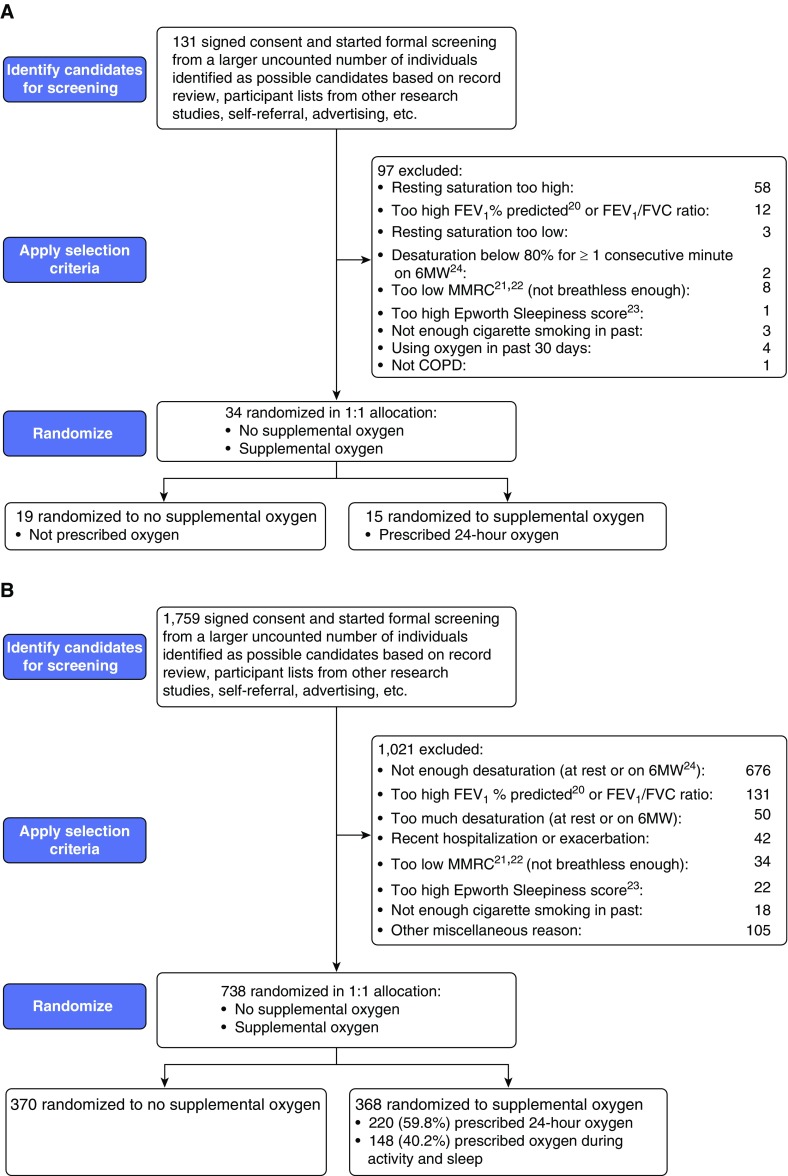
The LOTT required participants to have COPD as the primary pulmonary disorder, with an FEV1/FVC ratio less than 0.70 and a post-bronchodilator FEV1 not exceeding 70% of the predicted normal value (20) (see Figure 2 regarding protocol revisions). Eligibility also required the presence of dyspnea (i.e., modified Medical Research Council [MMRC] dyspnea scale score ≥ 1) (21, 22), a cigarette-smoking history of at least 10 pack-years, and age of at least 40 years (Table 1; Figures 2 and 3; and Appendices E1 and E2 in the online supplement). Participants had to be clinically stable (i.e., no treatment for a COPD exacerbation and no increase in dose of systemic corticosteroids within the last 30 d) to initiate screening. Eligibility determination at the screening visit required 1) a resting SpO2 of 89–93%, or 2) the combination of resting SpO2 greater than 93% and SpO2 not exceeding 90% for a duration of 10 seconds or more during a 6-minute walk test. However, the LOTT excluded patients who had an SpO2 less than 80% for 1 minute or longer during the 6-minute walk test.
Figure 3.
CONSORT (Consolidated Standards of Reporting Trials [18]) diagrams for the Long-Term Oxygen Treatment Trial: (A) through September 2009 (original protocol) and (B) through end of recruitment in August 2014 (final protocol). 6MW = 6-minute walk; COPD = chronic obstructive pulmonary disease; MMRC = Modified Medical Research Council. Panel B was adapted by permission from Reference 7.
Study staff assessed SpO2 (Appendices E1 and E2) with the Masimo Radical-7 pulse oximeter and Masimo Rainbow DCI-dc3 finger sensor or LNCS TF-1 forehead sensor. The trial did not use PaO2 for eligibility determination in an effort to make the trial protocol exportable to common practice and to avoid a painful procedure for prospective participants.
Each participating site had institutional review board approval of the LOTT. All participants provided written informed consent before undergoing screening.
Data Collection
To increase the acceptance of the LOTT program by both patients and satellite sites, the LOTT used a two-tier level of data collection requirements defined as Core and Expanded (Table 3; Appendices E1 and E2) (20–30). Expanded data collection included all Core data collection plus three additional questionnaires (i.e., Short Form-36 Quality of Life Scale [27], Hospital Anxiety and Depression Scale [29], Pittsburgh Sleep Quality Index [28]) at baseline and annual follow-up evaluations, additional procedures at baseline (collection of serum for banking and alpha-1 antitrypsin testing [30]), and an additional procedure in follow-up (i.e., spirometry). All regional clinical centers were expected to complete Expanded data collection; satellite sites could choose to complete Core or Expanded data collection.
Table 3.
Long-Term Oxygen Treatment Trial data collection schedule
| Follow-Up |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | RZ | Year 1 |
Year 2 |
Year 3* |
|||||||
| Months from Randomization: |
−2 |
0 |
4 |
8 |
12 |
16 |
20 |
24 |
28 |
32 |
36 |
| Type of Visit: | C | C | T, M | T | C | T, M | T | C | T | T | C |
| Core (all participants, all sites)† | |||||||||||
| History | X | X | XT | XT | X | XT | XT | X | XT | XT | X |
| Room air resting oximetry | X | X | X | X | |||||||
| Room air 6MW (24) with oximetry | X | X | X | X | |||||||
| Ambulatory dosing‡ | X | X | X | X | |||||||
| FEV1, FVC | X | ||||||||||
| Height | X | ||||||||||
| Weight and pretibial pitting edema | X | X | X | X | |||||||
| Hemoglobin and hematocrit | X | ||||||||||
| Cotinine (if not using nicotine products) | X | X | |||||||||
| Epworth Sleepiness Scale (23) | X | ||||||||||
| MMRC dyspnea score (21, 22) | X | X | X | X | |||||||
| St. George’s Respiratory Questionnaire (25) | X | XM | X | XM | X | X | |||||
| Quality of Well-Being Scale (26) | X | XM | X | XM | X | X | |||||
| Expanded (participants at selected sites) | |||||||||||
| Short Form-36 Quality of Life Scale (27) | X | X | X | X | |||||||
| Pittsburgh Sleep Quality Index (28) | X | X | X | X | |||||||
| Hospital Anxiety and Depression Scale (29) | X | X | X | X | |||||||
| FEV1, FVC | X | X | X | ||||||||
| Alpha-1 antitrypsin level and phenotype (30) | X | ||||||||||
Definition of abbreviations: 6MW = 6-minute walk; BL = baseline; C = clinic; M = mail; MMRC = Modified Medical Research Council; RZ = randomization; T = telephone.
Years 4, 5, and 6 follow the same pattern of data collection.
Table does not show adherence program schedule (see Table 4).
For participants randomized to supplemental oxygen.
Adapted by permission from Reference 7.
Screening
The screening/baseline visit included informed consent activities, protocol-required testing (Table 3), assessment of patient eligibility (Table 1), and participant characterization. The protocol required that a participant who regularly used supplemental oxygen during screening had to stop using it for the four calendar days before randomization and had to report before randomization that he/she had no problems functioning without the oxygen.
Randomization
The protocol required that study participants undergo randomization within 60 days of starting screening. The LOTT used a web-based data system to randomly assign participants to the supplemental oxygen or no supplemental oxygen group at a 1:1 ratio. The randomization schedule was stratified by regional clinical center, with randomly permuted blocks of sizes 2, 4, and 6. The data system generated the random treatment assignment for a participant only if the data entered passed the computerized checks for completeness and conformance with the eligibility criteria.
Postrandomization Visits and Follow-Up
Participants randomly assigned to supplemental oxygen underwent an oxygen education and titration visit within 1 week of being randomized. Study personnel asked participants who were already using portable oxygen to bring their own equipment for the ambulatory oxygen dose titration (see Interventions).
Trained adherence educators telephoned participants randomized to the supplemental oxygen treatment group weekly for the first month after randomization, monthly for the next 5 months, and then every 2 months until their first annual visit (Table 4; Appendix E2 [LOTT protocol]); the calls provided an opportunity to address barriers to use of oxygen and to assess adherence to oxygen treatment (see Interventions). The adherence educators also telephoned participants assigned to no supplemental oxygen within 1 week of randomization to address study participation issues and to address concerns about the treatment assignment.
Table 4.
Components of Long-Term Oxygen Treatment Trial adherence program for participants randomized to supplemental oxygen therapy
| Adherence educator |
| • Trained and certified in motivational interviewing |
| • Help participant realize/calculate/recognize/determine his/her personal level of importance of oxygen |
| • Help participant articulate/identify his/her personal barriers to adherence and devise solutions |
| • Problem solve/troubleshoot, aiming at participant’s problems with adherence |
| Adherence promotion contacts after treatment assignment |
| • Within first week (in person) |
| • Weekly thereafter through 1 mo (telephone) |
| • Monthly thereafter through 6 mo (telephone) |
| • Every 2 mo thereafter through 12 mo (telephone) |
| • Yearly thereafter (in person) |
| Supplemental oxygen usage reporting |
| • Logs filled out by participant (could be supplemented by information obtained from oxygen suppliers), from randomization through end of follow-up and collected every 2 mo |
| – Meter readings (oxygen concentrator) |
| – Tank counts (gas tanks or liquid tanks) |
| – Pounds of oxygen delivered (liquid systems) |
| • Interview report of average daily hours of use in last week, at each adherence promotion contact |
Postrandomization contacts for all participants also included 1) annual follow-up visits and 2) telephone visits in the intervening 4-month intervals (Table 3). Study coordinators encouraged participants to contact them if any changes in health occurred between these visits. Participants also underwent health-related quality of life assessment via mailed questionnaires at 4 and 16 months after randomization.
Interventions
LOTT investigators prescribed a stationary oxygen system and a portable oxygen system for each participant randomized to supplemental oxygen. The protocol specified that participants randomized to supplemental oxygen who had a moderate degree of resting desaturation (SpO2 89–93%) while breathing room air at screening use supplemental oxygen continuously; oxygen prescription at rest and during sleep consisted of a flow rate of 2 L/min. An oxygen titration assessment determined the flow prescription during physical activity. During the assessment, the study coordinator instructed each participant to walk at his/her usual pace. If the SpO2 dropped below 90% during the first 2 minutes, the coordinator increased the oxygen dose from a setting of 2 L/min (or an oxygen conserver setting of 2) by 1-L/min increments (or oxygen conserver increments of 1) until the dose kept the participant’s SpO2 at 90% or more for the next 2 minutes of walking. Thus, the titration required a minimum walking duration of 2 minutes and lasted until 2 minutes after the last oxygen flow rate change, to a maximum of 10 minutes.
Participants randomized to supplemental oxygen who had a resting SpO2 greater than 93% while breathing room air at screening (i.e., eligible under the exercise desaturation criterion) were instructed to use supplemental oxygen during physical activity (at the flow rate determined during the titration) and during sleep (at a flow rate of 2 L/min).
Participants randomized to supplemental oxygen received the LOTT adherence promotion program (Table 4 and Appendix E2). During the regularly scheduled contacts, the adherence educators provided assistance with questions and/or concerns about oxygen use. Thus, the study bundled the randomized supplemental oxygen therapy with adherence promotion.
The LOTT protocol (see Appendix E2) specified that each participant randomized to the no supplemental oxygen group avoid using supplemental oxygen unless he/she developed severe resting desaturation (SpO2 ≤ 88%) and/or severe desaturation with exercise (SpO2 < 80% for at least 1 min). If either of these severe desaturations developed, the participant was prescribed supplemental oxygen, and the study staff requested that the participant undergo a reassessment, after more than 30 days had elapsed, to determine the need for supplemental oxygen. If the severe desaturation persisted on reassessment, then staff asked the participant to continue using the supplemental oxygen until the next annual reassessment. For the participants assigned to the supplemental oxygen group, the protocol specified that supplemental oxygen use continue regardless of improvements in SpO2 subsequent to randomization.
The LOTT did not include a placebo group. The investigators felt that the objective primary outcome(s) used in the study (death and hospitalization) would not be significantly affected by the nonblinded treatment. Also, practically, the study did not have the budget to pay for sham oxygen and the associated supplies and services.
LOTT investigators encouraged all participants to avoid cigarette smoking. Before randomization, the protocol required each participant to sign an agreement not to smoke while using oxygen. LOTT investigators also encouraged optimization of medical therapy with treatments that included, but were not limited to, inhaled bronchodilators, vaccinations, and pulmonary rehabilitation.
Adherence Assessment
A study coordinator interviewed each participant regarding supplemental oxygen use, regardless of treatment assignment, at each study visit (Table 3). In addition, the scheduled telephone adherence promotion contacts completed by participants randomized to supplemental oxygen included the participant’s self-report of average hours of supplemental oxygen use in the last week. For each participant in the supplemental oxygen group, a study coordinator collected the written usage log (tailored to the participant’s type of oxygen equipment) every 2 months for the duration of the trial (Appendices E1 and E2).
Outcome Assessment
For the study’s composite primary outcome components of death and hospitalization, study personnel used multiple sources of information for verification (Appendices E1 and E2). The study did not require blinded assessment of outcomes because it used objective primary outcome components and self-completion questionnaires for secondary outcomes, and this approach would have required additional personnel and introduced additional costs. Study sites reported adverse events according to a standardized approach (Appendices E1 and E2).The study specifically assessed adverse events related to study participation and supplemental oxygen use.
Sample Size and Power
The original sample size for the LOTT of 3,108 participants was modified through two protocol amendments approved by the LOTT DSMB to a final sample size of 737 participants (Figures 2 and 3). Changing the primary outcome from time to all-cause mortality to the composite of time to all-cause hospitalization or all-cause mortality, whichever occurred first, allowed a sample size reduction from 3,108 to 1,134 participants. Recognition that the observed overall treatment crossover rates (11.7% crossovers from no supplemental oxygen to supplemental oxygen and 3.1% crossovers from supplemental oxygen to no supplemental oxygen) were much less than the overall rates assumed (21% and 50%, respectively) for the initial sample size allowed further refinement of the required sample size. The SIZE design software program (31) estimated that the study would require a final sample of 737 patients, based on a time to composite event survival model, the log rank test statistic, a hazard ratio of 0.60 (supplemental oxygen group vs. no supplemental oxygen group), a two-sided type I error rate of 0.05, statistical power of 0.90, and the treatment group crossover rates observed through January 2012. The hazard ratio of 0.60 corresponded to the smallest difference in mortality that the investigators judged clinically worthwhile (a 40% lower rate in the supplemental oxygen group than in the no supplemental oxygen group), on the basis of the number of patients needed to treat, as determined in a pretrial survey of the Steering Committee. Because of the cost and burden associated with supplemental oxygen therapy, the Steering Committee also deemed the hazard ratio of 0.60 as appropriate for the composite primary outcome. For the no supplemental oxygen group, we assumed a 33% annual hospitalization rate in those with and a 10% annual rate in those without a recent (i.e., past year) COPD hospitalization (Appendices E1 and E2). We also assumed a 7% annual mortality rate in those with, and a 6% rate in those without, a recent COPD hospitalization. We therefore estimated a 28% composite event rate per year in the no supplemental oxygen group. We assumed that the loss to composite outcome follow-up would only be 1% of those randomized, because we would confirm and supplement direct mortality and hospitalization ascertainment by clinic staff with searches of the Social Security Administration Master Death File (32).
The secondary objectives of the LOTT included the assessment of treatment group differences with respect to each component of the primary outcome: all-cause mortality (power, 0.39) and all-cause hospitalization (power, 0.82). Other objectives included a comparison of the treatment groups with respect to preference-weighted health-related quality of life, disease-specific quality of life, and other outcomes of interest (Tables 2 and 5). Overall, the LOTT had exceptionally high power to assess these outcomes, because they involve continuous or semicontinuous outcome measures, rather than time-to-event outcomes. The study protocol also specified that the LOTT would explore the consistency of the treatment effects across subgroups defined by baseline demographic and clinical characteristics. Subgroups to be examined included but were not limited to those defined by age, race/ethnicity, sex, oxygen saturation during exercise, lung function, and smoking status.
Table 5.
Long-Term Oxygen Treatment Trial outcomes
| Primary |
| • Death from any cause or first hospitalization from any cause |
| Secondary |
| • Death from any cause |
| • Hospitalization from any cause |
| • Quality of life and symptoms |
| – St. George’s Respiratory Questionnaire (25) |
| – Quality of Well-Being Scale (26) |
| – Modified Medical Research Council Dyspnea score (21, 22) |
| – SF-36 (27) (Expanded data collection) |
| – Hospital Anxiety and Depression Scale (29) (Expanded data collection) |
| – Pittsburgh Sleep Quality Index (28) (Expanded data collection) |
| • Medical and pulmonary |
| – COPD exacerbations |
| – Health care utilization |
| – Pretibial edema |
| – Nutritional status |
| – Development of severe resting desaturation |
| – Development of severe exercise desaturation |
| – Spirometry values (Expanded data collection) |
| • Exercise performance |
| – 6-min walk distance (24) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; SF-36 = Short Form-36 Quality of Life Scale.
Bold type item added as protocol amendment #1 (see Figure 2).
Statistical Monitoring
A multidisciplinary, independent DSMB appointed by the NHLBI had responsibility for monitoring the quality of the data and protecting the safety of patients enrolled in the LOTT. The responsibilities and operating characteristics of the DSMB followed the NHLBI guidelines for DSMBs (33).
The DSMB made recommendations to the NHLBI regarding continuation or discontinuation of the trial. To assist in the interpretation of the primary outcome, the protocol specified stopping guidelines using a total α error of 0.05. The α spending function approach of Lan and DeMets (34), combined with an O’Brien–Fleming (35) boundary for efficacy and a Fleming–Harrington–O’Brien (36) boundary for futility, was used to define asymmetric boundaries for efficacy and futility for a sequence of normalized z-scores from interim log rank statistics that compared time to the composite outcome in the supplemental oxygen group versus the no supplemental oxygen group (37). In January 2014, with 663 of 738 patients (90%) randomized, the DSMB changed from sequential asymmetric boundaries for both efficacy and futility to sequential symmetric Lan and DeMets/O’Brien–Fleming (34, 35) boundaries for efficacy and harm, and futility monitoring was stopped. The DSMB noted that extension of the recruitment period implied extension of the planned follow-up time, which meant more primary outcome events than originally determined were expected to occur (500 expected events, increased from 351 expected events). This time extension necessitated revision of the O’Brien–Fleming interim monitoring boundaries for efficacy. In addition, the DSMB was of the opinion that the trial should be allowed to finish given its uniqueness and the low likelihood of a similar trial being done in the future. The DSMB also believed that demonstration of a reduction in risk of the primary outcome of lower than the design parameter (i.e., 40% reduction for those prescribed supplemental oxygen vs. those not prescribed supplemental oxygen) would have value to the medical community. Hence, after discussion, the DSMB approved going forward with use of symmetric O’Brien–Fleming boundaries for both efficacy and harm that were corrected for efficacy α error already spent, and replacing futility monitoring with periodic calculations of conditional power (38).
The DSMB evaluated in a masked fashion, with the option to unmask on request, the observed data in relation to these guidelines for consideration of 1) early termination or modification of the trial due to demonstrated benefit associated with upper boundary crossing, 2) early termination or modification of the trial due to demonstrated harm associated with lower boundary crossing, or 3) unmodified continuation of the trial.
Quality Assurance
The LOTT utilized extensive quality control procedures. Site personnel underwent training and certification for many study-related procedures, including the conduct of adherence phone calls. The DCC implemented multiple data quality control procedures, monitored site performance, and provided feedback to sites.
Discussion
The LOTT further defined the benefits and risks of LTOT and its indications in patients who have COPD and resting and/or exercise-induced moderate desaturation. Specifically, the LOTT addressed whether the provision of supplemental oxygen treatment reduces time to hospital admission and/or death for these patients.
To address limitations of previous studies of LTOT, such as female underrepresentation, small sample size, limited ability to do appropriately powered subgroup analyses, and poor generalizability based on narrow eligibility criteria (6), the LOTT utilized a relatively broad set of eligibility criteria, a large sample size, and a fairly broad range of participants that allowed for meaningful subgroup analyses. The LOTT chose to use the practical and more common approach of pulse oximetry for arterial oxygenation assessment, instead of the more burdensome arterial blood gas analysis approach, and the protocol included multiple quality control procedures to ensure use of reliable and accurate oximetry data. The two primary inclusion criteria related to resting and exercise-associated moderate desaturation allowed the LOTT to answer important questions about two primary indications for LTOT. The LOTT had good power for assessing multiple important secondary endpoints.
For LOTT participants randomized to receive supplemental oxygen, the treatment also included an education/training/adherence promotion program. All participants had to commit to avoid cigarette smoking during oxygen use. The LOTT investigators believe that supplemental oxygen prescription in the “real world” should include such interventions.
The Steering Committee dealt with many challenges when designing and conducting the LOTT. The investigators spent much time debating eligibility criteria, specifics of the oxygen intervention, primary and secondary outcomes, and other aspects of the protocol. Some challenges became quite apparent during the conduct of the trial, and some led to important amendments in trial design.
The initial strict eligibility criteria limited study enrollment, so the protocol quickly underwent a revision to broaden the types of patients allowed into the trial. Because the revision occurred early in the trial, it did not affect the quality of the data or the ability to interpret the outcomes, and it improved the generalizability of the trial while also improving its feasibility.
The LOTT experience demonstrated the importance of palatability of the study to the potential participants and their health care providers because of its effects on study eligibility and related feasibility and efficiency implications. Investigators learned to better consider the mindset and willingness of the health care providers to support the conduct of the trial. Investigators also learned that the eligibility criteria may affect adherence to prescribed therapy based on the types of patients that enroll in trials.
The LOTT also demonstrated the negative effects of unrealistic investigator optimism regarding patient recruitment. The experience supports the approach of monitoring for, identifying, discussing, and addressing recruitment concerns as early as possible and throughout the trial. This approach would also allow investigators to anticipate criticism/skepticism from the sponsor and the DSMB. The experience suggested that development (and implementation) of a toolbox of recruitment methods, and nonreliance on a single “savior” method, would help to optimize recruitment. The investigators learned that trial design should be based on an understanding of patient concerns, and that programs should be implemented to improve patient engagement. In addition, a pilot study could have identified recruitment challenges and eligibility criteria problems, although the investigators felt that adequate data from other smaller studies already existed.
To address recruitment issues, instead of only utilizing primary clinical sites, the LOTT utilized satellite sites. The initial satellite network concept had the primary site as the hub and geographically close satellites as the spokes. However, the satellite concept became less geographically restrictive over time. Networks of primary and satellite sites developed on the basis of personal relationships between investigators or happenstance networking. The LOTT recruited and activated satellite sites throughout the 5.5 years of recruitment, although all certified sites did not successfully screen and randomize a participant. The LOTT satellite sites contributed 54% of the randomized population. From the satellite experience, the LOTT investigators learned to better engage the community, to provide meaningful involvement for community investigators, to be open to suggestions about developing flexible recruitment infrastructure, and to anticipate the long processes of negotiations and contracting and the need to address them early.
To improve study feasibility, knowing that hospitalization of patients with COPD significantly increases the risk of death (39), the investigators changed the primary outcome to a composite of time to hospital admission or death (whichever occurs first). This change occurred early in the LOTT timeline, and it allowed for a significant reduction in the required sample size. Lower than expected crossover rates during the trial allowed for a further reduction in the required sample size, although the LOTT methodology did not include an adaptive design clinical trial (40). Because none of the protocol amendments involved an evaluation of outcome data, these protocol changes did not introduce statistical biases into the trial (40).
The LOTT investigators made some incorrect assumptions about outcome event rates. During the design process, the Steering Committee reviewed published literature, solicited expert opinion, and reviewed local research databases for data on mortality in the COPD population of interest, and generated a 6% (range, 4–8%) mortality per year consensus estimate. The Steering Committee consisted of individuals with expertise in COPD, extensive experience in caring for patients who have COPD, and a track record of conducting clinical trials in patient who have COPD. The experience reiterated to investigators that people who enroll in trials (or who are advised to enroll in trials by caregivers) may differ in subtle ways from the general population, and the event rate estimates derived from general populations may not replicate in the trial population. The investigators learned a similar lesson regarding expected versus observed treatment crossover rates. Because the choice of the primary outcome and the estimates of the outcome event rates and the crossover rates have significant effects on sample size requirements and the power of the trial, investigators were reminded of the importance of pretrial scrutiny of these issues and their supporting data and the need for accurate rate estimates.
The LOTT did not mask study personnel or participants to randomized treatment assignment. The Steering Committee discussed possible ways to attempt masking, but ultimately decided that masking had a low likelihood of success and lacked feasibility. Because unmasked treatment assignment would likely not affect assessment of the trial’s relatively objective primary endpoint components of hospitalization and death, the Steering Committee also did not require masking of assessors (those collecting the data). The LOTT did not use masked adjudication of outcomes because the trial used objective criteria for determining their presence or absence. The nonmasked treatment assignment could have potentially affected some of the secondary outcomes, however.
In summary, the LOTT investigators conducted the largest randomized clinical trial of supplemental oxygen use to date. The results of the LOTT further clarified the indications for supplemental oxygen use. Lessons learned in the design and conduct of the LOTT will assist future trials that will assess the efficacy and safety of LTOT and other interventions.
Supplementary Material
Acknowledgments
Members of the LOTT Research Group (as of December 2015) are as follows:
Office of the Chair of the Steering Committee, University of Alabama, Birmingham: William C. Bailey, M.D.
Regional clinical centers:
Brigham and Women’s Hospital: Anne L. Fuhlbrigge, M.D.; Ernestina Sampong
Associated sites: Boston Medical Center: Karin Sloan, M.D.; Ashley Wagner; Susan Anderson
Boston VA: Marilyn Moy, M.D.; Osarenoma Okunbor
Cleveland Clinic Foundation: James K. Stoller, M.D., M.S. (Principal Investigator) ; Scott Marlow, R.R.T., Yvonne Meli, R.N., Richard Rice, R.R.T., M.Ed. (Study Coordinators); Loutfi S. Aboussouan, M.D., Robert Castele, M.D., Joseph Parambil, M.D., Sumita Khatri, M.D., Aman Pande, M.D., Joe Zein, M.D., Thomas Olbrych, M.D. (Co-Investigators)
Associated sites: Crouse Medical Practice: Stephan Alkins, M.D.; Christine Jocko, M.A.
Cleveland Clinic Florida: Franck Rahaghi, M.D., M.H. ; Jean Barton, M.B.A.
Denver Health and Hospital Authority: Richard K. Albert, M.D.; Jennifer Underwood
Associated sites: National Jewish Health: Barry Make, M.D., F.A.C.P., F.C.C.P., F.A.A.C.V.P.R.; Jennifer Underwood
Duke University: Neil MacIntyre, M.D.; John Davies
Kaiser Foundation Hospitals: Thomas Stibolt, M.D.; Richard Mularski, M.D.; Allison Naleway, Ph.D.; Sarah Vertrees
Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center: Richard Casaburi, Ph.D., M.D.; Janos Porszasz, M.D., Ph.D.; Peggy Walker, R.R.T.; Renee Indelicato
Associated sites: Loma Linda VA: Lennard Specht, M.D.; Kathleen Ellstrom, Ph.D., R.N.; Jamie Portillo, R.R.T.
City of Hope National Medical Center: David Horak, M.D.; Brian Tiep, M.D.; Mary Barnett, R.N.
Ohio State University: Philip Diaz, M.D.; Janice Drake; Mahasti Rittinger; Rachael Compton, Scott Miller
Associated site: University of Cincinnati: Ralph J. Panos, M.D.; Laura A. Lach, B.H.S.
Temple University: Gerard Criner, M.D.; Carla Grabianowski, B.S.N., R.N., C.C.R.P ; Francis Cordova, M.D.; Parag Desai, M.D.; Samuel Krachman, D.O.; James Mamary, M.D.; Nathaniel Marchetti, M.D.; Aditi Satti, M.D.; Eileen Mumm, C.R.N.P.; Michelle Vega-Olivo, C.R.N.P.; Jenny Hua; Vanna Tauch; Lii-Yoong Criner, R.N., C.C.R.C.; Michael Jacobs, Pharm.D.; Peter Rising, M.S.
Associated sites: Geisinger Institute: Paul Simonelli, M.D.; Michele Mitchell, B.S.N., R.N., C.C.R.C.
Louisiana State University: Matthew Lammi, M.D.; Connie Romaine, M.S.N., A.P.R.N.-N.P.-C.
Institute for Respiratory and Sleep Medicine: Howard Lee, M.D.; Mary Ianacone, D.O.
University of Maryland: Steven Scharf, M.D., Ph.D.; Wanda Bell-Farrell
Buffalo VA: M. Jeffery Mador, M.D.; Ayesha Rahman, M.S.
Respiratory Specialists: Mumtaz Zaman, M.D.; Lisa Hill, L.P.N., C.R.C.; Alec Platt, M.D.
University of Alabama: J. Allen Cooper, Jr., M.D.; Kathleen Harrington, Ph.D., M.P.H.; Mark Dransfield, M.D.; Patti Smith, R.N.; Donald Davis
Associated sites: Birmingham VA: J. Allen Cooper, Jr., M.D.; Patti Smith, R.N.
North Florida/South Georgia VA: Peruvemba Sriram, M.D.; Katherine Herring
University of Michigan: Steven Gay, M.D.; Fernando Martinez, M.D., M.S.; Meilan Han, M.D.; Kelly Rysso; Catherine Meldrum, Ph.D., R.N., M.S., C.C.R.C.
Associated sites: Beaumont Hospital: K. P. Ravikrishnan, M.D.; Daniel Keena, M.D.; Jennifer DeRidder, R.N.; Beth Kring, C.N.M., C.C.R.C.
San Antonio VA: Antonio Anzueto, M.D.; Alex Aguilera; Timothy Houlihan, R.N.
Spectrum Health: Reda Girgis, M.D.; Jennifer Cannestra, R.N., B.S.N.
University of Pittsburgh: Frank Sciurba, M.D.; Benjamin Kelly
University of Utah: Richard Kanner, M.D.; Mary Beth Scholand, M.D.; G. Martin Villegas; Judy Carle
University of Washington: David H. Au, M.D., M.S.; Edmunds Udris, M.P.H.
Associated sites: Harborview Medical Center: Randall Curtis, M.D., M.P.H.
VA Puget Sound HCS: David Au, M.D., M.S.; Laura C. Feemster, M.D, M.S.; Richard Goodman, M.D.; Brianna Moss, B.S.; Lynn Reinke, Ph.D., A.R.N.P. ; Edmunds Udris, M.P.H.
University of Washington Medical Center: Moira Aitken, M.D.; Bruce Culver, M.D.
Washington University: Roger D. Yusen, M.D., M.P.H.; Mario Castro, M.D., M.P.H. ; Brigitte Mittler, B.A.; Jeanne Heaghney, R.N.
Associated sites: Pulmonary Consultants/Christian Hospital: Myron Jacobs, M.D.
University of Illinois at Chicago: Min Joo, M.D., M.P.H.; Nina Bracken, A.P.N.
Suburban Lung Associates: Edward Diamond, M.D.; Mary K. Joseph, Ph.D.
University of California, San Diego: Xavier Soler, M.D., Ph.D.; Arianna Villa, B.S., R.R.T.
Central Florida Pulmonary Group: Daniel Layish, M.D.
Biospecimen Repository, Channing Division of Network Medicine, Brigham and Women’s Hospital: Edwin Silverman, M.D., Ph.D.; Roxanne Kelly, B.S., M.B.A.; Daniel Cossette, B.S.
Data Coordinating Center, Johns Hopkins University: James Tonascia, Ph.D.; Patricia Belt, Amanda Blackford, Sc.M.; Betty Collison; John Dodge; Michele Donithan, M.H.S.; Cathleen Ewing; Rosetta Jackson; K Patrick May, M.S.; Jill Meinert; Steven Piantadosi, M.D., Ph.D.; Girlie Reyes, B.S.; David Shade, J.D.; Michael Smith, B.S.; Alice L. Sternberg, Sc.M.; Mark Van Natta, M.H.S.; Laura Wilson, Sc.M.; Annette Wagoner; Robert Wise, M.D.; Katherine P. Yates, Sc.M.
Centers for Medicare and Medicaid Services: Rosemarie Hakim, Ph.D.
National Heart, Lung, and Blood Institute: Antonello Punturieri, M.D., Ph.D. ; Julie Bamdad, M.S.E.; Thomas Croxton, Ph.D., M.D.; Joanne Deshler; Pamela McCord-Reynolds; Mario Stylianou, Ph.D.; Gail Weinmann, M.D (DSMB executive secretary)
Data and Safety Monitoring Board: Gordon Bernard, M.D. (chair; Vanderbilt University); James Anderson, Ph.D. (2007–2015; Frontier Science); Bernard Lo, M.D. (2007–2013; University of California, San Francisco); Andrew Ries, M.D., M.P.H. (2007–2014; University of California, San Diego); Stuart Stoloff, M.D. (University of Nevada); Byron Thomashow, M.D. (Columbia University); Barbara Tilley, Ph.D. (University of Texas); Kevin Weiss, M.D. (Accreditation Council of Graduate Medical Education)
Acknowledgment
The LOTT Research Group thanks the Masimo Corporation (Irvine, CA) for technical support of the pulse oximeters used in the Long-term Oxygen Treatment Trial.
Footnotes
Supported by the National Heart, Lung, and Blood Institute (contracts HHSN268200736183C, HHSN268200736184C, HHSN268200736185C, HHSN268200736186C, HHSN268200736187C, HHSN268200736188C, HHSN268200736189C, HHSN268200736190C, HHSN268200736191C, HHSN268200736192C, HHSN268200736193C, HHSN268200736194C, HHSN268200736195C, Y1-HR-7019-01, Y1-HR-8076-01, HHSN268200736196C, HHSN268200736197C) and the Centers for Medicare and Medicaid Services.
This article is subject to the National Institutes of Health (NIH) Public Access policy (http://publicaccess.nih.gov/).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the LOTT Research Group, William C. Bailey, Anne L. Fuhlbrigge, Ernestina Sampong, Karin Sloan, Ashley Wagner, Susan Anderson, Marilyn Moy, Osarenoma Okunbor, James K. Stoller, Scott Marlow, Yvonne Meli, Richard Rice, Loutfi S. Aboussouan, Robert Castele, Joseph Parambil, Sumita Khatri, Aman Pande, Joe Zein, Thomas Olbrych, Stephan Alkins, Christine Jocko, Franck Rahaghi, Jean Barton, Richard K. Albert, Jennifer Underwood, Barry Make, Jennifer Underwood, Neil MacIntyre, John Davies, Thomas Stibolt, Richard Mularski, Allison Naleway, Sarah Vertrees, Richard Casaburi, Janos Porszasz, Peggy Walker, Renee Indelicato, Lennard Specht, Kathleen Ellstrom, Jamie Portillo, David Horak, Brian Tiep, Mary Barnett, Philip Diaz, Janice Drake, Mahasti Rittinger, Rachael Compton, Scott Miller, Ralph J. Panos, Laura A. Lach, Gerard Criner, Carla Grabianowski, Francis Cordova, Parag Desai, Samuel Krachman, James Mamary, Nathaniel Marchetti, Aditi Satti, Eileen Mumm, Michelle Vega-Olivo, Jenny Hua, Vanna Tauch, Lii-Yoong Criner, Michael Jacobs, Peter Rising, Paul Simonelli, Michele Mitchell, Matthew Lammi, Connie Romaine, Howard Lee, Mary Ianacone, Steven Scharf, Wanda Bell-Farrell, M. Jeffery Mador, Ayesha Rahman, Mumtaz Zaman, Lisa Hill, Alec Platt, J. Allen Cooper, Kathleen Harrington, Mark Dransfield, Patti Smith, Donald Davis, J. Allen Cooper, Patti Smith, Peruvemba Sriram, Katherine Herring, Steven Gay, Fernando Martinez, Meilan Han, Kelly Rysso, Catherine Meldrum, K. P. Ravikrishnan, Daniel Keena, Jennifer DeRidder, Beth Kring, Antonio Anzueto, Alex Aguilera, Timothy Houlihan, Reda Girgis, Jennifer Cannestra, Frank Sciurba, Benjamin Kelly, Richard Kanner, Mary Beth Scholand, G. Martin Villegas, Judy Carle, David H. Au, Edmunds Udris, Randall Curtis, David Au, Laura C. Feemster, Richard Goodman, Brianna Moss, Lynn Reinke, Edmunds Udris, Moira Aitken, Bruce Culver, Roger D. Yusen, Mario Castro, Brigitte Mittler, Jeanne Heaghney, Myron Jacobs, Min Joo, Nina Bracken, Edward Diamond, Mary K. Joseph, Xavier Soler, Arianna Villa, Daniel Layish, Edwin Silverman, Roxanne Kelly, Daniel Cossette, James Tonascia, Patricia Belt, Amanda Blackford, Betty Collison, John Dodge, Michele Donithan, Cathleen Ewing, Rosetta Jackson, K Patrick May, Jill Meinert, Steven Piantadosi, Girlie Reyes, David Shade, Michael Smith, Alice L. Sternberg, Mark Van Natta, Laura Wilson, Annette Wagoner, Robert Wise, Katherine P. Yates, Rosemarie Hakim, Antonello Punturieri, Julie Bamdad, Thomas Croxton, Joanne Deshler, Pamela McCord-Reynolds, Mario Stylianou, Gail Weinmann, Gordon Bernard, James Anderson, Bernard Lo, Andrew Ries, Stuart Stoloff, Byron Thomashow, Barbara Tilley, and Kevin Weiss
References
- 1.Centers for Disease Control and Prevention. Chronic pulmonary obstructive disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938–942. [PubMed] [Google Scholar]
- 2.Diaz-Guzman E, Mannino DM. Epidemiology and prevalence of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:7–16. doi: 10.1016/j.ccm.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93:391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 4.Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: report of the Medical Research Council Working Party. Lancet. 1981;1:681–686. [PubMed] [Google Scholar]
- 5.Górecka D, Gorzelak K, Sliwiński P, Tobiasz M, Zieliński J. Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxaemia. Thorax. 1997;52:674–679. doi: 10.1136/thx.52.8.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaouat A, Weitzenblum E, Kessler R, Charpentier C, Enrhart M, Schott R, et al. A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur Respir J. 1999;14:1002–1008. doi: 10.1183/09031936.99.14510029. [DOI] [PubMed] [Google Scholar]
- 7.Albert RK, Au DH, Blackford AL, Casaburi R, Cooper JA, Jr, Criner GJ, et al. Long-Term Oxygen Treatment Trial Research Group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375:1617–1627. doi: 10.1056/NEJMoa1604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoller JK, Aboussouan LS, Kanner RE, Wilson LA, Diaz P, Wise R LOTT Research Group. Characteristics of alpha-1 antitrypsin–deficient individuals in the Long-Term Oxygen Treatment Trial and comparison with other subjects with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12:1796–1804. doi: 10.1513/AnnalsATS.201506-389OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B Long-Term Oxygen Treatment Trial Research Group. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest. 2010;138:179–187. doi: 10.1378/chest.09-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Make B, Krachman S, Panos RJ, Doherty DE, Stoller JK. Oxygen therapy in advanced COPD: in whom does it work? Semin Respir Crit Care Med. 2010;31:334–342. doi: 10.1055/s-0030-1254073. [DOI] [PubMed] [Google Scholar]
- 11.Narewski ER, Blackford A, Desai P, Lammi MR, Fuhlbrigge A, Soler X, et al. Clinical differences in COPD patients with mild–moderate hypoxemia at rest ± exertion vs. those normoxemic at rest who desaturate only with exertion [abstract] Am J Respir Crit Care Med. 2014;189:A3053. [Google Scholar]
- 12.MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol. 2001;429:195–207. doi: 10.1016/s0014-2999(01)01320-6. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Government Accountability Office. Report to Congressional Requesters: Medicare Home Oxygen: Refining Payment Methodology Has Potential to Lower Program and Beneficiary Spending. GAO-11-56. January 2011. Available from: http://www.gao.gov/assets/320/315094.pdf (accessed 2017 Nov 15)
- 14.Office of Information Products and Data Analytics, Centers for Medicare & Medicaid Services. CMS Statistics Reference Booklet. CMS Pub. No. 03504, August 2013. Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMS-Statistics-Reference-Booklet/Downloads/CMS_Stats_2011.pdf (accessed 2017 Nov 15)
- 15.Drummond MB, Blackford AL, Benditt JO, Make BJ, Sciurba FC, McCormack MC, et al. NETT Investigators. Continuous oxygen use in nonhypoxemic emphysema patients identifies a high-risk subset of patients: retrospective analysis of the National Emphysema Treatment Trial. Chest. 2008;134:497–506. doi: 10.1378/chest.08-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croxton TL, Bailey WC NHLBI Working Group on Long-Term Oxygen Treatment in COPD. Long-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report. Am J Respir Crit Care Med. 2006;174:373–378. doi: 10.1164/rccm.200507-1161WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ClinicalTrials.gov. Long-Term Oxygen Treatment Trial. Available from: https://clinicaltrials.gov/ct2/show/NCT00692198 (accessed 2017 Nov 15)
- 18.Schulz KF, Altman DG, Moher D CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 19.CMS.gov. National Coverage Determination. Available from: https://www.cms.gov/Medicare/Medicare-General-Information/MedicareApprovedFacilitie/o2trial.html (accessed 2017 Nov 15)
- 20.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society. American Thoracic Society, medical section of the American Lung Association: evaluation of impairment/disability secondary to respiratory disease. Am Rev Respir Dis. 1982;126:945–951. [PubMed] [Google Scholar]
- 22.American Thoracic Society. Surveillance for respiratory hazards in the occupational setting [American Thoracic Society] Am Rev Respir Dis. 1982;126:952–956. [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 24.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 25.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan RM, Atkins CJ, Timms R. Validity of a quality of well-being scale as an outcome measure in chronic obstructive pulmonary disease. J Chronic Dis. 1984;37:85–95. doi: 10.1016/0021-9681(84)90050-x. [DOI] [PubMed] [Google Scholar]
- 27.Stewart AL, Hays RD, Ware JE., Jr The MOS Short-Form General Health Survey: reliability and validity in a patient population. Med Care. 1988;26:724–735. doi: 10.1097/00005650-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 29.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 30.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168:818–900. doi: 10.1164/rccm.168.7.818. [DOI] [PubMed] [Google Scholar]
- 31.Shih JH. Sample size calculation for complex clinical trials with survival endpoints. Control Clin Trials. 1995;16:395–407. doi: 10.1016/s0197-2456(95)00132-8. [DOI] [PubMed] [Google Scholar]
- 32.Social Security Administration. Limited Access Death Master File. National Technical Information Service, United States Department of Commerce, Alexandria, VA. [Accessed 2017 Nov 15]. Available from: https://classic.ntis.gov/products/ssa-dmf/
- 33.NIH-NHLBI DSMB charter. [Accessed 2017 Nov 15]. Available from: https://www.nhlbi.nih.gov/research/funding/human-subjects/data-safety-monitoring-policy.
- 34.Lan KK, DeMets DL. Changing frequency of interim analysis in sequential monitoring. Biometrics. 1989;45:1017–1020. [PubMed] [Google Scholar]
- 35.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 36.Fleming TR, Harrington DP, O’Brien PC. Designs for group sequential tests. Control Clin Trials. 1984;5:348–361. doi: 10.1016/s0197-2456(84)80014-8. [DOI] [PubMed] [Google Scholar]
- 37.DeMets DL, Ware JH. Asymmetric group sequential boundaries for monitoring clinical trials. Biometrika. 1982;69:661–663. [Google Scholar]
- 38.Lan KK, Wittes J. The B-value: a tool for monitoring data. Biometrics. 1988;44:579–585. [PubMed] [Google Scholar]
- 39.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67:957–963. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Food and Drug Administration Guidance for Industry Adaptive Design Clinical Trials for Drugs and Biologics. 2010. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM201790.pdf (accessed 2017 Nov 15).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.