Abstract
Graft-versus-host disease (GVHD) is initiated by antigen-presenting cells (APCs) that prime alloreactive donor T cells. In anti-pathogen responses antigen-bearing APCs receive signals though pattern-recognition receptors (PRRs), including TLRs, which induce the expression of costimulatory molecules and production of inflammatory cytokines, which mold the adaptive T cell response. However, in allogeneic stem cell transplantation (alloSCT), there is no specific pathogen, alloantigen is ubiquitous and signals that induce APC maturation are undefined. To investigate APC activation in GVHD, we used recipient mice with hematopoietic cells genetically deficient in pathways critical for APC maturation in models in which host APCs are absolutely required. Strikingly, CD8 and CD4-mediated GVHD were similar whether host APCs were wild type or deficient in MyD88, TRIF or MyD88 and TRIF, which excludes essential roles for TLRs and IL-1β, the key product of inflammasome activation. Th1 differentiation was if anything augmented when APCs were MyD88/TRIF−/− and T cell production of IFN-γ did not require host IL-12. GVHD was also intact when APCs lacked the type I IFN receptor, which amplifies APC activation pathways that induce type I IFNs. Thus in GVHD alloreactive T cells can be activated when pathways critical for anti-pathogen T cell responses are impaired.
Introduction
Allogeneic hematopoietic stem cell transplantation (alloSCT) can be a life-saving therapy for hematologic malignancies and nonmalignant disorders of blood cells such as sickle cell anemia and aplastic anemia. Donor T cells in allografts contribute to the efficacy of alloSCT. They are pivotal for reconstituting T cell immunity, particularly in adult patients who have incomplete and delayed generation of progenitor-derived T cells. They also mediate a potent anti-neoplastic effect called graft-versus-leukemia (GVL). Unfortunately, donor T cells can broadly target nonmalignant host tissues in a process called graft-vs.-host disease (GVHD) (1). Because of GVHD, all patients receive some type of prophylactic immunosuppression, either by depleting T cells from the allograft, or more commonly, with pharmacologic agents that inhibit T cell function. However, pharmacologic immunosuppression is incompletely effective and also renders recipients susceptible to serious pathogen infections.
GVHD is initiated by antigen presenting cells (APCs) that prime alloreactive donor T cells (1–6). Recipient APCs that survive conditioning are essential for GVHD in MHC-mismatched transplants and in CD8-mediated GVHD across only minor histocompatibility antigens (miHAs) (1–4, 7, 8). They also have an important and nonredundant role in CD4-mediated GVHD across miHAs (6). Thus, recipient APCs would be a logical target for suppressing GVHD.
APCs, which include dendritic cells (DCs), B cells, macrophages and basophils, are diverse cells that have in common the ability to prime T cells. Among APCs, DCs are perhaps the most efficacious in priming naïve T cells, which are potent inducers of GVHD (9). Consistent with this, in an MHC-mismatched alloBMT model, infusing wild type recipient splenic or plasmacytoid DCs can partially restore GVHD to mice with MHC-deficient hematopoietic cells (3, 4). Therefore DCs would be attractive targets for GVHD prevention.
In anti-pathogen responses, the nature of the pathogen challenge can in part be decoded by pattern recognition receptors (PRRs) expressed by APCs, which induce maturation programs characterized by upregulation of MHCII and costimulatory molecules and production of proinflammatory cytokines, all of which contribute to molding the innate and adaptive T cell responses appropriate to the invading pathogen (10–12). Indeed, it appears critical for effective pathogen responses that APCs recognize the pathogen by one or another innate immune receptors, leading to pathogen uptake, APC activation, and effective Ag presentation to T cells (13, 14).
Based on the need for host APCs in GVHD, and the practically universal requirement that in pathogen responses these APCs become activated by means of innate immune receptor recognition and/or via T cell-derived signals such as CD40L, we hypothesized that a similar mode of APC activation would be required for effective GVHD. However, in alloSCT, there is no specific pathogen. Nonetheless, there are a number of means by which APCs could become activated via innate immune or CD40L signals. First, host APCs could be activated by the same PRRs that are engaged in pathogen infections, stimulated by products from commensal bacteria, such as those in the bowel. Based on this rationale, translocation of intestinal bacteria and their products, in particular LPS, with subsequent activation of host APCs has been proposed as an essential component of GVHD initiation (15). Such a model would predict that without functional receptors for detecting these bacteria, GVHD would be ameliorated, as is the case for responses against bacterial themselves. Such a mechanism has recently been implicated in autoimmune diabetes in NOD mice (16). Alternatively, it is possible that PRRs respond to endogenous ligands, some of which signal via TLRs (13, 17–21). This mode of APC activation has been shown to be essential in lupus (22–24). On the other hand, as this hypothesis has not yet been tested, a distinct possibility is that specific APC activation is not necessary and that constitutive levels of MHC, costimulatory molecules and inflammatory cytokines are sufficient to activate T cells in the GHVD setting.
Here we have examined the roles of relevant, candidate PRRs and their associated signaling pathways and critical cytokine mediators. Among PRRs, the Toll-like receptors (TLRs) have been the most intensively studied (25). All TLRs, except TLR3, signal through the adaptor protein MyD88. TLR3 signals exclusively via the adaptor molecule TRIF whereas TLR4 signals via both MyD88 and TRIF. A second category of PRRs are the cytoplasmic RIG-I-like receptors (retinoic acid inducible gene like receptors; RLRs) (26) which recognize 5’triphosphate-capped RNA and dsRNA. RLRs signal via IPS1 and induce type I IFNs. A less defined set of receptors recognizes cytoplasmic DNA and signals via TBK-1 and IKKε to also induce type I IFN production (27). In general, the type I IFN response is amplified via type I IFN receptor signaling in an autocrine/paracrine fashion (28). A third class of sensors are the cytoplasmic NACHT-LRR (NOD-like receptors; NLR) proteins, which include NODs and NALPs (10, 29). The NOD members signal through RICK-2 to induce NF-κB and MAPK activation. In contrast, other NLR family members including the NALPs, signal through the adaptor ASC to induce inflammasome activation, for which an important outcome is activation of caspase 1 and processing of pro-IL-1β to IL-1β. Inflammasomes can also be activated by endosomal and cytoplasmic DNA (30) (31).
We report here our findings from a comprehensive genetic approach to assessing these modes of immune recognition, using recipient mice with APCs genetically deficient in subsets of the aforementioned pathways in GVHD models in which host APCs are absolutely required (2, 8). To address APC activation by TLRs we used recipients with APCs lacking MyD88, TRIF (32), or MyD88 and TRIF. MyD88 is also essential for IL-1R signaling; therefore MyD88−/− APCs cannot respond to IL-1β generated by multiple pathways, including inflammasome activation. This is an especially broad impairment as innate responses to nearly all bacteria, including enteric bacteria, and many viruses engage TLRs, the inflammasome or both (33–60). To study activators of type I IFNs, we used recipient mice lacking IFNAR1 (61), an essential component of the receptor for all type I IFNs (28). We also studied recipients lacking CD40, B71/B72 and p35, a component of IL-12. Taken together, the results of these studies suggest that GVHD is fundamentally different from most anti-pathogen responses, tend to counter the prevailing models for GVHD initiation (15), and have implications for strategies to prevent GVHD in the clinic.
Materials and methods
Mice
B6 CD45.1 mice were purchased from Taconic/NCI Frederick (Frederick, MD). C3H.SW and B6bm12 mice were purchased from the Jackson Labs (Bar Harbor, ME) and bred at Yale. MyD88−/− mice (62) backcrossed at least 10 generations to B6 and MyD88−/−/IFNAR1−/− mice were obtained from Ruslan Medzhitov (Yale University School of Medicine, New Haven, CT). B6 LPS2 mice (TRIF-deficient; TRIF−/ (32)) were obtained from Bruce Beutler (Scripps Research Institute, La Jolla, CA). MyD88/TRIF−/− (double knockout; DKO) mice were generated by crossing MyD88−/− and LPS2 mice. Typing for the MyD88−/− and LPS2 alleles were performed as published (32, 62). IFNAR1−/− mice (61) were provided by Helene Rosenberg (NIH, Bethesda, MD). p35-deficient and CD40-deficient mice were purchased from the Jackson Labs. B71/B72−/− mice (63) were provided by Bruce Blazar (University of Minnesota, Minneapolis, MN). B10/J mice were obtained from the Jackson Labs and B10.ScNCr mice from the NCI.
Cell purifications
CD8 cells were purified via depletion from lymph node (LN) using biotin-conjugated antibodies, streptavidin microbeads and an AutoMACs cell separator (Miltenyi Biotec, Auburn, CA) (5). CD8 cells were >90% pure with CD4+ T cell contamination of less than 3%. All donor bone marrow (BM) was depleted of T cells using anti-Thy1.2 microbeads and the AutoMACs (Miltenyi Biotec). Naïve B6bm12 CD4 cells were sorted by staining splenocytes with antibodies against CD4 (pacific blue; RM4-5, Biolegend), CD44 (FITC; IM7, BD Pharmingen), CD25(PE; 3C7, Southern Biotechnology, Birmingham, AL), CD62L (PE-Cy7; Mel-14; eBioscience)
Bone marrow transplantation
All transplants were performed according to protocols approved by the Yale University Institutional Animal Care and Use Committee. To create bone marrow chimeras, mice received 2×500cGy or a single fraction of 1000cGy and were reconstituted with 107 donor BM cells. To induce GVHD, chimeras were reirradiated (2×450cGy) and reconstituted with T cell-depleted donor BM with or without T cells. In the C3H.SW→B6 system, GVHD was induced by 2–3×106 CD8 cells. In the B6bm12→B6 model, GVHD was induced by splenocytes containing 106 CD4 cells or sorted naïve CD4 cells as specified in the text. Mice were weighed 2–3×/week; weights from mice that died or that were sacrificed due to predefined measures of morbidity were included in averages for subsequent time points at the last value recorded. Skin disease was scored when mice were weighed; minimal criteria for clinical skin disease was fur loss in an area > 1cm2.
Intracellular cytokine staining
Intracellular cytokine staining was performed as described (64). Splenocytes were harvested and cultured with phorbol myristic acid (PMA; Sigma Chemical Co., St. Louis, MO) and ionomycin (Calbiochem-Novabiochem Corp., La Jolla, CA) for 5 hours; GolgiPlug (BD Pharmingen, San Diego, CA) was added for the final 2 hours. Prior to permeabilization, cells were incubated with ethidium monoazide (EMA, Invitrogen, Carlsbad, CA). Cells were stained with antibodies against CD4 (Alexa488; GK1.5; lab-grown and conjugated) and CD45.1 (PE, A20; BD Pharmingen), permeabilized, and then stained for IFN-γ (APC; XMG1.2; BD Pharmingen).
APC engraftment in BM chimeras
Spleens and LNs were digested with collagenase as described (5, 8). To distinguish residual recipient (CD45.1+) and donor-derived (CD45.2+) DCs in initial BM chimeras, cells were stained with antibodies against CD45.1 or CD45.2 (FITC; clones A20 and 104; BD Pharmingen), CD11c (APC; clone HL3; BD Pharmingen), a cocktail of biotin-conjugated antibodies against Gr-1 (RB6-8C5; BD Pharmingen), CD19 (1D3; BD Pharmingen), Ly76 (TER119; BD Pharmingen), and Thy1.2 (30H12; lab-prepared). Cells were stained with streptavidin-PerCP (BD Pharmingen). DCs were identified as CD11c+lineage−.
Measurement of serum cytokines
Serum cytokines were measured using the Bioplex kit Th1/Th2 panel (Bio-Rad; Hercules, CA) and a Luminex 100 system (Luminex; Austin, TX) as previously described (64).
Scoring of pathology
Because our transplant conditions were generally not lethal, we were able to take pathology specimens at the conclusion of each experiment (see weight curves for time points). In the C3H.SW→B6 strain pairing a small minority of CD8 recipients reached endpoints for extent of skin disease that required earlier sacrifice and pathology was harvested at that time. Skin pathology was scored as previously described (J.M)(5). In experiments with TLR4−/−, B71/B72−/− and CD40−/−, gastrointestinal pathology was scored by D.J. as previously described (5). In all other experiments gastrointestinal pathology was scored by A.J.D. follows. For liver, portal inflammation, bile duct damage, central perivenulitis, and lobular necro-inflammation were semi-quantitatively evaluated on a scale of 0 to 3. Weighted scores were calculated by multiplying the scores of portal inflammation and central perivenulitis by 1.0 and bile duct injury and lobular necro-inflammatory scores by 0.5. The total score was the sum of weighted scores. In bowel, inflammation/cryptitis, number of apoptotic bodies per 10 crypts in the most severely affected areas, crypt abscesses, crypt loss and ulceration were scored 0 to 3. Scores were weighted as follows: inflammation/cryptitis × 0.9; apoptotic bodies per 10 crypts × 0.1; crypt abscess × 0.4; crypt loss × 1 and ulceration × 2. The total score was the sum of weighted scores.
Statistics
Significance for differences in weight loss and serum cytokine levels was calculated by an unpaired t test. P values for differences in survival and the incidence of skin disease were calculated by a log-rank test. P values for histology were calculated by Mann-Whitney. Error bars for weights represent standard error measurements (GraphPad Prism)
Results
GVHD in TLR4-deficient mice
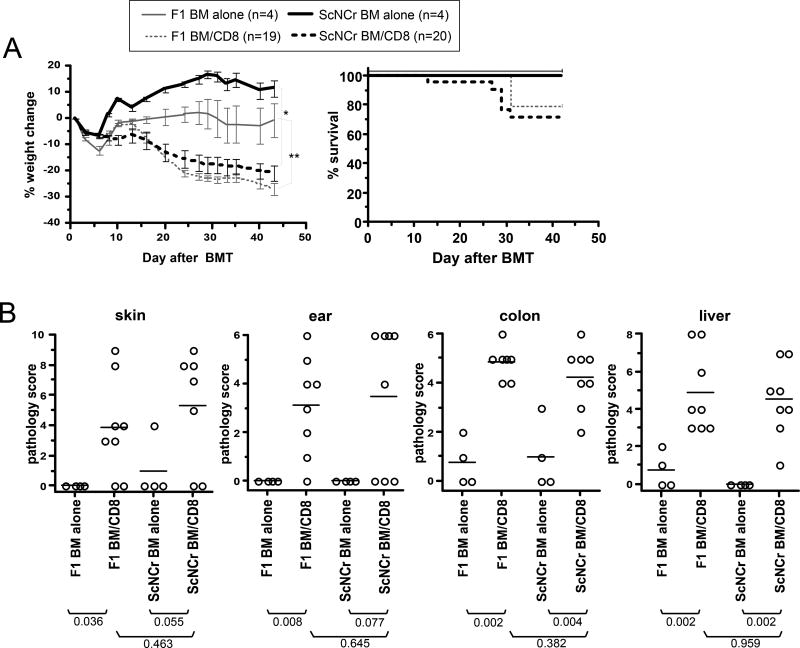
Serum LPS is increased in irradiated mice, presumably secondary to translocation from the bowel (65). We therefore considered the hypothesis that LPS is an important APC maturation factor. To test this we compared GVHD in B10.ScNCr mice, which have a naturally occurring deletion of TLR4, to that in B10.ScNCrxB10/J F1 (TLR4+/−) mice. We used F1 control recipients to minimize the contribution from additional genetic differences between B10.ScNCr and B10/J (TLR4+/+) mice. We utilized the C3H.SW (H-2b)→B10 (H-2b) CD8-mediated GVHD model in which host APCs are absolutely required for GVHD. GVHD was similar in B10.ScNCr and F1 CD8-recipients as measured by survival, weight loss and pathology in skin, ear, colon and liver (Figure 1). GVHD was also comparable in B10.ScNCr and B10/J (TLR4+/+) mice (not shown). Thus, TLR4 ligands, be they exogenous or endogenous, are not essential APC maturation factors for GVHD in the C3H.SW→B10 strain pairing.
Figure 1. Signaling by host TLR4 is not required for GVHD.
Lethally irradiated B10.ScNCr (TLR4−/−) and B10.ScNCrXB10/J F1 (TLR4+/−) mice were reconstituted with C3H.SW BM with or without 2×106 purified C3H.SW CD8 cells. (A) Percent weight change. *P<0.05, F1 BM alone vs F1 BM plus CD8 from day 19 onward; **P<0.05 B10.ScNCr BM alone vs B10.ScNCr BM plus CD8 from day 7 onward; no significant difference comparing F1 and B10.ScNCr CD8 recipients at all time points except day 9. (B) Pathology scores. Each symbol is the score from an individual mouse. Horizontal lines represent mean scores. P values are shown below the group labels. Data are from 1 experiment.
GVHD in CD40−/− mice
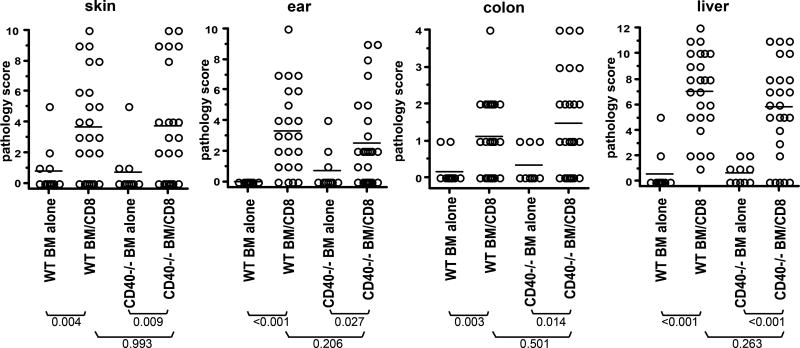
We also determined whether host CD40 contributed to GVHD in the C3H.SW→B6 model. CD40L could be expressed on donor CD8+ cells (66) or by the small number of contaminating donor CD4 cells. Moreover, as all recipient APCs express miHAs, those activated for any reason, including by recipient CD40L+ T cells responding to pathogens or allergens prior to transplantation, could license recipient DCs. However, CD40−/− and wt control recipients of CD8 cells developed similar weight change (Supplemental Figure 1) and pathologic GVHD in skin, ear, liver and colon (Figure 2; P≥0.2 comparing wt and CD40−/− CD8 recipients).
Figure 2. Signaling by host CD40 is not required for GVHD.
B6 CD40−/− and control B6 mice were irradiated and reconstituted with C3H.SW BM with or without 2×106 purified CD8 cells. Shown are pathology scores of skin, ear, colon and liver. There was significant pathology in all tissues in both wt and CD40−/− recipients, relative to their BM only controls; however there was no difference in any tissue comparing wt and CD40−/− CD8 recipients. Data are combined from 2 independent experiments with similar results.
GVHD in mice with APCs deficient in MyD88, TRIF or MyD88 and TRIF (double knockout; DKO)
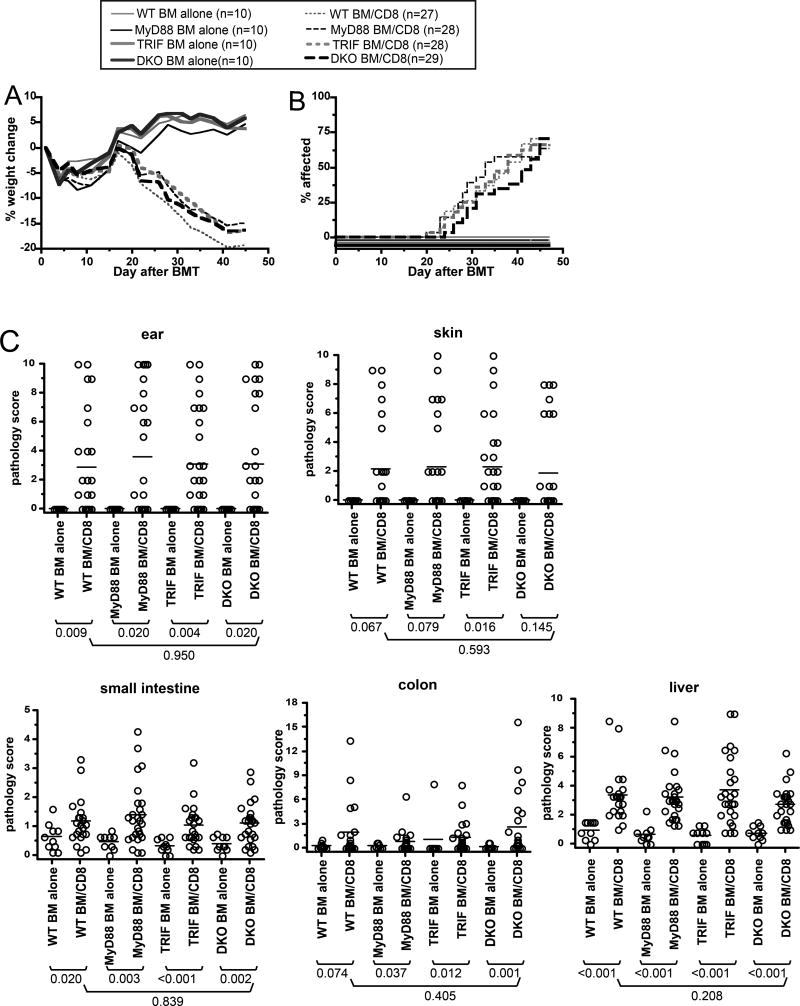
To broadly target TLRs we employed mice deficient in MyD88, TRIF or both. TLRs are not only expressed by hematopoietic cells including APCs, but also function in nonhematopoietic cells. Because of this, the interpretation of GVHD experiments in mice unable to signal via TLRs could be confounded by effects on nonhematopoietic tissues. We therefore used as recipients in GVHD-inducing experiments mice in which nonhematopoietic cells were wt but the hematopoietic system, including APCs, was gene-deficient. We created such mice as BM chimeras. Wt CD45.1+ B6 mice were irradiated and reconstituted with BM from B6 wt, MyD88−/−, TRIF−/ or DKO mice (all CD45.2). Cohorts of at least 3 mice were analyzed to confirm donor APC engraftment. DCs in spleen and LN were at least 97% donor-derived in all experiments (Supplemental Figure 2). Approximately 3 months after the first transplant, chimeras were reirradiated and reconstituted with C3H.SW BM, with or without 2–3×106 purified C3H.SW CD8+ T cells.
All groups transplanted with donor CD8 cells developed comparable GVHD as manifest by weight loss, (Figure 3A) and incidence of clinical skin disease (Figure 3B). Wt→wt, MyD88−/−→wt, TRIF−/−→wt and DKO→wt CD8-recipients developed histopathologic GVHD of the ear, liver, small intestine and colon relative to their respective BM only controls (Figure 3C). Skin pathology scores were of borderline significance in all groups except for TRIF−/− CD8-recipients (P=0.016); however when compared to the pooled scores of all of the BM alone mice, pathology scores were significant for each CD8 recipient group (P<0.009). Importantly, the pathology scores did not differ in wt→wt and DKO→wt CD8 recipients in any tissue (P>0.2). Thus, in this CD8-dependent GVHD model wherein host APCs are essential, signaling via MyD88 and/or TRIF in host APCs was not required.
Figure 3. TLR signaling on recipient APCs is not required for CD8-mediated GVHD.
Wt→wt, MyD88−/−→wt, TRIF−/−→wt and DKO→wt BM chimeras were reirradiated and reconstituted with C3H.SW BM with or without 2–3×106 purified C3H.SW CD8+ T cells. (A) Percent weight change (P≥0.5 comparing all CD8 recipient groups at all time points; P<0.05 comparing the percent weight change in wt, MyD88−/−, TRIF−/− or DKO chimera CD8-recipients with the respective BM alone groups beginning on day +26); (B) incidence of skin disease (P≥0.29 comparing any CD8 group to any other CD8 group; P<0.003 comparing incidence of skin disease of each CD8 recipient group with its respective BM alone group); (C) Pathology scores. Data are combined from two independent experiments with similar results.
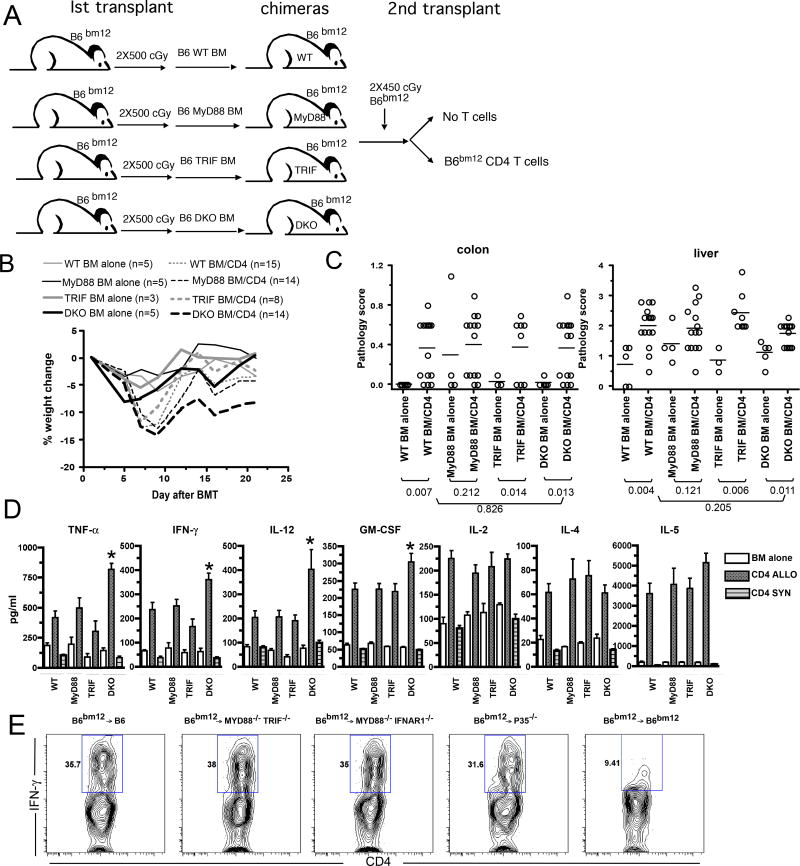
Although APCs in the chimeras used in the second GVHD-inducing BMT were nearly completely donor-derived (ie MyD88−/−, TRIF−/− or DKO) and we have previously found β2M−/−→B6 and C3H.SW→B6 chimeras to be resistant to GVHD in the same strain pairing thereby validating this approach (8), we considered the possibility that the small number of residual host wt B6 APCs was sufficient to induce GVHD. To exclude this, we compared GVHD in MyD88−/−, TRIF−/−, DKO or wt→B6bm12 BM chimeras retransplanted with B6bm12 BM with or without B6bm12 CD4 cells (experimental design, Figure 4A). GVHD in the MHCII-mismatched B6bm12→B6 strain pairing occurs even when the recipient parenchyma is MHCII− (2) and therefore allogeneic host hematopoietic cells alone are sufficient. In our retransplanted B6→B6bm12 chimeras, any residual host APCs were B6bm12 and syngeneic to the donor CD4 cells in the GVHD inducing transplant. Consequently these APCs could not contribute to donor T cell priming. Moreover, because B6bm12 mice are B6 in origin, there are no miHA differences between donor and recipient, which precludes GVHD directed at miHAs indirectly presented by donor APCs. As was the case in the C3H.SW→B6 strain pairing, GVHD as measured by weight loss occurred in all CD4 recipient groups (Figure 4B). Surprisingly, DKO→B6bm12 CD4 recipients had the greatest weight loss. Similar pathologic colon and liver GVHD were present in wt→B6bm12 and DKO→B6bm12 CD4 recipients. Neither liver nor colon pathology scores were significant in MyD88−/−→wt CD4 recipients, likely due to background inflammation in the MyD88−/− BM alone mice. However, as compared to the combined scores of all BM alone recipients, there was significant pathology in both colon and liver (P≤0.0009).
Figure 4. TLR signaling on recipient APCs is not required for CD4 mediated MHC-mismatched GVHD.
(A) Experimental design for B–D. B6bm12 mice were irradiated and reconstituted with 5×106 T cell depleted BM from B6 wt, MyD88−/−, TRIF−/− or DKO donors. Three months later, the chimeras were reirradiated and reconstituted with T cell depleted B6bm12 BM and B6bm12 splenocytes containing 106 CD4 cells. (B) Percent weight change. P values comparing BM alone vs CD4 recipients by host: wt, P<0.05 at day 7; MyD88−/−, P<0.05 from day 7 onward; TRIF−/−, P<0.05 at day 7; DKO, P<0.05 from day 5 onward except day 14 and 16. P<0.05 comparing DKO and wt CD4 recipients, beginning on day 12. (C) Pathology scores from colon and liver. No significant difference comparing wt→B6bm12 CD4 recipients to any other CD4 recipient group. Data in B and C were from one experiment; the number of mice/group is shown in the figure. (D) Serum cytokine levels and representative intracellular IFN-γ staining from chimeric mice transplanted as in (A) at day 7 after BMT. Data from 3 mice per BM alone group. The numbers of mice per CD4 group were as follows: wt, n=10; MyD88−/−, n=5; TRIF−/−, n=5; DKO, n=9. P<0.05, comparing wt, MyD88−/−, TRIF−/− or DKO CD4 recipients to their respective BM only controls for all cytokines. Levels of TNF-α, IFN- γ, GM-CSF and IL-12 were greater in DKO recipients as compared to wt controls (* P<0.05). Data for DKO and wt recipients are combined from two experiments with similar results. B6 wt, DKO, p35−/− and MyD88−/−/IFNAR1−/− mice were irradiated and reconstituted with B6bm12 BM and 5×105 B6bm12 CD4+CD62L+CD44−CD25− T cells. Intracellular IFN-γ staining was performed on splenocytes harvested from mice on day +7 (E; representative data from 6–10 mice per group from at least 2 independent experiments).
Because signaling through MyD88/TRIF can promote Th1 responses, we investigated whether cytokine production was affected by the absence of TLR signaling. We harvested serum 7 days post BMT from wt→B6bm12, MyD88→B6bm12 and DKO→B6bm12 chimeras retransplanted with B6bm12 BM and CD4 cells. In all CD4 recipients, serum IFN-γ, IL-12, TNF-α, GM-CSF, IL-2, IL-4 and IL-5 were elevated relative to their BM alone controls (Figure 4D). Cytokine levels in syngeneic B6→B6 CD4 recipients were similar to that in BM alone controls (Figure 4 and (64)). Surprisingly, serum levels of the Th1 cytokines IFN-γ and TNF-α were higher in DKO→B6bm12 as compared to wt→B6bm12 CD4 recipients, as were IL-12 and GM-CSF. IL-4 and IL-5 were similar in retransplanted wt→B6bm12 and DKO→B6bm12 recipients (P>0.29).
It was possible that the elevated levels of Th1 cytokines in DKO→B6bm12 CD4 recipients were due to cytokine production by cells other than donor CD4 cells or by a relatively small number of donor CD4 cells. To address this wt B6 or DKO mice were irradiated and reconstituted with B6bm12 BM and sort-purified B6bm12 CD4+CD62L+CD44− CD25− naïve T cells. Mice were sacrificed on day +7 and CD4+IFN-γ+ cells were quantitated. A similar percentage of splenic CD4 cells produced IFN-γ in wt and DKO recipients (Figure 4E) which confirms that donor CD4 cells were polarized when primed by DKO recipient APCs. Parallel data were obtained with retransplanted B6→B6bm12 and DKO→B6bm12 mice (Supplemental Figure 3A). Even when recipients were MyD88−/− /IFNAR1−/− mice, a similar percentage (Figure 4E, representative flow cytometry) and total number of donor CD4 cells (Supplemental Figure 3B) produced IFN-γ. Thus even when there was no TLR or IL-1R signaling or no MyD88 and IFNAR1−/− signaling in host APCs, Th1 polarization was robust.
IL-12 induced by TLR-engagement, while not required for Th1 commitment (67, 68), potentiates the expansion of Th1 cells. We hypothesized that a MyD88/TRIF-independent signal was inducing host IL-12, which promoted Th1 differentiation. To test this we used the B6bm12→B6 system to determine whether host IL-12 production is required for the expansion of Th1 alloreactive CD4 cells. B6 or B6 p35−/− (IL-12−/−) mice were irradiated and reconstituted with B6bm12 BM and sort-purified B6bm12 CD4+CD62L+CD44− CD25− naïve T cells. Seven days later, mice were sacrificed, and IFN-γ-producing CD4 cells were enumerated. Surprisingly, the percentage of CD4 cells that produced IFN-γ (Figure 4E, representative flow cytometry), the number of splenic CD4 and the number of IFN-γ+ CD4 cells were similar in p35−/− and wt recipients (Supplemental Figure 3C). Therefore donor CD4 cells can polarize even when the presenting APC cannot produce IL-12.
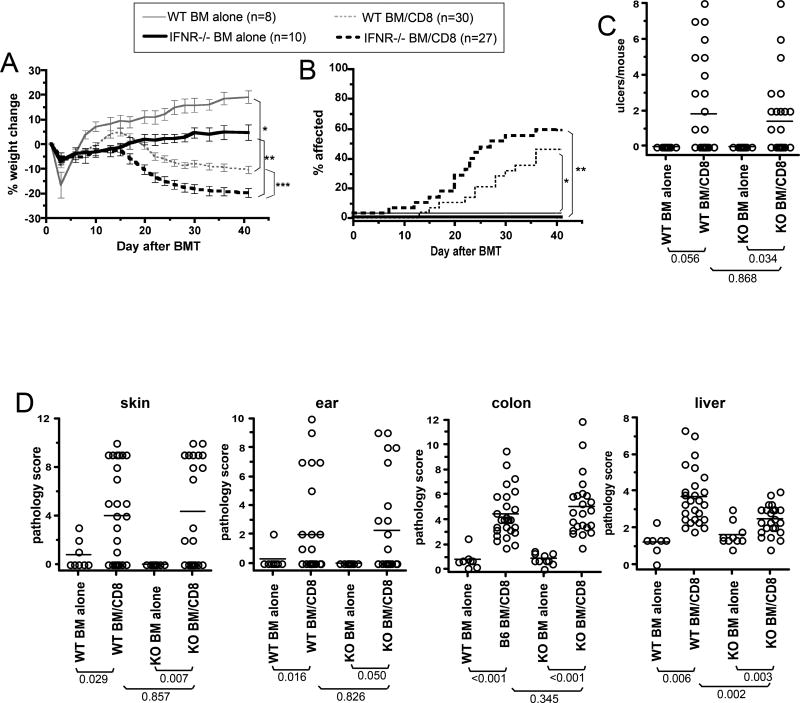
GVHD in IFNAR1−/− mice
To determine whether type I IFNs have a nonredundant role in recipient APC maturation in GVHD, we compared GVHD in wt and IFNAR1−/− recipients in the C3H.SW→B6 strain pairing. Both wt and IFNAR1−/− CD8-recipients lost weight relative to their respective BM alone controls (Figure 5A). IFNAR1−/− CD8-recipients lost more weight than did wt CD8-recipients; however, IFNAR1−/− BM alone recipients failed to gain weight as did wt BM alone recipients. Both wt and IFNAR1−/− CD8 recipients developed clinical skin disease (Figure 5B, incidence; Figure 5C, number of ulcers), which was similar in both CD8 groups. Both CD8-recipient-groups developed pathologic GVHD of the skin, ear, colon and liver (Figure 5D). However, liver GVHD was modestly less severe in IFNAR1−/− hosts.
Figure 5. Host IFNAR1 is not required for CD8-mediated GVHD in the C3H.SW→B6 strain pair.
(A) Percent weight change. *P<0.03, comparing wt BM alone vs CD8 recipients from day 17 onward; **P<0.001, comparing IFNAR1−/− BM alone vs IFNAR1−/− CD8 from day 20 onward; *** P<0.01 comparing wt and IFNAR 1 CD8 recipients from day 13 onward. (B) Incidence of skin disease. P<0.03 comparing wt and IFNAR1−/− CD8 recipients to their BM alone controls; P>0.24 comparing wt and IFNAR1−/− CD8 recipients. (C) Number of skin ulcers. and (D) pathology scores. CD8 recipients had significant pathology in skin, ear, colon and liver relative to their BM alone controls. Data are combined from two independent experiments with similar results.
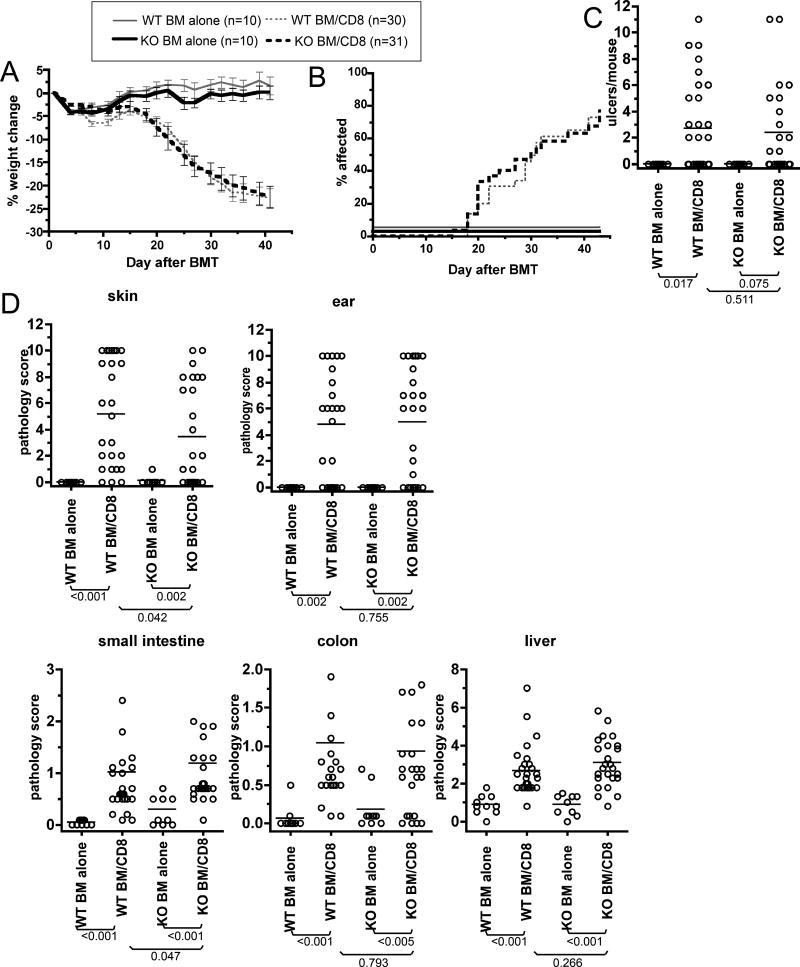
To focus on type I IFN action on recipient APCs, we took the BM chimera approach and compared GVHD in retransplanted wt→wt and IFNAR1−/−→wt BM chimeras, again in the C3H.SW→B6 strain pairing (Figure 6; data combined from two independent experiments). As manifest by weight loss (Figure 6A) and clinical skin disease (Figure 6B) GVHD was similar in wt→wt and IFNAR1−/−→wt mice. Similar histopathologic GVHD of the skin, ear, small intestine, colon and liver developed in both CD8 recipient groups, though in IFNAR1−/−→wt CD8 recipients skin pathology was modestly decreased whereas small intestine scores were higher.
Figure 6. Comparable GVHD developed in retransplanted IFNAR1−/−→wt and wt→wt BM chimeras.
B6 mice were irradiated and reconstituted with BM from wt or IFNAR1−/− mice. Three months later, chimeras were reirradiated and reconstituted with C3H.SW BM with or without purified C3H.SW CD8 cells. (A) Percent weight change. P<0.05 from day +8 onwards comparing wt→wt BM alone vs CD8 recipients. P<0.05 from day +20 onwards comparing IFNAR1−/−→wt BM alone vs CD8 recipients. (B) Incidence of skin disease. P<0.001 comparing wt→wt and IFNAR1−/−→wt CD8 recipients to their respective BM alone controls. P>0.86 comparing CD8 recipient groups. (C) Number of skin ulcers and (D) pathology scores. CD8 recipients had significant pathology in skin, ear, small intestine, colon and liver relative to their BM alone controls. There was no significant differences comparing CD8 recipient groups except for modestly more severe skin and small intestine GVHD in wt→wt and IFNAR1−/−→wt CD8 recipients, respectively. Data are combined from two independent experiments with similar results.
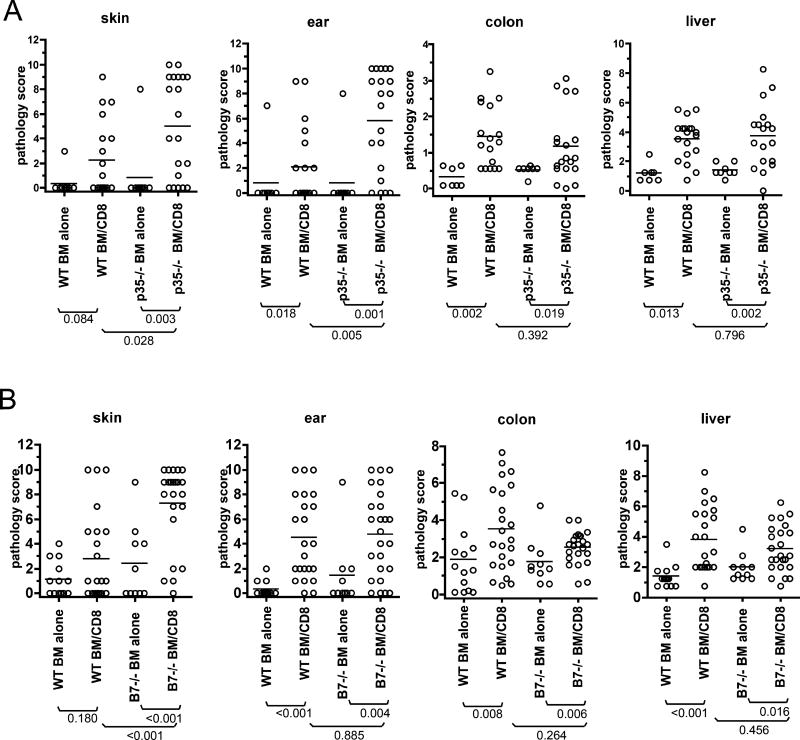
GVHD in B71/B72−/− and p35−/− mice
In response to maturation stimuli, DCs upregulate B71 and B72 and produce IL-12. We therefore determined whether CD8-mediated GVHD in the C3H.SW→B6 model is reduced in B71/B72−/− or IL-12−/− hosts. Pathologic skin and ear GVHD were more severe in p35−/− CD8-recipients (Figure 7A) whereas liver and colon GVHD were similar in wt and p35−/− CD8 recipients. Histopathologic skin GVHD was only significant in B71/B72−/− CD8-recipients (Figure 7B) and was significantly more severe than in wt CD8-recipients. Pathologic GVHD of the ear, colon and liver were present in both wt and B71/B72−/− recipients and the scores did not significantly differ (Figure 7B). Thus GVHD was intact and if anything was more severe in B71/B72−/− mice.
Figure 7. Host B71/B72 or IL-12 are not required for CD8-mediated GVHD in the C3H.SW→B6 model.
Pathology scores comparing GVHD in p35−/− (A) and B71/B72−/− (B) as compared to wild type recipients. Shown are data combined from two independent experiments for each in A and B. There were no significant differences comparing wt and p35−/− CD8 recipients except more severe skin (P=0.028) and ear disease (P=0.005) in p35−/− mice. There were no significant differences comparing wt and B71/B72−/− CD8 recipients except more severe skin disease in B71/B72−/− mice (P<0.001).
Discussion
In pathogen models optimal adaptive immune responses require APCs to be activated by pathogen-derived molecules which engage PRRs (12, 13). The goal of the present work was to understand how host APCs, which have essential roles in GVHD induction, are activated to initiate GVHD. Surprisingly and contrary to our expectations, we found GVHD to be unaffected and not even partially ameliorated in mice with APCs unable to respond to the major PRRs. Therefore T cell activation in alloSCT is not equivalent to that in many pathogen systems. Since most bacteria are recognized by TLRs that transduce signals via MyD88 and TRIF and in some cases with concomitant inflammasome activation and generation of IL-1β (which also signals through MyD88), these data make it unlikely that activation of host APCs by translocation of bacteria and bacterial products from the bowel is a pivotal step in GVHD initiation, as has been long been thought (69, 70) and proposed in recent schemas of GVHD initiation (15, 71). Nor did we observe reduced GVHD when host APCs were IFNAR1−/− or reduced alloreactive T cell activation when APCs were IFNAR1−/−/MyD88−/−. The present data do not exclude a role for PRR ligands—however they make it unlikely that TLR, IL-1R or type I IFN-receptor engagement on host APCs are decisive events in triggering GVHD.
In denying our primary hypothesis, our studies were comprehensive and used definitive genetic approaches to ensure lack of residual signaling that could have confounded interpretations. For example, in experiments with DKO→wt recipients, APCs could not respond to any TLR ligands, be they exogenous and pathogen-derived or endogenous and stress-induced. Importantly, in the B6bm12→(B6→B6bm12) system we were able to exclude any priming by residual wild type host APCs or donor APCs, as residual recipient APCs were syngeneic to donor B6bm12 CD4 cells, and there were no miHAs for indirect presentation by donor APCs since both donor and recipient were B6 background. We studied both CD4 and CD8-mediated GVHD in independent model with concordant results. Our clinical assessments were supported by a blinded analysis of organ pathology and quantitative measurements of T cell activation, providing sensitivity to pick up even subtle effects.
It would not have been surprising to find organ-specific differences in GVHD in at least some of the knockout recipients. To screen for this we performed detailed histologic analyses in all experiments. Yet, other than decreased liver GVHD in IFNAR1−/− mice, decreased skin and increased small bowel GVHD in IFNAR1−/−→wt recipients, and increased skin disease in B71/B72−/− mice, we did not detect organ-specific differences in GVHD pathology between any KO mice and wild type controls. Thus, if specific APC maturation signals contributed to distinct patterns of organ injury, aside from the aforementioned differences, the effects were sufficiently subtle such that we could not identify them.
The genetic deficiencies studied had pleiotropic effects on immune activation pathways. Though we did not directly study IL-1/18 signaling and the inflammasome pathway, it is important to point out that MyD88 deficiency also rendered APCs unresponsive to IL-1β (and IL-18), an important end product of inflammasome activation (10). Moreover, pro-IL-1β generation is at least in-part TLR-dependent. Not only did the absence of MyD88 and TRIF fail to block GVHD, it also did not impair polarization of naïve donor CD4 cells into IFN-γ-producers and the induction of elevated serum levels of the canonical Th1 cytokines IFN-γ, IL-2 and TNF-α. This was surprising as TLR ligation promotes Th1 polarization of naïve CD4 cells in a variety of experimental models (68, 72, 73, 74. Depletion of CD4+CD25+ Tregs can in part restore CD4 T cell responses in MyD88−/− mice, including IFN-γ production (73, 74). Although IL-12 is not required for initial Th1 commitment (67, 68), we thought it likely that host-derived IL-12 would have been important for expansion of IFN-γ-producing alloreactive CD4 cells. However, contrary to this expectation, host IL-12 was not required for alloreactive Th1 polarization. Importantly, because we infused naïve T cells, IFN-γ+ cells were not derived from cells that had already been polarized.
Pathways other than those dependent on MyD88 and TRIF can induce APC maturation. A critical component of some of these, in particular those that detect cytoplasmic nucleic acids, is the elaboration of type I IFNs, which then directly promote DC activation (11, 26, 28). In this way type I IFNs amplify the signals that originally induced their production. Yet, CD8-mediated GVHD in IFNAR1−/− mice and IFNAR1−/−→wt chimeras was similar to that in wt controls and alloreactive CD4 expansion and polarization was intact in MyD88/IFNAR1−/− hosts. CD40 signaling is also MyD88/TRIF-independent, and CD8-mediated GVHD was intact in CD40−/− mice.
Stimulation through TLRs has been previously examined in alloBMT models. Mice that have undergone an alloBMT with donor T cells are more sensitive to LPS (70). Administration of CpG or imiquimod can augment GVHD and graft rejection (75, 76). On the other hand, stimulation by LPS was found by one group to decrease GVHD (77). Less GVHD was also observed in an MHC-mismatched GVHD model when donor BM cells were TLR4-deficient (78). However, why differences in donor BM cells would impact early alloreactivity targeted to recipient allogeneic MHC was not explained. Our data make it clear that while stimulation through MyD88/TRIF-dependent pathways may modify the alloimmune response and could promote GVHD, in the absence of such signals, GVHD can nonetheless proceed.
Recipient regulatory T cells (Tregs) can survive irradiation and suppress GVHD (79), and in our experiments they would have also been unable to signal via the affected PRRs. Tregs express TLRs (reviewed in (80)) and in general TLR ligands have been found to promote Treg proliferation and suppression. If radiation-resistant host Tregs modify GVHD in the models we employed (which has not been tested), the absence of TLR-stimulation could have diminished their activity thereby augmenting GVHD. On the other hand, TLR engagement on APCs has been shown to inhibit Treg activation (73, 74). If Tregs are activated by host APCs, the absence of TLR signaling on host APCs could have augmented Treg function. Whether or not there was any influence of host Tregs in our studies, PRR-defective APCs were fully capable of initiating donor T cell activation and GVHD.
In summary, these studies demonstrate that APC activation pathways that contribute to T cell responses in pathogen models are not required for GVHD induction by host APCs. GVHD thus differs quite fundamentally from anti-pathogen responses. Why is it that innate immune signaling and host APC activation do not appear to be as important as in responses to bacteria, fungi and viruses? It is possible that, in the context of alloSCT, pathways that induce APC activation could be massively redundant, owing to the ubiquitous and abundant availability of alloantigen, such that any APC licensed by any activating ligand can contribute to GVHD induction. Although we took the broadest approaches available to impair PRRs, we could not block all potential pathways simultaneously. It is possible that independence from PRRs was owing to MyD88/TRIF-independent activation pathways engaged by mechanisms reliant on the conditioning regimen. To address this would require crossing the knockouts we employed to RAG−/− or TCR-deficient backgrounds or breeding them to equivalent KOs on another background (such as BALB/c) to allow parent→F1 GVHD-inducing transplants without conditioning. Such breeding was beyond the scope of the present work, but if such mice were available, they could be used in experiments to determine the role of conditioning, though from a clinical standpoint, conditioning is nearly always employed.
It is important to realize that, with respect to pathogen responses, most of the single and doubly deficient mice we have studied did indeed have striking phenotypes, suggesting at the least a much more narrow level of redundancy in pathogen resistance than in GVHD. Alternatively, instructive APC maturation may not be required. Baseline levels of costimulatory molecules and inflammatory cytokines might be sufficient to initiate GVHD given the relatively high frequency of host reactive T cells and, especially, the ubiquitous and high levels of target antigens that cannot be cleared. In this regard, PRR induction of APC migration, enhanced phagosome and lysosome maturation (81, 82), and cross-presentation (83) (84) may not be required. At a practical level, it will be difficult to study yet further compound “knockout” mice to test for multi-layered redundancy. This may not be so important, because from the point of view of clinical application, which is paramount in the study of GHVD, our data suggest that blockade of specific PRRs at the time of transplant are unlikely to completely prevent GVHD.
Supplementary Material
Acknowledgments
We thank the Yale Animal Resources Center for expert animal care. We also thank Mark Shlomchik for his critical reading of the manuscript.
Footnotes
This work was supported by NIH HL-083072 and P01 AI064343. W.D.S is a recipient of a Clinical Scholar award from the Leukemia and Lymphoma Society.
Authorship
H.L. designed and executed experiments, analyzed results and wrote the paper. H.S.T. and S.V. assisted in experiments. J.M., A.J.D. and D.J. analyzed pathology. F.L. and D.R. helped with data interpretation, experimental design and edited the manuscript. W.D.S. designed experiments, analyzed results and wrote the paper.
References
- 1.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 2.Teshima T, Ordemann R, Reddy P, Gagin S, Liu C, Cooke KR, Ferrara JL. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–581. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 3.Duffner UA, Maeda Y, Cooke KR, Reddy P, Ordemann R, Liu C, Ferrara JL, Teshima T. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172:7393–7398. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 4.Koyama M, Hashimoto D, Aoyama K, Matsuoka K, Karube K, Niiro H, Harada M, Tanimoto M, Akashi K, Teshima T. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 2009;113:2088–2095. doi: 10.1182/blood-2008-07-168609. [DOI] [PubMed] [Google Scholar]
- 5.Matte CC, Liu J, Cormier J, Anderson BE, Athanasiadis I, Jain D, McNiff J, Shlomchik WD. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10:987–992. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]
- 6.Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105:2227–2234. doi: 10.1182/blood-2004-08-3032. [DOI] [PubMed] [Google Scholar]
- 7.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 8.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. Prevention of graft versus host disease by inactivation of host antigen- presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 9.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 10.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joffre O, Nolte MA, Sporri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 2009;227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 18.Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Grone HJ, Schaefer L. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284:24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M, Malle E, Schaefer RM, Grone HJ. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 22.Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beutler BA. TLRs and innate immunity. Blood. 2009;113:1399–1407. doi: 10.1182/blood-2008-07-019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakhaei P, Genin P, Civas A, Hiscott J. RIG-I-like receptors: sensing and responding to RNA virus infection. Semin Immunol. 2009;21:215–222. doi: 10.1016/j.smim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 28.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 29.Ting JP, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. Nat Rev Immunol. 2008;8:372–379. doi: 10.1038/nri2296. [DOI] [PubMed] [Google Scholar]
- 30.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 31.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009;10:1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 33.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 35.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 36.Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, Futatsugi-Yumikura S, Takeuchi O, Hoshino K, Akira S, Fujimoto J, Nakanishi K. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Moser C, Louboutin JP, Lysenko ES, Weiner DJ, Weiser JN, Wilson JM. Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J Immunol. 2002;168:810–815. doi: 10.4049/jimmunol.168.2.810. [DOI] [PubMed] [Google Scholar]
- 38.Feng CG, Scanga CA, Collazo-Custodio CM, Cheever AW, Hieny S, Caspar P, Sher A. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J Immunol. 2003;171:4758–4764. doi: 10.4049/jimmunol.171.9.4758. [DOI] [PubMed] [Google Scholar]
- 39.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mun HS, Aosai F, Norose K, Chen M, Piao LX, Takeuchi O, Akira S, Ishikura H, Yano A. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int Immunol. 2003;15:1081–1087. doi: 10.1093/intimm/dxg108. [DOI] [PubMed] [Google Scholar]
- 41.Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19:891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 42.Power MR, Peng Y, Maydanski E, Marshall JS, Lin TJ. The development of early host response to Pseudomonas aeruginosa lung infection is critically dependent on myeloid differentiation factor 88 in mice. J Biol Chem. 2004;279:49315–49322. doi: 10.1074/jbc.M402111200. [DOI] [PubMed] [Google Scholar]
- 43.Scanga CA, Bafica A, Feng CG, Cheever AW, Hieny S, Sher A. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect Immun. 2004;72:2400–2404. doi: 10.1128/IAI.72.4.2400-2404.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vazquez-Torres A, Vallance BA, Bergman MA, Finlay BB, Cookson BT, Jones-Carson J, Fang FC. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J Immunol. 2004;172:6202–6208. doi: 10.4049/jimmunol.172.10.6202. [DOI] [PubMed] [Google Scholar]
- 46.Albiger B, Sandgren A, Katsuragi H, Meyer-Hoffert U, Beiter K, Wartha F, Hornef M, Normark S, Normark BH. Myeloid differentiation factor 88-dependent signalling controls bacterial growth during colonization and systemic pneumococcal disease in mice. Cell Microbiol. 2005;7:1603–1615. doi: 10.1111/j.1462-5822.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 47.Delale T, Paquin A, Asselin-Paturel C, Dalod M, Brizard G, Bates EE, Kastner P, Chan S, Akira S, Vicari A, Biron CA, Trinchieri G, Briere F. MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo. J Immunol. 2005;175:6723–6732. doi: 10.4049/jimmunol.175.10.6723. [DOI] [PubMed] [Google Scholar]
- 48.Khan AQ, Chen Q, Wu ZQ, Paton JC, Snapper CM. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on toll-like receptor 2. Infect Immun. 2005;73:298–307. doi: 10.1128/IAI.73.1.298-307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naiki Y, Michelsen KS, Schroder NW, Alsabeh R, Slepenkin A, Zhang W, Chen S, Wei B, Bulut Y, Wong MH, Peterson EM, Arditi M. MyD88 is pivotal for the early inflammatory response and subsequent bacterial clearance and survival in a mouse model of Chlamydia pneumoniae pneumonia. J Biol Chem. 2005;280:29242–29249. doi: 10.1074/jbc.M503225200. [DOI] [PubMed] [Google Scholar]
- 51.Zhou S, Kurt-Jones EA, Mandell L, Cerny A, Chan M, Golenbock DT, Finberg RW. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur J Immunol. 2005;35:822–830. doi: 10.1002/eji.200425730. [DOI] [PubMed] [Google Scholar]
- 52.Archer KA, Roy CR. MyD88-dependent responses involving toll-like receptor 2 are important for protection and clearance of Legionella pneumophila in a mouse model of Legionnaires' disease. Infect Immun. 2006;74:3325–3333. doi: 10.1128/IAI.02049-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhan U, Lukacs NW, Osterholzer JJ, Newstead MW, Zeng X, Moore TA, McMillan TR, Krieg AM, Akira S, Standiford TJ. TLR9 is required for protective innate immunity in Gram-negative bacterial pneumonia: role of dendritic cells. J Immunol. 2007;179:3937–3946. doi: 10.4049/jimmunol.179.6.3937. [DOI] [PubMed] [Google Scholar]
- 54.Zhou S, Kurt-Jones EA, Fitzgerald KA, Wang JP, Cerny AM, Chan M, Finberg RW. Role of MyD88 in route-dependent susceptibility to vesicular stomatitis virus infection. J Immunol. 2007;178:5173–5181. doi: 10.4049/jimmunol.178.8.5173. [DOI] [PubMed] [Google Scholar]
- 55.Bhoj VG, Sun Q, Bhoj EJ, Somers C, Chen X, Torres JP, Mejias A, Gomez AM, Jafri H, Ramilo O, Chen ZJ. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2008;105:14046–14051. doi: 10.1073/pnas.0804717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung A, Kato H, Kumagai Y, Kumar H, Kawai T, Takeuchi O, Akira S. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88. J Virol. 2008;82:196–206. doi: 10.1128/JVI.01640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abplanalp AL, Morris IR, Parida BK, Teale JM, Berton MT. TLR-dependent control of Francisella tularensis infection and host inflammatory responses. PLoS ONE. 2009;4:e7920. doi: 10.1371/journal.pone.0007920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Archer KA, Ader F, Kobayashi KS, Flavell RA, Roy CR. Cooperation between multiple microbial pattern recognition systems is important for host protection against the intracellular pathogen Legionella pneumophila. Infect Immun. 2010;78:2477–2487. doi: 10.1128/IAI.00243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhan U, Ballinger MN, Zeng X, Newstead MJ, Cornicelli MD, Standiford TJ. Cooperative interactions between TLR4 and TLR9 regulate interleukin 23 and 17 production in a murine model of gram negative bacterial pneumonia. PLoS ONE. 2010;5:e9896. doi: 10.1371/journal.pone.0009896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 62.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 63.Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 64.Zheng H, Matte-Martone C, Li H, Anderson BE, Venketesan S, Sheng Tan H, Jain D, McNiff J, Shlomchik WD. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111:2476–2484. doi: 10.1182/blood-2007-08-109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 66.Wong KL, Lew FC, MacAry PA, Kemeny DM. CD40L-expressing CD8 T cells prime CD8alpha(+) DC for IL-12p70 production. Eur J Immunol. 2008;38:2251–2262. doi: 10.1002/eji.200838199. [DOI] [PubMed] [Google Scholar]
- 67.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 68.Jankovic D, Kullberg MC, Hieny S, Caspar P, Collazo CM, Sher A. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity. 2002;16:429–439. doi: 10.1016/s1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 69.Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–2968. [PubMed] [Google Scholar]
- 70.Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. J. Exp. Med. 1992;175:405–413. doi: 10.1084/jem.175.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Penack O, Holler E, van den Brink MR. Graft-versus-host disease: regulation by microbe-associated molecules and innate immune receptors. Blood. 2010;115:1865–1872. doi: 10.1182/blood-2009-09-242784. [DOI] [PubMed] [Google Scholar]
- 72.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 73.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 74.Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 75.Chakraverty R, Cote D, Buchli J, Cotter P, Hsu R, Zhao G, Sachs T, Pitsillides CM, Bronson R, Means T, Lin C, Sykes M. An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues. J Exp Med. 2006;203:2021–2031. doi: 10.1084/jem.20060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taylor PA, Ehrhardt MJ, Lees CJ, Panoskaltsis-Mortari A, Krieg AM, Sharpe AH, Murphy WJ, Serody JS, Hemmi H, Akira S, Levy RB, Blazar BR. TLR agonists regulate alloresponses and uncover a critical role for donor APCs in allogeneic bone marrow rejection. Blood. 2008;112:3508–3516. doi: 10.1182/blood-2007-09-113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abdul-Hai A, Weiss L, Slavin S, Or R. Improved survival following induction of GVHD following lipopolysaccharide immunization. Exp Hematol. 2006;34:549–553. doi: 10.1016/j.exphem.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 78.Cooke KR, Hill GR, Crawford JM, Bungard D, Brinson YS, Delmonte J, Jr, Ferrara JL. Tumor necrosis factor- alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. Journal of Clinical Investigation. 1998;102:1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anderson BE, McNiff J, Matte C, Athanasiadis I, Shlomchik WD, Shlomchik MJ. Recipient CD4+ T Cells That Survive Irradiation Regulate Chronic Graft-vs.-Host Disease. Blood. 2004 doi: 10.1182/blood-2004-01-0328. In Press. [DOI] [PubMed] [Google Scholar]
- 80.Conroy H, Marshall NA, Mills KH. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumours. Oncogene. 2008;27:168–180. doi: 10.1038/sj.onc.1210910. [DOI] [PubMed] [Google Scholar]
- 81.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 82.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 83.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 84.Datta SK, Redecke V, Prilliman KR, Takabayashi K, Corr M, Tallant T, DiDonato J, Dziarski R, Akira S, Schoenberger SP, Raz E. A subset of Toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. Journal of Immunology. 2003;170:4102–4110. doi: 10.4049/jimmunol.170.8.4102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.