Abstract
Many studies have noted similarities between atherosclerosis and cancer including pronounced cellular plasticity, clonal expansion of cellular subtypes, increased DNA mutations, defective efferocytosis pathways, and an important role for proto-oncogenes in disease development. Although it is clear that these 2 diseases have disparate causes, noting the parallels between atherosclerosis and cancer may help us identify unique, targeted therapeutic strategies.
Keywords: atherosclerosis, efferocytosis, macrophages, myocytes, smooth muscle, neoplasms, oncogenes
Origins of Atherosclerotic Plaque Cells: A Theme of Plasticity
Early articles described atheromas as benign neoplasms of the blood vessel comprised primarily of fibrous smooth muscle cells (SMCs). However, additional studies soon noted the presence of macrophages and other cell types, thereby promoting controversy as to which cells actually give rise to the atherosclerotic plaque. Much of this controversy results from the promiscuity of the so-called lineage-specific markers.1 For example, bone marrow–derived cells can migrate into plaques and begin to express certain SMC markers,2 whereas SMCs can upregulate macrophage-specific markers such as galectin-3, CD11b, and F4/80 as they migrate into the plaque.3,4 In addition, recent lineage tracing studies suggest that endothelial cells can acquire mesenchymal cell characteristics, and some adventitial cells may have the capability of forming SMC-like cells. Collectively, these studies demonstrate the impressive capacity of many vascular cell types to dedifferentiate or transdifferentiate in response to the atherosclerotic environment and suggest that plaques may arise from multiple vascular and circulating cell types. Clearly, cellular plasticity is a major theme in atherosclerotic plaque development—as it is in cancer—and methods to reprogram proatherosclerotic cells could have tremendous therapeutic potential.
Clonal Origins for Plaque-Resident Cells?
The clonal evolution theory of cancer development posits that certain tumors arise from a single cell that expands in response to a series of acquired mutations. In 1973, Benditt and Benditt5 performed X-inactivation studies on human atherosclerotic lesions and found evidence that atherosclerotic plaques could be derived from a single cell that underwent monoclonal expansion, possibly through the acquisition of mutations in a manner similar to cancer development. Additional study by Pearson and Lee would go on to show that a large portion of human plaques showed some monoclonal foci, but that it was exceedingly rare for an entire plaque to exhibit a single X-inactivation pattern.6 Although these studies cast doubt on the hypothesis that atherosclerosis is a truly monoclonal process, it is now clear that atheromas do not simply grow through the simultaneous division of all cells at the same rate, as in the case of growing scar (a process known to be polyclonal). Rather, it is likely that there are pockets of proliferative, monotypic cells that contribute to expansion of the atherosclerotic lesion.
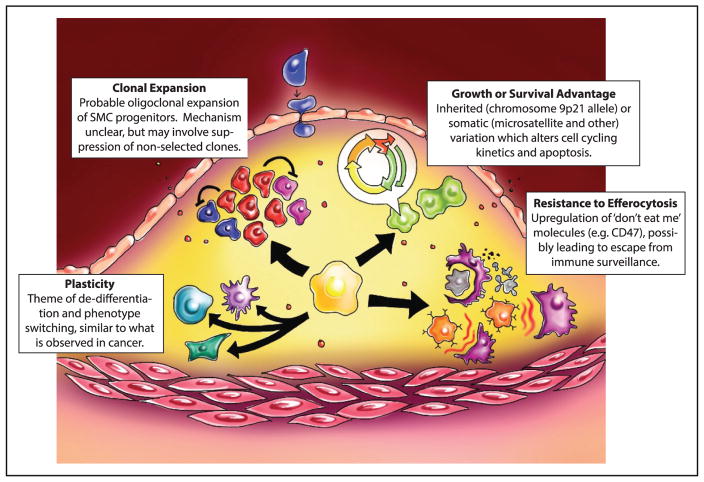
Interestingly, data now exist showing that local macrophage and clonal T-cell expansion occurs within the plaque, suggesting that these cells may contribute to the observed monoclonal foci.7 However, several recent studies have noted the presence of progenitor cells in atherosclerotic plaque development, possibly including a media-resident Sca1+ progenitor.8 These results are intriguing, but several emerging studies suggest that if an atherosclerosis clone truly exists, it may originate from the differentiated SMC. First, recent studies from the Owens group have revealed that mature, SM-MHC (smooth muscle-myosin heavy chain)–expressing SMCs can upregulate Sca1 and require key stem cell genes for migration and survival in the plaque.4 Furthermore, limited labeling of SMCs has shown the existence of intralesional clonal patches that arise from SMCs.3 This study was recently extended by Chappell et al9 who used an elegant multicolor lineage tracing reporter to conclusively demonstrate the clonal expansion of SMC-derived cells. Although mechanistic studies remain to be completed, it is plausible that these monoclonal foci of dedifferentiated SMCs arise from either (1) overgrowth of a hyperproliferative cell that simply outcompetes other cellular populations, (2) expansion of a clone, which is able to tolerate a lesional environment that is toxic to other cell types, or (3) active suppression of neighboring cells (eg, via juxtacrine factors) by the dominant/selected clone (Figure 1). If found to be true, the third option may have arisen as an evolutionary mechanism to prevent the overexpansion of cells in response to injury and to retain contractile vessel function. Regardless the fact that atherosclerosis is at least oligoclonal in nature suggests that molecular pathways exist, which could be leveraged to prevent the competitive advantage enjoyed by these clones during lesion expansion or even to selectively promote the expansion of beneficial SMC clones that favor the formation of an atheroprotective fibrous cap. Although it is well established that SMC-rich lesions are more stable, many individuals in our field mistakenly think that inhibiting SMC migration and proliferation is a viable therapeutic strategy for reducing plaque rupture. Therefore, it is critical to recognize that lesional SMC may have either atheroprotective or atheropromoting properties depending on the nature of their phenotypic transitions10 and we currently know little about what controls these respective transitions.
Figure. Similarities between cancer and atherosclerosis.
Although clearly separate entities, atherogenesis and malignant transformation share a number of overlapping features, including cellular plasticity, clonal expansion, genomic variation, and resistance to efferocytosis (clockwise from lower left).
Similarities Between Atherosclerosis and Cancer
As described above, atherosclerosis is unlikely to originate through a mechanism indistinguishable from the clonal theory of cancer development. It is important to note, however, that atherogenesis does share several other features with cancer, and studies predating the genome-wide association study-era have implicated variation in microsatellites, mutations in proto-oncogenes, and loss of genetic heterozygosity in vascular disease progression.11 In atherosclerosis, these mutations do not seem to drive cell proliferation to the extent they might during cancer development, but instead may have more subtle effects on DNA repair, apoptosis, and cellular senescence within the plaque. Mutation and mitosis are not necessarily the driving force behind plaque progression, but the 2 processes are not mutually exclusive.
Although these two diseases are, of course, not identical in a classic sense, several other commonalities between atherosclerosis and malignancy have been recently described. First, the top genome-wide association study locus for coronary artery disease resides adjacent to a collection of tumor suppressor genes that have been incontrovertibly linked to cancer progression. Although there is ongoing debate about the true causal gene at this locus, there is strong evidence that the key cell cycle regulator, CDKN2B, is at least one of the genes responsible for lesion development. Importantly, initial experimental studies suggest that loss of CDKN2B promotes vascular disease via mechanisms other than a simple increase in cellular proliferation.12,13 Indeed, other factors relevant to cancer have also been linked to atherosclerosis outside of genome-wide association study, including critical tumor suppressor genes (p53, Rb, and cyclins), cell adhesion molecules (cadherin proteins), and signaling pathways (MAPK [mitogen-activated protein kinase], Akt, and NFκB [nuclear factor kappa-light-chain-enhancer of activated B cells]).11 Although admittedly a distinct clinical entity, it is important to at least note that one of the most effective therapies currently available to prevent the restenosis of mechanically treated atherosclerotic lesions includes drug-eluting stents that deliver potent chemotherapeutics (eg, paclitaxel) to the vessel wall.
The second, and perhaps most striking, parallel is the recent discovery that atherosclerosis and cancer share an impairment in the clearance of diseased and dying cells (efferocytosis).14 In malignancy, cancer stem cells are known to upregulate so-called don’t-eat-me molecules such as CD47. This renders them resistant to phagocytic clearance by tumoricidal macrophages, thereby allowing them to continue to proliferate.15 Recently, this same pathway has been shown to be upregulated in the atherosclerotic plaque, which likely promotes the accumulation of diseased cells in and near the necrotic core. Future studies will determine if these pathways allow vascular cells to escape immune surveillance in a manner similar to that which occurs in the cancer stem cell.
Summary
In addition to similar molecular and cellular mechanisms, cancer and atherosclerosis share epidemiological risk factors such as smoking, advanced age, and exposure to ionizing radiation. These correlations are, of course, not indicative of a common cause. However, with the emerging evidence that (1) the most significantly associated heritable locus for coronary artery disease affects a key tumor suppressor gene, (2) there exists a clonal component to plaque development, and (3) those clones may resist phagocytic clearance, we must consider the possibility that atherosclerosis may share more of its mechanistic origins with cancer than previously thought. Clearly, atherosclerosis is not merely a cancer of the blood vessel and multiple cell types contribute to plaque growth rather than to the proliferation of a single mutated cell. On the other hand, atherosclerosis is not purely polyclonal and substantial evidence suggests the presence of monoclonal foci that enjoy a significant growth advantage. These cells have found a way to survive in a toxic plaque environment and theoretically may manipulate the immune system by suppressing efferocytosis to drive further plaque development. There is a strong need to identify these proliferating clones, determine their atherogenic potential, and identify mechanisms to prevent or even promote their proliferation (if they are beneficial to plaque stability). Future experiments using sophisticated lineage tracing models with multicolor reporters coupled with single-cell gene expression studies could identify pathways and targets that can be exploited for therapeutic use. Given the emerging availability of targeted proefferocytic therapies that seem to weed out the root cause of cancer, we may be able to exploit these techniques to stop the attack of the clones in atherosclerosis and target the critical cell types in plaque development.
Acknowledgments
We gratefully acknowledge Sophia Xiao for creating the Figure.
Sources of Funding
This study was supported by the National Institutes of Health (R01HL12337001 to Dr Leeper) and the American Heart Association (16POST30180018 to D. DiRenzo).
Footnotes
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
Disclosures
None.
References
- 1.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95:156–164. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci U S A. 2003;100:4754–4759. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 4.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973;70:1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murry CE, Gipaya CT, Bartosek T, Benditt EP, Schwartz SM. Monoclonality of smooth muscle cells in human atherosclerosis. Am J Pathol. 1997;151:697–705. [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, Helms JA, Li S. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD, et al. Extensive proliferation of a subset of differentiated, yet plastic, medial vascular smooth muscle cells contribute to neointimal formation in mouse injury and atherosclerosis models. Circ Res. 2016;116:309799. doi: 10.1161/CIRCRESAHA.116.309799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross JS, Stagliano NE, Donovan MJ, Breitbart RE, Ginsburg GS. Atherosclerosis and cancer: common molecular pathways of disease development and progression. Ann N Y Acad Sci. 2001;947:271–292. [PubMed] [Google Scholar]
- 12.Kojima Y, Downing K, Kundu R, Miller C, Dewey F, Lancero H, Raaz U, Perisic L, Hedin U, Schadt E, Maegdefessel L, Quertermous T, Leeper NJ. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J Clin Invest. 2014;124:1083–1097. doi: 10.1172/JCI70391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leeper NJ, Raiesdana A, Kojima Y, et al. Loss of CDKN2B promotes p53-dependent smooth muscle cell apoptosis and aneurysm formation. Arterioscler Thromb Vasc Biol. 2013;33:e1–e10. doi: 10.1161/ATVBAHA.112.300399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima Y, Volkmer JP, McKenna K, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiskopf K, Weissman IL. Macrophages are critical effectors of antibody therapies for cancer. MAbs. 2015;7:303–310. doi: 10.1080/19420862.2015.1011450. [DOI] [PMC free article] [PubMed] [Google Scholar]