Abstract
Background and Objective
Mineral trioxide aggregate (MTA) is a biomaterial used in endodontic procedures as it exerts beneficial effects on regenerative processes. In this study, we evaluate the effect of MTA on healing of periodontal ligament (PDL) and surrounding tissue, following injury, in a transgenic mouse model and on the differentiation of murine mesenchymal progenitor cells in vitro.
Material and Methods
We used an inducible Cre-loxP in vivo fate mapping approach to examine the effects of MTA on the contributions of descendants of cells expressing the αSMA-CreERT2 transgene (SMA9+) to the PDL and alveolar bone after experimental injury to the root furcation on the maxillary first molars. Col2.3GFP was used as a marker to identify mature osteoblasts, cementoblasts and PDL fibroblasts. The effects of MTA were examined 2, 17 and 30 days after injury and compared histologically with sealing using an adhesive system. The effects of two dilutions of medium conditioned with MTA on proliferation and differentiation of mesenchymal progenitor cells derived from bone marrow (BMSC) and periodontal ligament (PDLC) in vitro were examined using the PrestoBlue viability assay, alkaline phosphatase and Von Kossa staining. The expression of markers of differentiation was assessed using real-time PCR.
Results
Histological analyses showed better repair in teeth restored with MTA, as shown by greater expansion of SMA9+ progenitor cells and Col2.3GFP+ osteoblasts compared with control teeth. We also observed a positive effect on differentiation of SMA9+ progenitors into osteoblasts and cementoblasts in the apical region distant from the site of injury. The in vitro data showed that MTA-conditioned medium reduced cell viability and osteogenic differentiation in both PDLC and BMSC, indicated by reduced von Kossa staining and lower expression of osteocalcin and bone sialoprotein. In addition, cultures grown in the presence of MTA had marked decreases in SMA9+ and Col2.3GFP+ areas as compared with osteogenic medium, confirming reduced osteogenesis.
Conclusion
MTA promotes regeneration of injured PDL and alveolar bone, reflected as contribution of progenitors (SMA9+ cells) into osteoblasts (Col2.3GFP+ cells). In vitro, MTA-conditioned medium fails to promote osteogenic differentiation of both PDLC and BMSC.
Keywords: differentiation, injury, MTA, periodontal ligament, progenitor cells
1 | INTRODUCTION
Mineral trioxide aggregate (MTA), a calcium silicate-based cement, is a biomaterial used in almost all endodontic therapies, including root perforation repair, apexification, apexogenesis, pulpotomy and root-end filling.1 MTA has been used extensively in regenerative endodontic procedures because of its low cytotoxicity and ability to promote proliferation and differentiation of stem/progenitor cells, resulting in cementogenesis, dentinogenesis and osteogenesis.2–9 Studies using MTA for repair of perforated roots have reported continuous hard-tissue formation, formation of root cementum without an inflammatory reaction and regeneration of periodontal ligament attached to cementum.10–12 It has been shown that re-establishment of the periodontal tissue is dependent on regeneration of cementum.13 While other materials used to repair root perforations have been shown to result in the formation of a nonmineralized fibrous connective tissue capsule, MTA promoted regeneration of mineralized cementum and facilitated regeneration of the periodontal tissue.13
Dental tissues are a rich source of mesenchymal stem cells (MSCs) that participate in healing and regeneration following injury or infection.14–16 Previous in vivo lineage tracing studies in our laboratory showed that alpha-smooth muscle actin (αSMA)-expressing cells reside in perivascular areas within a number of tissues, including periodontal ligament (PDL), dental pulp, bone marrow and periosteum, and represent a population of mesenchymal progenitor cells.16–20 Following periodontal injury, αSMA+ cells expand and differentiate into osteoblasts in the alveolar bone, fibroblasts in the PDL and cementoblasts.20 Previous studies also demonstrated that αSMA+ cells in the dental pulp are capable of giving rise to a second generation of odontoblasts during reparative dentinogenesis.19
One of the main challenges of tissue regeneration is the re-establishment of tissues damaged by disease or trauma. Optimal regeneration or repair involves recruitment or activation of MSCs and their proliferation and differentiation. Despite numerous studies on the effect of MTA on stem/progenitor cells in various dental tissues, the underlying mechanisms of the effects of MTA on regeneration of periodontal tissues and surrounding alveolar bone and its effects on differentiation of stem/progenitor cells are not fully understood.2,4,21 The present study was designed to gain insight into the effects of MTA on differentiation of periodontal progenitor cells. αSMACreERT2;Ai9/ Col2.3GFP transgenic mice, in which αSMA serves as a marker of progenitor cells in PDL, were utilized in this study.17 The effects of MTA were examined in vivo during repair of the periodontium and surrounding alveolar bone using cell lineage-tracing experiments following an experimental injury to the root furcation of mouse molars, and in in vitro studies.
2 | MATERIAL AND METHODS
2.1 | Transgenic mice
The αSMACreERT2;Ai9/Col2.3GFP triple transgenic mice have been described previously.17 In αSMACreERT2 mice, expression of Cre recombinase is controlled by the αSMA promoter. The administration of tamoxifen activates CreERT2 in cells expressing the transgene. To detect the recombination event, αSMACreERT2 mice were crossed with Cre-dependent Ai9 reporter mice (Rosa26-tdTomato) enabling the visualization of αSMA+ cells and their progenies by expression of the tdTomato (red) fluorescent protein. Then we crossed αSMACreERT2;Ai9 mice with Col2.3GFP, where green fluorescent protein (GFP) is expressed in differentiated osteoblasts, cementoblasts and PDL fibroblasts. Animal protocols were approved by the Institutional Animal Care Committee.
2.2 | Tooth injury in vivo
Tooth injury was performed in 4- to 6-week-old αSMACreERT2;Ai9/ Col2.3GFP mice. In order to label αSMA-expressing progenitors, mice were injected with tamoxifen (75 μg/g body weight), twice, at 24-hour intervals. Two days later, the mice were anesthetized with an intraperitoneal injection of ketamine (87 mg/kg) and xylazine (13 mg/kg) and experimental pulp perforations on maxillary first molars were performed using a stereo microscope (Nikon SZM800; Nikon, Melville, NY, USA), as previously described.22 Briefly, a class I cavity was prepared with a carbide round burr (diameter 0.40 mm) on the occlusal surface of the first maxillary molars. Pulp chambers were opened, and coronal pulp tissues were removed with a pulp extractor (VDW® STERILE Barbed Broaches; VDW GmbH, Munich, Germany) up to the root canal orifices. A perforation was created in the center of the floor of the pulp chamber using an endodontic hand file, #15 (Dentsply, Tulsa, OK, USA) (Fig. S1).
The perforation area was filled with one-step self-etching Adhesive System (AS) (Clearfil SE Bond; Kuraray, Okayama, Japan) (control) or White ProRoot MTA (Dentsply), prepared according to the manufacturer’s recommendations. The cavities were then sealed with a light-cured composite resin (SDI wave restorative system; SDI Inc., Itasca, IL, USA) in both groups. The mice were killed by intracardiac perfusion with 4% paraformaldehyde in phosphate-buffered saline (PBS)22 at various study time points (2, 17 and 30 days). The maxillary arches were isolated, cleaned of soft tissue, fixed in 4% paraformaldehyde solution for an additional 24 hours, then decalcified with 14% EDTA for 7 days. Decalcified tissues were placed in 30% sucrose solution overnight and embedded in cryomatrix (Thermo Shandon, Pittsburg, PA, USA). Seven-micrometer thick sections were obtained using a Leica cryostat (Leica Biosystems Inc, Buffalo Grove, Il, USA) and mounted using a CryoJane tape transfer system (Instrumedics, St Louis, MO, USA). Sections were imaged using an AxioScan.Z1 (Carl Zeiss Microscopy LLC, Pebody, MA, USA). Adjacent sections were processed for hematoxylin/eosin staining and analyzed by light microscopy. For evaluation of injury and repair, at least three animals were analysed per time point.
2.3 | Cell isolation and culture
Primary bone marrow stromal cells (BMSC) were prepared from the hind limbs of 4- to 6-week-old αSMACreERT2;Ai9/Col2.3GFP mice, as previously described 23,24. The cells were plated in 12-well culture plates at a density of 106cells/cm2 for 7 days in basal medium containing α-modified essential medium (α-MEM), 10% fetal bovine serum (FBS; Life Technologies, Carlsbad, CA, USA) and 100 U/mL of penicillin/100 mg/mL of streptomycin (1% PS). Cre recombinase activity was induced by addition of 1 μmol/L of 4-OH-Tamoxifen at days 2 and 4 of culture.
Beginning on day 7, cells were grown in four different culture conditions: control basal medium, two dilutions of MTA-conditioned medium (MTA-CM) in basal medium and osteogenic media (OM) (α-MEM + 10% FBS + 50 μg/mL ascorbic acid + 8 mmol/L β-glycerophosphate). Medium was changed every 2 days.
PDL cells (PDLC) were isolated from 4- to 6-week-old αSMAC-reERT2;Ai9/Col2.3GFP mice, as previously described.16 Briefly, the mandibles and maxillae were dissected from the surrounding tissues, rinsed in 0.12% chlorhexidine digluconate (Clorhexidina; Villevie, Joinville, SC, Brazil) for 30 seconds and washed in PBS. Molars with the adherent PDL were removed from the surrounding alveolar bone and digested in Dulbecco’s modified Eagle’s medium (DMEM) containing 2 mg/mL of Collagenase P (Sigma-Aldrich, Saint Louis, MO, USA) and 0.25% trypsin (Life Technologies) and digested for at least 2 hours at 37°C. Following washing, PDLC were seeded in DMEM containing 20% FBS + 1% PS and cultured in 5% oxygen for 7 days. The medium was changed every 2 days and Cre recombinase activity was induced by addition of 1 μmol/L of 4-OH-Tamoxifen at days 3 and 5 of culture. At day 7, the cells were transferred to normoxic conditions until confluent (around day 11) and then trypsinized and plated on 24-well plates at a density 105 cells/cm2. Medium was changed to different treatments the following day. The experiments were performed in triplicate.
2.4 | MTA-Conditioned Medium
White ProRoot MTA was mixed with sterile water according to the manufacturer’s instructions. MTA discs were prepared under aseptic conditions, as described previously with minor modifications.25,26 Briefly, the discs were created using a sterile cylindrical polyethylene tube (diameter: 5 mm; height: 3 mm). The MTA discs were kept in a 5% CO2 incubator at 37°C for 6 hours to set, then sterilized by ultraviolet light for 1 hour. The discs were incubated for 3 days in α-MEM + 10% FBS at 37°C in a humidified atmosphere containing 5% CO2 (1 mL of α-MEM + 10% FBS for each disc). The supernatants, referred to as MTA-CM, were collected and passed through a sterile 0.22-μm pore-size-diameter filter. Two different dilutions of MTA-CM were used: 1:50 and 1:5.
2.5 | Cell viability assay
Cell viability was determined using a PrestoBlue assay (Thermo Fisher Scientific, Waltham, MA, USA). At various time points the PrestoBlue reagent was added to the cell medium, incubated for 2 hours and the fluorescence intensity was measured (560 nm excitation/590 nm emission).
2.6 | Histochemical analysis of cell cultures
Staining for alkaline phosphatase (ALP) was performed on cultures fixed in 10% formalin for 5 minutes; an 86-R Alkaline Phosphatase kit (Sigma Aldrich) was used, according to the manufacturer’s instructions. The number of ALP-positive colonies per well was counted. Mineralization was assessed after 21 days of culture using von Kossa staining, as described previously.27 Plates were imaged on a flatbed scanner and the mineralized area was quantified using ImageJ (NIH, Bethesda, MD, USA).
2.7 | Detection of epifluorescence
Expression of GFP and tdTomato was imaged on an inverted Observer Z1 microscope (Carl Zeiss). The region scanned covered approximately half the well area. The proportion of fluorescent area for each channel was quantified using ImageJ.
2.8 | RNA extraction and gene expression
RNA was extracted using Trizol reagent (Life Technologies).24 Reverse transcription was performed using the iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). The expression of osteogenic genes osteocalcin (Oc) (Mm03413826_mH) and bone sialoprotein (Bsp) (Mm00492555_m1) was assessed by RT-qPCR using Taqman primer-probe sets (Life Technologies).18 Gene expression was normalized to expression of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (Gapdh).
2.9 | Statistics
Data were subjected to statistical analysis using GraphPad Prism (version 5.0) software (GraphPad Software Inc., La Jolla, CA, USA). For all parametric data, ANOVA followed by Tukey′s test was used. A value of P <.05 was considered significant.
3 | RESULTS
3.1 | Effects of MTA on periodontal tissue regeneration
In order to examine the effects of MTA on regeneration of periodontal tissue following injury, and its effects on αSMA-expressing progenitor cells, we used a previously established lineage tracing model: αSMACreERT2;Ai9/Col2.3GFP.17,19,20 This enables permanent labeling and tracing of αSMA+ cells (referred to as SMA9+). To identify mature cells, we bred in the Col2.3GFP reporter that is expressed in mature osteoblasts, cementoblasts and PDL fibroblasts.20
An AS without MTA was used as a control for the effects of MTA on healing. In these experiments perforation of the pulp floor in the furcation area in the first maxillary molars was performed on 4- to 6-week-old tamoxifen-injected αSMACreERT2;Ai9/Col2.3GFP mice. In uninjured tissue, expression of Col2.3GFP (referred to as 2.3GFP) was detected in osteoblasts, osteocytes within the alveolar bone, cementoblasts on the root surface, odontoblasts lining the pulp chamber and roots and in PDL fibroblasts surrounding the roots in the remaining areas of the teeth, consistent with previous studies (Figure 1A–E). In uninjured teeth, a small number of SMA9+ cells were present in the PDL, but rarely coexpressed 2.3GFP, suggesting that they are not mature cell types (Figure 1A–E). Histology on day 2 following injury was used to evaluate the extent of PDL and alveolar bone injury. Later time points (days 17 and 30) were used to evaluate the repair process. Histological analysis of the injured area showed that perforation at the pulpal floor resulted in local destruction of dentin, odontoblasts, PDL in the furcation area and the underlying alveolar bone, as evident by the lack of 2.3GFP expression in these locations. Examination of the area underneath the injury showed the presence of a few SMA9+ and 2.3GFP+ cells in teeth filled with AS and MTA (Figure 1). At this time the number of SMA9+ cells in bone marrow of the alveolar bone and in dental pulp were increased compared with uninjured controls (Figure 1).
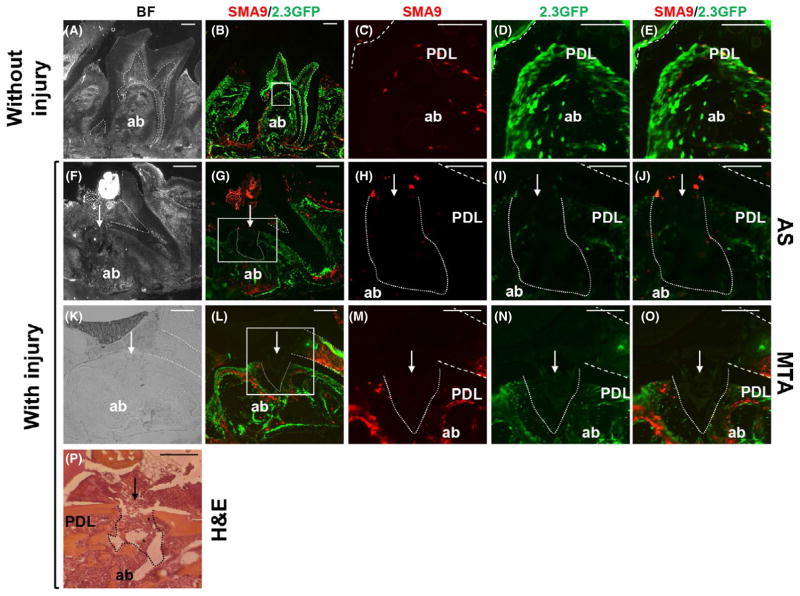
FIGURE 1.
Effects of experimental perforation of the integrity of periodontal ligament (PDL) and alveolar bone (ab). Representative images of sagittal sections through maxillary molars from αSMACreERT2;Ai9/Col2.3GFP mice. In all images the dental pulp is denoted by dashed lines, the site of injury by an arrow and the injury to the PDL and underlying ab by dotted lines. Bright-field (A) and epifluorescence (B) images of intact molars isolated 14 days after tamoxifen injection. (C–E) Higher magnification of boxed area shown in (B). Note the expression of SMA9+ cells (red) and 2.3GFP+ cells (green) in dental pulp, PDL and ab. Also note a few cells coexpressing SMA9/Col2.3-GFP (yellow) in PDL, indicating the differentiation of SMA9 into PDL fibroblasts. Bright-field (F, K) and epifluorescence (G, L) images of sections through injured maxillary molars filled with one-step self-etch Adhesive System (AS) (F–J) and mineral trioxide aggregate (MTA) (K–O) isolated 2 days after PDL injury. (H–J) Higher magnification of boxed area shown in (G). (M–O) Higher magnification of boxed area shown in (L). Note the lack of detectable SMA9+ and 2.3GFP+ cells at the sites of injury. Also note expansion of SMA9+ and 2.3GFP+ cells in bone marrow of the ab surrounding injury in molars restored with MTA (L–O). (P) Hematoxylin and eosin (H&E)-stained section of a maxillary molar isolated 2 days after injury (indicated by arrow). Note the destruction of dentin in the pulpal floor, PDL and ab at the site of injury. Also note residual dentin chips (*). Scale bars=100 μm
Examination of the region 17–30 days after injury showed increased numbers of SMA9+ and 2.3GFP+ cells and of cells coexpressing SMA9+ and 2.3GFP+ at the site of injury compared with day 2 (Figure 2). In teeth filled with AS, the area underneath the injury contained necrotic tissue that was separated from alveolar bone with a relatively large fibrous area filled with small and elongated 2.3GFP+ fibroblasts, some of which were also SMA9+, oriented parallel to the bone surface (Figure 2A). A few cells coexpressing both transgenes were detected at day 17 but not at day 30 after injury (Figure 2B).
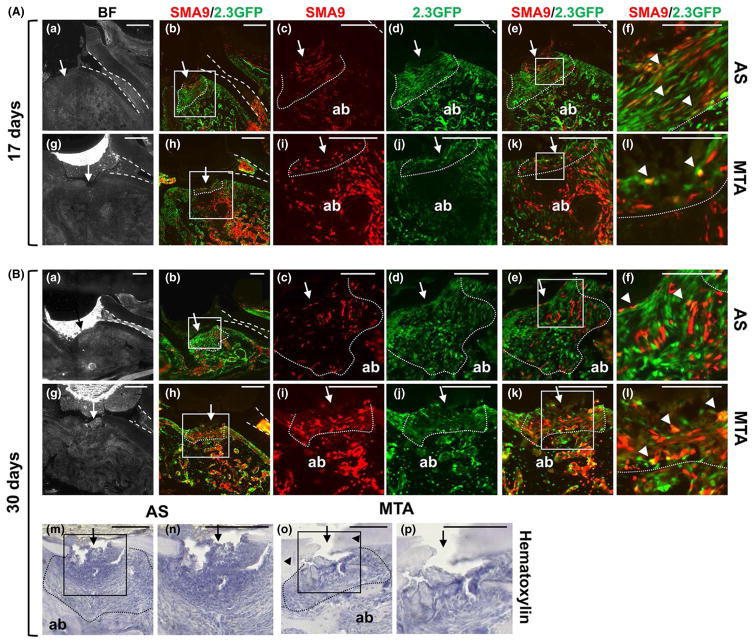
FIGURE 2.
Effect of mineral trioxide aggregate (MTA) on regeneration of periodontal ligament (PDL) and the underlying alveolar bone (ab). Representative bright-field and epifluorescence images of sagittal sections through injured maxillary molars from αSMACreERT2;Ai9/ Col2.3GFP mice. In all images the dental pulp is denoted by dashed lines, the site of injury by an arrow and the sites of repair by dotted lines. (A) Histology of injured molars 17 days after injury and restoration with one-step self-etch Adhesive System (AS) (a–f) and MTA (g–l). (c–f) Higher magnification of the boxed area in (b); (i–l) higher magnification of the boxed area in (h). SMA9+ cells are present in the PDL (outlined by dotted lines) and ab in both groups (c, i). A thick layer of cells expressing 2.3GFP are evident in AS-restored molars (d). Coexpression of SMA9 and 2.3GFP (yellow, arrowheads) is indicated. (B). Images of molars 30 days after injury and restoration with AS (a–f) and MTA (g–l). (c–f) Higher magnification of the boxed area in (b); (i–l) higher magnification of the boxed area in (h). Note the increase in number of SMA9+ cells in repaired PDL and ab in molars restored with MTA (i) than in molars restored with AS (c). Also note the increase in coexpression of SMA9+ and 2.3GFP+ cells (arrowheads) in repaired PDL of molars restored with MTA (f) than in molars restored with AS (l). Scale bar=50 μm (f and l) in A and 25 μm in B. (m–p) Hematoxylin-stained section of maxillary molars 30 days after injury (indicated by arrow). (n, p) Higher magnifications of boxed areas in (m) and (o), respectively. Repaired PDL and PDL region are outlined with dotted line. Note the lack of ab repair and large fibrous area containing fibroblastic cells in the PLD region in molars restored with AS (m, n). Also note well-organized repaired ab, PDL with PDL-like fibroblasts and partial dentin repair (arrowheads) in molars restored with MTA (o, p). Scale bar=100 μm
Histological examination of teeth filled with MTA showed that the area underneath the injury contained dentin chips, PDL-like fibroblasts and well-organized alveolar bone. SMA9+ and 2.3GFP+ cells were detected in the PDL-like fibroblasts and the alveolar bone in the area of repair. A few cells coexpressing both transgenes (SMA9+/2.3GFP+) were detected in the area of repair and underlying alveolar bone at day 17 (Figure 2A). The number of SMA9+/2.3GFP+ cells in PDL-like cells and alveolar bone increased at day 30 (Figure 2B). These observations together indicated contribution of SMA9+ cells to the organized PDL-like fibroblasts and underlying alveolar bone in teeth filled with MTA, but limited differentiation of SMA9+ cells to mature lineages in injuries filled with AS (Figure 2).
Further examination of these teeth showed that injury at the pulp floor and restorative materials also had a significant effect on the expression of both transgenes in the apical regions (Figure 3). Examination at day 30 showed significant expansion of the SMA9+ cells in the apical regions in injured teeth compared with uninjured teeth (Figure 3). The apical region of teeth filled with MTA showed organized structure containing SMA9+ cells and 2.3GFP+ osteoblasts and cementoblasts. There were numerous double-labeled cells in this area, indicating differentiation of SMA9+ progenitor cells to osteoblasts and cementoblasts during the healing process. In contrast, the periapical regions of teeth filled with AS were very disorganized, with significantly lower numbers of SMA9+ and 2.3GFP+ cells, and no double-labeled cells. These data indicate that application of MTA resulted in better repair of the PDL in the area under the injury as well as in the apical region, and promoted differentiation of SMA9+ mesenchymal progenitors in the tissue into mature cell types, including osteoblasts and cementoblasts.
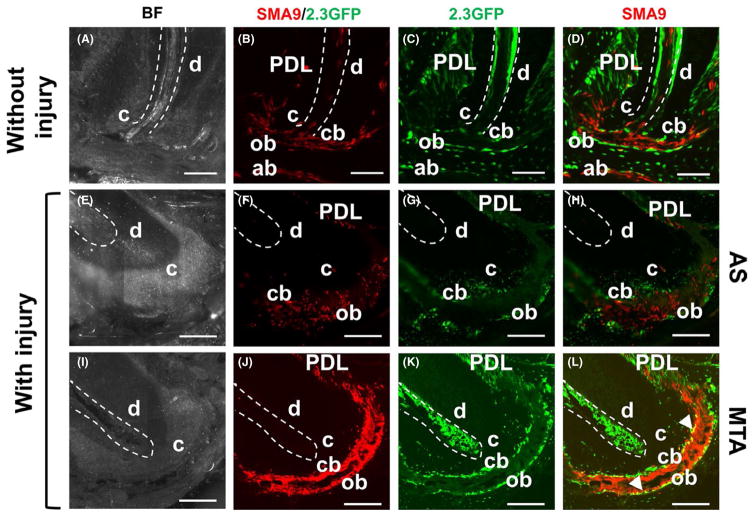
FIGURE 3.
Effect of mineral trioxide aggregate (MTA) on apical region. (A–D) Representative bright-field and fluorescent images of a sagittal section through the root of an uninjured maxillary molar at day 30 after tamoxifen injection, showing expression of SMA9+ (B) and 2.3GFP+ (C) cells in dental pulp (outlined with dotted line), alveolar bone (ab), periodontal ligament (PDL), cementoblasts (cb) and osteoblasts (ob) in the apical area. Note coexpressing SMA9/Col2.3-GFP (yellow) detected in cementoblasts and osteoblasts. Scale bar=100 μm. (E–L) Representative images of the apical root region at day 30 post-injury restored with AS (E–H) and MTA (I–L). Note significant expansion of SMA9+ (J) and 2.3GFP+ (K) cells in the apical area of MTA-restored molars compared with AS-restored molars (F,G). Also note lack of coexpressing cells in apical cementoblasts and osteoblasts in molars restored with AS (H) compared with MTA-restored molars in which coexpression of SMA9 and 2.3GFP is present in both cementoblasts and osteoblasts (L). Note increase in coexpressing cells in the apical region of molars restored with MTA compared with control without injury. Scale bar=50 μm
3.2 | Effect of MTA-CM on PDLC in vitro
To gain a better understanding of the underlying process mediated by MTA, we examined the effects of media conditioned with MTA (MTA-CM) on cell viability and the differentiation of PDL progenitors. In these experiments the effects of two different concentrations of MTA-CM were compared with OM and control basal medium. PrestoBlue assay showed that, compared with control medium, OM increased the viability of the PDLC at day 7. However, at both concentrations, MTA had negative effects on cell viability at day 7, although this effect was greater at the lower concentration (Figure 4A).
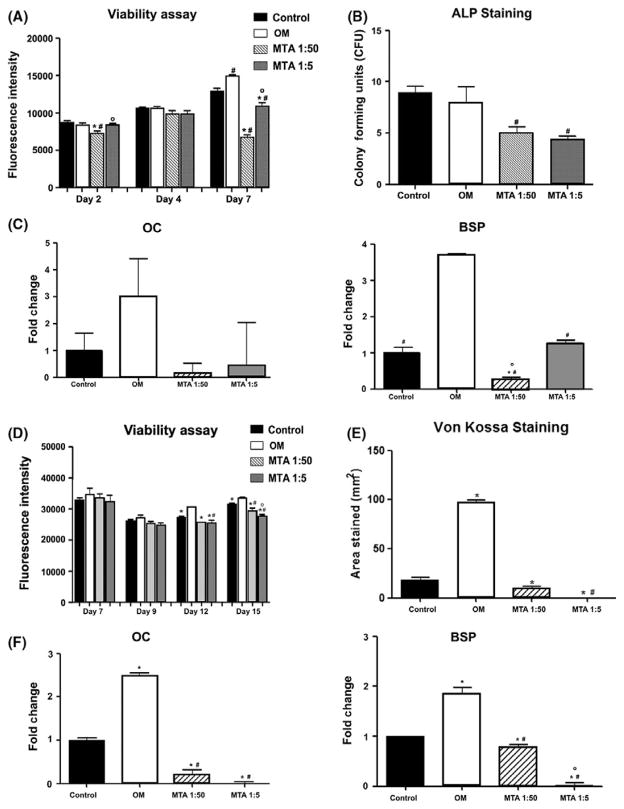
FIGURE 4.
Effect of mineral trioxide aggregate-conditioned medium (MTA-CM) on cell viability and osteogenesis of periodontal ligament cells (PDLC) and bone marrow stromal cells (BMSC). PDLC (A–C) and BMSC (D–E) were cultured in control medium, osteogenic medium (OM) or MTA-CM diluted 1:50 or 1:5. (A) Cell viability was determined using PrestoBlue reagent at days 2, 4 and 7 after treatment initiation. (B) Alkaline phosphatase (ALP) staining was performed on day 7 of treatment and the number of positive colonies were counted. (C) Expression of osteocalcin (Oc) and bone sialoprotein (Bsp) was determined at day 7 of treatment, normalized to expression of glyceraldehyde-3-phosphate dehydrogenase (Gapdh). Results represent mean ± standard error of the mean values from three independent experiments. #P ≤.05 vs OM; *P<.05 vs Control; 0P<.05 vs MTA 1:50. (D) Cell viability was determined using PrestoBlue reagent at days 7 (before MTA-CM), 9, 12 and 15. (E) Mineralization by von Kossa staining was assessed on day 21 and expressed as area stained. (F) Expression of Oc and Bsp was determined at day 21 of culture, normalized to expression of Gapdh. Results represent mean ± standard error of the mean from three independent experiments. #P ≤.05 vs OM; *P<.05 vs Control; 0P<.05 vs MTA 1:50
We also examined the effect of MTA-CM on osteogenic differentiation. In PDLC, OM did not affect the ALP staining (Figure 4B) but resulted in increased levels of expression of Oc and Bsp compared with control medium. Both high and low concentrations of MTA-CM decreased the number of ALP+ colonies compared with both control medium and OM. MTA-CM also failed to induce expression of Oc and Bsp compared with control medium (Figure 4C). These observations show that MTA-CM had negative effects on both the viability and differentiation of PDL fibroblasts.
3.3 | Effect of MTA-CM on BMSC in vitro
As we observed significant effects of MTA on the alveolar bone underneath the site of injury as well as in the apical region, we also examined the effects of MTA-CM on BMSC. Our results showed that OM increased viability or cell number in cultures, while both concentrations of MTA-CM had a slight, but significantly negative, effect on viability (Figure 4D). Treatment with OM increased von Kossa staining and increased the levels of expression of both Oc and Bsp in BMSC compared with controls. Both concentrations of MTA-CM resulted in significant decreases in von Kossa staining and levels of expression of Oc and Bsp compared with OM and control medium (Figure 4E,F).
3.4 | Effects of MTA-CM on SMA9+ progenitors and their osteogenic differentiation in vitro
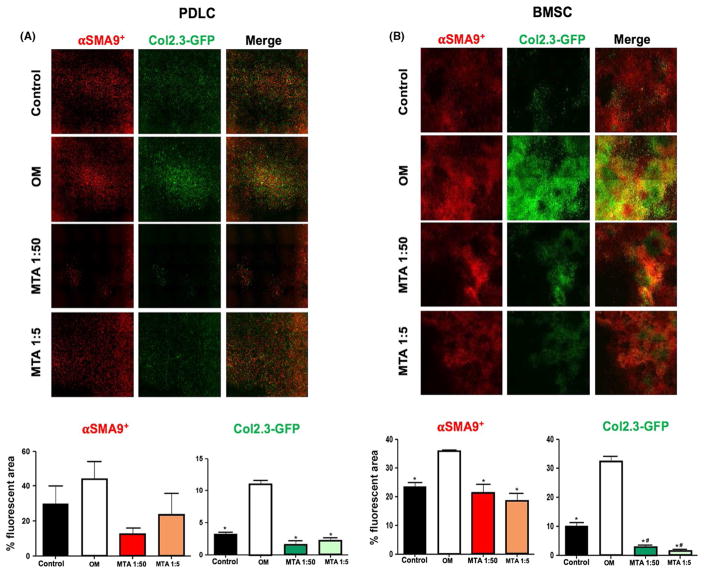
BMSC and PDLC grown in OM showed marked increases in SMA9+ and 2.3GFP+ areas compared with controls, indicating the positive roles of OM on progenitor cell expansion and differentiation (Figure 5). In BMSC and PDLC grown in both concentrations of MTA-CM, SMA9+ and 2.3GFP+ areas were significantly lower compared with BMSC and PDLC grown on OM, and the 2.3GFP+ area was reduced compared with BMSC and PDLC grown on control medium (Figure 5). Reduced levels of expression of markers of osteogenesis in these cultures were therefore related to the reduced number of progenitor cells giving rise to osteoblasts.
FIGURE 5.
Effects of mineral trioxide aggregate-conditioned medium (MTA-CM) on the SMA9+ progenitors and their osteogenic differentiation in vitro. (A,B) Images of scanned periodontal ligament cells (PDLC) and bone marrow stromal cell (BMSC) cultures analyzed at the end point (day 7 for PDLC and day 21 for BMSC). Note increases in the SMA9+ and 2.3GFP+ areas in the OM group compared with the control group in both PDLC and BMSC cultures. Also, note significant decreases in SMA9+ and 2.3GFP+ areas in both concentrations of MTA-CM compared with OM in both cultures. Image analysis was performed on whole cell-culture wells and area of fluorescence was measured. The images are generated from one representative of three biological replicates
4 | DISCUSSION
Despite the progress made in understanding the biological effects of MTA, the mechanism of its effects on wound healing and the nature of hard-tissue formation remain unclear. Therefore, our study focused on the effect of MTA on mesenchymal progenitor cells during repair of PDL and surrounding tissues. We showed that placement of MTA in the site of injury in the root furcation area can significantly improve the healing process. Compared with AS-filled teeth, where the injured area contained necrotic tissue and a large fibrous layer typical of scarring, in MTA-filled teeth, PDL-like fibroblasts and well-organized tissue were present. These observations are consistent with previous observations that have shown bacterial invasion and necrosis of tissues in teeth filled with AS as a result of its inadequate sealing properties.28,29 On the other hand, it is well documented that MTA is a commonly used restorative material because of its biological and sealing properties that reduce bacterial invasion.30
A fate mapping approach allowed us to identify a population of mesenchymal progenitor cells expressing αSMA and track contribution of their progeny to repair. Previous studies showed that SMA9+ cells contribute to healing and production of mineralized and nonmineralized connective tissues during fracture healing, tendon injury and pulp exposure in molars.17–20,31 In the PDL, SMA9+ cells differentiated into mature cell types, including PDL fibroblasts, osteoblasts and cementoblasts, during postnatal growth and in repair after PDL injury.20 In the current study, we performed a more extensive injury that included damage to the tooth and pulp removal as well as injury to the PDL. Our lineage tracing study showed significant expansion of SMA9+ cells, and appearance of cells co-expressing SMA9/2.3GFP at the perforation site in MTA-treated injuries. Differentiation of SMA9+ cells into mature 2.3GFP+ cells in the PDL and on bone and root surfaces was not observed when injuries were filled with AS. This confirms that the healing process was promoted by MTA. Furthermore, MTA induced contribution of SMA9+ cells to repair of alveolar bone underlying injured PDL, indicating that MTA promotes bone repair. Following PDL regeneration and alveolar bone healing, MTA showed a positive effect on distant root periodontium. Unlike the disorganized apical region in AS capped teeth, in teeth filled with MTA this area showed organized structure containing differentiated SMA9+/2.3GFP+ osteoblasts and cementoblasts. These observations suggest that MTA mediates regeneration by promoting differentiation of αSMA+ progenitor/stem cells within periodontal ligament and alveolar bone progenitor/stem cells.
In contrast to the in vivo observations, the in vitro results from this study show that MTA-CM inhibits differentiation of BMSC and PDLC, and if anything, had an inhibitory effect on viability. The majority of studies have shown inhibitory effects of MTA-CM, particularly on cell viability when applied at high concentrations.4,25,32–34 However, at lower doses, MTA-CM has either no effect, or in some studies even promoted cell growth.32,35 Given the variation in methods to prepare MTA-CM, it is not easy to compare doses used in different studies or determine what components of the MTA-CM are eliciting a biological effect. It is likely that in vivo concentrations of MTA-related compounds are lower for the majority of cells responding to a repair. Culturing cells directly on MTA has been reported in fewer studies, but human dental pulp cells showed improved viability when cultured on MTA than did controls.36 Cells cultured in a three-dimensional scaffold in a well containing MTA showed reduced viability but improved differentiation.37
The effects of MTA on differentiation also vary depending on the concentration used; however, dilute MTA-CM has been reported to promote differentiation/mineralization in OCCM-30 cementoblast cells, human dental pulp cells and rat craniofacial BMSC.4,25,32,33 Mediators of MTA-CM effects may include calcium, aluminum, bismuth and silicon ions that are released into the medium,32,36,38,39 and enhanced Wnt, JNK and ERK signaling have been implicated in the osteogenic effects of MTA.4,32 In the current study, we observed clear inhibition of osteogenic differentiation in both cell types evaluated. Addition of the osteogenic factors ascorbic acid and β-glycerol phosphate may have improved differentiation in the presence of MTA; however, given the reduction in number of ALP+ colonies, which can form even in the absence of osteogenic stimuli, it is likely that the effects of the MTA-CM would not be overcome by these compounds. Many of the published studies were performed in human cells, and it is also possible that mouse cells are more sensitive to components of MTA-CM. Overall it appears that the in vitro conditions employed in this study did not effectively represent the in vivo response of osteoprogenitors to MTA.
Although in vitro studies are useful for defining mechanisms, it is not possible to assess the complex interactions between materials and host using such a simplified system. Therefore, we primarily evaluated the in vivo effects of the MTA on the progenitor lineages. Collectively, our findings showed that SMA9+ progenitor cells contributed to bone, cementum and PDL formation following injury, an effect that was promoted by the application of MTA as part of the repair.
Supplementary Material
Acknowledgments
Funding information
This work has been supported by R01-AR055607 NIH/NIAMS to I.K and by R01-DE016689 & R90-DE022526 to M.M. BGM is supported by Connecticut Innovations grant 14-SCA-UCHC. I.O.A.Q. is supported by fellowship from São Paulo Research Foundation (FAPESP) #2014/13750-0
We would like to thank all individuals who provided reagents, valuable input and technical assistance in various aspects of this study, including Drs Emilie Roeder and Xi Wang. This work was supported by R01-AR055607 (IK), R01-DE016689 (MM) and R90-DE022526 grants from the National Institutes of Health. BGM was supported by Connecticut Innovations grant 14-SCA-UCHC. IOAQ was supported by fellowship from São Paulo Research Foundation (FAPESP) #2014/13750-0.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review–part II: leakage and biocompatibility investigations. J Endod. 2010;36:190–202. doi: 10.1016/j.joen.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Zhao X, He W, Song Z, Tong Z, Li S, Ni L. Mineral trioxide aggregate promotes odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp stem cells. Mol Biol Rep. 2012;39:215–220. doi: 10.1007/s11033-011-0728-z. [DOI] [PubMed] [Google Scholar]
- 3.Yan M, Wu J, Yu Y, et al. Mineral trioxide aggregate promotes the odonto/osteogenic differentiation and dentinogenesis of stem cells from apical papilla via nuclear factor kappa B signaling pathway. J Endod. 2014;40:640–647. doi: 10.1016/j.joen.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Li J, Song W, Yu J. Mineral trioxide aggregate upregulates odonto/osteogenic capacity of bone marrow stromal cells from craniofacial bones via JNK and ERK MAPK signalling pathways. Cell Prolif. 2014;47:241–248. doi: 10.1111/cpr.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chueh LH, Ho YC, Kuo TC, Lai WH, Chen YH, Chiang CP. Regenerative endodontic treatment for necrotic immature permanent teeth. J Endod. 2009;35:160–164. doi: 10.1016/j.joen.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Paryani K, Kim SG. Regenerative endodontic treatment of permanent teeth after completion of root development: a report of 2 cases. J Endod. 2013;39:929–934. doi: 10.1016/j.joen.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Maroto M, Barberia E, Vera V, Garcia-Godoy F. Dentin bridge formation after white mineral trioxide aggregate (white MTA) pulpotomies in primary molars. Am J Dent. 2006;19:75–79. [PubMed] [Google Scholar]
- 8.Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review–Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–413. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Gandolfi MG, Prati C. MTA and F-doped MTA cements used as sealers with warm gutta-percha. Long-term study of sealing ability. Int Endod J. 2010;43:889–901. doi: 10.1111/j.1365-2591.2010.01763.x. [DOI] [PubMed] [Google Scholar]
- 10.Ford TR, Torabinejad M, McKendry DJ, Hong CU, Kariyawasam SP. Use of mineral trioxide aggregate for repair of furcal perforations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:756–763. doi: 10.1016/s1079-2104(05)80313-0. [DOI] [PubMed] [Google Scholar]
- 11.Holland R, Filho JA, de Souza V, Nery MJ, Bernabe PF, Junior ED. Mineral trioxide aggregate repair of lateral root perforations. J Endod. 2001;27:281–284. doi: 10.1097/00004770-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Regan JD, Gutmann JL, Witherspoon DE. Comparison of Diaket and MTA when used as root-end filling materials to support regeneration of the periradicular tissues. Int Endod J. 2002;35:840–847. doi: 10.1046/j.1365-2591.2002.00582.x. [DOI] [PubMed] [Google Scholar]
- 13.Main C, Mirzayan N, Shabahang S, Torabinejad M. Repair of root perforations using mineral trioxide aggregate: a long-term study. J Endod. 2004;30:80–83. doi: 10.1097/00004770-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Sharpe PT. Dental mesenchymal stem cells. Development. 2016;143:2273–2280. doi: 10.1242/dev.134189. [DOI] [PubMed] [Google Scholar]
- 15.Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8:191–199. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 16.San Miguel SM, Fatahi MR, Li H, Igwe JC, Aguila HL, Kalajzic I. Defining a visual marker of osteoprogenitor cells within the periodontium. J Periodontal Res. 2010;45:60–70. doi: 10.1111/j.1600-0765.2009.01201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grcevic D, Pejda S, Matthews BG, et al. In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells. 2012;30:187–196. doi: 10.1002/stem.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews BG, Grcevic D, Wang L, et al. Analysis of alphaSMA-labeled progenitor cell commitment identifies notch signaling as an important pathway in fracture healing. J Bone Miner Res. 2014;29:1283–1294. doi: 10.1002/jbmr.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidovic I, Banerjee A, Fatahi R, et al. alphaSMA-expressing perivascular cells represent dental pulp progenitors in vivo. J Dent Res. 2017;96:323–330. doi: 10.1177/0022034516678208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roguljic H, Matthews BG, Yang W, Cvija H, Mina M, Kalajzic I. In vivo identification of periodontal progenitor cells. J Dent Res. 2013;92:709–715. doi: 10.1177/0022034513493434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan P, Yuan Z, Jiang H, Peng B, Bian Z. Effect of bioaggregate on differentiation of human periodontal ligament fibroblasts. Int Endod J. 2010;43:1116–1121. doi: 10.1111/j.1365-2591.2010.01786.x. [DOI] [PubMed] [Google Scholar]
- 22.Frozoni M, Balic A, Sagomonyants K, Zaia AA, Line SR, Mina M. A feasibility study for the analysis of reparative dentinogenesis in pOB-Col3.6GFPtpz transgenic mice. Int Endod J. 2012;45:907–914. doi: 10.1111/j.1365-2591.2012.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalajzic I, Kalajzic Z, Kaliterna M, et al. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res. 2002;17:15–25. doi: 10.1359/jbmr.2002.17.1.15. [DOI] [PubMed] [Google Scholar]
- 24.Repic D, Torreggiani E, Franceschetti T, et al. Utilization of transgenic models in the evaluation of osteogenic differentiation of embryonic stem cells. Connect Tissue Res. 2013;54:296–304. doi: 10.3109/03008207.2013.814646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshino P, Nishiyama CK, Modena KC, Santos CF, Sipert CR. In vitro cytotoxicity of white MTA, MTA Fillapex(R) and Portland cement on human periodontal ligament fibroblasts. Braz Dent J. 2013;24:111–116. doi: 10.1590/0103-6440201302115. [DOI] [PubMed] [Google Scholar]
- 26.Lee BN, Kim HJ, Chang HS, et al. Effects of mineral trioxide aggregate mixed with hydration accelerators on osteoblastic differentiation. J Endod. 2014;40:2019–2023. doi: 10.1016/j.joen.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Kalajzic Z, Li H, Wang LP, et al. Use of an alpha-smooth muscle actin GFP reporter to identify an osteoprogenitor population. Bone. 2008;43:501–510. doi: 10.1016/j.bone.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsatsas DV, Meliou HA, Kerezoudis NP. Sealing effectiveness of materials used in furcation perforation in vitro. Int Dent J. 2005;55:133–141. doi: 10.1111/j.1875-595x.2005.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 29.Lodiene G, Kleivmyr M, Bruzell E, Orstavik D. Sealing ability of mineral trioxide aggregate, glass ionomer cement and composite resin when repairing large furcal perforations. Br Dent J. 2011;210:E7. doi: 10.1038/sj.bdj.2011.198. [DOI] [PubMed] [Google Scholar]
- 30.Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993;19:591–595. doi: 10.1016/S0099-2399(06)80271-2. [DOI] [PubMed] [Google Scholar]
- 31.Dyment NA, Hagiwara Y, Matthews BG, Li Y, Kalajzic I, Rowe DW. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS ONE. 2014;9:e96113. doi: 10.1371/journal.pone.0096113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YW, Ho CC, Huang TH, Hsu TT, Shie MY. The ionic products from mineral trioxide aggregate-induced odontogenic differentiation of dental pulp cells via activation of the wnt/beta-catenin signaling pathway. J Endod. 2016;42:1062–1069. doi: 10.1016/j.joen.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Hakki SS, Bozkurt SB, Hakki EE, Belli S. Effects of mineral trioxide aggregate on cell survival, gene expression associated with mineralized tissues, and biomineralization of cementoblasts. J Endod. 2009;35:513–519. doi: 10.1016/j.joen.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Keiser K, Johnson CC, Tipton DA. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblasts. J Endod. 2000;26:288–291. doi: 10.1097/00004770-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Takita T, Hayashi M, Takeichi O, et al. Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int Endod J. 2006;39:415–422. doi: 10.1111/j.1365-2591.2006.01097.x. [DOI] [PubMed] [Google Scholar]
- 36.Wu BC, Kao CT, Huang TH, Hung CJ, Shie MY, Chung HY. Effect of verapamil, a calcium channel blocker, on the odontogenic activity of human dental pulp cells cultured with silicate-based materials. J Endod. 2014;40:1105–1111. doi: 10.1016/j.joen.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Rifaey HS, Villa M, Zhu Q, Wang YH, Safavi K, Chen IP. Comparison of the Osteogenic Potential of Mineral Trioxide Aggregate and Endosequence Root Repair Material in a 3-dimensional Culture System. J Endod. 2016;42:760–765. doi: 10.1016/j.joen.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Hoppe A, Guldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Duarte MA, Demarchi AC, Yamashita JC, Kuga MC, de Fraga SC. pH and calcium ion release of 2 root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:345–347. doi: 10.1067/moe.2003.12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.