Abstract
Rationale: Lung cancer screening has a mortality benefit to high-risk smokers, but implementation remains suboptimal. Providers represent the key entry point to screening, and an understanding of provider perspectives on lung cancer screening is necessary to improve referral and overall implementation.
Objectives: The objective of this study was to understand knowledge, beliefs, attitudes, barriers, and facilitators to screening in a diverse group of referring pulmonologists and primary care providers.
Methods: We conducted an electronic survey of primary care and pulmonary providers within a tertiary care medical center across different practice sites. The survey covered the following domains: 1) beliefs and assessment of evidence, 2) knowledge of lung cancer screening and guidelines, 3) current screening practices, 4) barriers and facilitators, and 5) demographic and practice characteristics.
Results: The 196 participants included 80% primary care clinicians and 19% pulmonologists (1% others). Forty-one percent practiced at university-based or affiliated clinics, 47% at county hospital–based clinics, and 12% at other or unidentified sites. The majority endorsed lung cancer screening effectiveness (74%); however, performance on knowledge-based assessments of screening eligibility, documentation, and nodule management was suboptimal. Key barriers included inadequate time (36%), inadequate staffing (36%), and patients having too many other illnesses to address screening (38%). Decision aids, which are used at the point of referral, were commonly identified both as important lung cancer screening clinical facilitators (51%) and as provider knowledge facilitators (59%). There were several differences by provider specialty, including primary care providers more frequently reporting time constraints and their patients having too many other illnesses to address screening as significant barriers to lung cancer screening.
Conclusions: Providers endorsed the benefits of lung cancer screening, but there are limitations in provider knowledge of key screening components. The most frequently reported barriers to screening represent a lack of clinical time or resources to address lung cancer screening in clinical practice. Facilitators for nodule management as well as point-of-care referral materials may be helpful in reducing knowledge gaps and the clinical burden of referral. These are all modifiable factors, which could be addressed to increase screening referral. Differences in attitudes and barriers by specialty should also be considered to optimize screening implementation.
Keywords: lung cancer, early detection of cancer, pulmonary nodule, attitudes of health personnel
Lung cancer is the leading cause of cancer death in the United States and will account for an estimated 158,000 deaths in 2017 (1). The National Lung Screening Trial was the first study to show a mortality benefit for a lung cancer screening protocol, demonstrating a 20% reduction in lung cancer mortality in high-risk current or former smokers undergoing three annual low-dose chest computed tomography examinations compared with a control group undergoing chest radiographs (2). The U.S. Preventive Services Task Force, the American Thoracic Society, and other professional societies now recommend annual low-dose chest computed tomography–based lung cancer screening for similar high-risk patient populations (3–5).
Lung cancer screening is in a period of early and incomplete implementation (6–9). Understanding referring provider perspectives during this period is important, particularly given unique aspects of lung cancer screening compared with other preventive modalities. Lung cancer screening may require more provider knowledge, as eligibility is based on factors beyond age and sex, including a detailed smoking assessment (10). Moreover, the U.S. Centers for Medicare and Medicaid Services (CMS) requires documentation of shared decision-making for payment of lung cancer screening services, requiring providers to communicate harms and benefits and help patients make personalized decisions (11). The process of lung cancer screening may take more clinical time and resources, given requirements for shared decision-making and complexities of nodule management. Finally, limited data exist on optimal lung cancer screening implementation.
Providers represent the key entry point to screening, and the few studies of provider use of lung cancer screening demonstrate limitations in knowledge and uptake (12–14). The optimization of screening practices requires understanding referring provider perspectives and must address key barriers and facilitators to screening. Because providers initiating lung cancer screening practice in a variety of specialties and locations, these factors likely have an impact on screening attitudes and practices. To better understand provider perspectives on lung cancer screening, we conducted a survey-based study of providers within a diverse medical system with goals to: 1) understand beliefs and knowledge of lung cancer screening, 2) assess perceived barriers and facilitators to screening, and 3) compare differences between the two most prominent referring specialists, pulmonologists and primary care providers (PCPs). A portion of this work was presented at the American Thoracic Society International Conference in May 2017 (15).
Methods
Study Population
The survey targeted PCPs (practitioners in general internal medicine and family medicine as well as other subspecialists who work in primary outpatient care, such as geriatricians) and pulmonologists within the University of Washington medical system in Seattle, Washington. The medical system includes a university medical center with neighborhood-based affiliated clinics, a county medical center that serves as both the safety net hospital and the level 1 trauma center for the region (Harborview Medical Center), and a Veterans Affairs medical center (Veterans Affairs Puget Sound, Seattle, WA). Twenty-four individual clinics were identified for participation, ranging in size from 1 to 64 providers. Fourteen of the clinics were affiliated with the university medical center, nine with the county hospital, and one with the Veterans Affairs medical center. During the conduct of this study, there was not a universal system-wide screening program. However, providers had the following options for screening: lung cancer screening could be performed directly by providers by ordering screening computed tomography scans at their discretion or through referral to a central location (the University of Washington–affiliated Seattle Cancer Care Alliance) for a clinical visit and subsequent lung cancer screening initiation and management.
Eligible participants included attending and resident/fellow physicians, nurse practitioners, and physician assistants. Participants were identified and approached by e-mail through: 1) individual clinic medical directors, 2) an internal medicine residency trainee roster, and 3) a pulmonary physician roster. Medical directors were given the option of either sharing provider e-mails for individual contact or sharing the participation request with providers at their respective site(s). Only one response per participant was allowed.
Eligible participants were sent an e-mail invitation to participate with details of the study, survey instructions, and an electronic survey web link. In the event of nonresponse, two subsequent e-mail–based reminders were sent. Participants provided informed consent as part of the survey, and responses were confidential and recorded in an encrypted database. A $5 gift card was provided for participation. The Institutional Review Board of the University of Washington/Fred Hutchinson Cancer Research Center (#9221) approved this study.
Survey Instrument and Administration
The survey was conducted from March to June 2016 using REDCap (Research Electronic Data Capture, Vanderbilt University), a web-based application serving as a data capture instrument and repository. The survey consisted of 30 questions, some with multiple parts (see online supplement). The survey was piloted and reviewed for content and clarity by research staff in the Department of Medicine at the University of Washington.
The survey included questions in five domains: 1) beliefs on lung cancer screening and assessment of the evidence (seven questions); 2) knowledge of lung cancer screening, including a question on required elements in shared decision-making documentation (per CMS), a question on eligibility criteria, and a question on risk of malignancy with specific positive screening scenarios (three multipart questions); 3) assessment of current screening practices (seven questions); 4) identification of key barriers and facilitators to screening (five questions); and 5) demographic characteristics and details of clinical practice (eight questions). Three questions were used/adapted from a survey on implementation of lung cancer screening among pulmonologists (16). Questions on lung cancer screening eligibility criteria were based on both CMS and U.S. Preventive Services Task Force criteria (3, 11). Questions on 5-year risk of lung cancer patient scenarios were informed by the Brock model (17).
Analysis
Questions with Likert scale responses were categorized in logical ways. We compared pulmonologists versus PCPs using chi-squared testing. All respondents were included in the entire cohort analysis. Two providers, who identified as “critical care” and “sleep medicine” specialists, were excluded from the specialty comparison. P values < 0.05 were considered significant. Missing responses to individual questions were treated as missing at random and not included. Both SAS 9.4 (SAS Institute) and STATA 14.0 (STATACorp) were used for statistical analysis.
Results
Participant Characteristics
The survey was delivered to approximately 551 unique individuals. Of these, 207 completed any portion of the survey (38% individual response rate). Only 196 provider surveys were included in the analytic cohort (36% completed survey response rate), as 11 respondents had invalid surveys (no questions answered other than demographics). At least one provider responded from 19 of the 24 clinics (79% clinic response rate). Participants were similar to nonrespondents by specialty and primary clinic location. For example, PCPs represented 80% of participants and 75% of nonrespondents; university-based providers represented 41% of participants and 43% of nonrespondents.
The majority of survey participants were female (67%) and white (79%) (Table 1). Participants included attending physicians (65%), trainee physicians (25%), and advanced practice providers (9.2%). Forty-nine percent of participants practiced general internal medicine, 22% family medicine, and 19% pulmonary medicine, with 9.7% practicing a different specialty (geriatrics, infectious diseases [human immunodeficiency virus primary care], and gynecology) in which they provided primary care for patients. Forty-seven percent primarily practiced at a county hospital–based clinic, 41% at a university-affiliated clinic, and 12% at either the Veterans Affairs medical center or another location or they did not identify a clinic. Twenty-two percent had discussed lung cancer screening with more than five patients in the past year.
Table 1.
Characteristics of survey participants (N = 196)
| Characteristic | % (n) |
|---|---|
| Female | 67 (132) |
| Race | |
| White | 79 (155) |
| Asian | 15 (30) |
| Black | 0.5 (1) |
| Other | 5.1 (10) |
| Hispanic ethnicity | 2.6 (5) |
| Age group, years | |
| <30 | 8.5 (16) |
| 30–39 | 42 (79) |
| 40–49 | 30 (43) |
| 50–59 | 16 (30) |
| >59 | 11 (20) |
| Rank/position | |
| Attending physician | 65 (127) |
| Resident/Fellow physician | 25 (51) |
| Advanced practice provider | 9.2 (18) |
| Specialty | |
| General internal medicine | 49 (95) |
| Family medicine | 22 (44) |
| Pulmonary medicine | 19 (38) |
| Other | 9.7 (19) |
| Primary clinic location | |
| County hospital–based clinic | 47 (92) |
| University-affiliated clinic | 41 (81) |
| Other/unidentified | 12 (23) |
| Average clinic days per month | |
| <1 full day | 9.7 (19) |
| 1–5 full days | 58 (114) |
| 6–10 full days | 7.1 (14) |
| >10 full days | 25 (49) |
| Current screening practices in past year | |
| Discussed lung cancer screening with >5 patients | 22 (44) |
| Ordered low-dose chest computed tomography scan for lung cancer screening for >5 patients | 14 (28) |
| Referred to other provider for lung cancer screening for >5 patients | 2.1 (4) |
Lung Cancer Screening Beliefs and Knowledge
The majority of participants believe that screening is at least moderately effective at preventing death from lung cancer (74%), believe the research evidence for screening is strong (57%), and agree screening is supported by randomized clinical trial evidence (75%). Although 66% agree screening may expose patients to harm, and 56% agree screening may place a burden on the healthcare system, more than half (58%) agree screening has more advantages than disadvantages for patients.
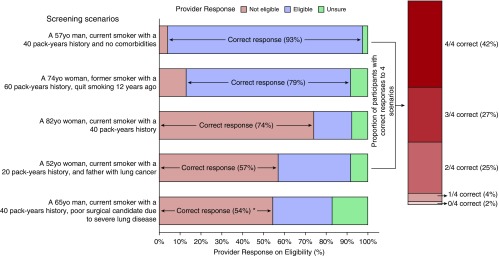
To assess provider knowledge, we asked participants to identify elements of lung cancer screening documentation required by CMS. Few (10%) providers correctly identified all elements, 57% identified at least six out of seven correct elements, and 90% identified at least five out of seven correct elements. We asked providers to identify patient screening eligibility in five screening scenarios (Figure 1). Only 69% of providers correctly assessed eligibility in at least three of the initial four scenarios. The fifth scenario, “A 65-year-old man, current smoker with a 40 pack-year history, and a poor surgical candidate because of severe lung disease (forced expiratory volume in 1 second < 30% predicted),” was created to assess provider knowledge regarding ineligibility of poor surgical candidates, per U.S. Preventive Services Task Force guidelines (3). Given potential controversy with this question, it was not included in the overall summary of correctness. Twenty-nine percent believed this patient was eligible, 54% believed he was not, and 17% were unsure. Finally, we asked providers to estimate lung cancer risk over a range (<5% risk, 5–10% risk, 11–20% risk, 21–50% risk, >50% risk), given patient scenarios with screening results. Few providers (5.1%) correctly estimated risk in all scenarios, and 33% correctly estimated risk in two out of three scenarios, with most incorrect responses overestimating risk. We did not note differences in knowledge by rank (attending versus trainee) or days in clinic per month.
Figure 1.
Provider responses on patient eligibility in five lung cancer screening scenarios. The scenarios presented to participants are listed on the left. The response options included “not eligible,” “eligible,” and “unsure.” The percent of participants with each response is illustrated on the bar graph, as are the correct answers. The proportion of participants by numbers of correct responses to the first four scenarios is illustrated in the vertical bar graph on the right. The final scenario was not included in this summary. We consider the correct response “not eligible,” as a poor surgical candidate should not be screened per U.S. Preventive Services Task Force guidelines; however, this is marked by an * as there is no definitive consensus contained in other guidelines. yo = year old.
Key Barriers, Facilitators, and Resources
Providers were asked whether certain patient-, practice-, or system-related factors were barriers to lung cancer screening in their practices (Table 2). The most frequently identified “big barriers” were patients having too many other illnesses to address screening (38%), lack of time (36%), and lack of staff to maintain and follow up screening (36%). Patient adherence with follow-up was also commonly (26%) identified. The most commonly identified potential facilitator was a registry system to track nodules, believed to be “very” or “extremely” helpful by 67% of providers. The additional information/knowledge most commonly identified as “needed” was follow-up recommendations for nodules (68%) and referral guidelines for specialty services (55%). The best format for additional provider training selected by more than 50% of providers included a high-quality decision aid (59%) and a pocket guide/checklist (55%).
Table 2.
Participant’s identification of key barriers, facilitators, and resources for lung cancer screening (N = 196)
| Category/Survey Question | % (n) |
|---|---|
| Percentage who considered the following “a big barrier” to lung cancer screening implementation in his/her current clinical practice (descending order) |
|
| Patients have too many other illnesses to address lung cancer screening | 38 (73) |
| Not enough time to address lung cancer screening in clinical practice | 36 (71) |
| Not enough staff to maintain and follow up lung cancer screening | 36 (71) |
| Patients unlikely to adhere to annual lung cancer screening and follow-up recommendation | 26 (50) |
| Lack of reimbursement and financial concerns | 24 (46) |
| Unavailability of a multidisciplinary team | 23 (44) |
| No current lung cancer screening guidelines at practice location | 19 (38) |
| Patients are not able to understand harms and benefits of lung cancer screening | 17 (33) |
| Patients are not interested in lung cancer screening | 15 (30) |
| Insufficient evidence to warrant a screening program | 9.3 (18) |
| Leadership at practice not supportive of lung cancer screening | 7.7 (15) |
| Legal concerns | 2.1 (4) |
| Percentage who believed the following would be “very” or “extremely” helpful in implementing lung cancer screening in his/her clinic (descending order) | |
| A registry system to track nodules | 67 (131) |
| An electronic health record system that includes smoking pack-years and quit date | 52 (101) |
| Easily available web-based decision aids | 51 (100) |
| Electronic health record–based clinical reminders of patients who may be eligible | 49 (95) |
| A dedicated person to provide screening results | 48 (94) |
| A dedicated person to perform shared decision-making counseling | 47 (91) |
| A note template to document a shared decision-making visit | 46 (89) |
| Easily available paper-based decision aids | 44 (86) |
| Percentage who believed they needed additional information in the following domains to successfully provide lung cancer screening (descending order) | |
| Follow-up recommendations for nodules | 68 (133) |
| Referral guidelines for specialty services (pulmonary or thoracic surgery) | 55 (108) |
| Insurance, billing, and reimbursement | 46 (91) |
| Scientific evidence | 44 (87) |
| Eligibility criteria | 43 (85) |
| Medical record documentation requirements | 41 (81) |
| Shared decision-making | 38 (74) |
| No additional knowledge or training needed | 10 (20) |
| Percentage who believed the following would provide the best format for additional training or resources for lung cancer screening (instructed to check all that apply) | |
| High-quality decision aid | 59 (116) |
| Pocket guide or checklist | 55 (107) |
| Online module | 45 (88) |
| In-clinic training session | 40 (79) |
| Review article | 21 (41) |
| Other | 3.1 (6) |
Primary Care versus Pulmonary Providers
Knowledge and beliefs about lung cancer screening were similar between pulmonologists and PCPs, but there were key differences in other domains (Table 3). Pulmonologists were more likely to agree that screening was requested by patients (32 vs. 12%, P = 0.002). PCPs were more likely to believe that primary care should be responsible for screening initiation (92 vs. 50%, P < 0.001) and implementation and follow-up (83 vs. 32%, P < 0.001). PCPs were more likely to identify not having enough time to address screening and patients having too many other illnesses to address screening as significant barriers. PCPs were less likely to believe they did not need additional training to provide screening (5 vs. 29%, P < 0.001).
Table 3.
Comparing pulmonologists to primary care providers on key questions (N = 194)
| Survey Question | Pulmonary Providers (n = 38) | Primary Care Providers (n = 156) | P Value |
|---|---|---|---|
| I am somewhat or extremely familiar with lung cancer screening. | 82 | 77 | 0.58 |
| Screening with annual low-dose chest computed tomography is moderately or very effective. | 66 | 76 | 0.22 |
| The research evidence for lung cancer screening is strong or very strong. | 55 | 58 | 0.75 |
| I agree or strongly agree that lung cancer screening is requested by patients at my practice. | 32 | 14 | 0.009 |
| I agree or strongly agree that lung cancer screening will place a burden on the healthcare system. | 84 | 49 | <0.001 |
| The primary care provider should be primarily responsible for initiating discussion with patients about lung cancer screening. | 50 | 92 | <0.001 |
| The primary care provider should be primarily responsible for implementing lung cancer screening and managing follow-up. | 32 | 83 | <0.001 |
| Not having enough time to address lung cancer screening in my practice is a “big barrier” to screening. | 8 | 44 | <0.001 |
| Patients having too many other illnesses is a “big barrier” to screening. | 18 | 42 | <0.001 |
| I need more information or knowledge on follow-up recommendations for nodules. | 34 | 76 | <0.001 |
| I need more information for referral guidelines to specialty services after lung cancer screening. | 13 | 65 | <0.001 |
| I need no additional knowledge or training for lung cancer screening. | 29 | 5 | <0.001 |
Data show percentage who gave that response. Two providers were not included in this comparison because of primary reported specialty (sleep and critical care medicine).
Discussion
In this study, we sought to better understand current knowledge, beliefs, practices, barriers, and facilitators to lung cancer screening among primary care providers and pulmonologists—those most likely to initiate the lung cancer screening process—in a diverse medical system before the roll-out of a formal system-wide lung cancer screening program. In addition, we explored differences between pulmonologists and primary care providers, and this study is the first we are aware of to examine differences in lung cancer screening perceptions and knowledge by specialty.
There were three important findings regarding attitudes and knowledge that emerged from the overall provider responses. First, the majority of providers (>70%) had a positive view of lung cancer screening effectiveness, both in the quality of supporting research and in the perceived effectiveness in preventing death. Second, providers acknowledge that lung cancer screening is associated with potential harms and is a burden on the healthcare system, and only a small majority (58%) believed benefits outweighed these harms. Finally, there are gaps in knowledge about key elements of lung cancer screening, including knowledge of documentation (required by U.S. Centers for Medicare and Medicaid Services for payment), patient eligibility, and patient risk after screening.
We also noted three findings regarding perceived barriers and facilitators to lung cancer screening. First, the most frequently identified barriers to lung cancer screening implementation were: patients have too many other illnesses to address screening, providers do not have enough time to address screening, and clinics lack staffing. These largely reflect time and resource constraints. Second, the most useful training facilitators are those designed to improve provider and patient knowledge at the point of care, such as a decision aid or pocket guide. Finally, nodule management seems to be an area of uncertainty for providers, and methods to improve knowledge of nodule management and tracking (a registry system) may be helpful.
Exploring differences between provider type was revealing. Pulmonologists believed that they needed less information on nodule management but performed similarly to primary care providers on lung cancer screening knowledge assessments. Other studies have shown similar discomfort with nodule management by primary care providers (18), and clinician adherence to nodule management guidelines and recommendations is likely suboptimal among both primary care providers and pulmonologists in practice (19–21). Primary care providers are more likely to believe they should be both initiating and managing screening, although our results suggest pulmonologists may perceive fewer barriers regarding patient comorbidities and time constraints.
We believe there are several lessons for implementation of lung cancer screening that can be taken from this study, most importantly in the realms of provider education and understanding and addressing modifiable barriers to screening. Provider-based education appears to be needed in lung cancer screening implementation, as a lack of adequate screening knowledge was seen in this and other studies of providers (12–14). Education on documentation, eligibility, nodule risk, and management all appear to be important areas where providers may need more information and guidance. Providers identified decision aids and pocket guides as desirable facilitators to this education and could provide this in real time by reinforcing eligibility and documentation requirements for the provider and patient at the point of referral. A recently published study demonstrated significant improvement in patient knowledge after shared decision-making performed with a decision aid (22). Our findings are novel, as they suggest that providers believe that a decision aid, despite being intended for patient-centered decision-making, can also improve provider knowledge. Decision aids should be evaluated for their ability to improve provider knowledge and could be adapted to this purpose. Moreover, decision aids, particularly when web-based (which was the preferred modality among our respondents), could be coupled with other web-based learning tools to effectively educate providers (23, 24).
The identified barriers of lack of clinical time and resources are modifiable barriers, indicating that lung cancer screening implementation may improve with more dedicated resources. Specific resources requested by providers, such as dedicated staff and a nodule registry, are highlighted features used by successful early-adopting lung cancer screening programs (25). These barriers also suggest that lung cancer screening may have limited success as part of a traditional problem-based clinical encounter (particularly for complex primary care visits), where schedule constraints and patient-illness burden may limit the ability of the provider to initiate screening. This has been seen in another study of primary care providers, with providers citing time constraints as a barrier, particularly for providing comprehensive shared decision-making and smoking cessation counseling (14). Interestingly, the most frequently identified barrier overall, which was reported more often by primary care providers than pulmonologists, was that “patients may have too many other illnesses to address lung cancer screening.” Although we assume this reflects a time-related barrier in many cases, this may also reflect provider acknowledgment of the complexities of screening decisions in patients who may have competing risks for death. These patients may not benefit from lung cancer screening, particularly if unable to undergo surgical resection (3). There is little research in provider decision-making in such cases, and future study is needed to determine how referring providers consider screening referral in medically complex patients.
Finally, this study highlights differences that exist between referring pulmonologists and primary care providers but does not suggest that either primary care providers or pulmonologists are the “best” provider to initiate screening. On the basis of our results and others, primary care providers and pulmonologists may have different strengths: pulmonologists may be more knowledgeable about nodule management and feel more skilled to initiate screening, but primary care providers may be more equipped to understand lung cancer screening in the context of the patient’s health experience (26–28). Limitations of each specialty emphasize the importance of multidisciplinary team involvement in all aspects of screening, as advocated by major screening guidelines (4, 5, 29).
There are limitations to this study. Our response rate was somewhat low; however, respondents were similar to nonrespondents. Also, although participants were recruited from a large and diverse medical system, recruitment from a single system may limit generalizability. Because the survey contained largely closed-ended questions, there were limited opportunities for respondents to convey unique responses; therefore, all key barriers and facilitators from the provider perspective may not have been captured. Finally, comparisons by specialty may be confounded by other differences in these groups.
In summary, although providers appear to believe in the benefits of lung cancer screening, there are limitations in provider knowledge of key screening components. There are also important barriers and facilitators that are modifiable but will need to be addressed to improve screening implementation, and these include adequate time and resources to perform screening. Some of these barriers and facilitators differ by provider specialty, and unique approaches to improve screening practices may need to be developed in different settings and different provider groups.
Supplementary Material
Footnotes
Supported by the American Lung Association Lung Cancer Discovery Grant (K.C.), National Heart, Lung, and Blood Institute (NHLBI) grant T32 HL007287 (supporting M.T. under Drs. Robb Glenny and J. Randall Curtis), and National Cancer Institute grant 1R01CA173754. C.G.S. is supported by resources from the VA Portland Health Care System, Portland, Oregon.
The Department of Veterans Affairs did not have a role in the conduct of the study; in the collection, management, analysis, interpretation of data; or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the U.S. Government.
Author Contributions: M.T., E.K.K., and K.C. contributed to conception and design, acquisition of the data, analysis and interpretation of the data, and drafted and revised the article. B.A.M. and J.G.E. contributed to the conception and design and revised the article for important intellectual content. C.G.S. and P.E.R. contributed to interpretation of the data and revised the article. S.S. contributed to conception and design, acquisition of the data, and revised the article. P.D.F. contributed to the data analysis and revised the article. All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.American Cancer Society. Cancer facts & figures. 2017 [accessed 2017 Mar 15]. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html.
- 2.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyer VA U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 4.Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e78S–e92S. doi: 10.1378/chest.12-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiener RS, Gould MK, Arenberg DA, Au DH, Fennig K, Lamb CR, et al. ATS/ACCP Committee on Low-Dose CT Lung Cancer Screening in Clinical Practice. An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med. 2015;192:881–891. doi: 10.1164/rccm.201508-1671ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jemal A, Fedewa SA. Lung cancer screening with low-dose computed tomography in the United States-2010 to 2015. JAMA Oncol. 2017;3:1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton MK. Integration of lung cancer screening into practice is lacking. CA Cancer J Clin. 2015;65:255–256. doi: 10.3322/caac.21282. [DOI] [PubMed] [Google Scholar]
- 8.Carter-Harris L, Tan AS, Salloum RG, Young-Wolff KC. Patient-provider discussions about lung cancer screening pre- and post-guidelines: Health Information National Trends Survey (HINTS) Patient Educ Couns. 2016;99:1772–1777. doi: 10.1016/j.pec.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raz DJ, Wu GX, Consunji M, Nelson R, Sun C, Erhunmwunsee L, et al. Perceptions and utilization of lung cancer screening among primary care physicians. J Thorac Oncol. 2016;11:1856–1862. doi: 10.1016/j.jtho.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Field JK, Duffy SW, Devaraj A, Baldwin DR. Implementation planning for lung cancer screening: five major challenges. Lancet Respir Med. 2016;4:685–687. doi: 10.1016/S2213-2600(16)30233-8. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Medicare and Medicaid Services. Decision memo for screening for lung cancer with low dose computer tomography. [created 2015 Feb 5; accessed 2017 Feb 1]. Available from: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274.
- 12.Lewis JA, Petty WJ, Tooze JA, Miller DP, Chiles C, Miller AA, et al. Low-dose CT lung cancer screening practices and attitudes among primary care providers at an academic medical center. Cancer Epidemiol Biomarkers Prev. 2015;24:664–670. doi: 10.1158/1055-9965.EPI-14-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ersek JL, Eberth JM, McDonnell KK, Strayer SM, Sercy E, Cartmell KB, et al. Knowledge of, attitudes toward, and use of low-dose computed tomography for lung cancer screening among family physicians. Cancer. 2016;122:2324–2331. doi: 10.1002/cncr.29944. [DOI] [PubMed] [Google Scholar]
- 14.Kanodra NM, Pope C, Halbert CH, Silvestri GA, Rice LJ, Tanner NT. Primary care provider and patient perspectives on lung cancer screening: a qualitative study. Ann Am Thorac Soc. 2016;13:1977–1982. doi: 10.1513/AnnalsATS.201604-286OC. [DOI] [PubMed] [Google Scholar]
- 15.Triplette M, Kross EK, Mann B, Elmore JG, Slatore CG, Shahrir S, et al. Assessing knowledge, barriers and facilitators to lung cancer screening among primary care and pulmonary providers [abstract] Am J Respir Crit Care Med. 2017;195:A5185. [Google Scholar]
- 16.Iaccarino JM, Clark J, Bolton R, Kinsinger L, Kelley M, Slatore CG, et al. A national survey of pulmonologists’ views on low-dose computed tomography screening for lung cancer. Ann Am Thorac Soc. 2015;12:1667–1675. doi: 10.1513/AnnalsATS.201507-467OC. [DOI] [PubMed] [Google Scholar]
- 17.McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369:910–919. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golden SE, Wiener RS, Sullivan D, Ganzini L, Slatore CG. primary care providers and a system problem: a qualitative study of clinicians caring for patients with incidental pulmonary nodules. Chest. 2015;148:1422–1429. doi: 10.1378/chest.14-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moseson EM, Wiener RS, Golden SE, Au DH, Gorman JD, Laing AD, et al. Patient and clinician characteristics associated with adherence: a cohort study of veterans with incidental pulmonary nodules. Ann Am Thorac Soc. 2016;13:651–659. doi: 10.1513/AnnalsATS.201511-745OC. [DOI] [PubMed] [Google Scholar]
- 20.Tanner NT, Aggarwal J, Gould MK, Kearney P, Diette G, Vachani A, et al. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148:1405–1414. doi: 10.1378/chest.15-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiener RS, Gould MK, Slatore CG, Fincke BG, Schwartz LM, Woloshin S. Resource use and guideline concordance in evaluation of pulmonary nodules for cancer: too much and too little care. JAMA Intern Med. 2014;174:871–880. doi: 10.1001/jamainternmed.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzone PJ, Tenenbaum A, Seeley M, Petersen H, Lyon C, Han X, et al. Impact of a lung cancer screening counseling and shared decision-making visit. Chest. 2017;151:572–578. doi: 10.1016/j.chest.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Cook DA, Levinson AJ, Garside S, Dupras DM, Erwin PJ, Montori VM. Internet-based learning in the health professions: a meta-analysis. JAMA. 2008;300:1181–1196. doi: 10.1001/jama.300.10.1181. [DOI] [PubMed] [Google Scholar]
- 24.Cook DA. Web-based learning: pros, cons and controversies. Clin Med (Lond) 2007;7:37–42. doi: 10.7861/clinmedicine.7-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gesthalter YB, Koppelman E, Bolton R, Slatore CG, Yoon SH, Cain HC, et al. Evaluations of implementation at early-adopting lung cancer screening programs: lessons learned. Chest. 2017;152:70–80. doi: 10.1016/j.chest.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Simmons VN, Gray JE, Schabath MB, Wilson LE, Quinn GP. High-risk community and primary care providers knowledge about and barriers to low-dose computed topography lung cancer screening. Lung Cancer. 2017;106:42–49. doi: 10.1016/j.lungcan.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Goodson JD. POINT: should only primary care physicians provide shared decision-making services to discuss the risks/benefits of a low-dose chest CT scan for lung cancer screening? Yes. Chest. 2017;151:1213–1215. doi: 10.1016/j.chest.2016.11.057. [DOI] [PubMed] [Google Scholar]
- 28.Powell CA. COUNTERPOINT: should only primary care physicians provide shared decision-making services to discuss the risks/benefits of a low-dose chest CT scan for lung cancer screening? No. Chest. 2017;151:1215–1217. doi: 10.1016/j.chest.2016.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzone P, Powell CA, Arenberg D, Bach P, Detterbeck F, Gould MK, et al. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest. 2015;147:295–303. doi: 10.1378/chest.14-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.