Abstract
Purpose
Our goal was to determine whether tumor radiosensitivity is associated with activation of the immune system across all tumor types as measured by two gene expression signatures (GES).
Methods
We identified 10,240 genomically profiled distinct solid primary tumors with gene expression analysis available from an institutional de-identified database. Two separate GES were included in the analysis, the radiosensitivity index (RSI) GES (a 10-gene GES as a measure of radiosensitivity), and the 12-chemokine (12-CK) signature (a 12-gene GES as a measure of immune activation). We tested whether the RSI and 12-CK were associated with each other across all tumor samples, and in an exploratory analysis, their prognostic significance in predicting distant metastasis-free survival (DMFS) among a well-characterized, independent cohort of 282 early-stage breast cancer cases treated with surgery and post-operative radiation alone without systemic therapy. The lower the RSI score, the higher the tumor radiosensitivity; whereas, the higher the 12-CK score the higher the immune activation.
Results
Using an RSI cut-point of ≤0.3745, RSI-low tumors (n=4,291, 41.9%) had a significantly higher median 12-CK GES value (0.54 [range −0.136,1.095]) compared with RSI-high tumors (−0.17 [−0.82,0.42]; p<0.001) across all tumor samples, indicating that radiosensitivity is associated with immune activation. In an exploratory analysis of early stage breast cancer cases, a multivariable model with patient age, RSI and 12-CK provided a strong composite model for DMFS (p=0.02), with RSI (HR 0.63 [95%CI 0.36,1.09]) and 12-CK (HR 0.66 [0.41,1.04]) each providing comparable contributions.
Conclusions
Tumor radiosensitivity is associated with immune activation as measured by two GES.
Keywords: immune, cancer, radiation, radiosensitivity, survival
Introduction
Over the past few decades, it has been well established that specific cancers are exquisitely radiosensitive, resulting in high locoregional control rates following treatment. HPV-positive oropharyngeal squamous cell carcinoma is a radiosensitive tumor, which exhibits a high cure rate following radiotherapy with or without concurrent chemotherapy.(1,2) Using daily or weekly 3D image guidance, HPV-positive oropharyngeal tumors often demonstrate a complete radiologic response shortly after completing definitive radiation therapy.(3) However, the pre-treatment identification of radiosensitive tumors and host factors associated with radiosensitivity remains a fragmented story for other tumor types.
The tumor microenvironment, including the immune system and function of the host, has been shown to play an important role in mediating both tumor growth and responses to radiation therapy.(4) Tumors frequently down-regulate the host’s adaptive immune system to avoid cell-mediated death and, in response, immune modulators have become a promising tool to combat this effect. Radiation therapy has been shown to have both enhancing and inhibitory effects on host immune function depending largely on the radiation dose and target.(5) A large radiation field targeting multiple vertebral bodies as palliative therapy for spinal metastases, for instances, can reduce a patient’s white blood cell count and potentially act as an immunosuppressive agent.(5,6) However, when radiation is highly conformal, with the use of modern treatment planning and delivery, and when it is delivered in higher doses per fraction, radiation has the potential to act as an immune stimulatory agent.(5)
Additionally, when combined with immune modulators, pre-clinical studies and case reports have shown a potential synergy between the radiation and immunotherapy via the abscopal effect. (7–9) How often the abscopal effect occurs and whether it can be triggered by a pre-defined strategy via combining radiotherapy and immunotherapy remains a critical clinical question.(9) Radiation has been shown to have a mixed response on PD-L1 expression on the surface of tumor cells, by upregulating, or even down-regulating PD-L1 expression(10,11), with differing responses possibly driven by tumor site, histology, and/or radiation dose per fraction.(5) Thus the identification of biomarker-based approaches is central to the development of clinical strategies to combine radiation therapy and immunotherapy.
To address this, our group recently developed two gene-expression signatures (GES) of radiosensitivity and immune-activation. The radiosensitivity index (RSI) is a 10-gene based signature developed as a marker of cellular radiosensitivity that has been independently validated as pan-tissue biomarker of radiosensitivity in multiple disease sites.(12–16) The 12-Chemokine (12-CK) GES is based on 12 chemokine genes (CCL2, CCL3, CCL4, CCL5, CCL8, CCL18, CCL19, CCL21, CXCL9, CXCL10, CXCL11, and CXCL13) chosen from a metagene grouping of immune-related and inflammation-related genes.(17) The 12-CK GES has been shown to be associated with the presence of tumor localized ectopic lymph node like structures (TL-ELNs) in both colorectal cancer and metastatic melanoma patients and was associated with improved survival outcomes in both patient populations.(17–19)
In the present study, we hypothesize that RSI and 12-CK are associated and, when combined, will provide an improved prognostic tool for patient outcomes. Combining RSI and 12-CK also serves as a possible clinical strategy to explore the relationship between tumor radiosensitivity and patient immune activation across many unique tumor types.
Methods
Gene Profile Analysis of Archived Tumors -Total Cancer Care (TCC) Database
We identified 10,240 genomically profiled distinct solid, primary, non-metastatic tumors from the TCC database, a prospective IRB-approved tissue collection protocol active at the Moffitt Cancer Center and 17 other institutions since 2006.(20) Tumors from patients enrolled in the TCC protocol were arrayed on Affymetrix Hu-RSTA-2a520709 Gene Chips (Affymetrix, Santa Clara, CA). The chip contains roughly 60,000 probesets representing 20,155 unique genes. Iterative Rank-Ordered Normalization (IRON) was used to normalize all samples and log2 values were calculated.(21). A RNA quality batch effect was removed using Partial Least Squares. Both the normalized and de-batched expression values for 10,240 tumor samples from the ten RSI genes and 12 chemokine genes were extracted from the TCC database.
Radiosensitivity Index (RSI) GES
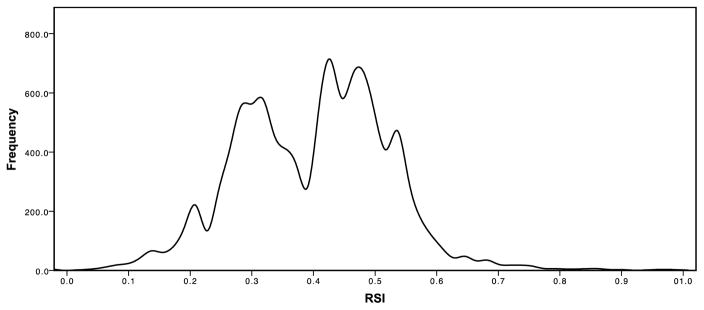
RSI was previously trained in 48 cancer cell lines to predict cellular radiosensitivity as determined by survival fraction at 2 Gy (SF2). Each of ten genes in the algorithm was ranked based on gene expression (highest expressed gene is ranked at 10 and lowest at 1) as previously described by Eschrich et al.(12). To make RSI comparisons across the entire dataset of 10,240 tumors across 62 disease sites, we used RSI=0.3745 as the cut-point, as it represents the value for the local minimum in the bimodal RSI density function (Figure 1). RSI-low tumors (i.e. more sensitive) were defined as having an RSI GES <0.3745 and RSI-high tumors (i.e. less sensitive) as having an RSI GES ≥0.3745.
Figure 1.
Histogram demonstrating RSI values across 10,240 solid tumors.
12-Chemokine (12-CK) GES
The 12-CK GES was defined as the first principal component (PC1) from a PCA model using all 12 genes in all 10,240 samples. The sample scores from the PCA model were scaled to have a variance of 1.
Erasmus Breast Cancer Cohort
The Erasmus Cancer Cohort has been previously described.(13) It includes 282 lymph node negative breast cancer patients treated with loco-regional therapy (surgery and RT) and no adjuvant systemic therapy (i.e. chemotherapy or hormonal therapy).(22) Patient exclusion characteristics and tumor RNA preparation and gene expression profiling were previously described.(22–24) Raw gene expression data from this cohort are publicly available in GEO (Erasmus – GSE2034, GSE5327). A robust multi-array (RMA) normalization method was previously applied to the Affymetrix U133A CEL files.(25–27) The study was approved by the Medical Ethics Committee of the Erasmus Medical Center. Radiation dose to the tumor cavity in the cohort ranged from 40–74 Gy delivered in standard fractionation (1.8–2 Gy per fraction). The endpoint defined by the Erasmus investigators was distant metastasis free survival, defined as an early distant recurrence in the first five years following completion of primary treatment, or death. RSI was previously generated for this cohort and RSI-low and RSI-high were previously defined and specific to this dataset.(13) The RSI cut point previously used with the ERASMUS dataset (13) and in the present analysis was 0.34; this is similar to the RSI cut point of 0.3745 for the overall analysis although the normalization methods were different making it difficult to directly compare with the two RSI cut points. We explored five alternative cut-points for dichotomization of the 12-CK GES, including 12-CK-high (immune-active) vs. 12-CK low (immune-inactive) in the Erasmus cohort. The cut-point used for testing outcomes was the top 75% of 12-CK patients (12-CK-high, 212 patients) vs. the bottom 25% (12-CK-low, 70 patients). This was the optimal cut-point of the five tested on univariate analysis for DMFS (p=0.08).
Statistical analysis
The primary endpoint of the study was to assess the association of RSI GES with 12-CK GES among 10,240 solid primary tumors in the TCC de-identified database. A secondary endpoint was to test whether the RSI and 12-CK GES could be prognostically important for distant metastasis free survival outcomes, using the Erasmus cohort of 282 breast cancer patients treated with breast conservation therapy as the validation dataset.
Statistical analysis was performed using SPSS® version 22.0 (IBM®, Chicago, IL) and R. For the TCC analysis of 10,240 solid tumors, differences in 12-CK values by RSI group were assessed using the Wilcoxon rank-sum test among all patients, and between individual tumor types with ≥10 samples in both RSI groups (n=17 tumor types compared). The associations between RSI and 12-CK GES were assessed using the non-parametric Spearman correlation coefficients in the full TCC cohort and within individual tumor types with ≥10 samples (n=42 tumor types included).
For the Erasmus dataset analysis, clinicopathologic differences between the RSI-low (radiosensitive)/RSI-high (less-radiosensitive) and 12-CK-high/12-CK-low patient populations were compared using the Wilcoxon rank-sum test and Pearson’s Chi-square test for continuous and discrete variables, respectively. Distant metastasis free survival rates were then calculated using the Kaplan-Meier method and compared using the log-rank test. Cox regression main-effects models for DMFS were performed to assess the prognostic significance of RSI, and the 12-CK score individually and together adjusted for age.
Results
Association between tumor radiosensitivity and immune-activation
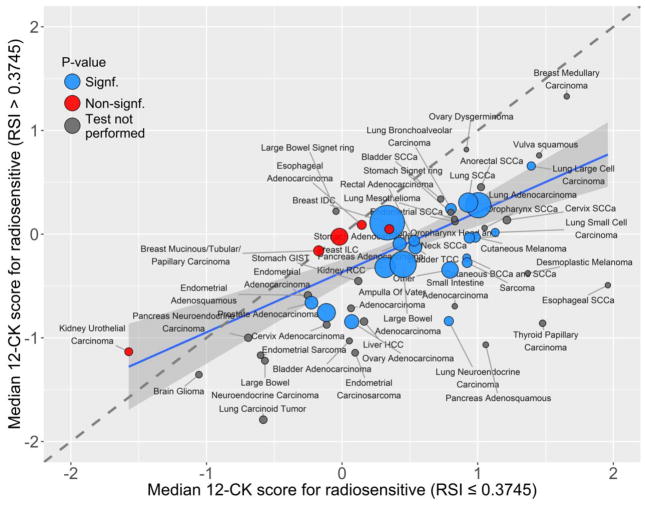
Table 1 and figure 2 show the association between tumor radiosensitivity and immune activation across the TCC cohort (n=10,240). Among 10,240 unique non-metastatic human tumors, RSI-low tumor samples (more radiosensitive) had a significantly higher median 12-CK GES (0.537 [range −0.136, 1.095]) compared with RSI-high tumors (−0.167 [range −0.816, 0.415]; p<0.001) across all tumor samples. This suggests that tumors with increased radiosensitivity also have increased immune activation and visa-versa. This observation was confirmed for the majority of unique tumor types on subset analysis (Table 1).
Table 1.
Comparison of tumor types and 12-CK gene expression signature (GES) scores by RSI-low (more radiosensitive, RSI≤0.3745) and RSI-high (less radiosensitive, RSI>0.3745) groups. Tumor types are sorted from highest to lowest 12-CK GES (most to least immune-active) within the RSI-low group. Tumor types with <10 samples in either RSI group were not compared.
| Tumor site and histology | RSI GES ≤ 0.3745 | RSI GES > 0.3745 | p | ||
|---|---|---|---|---|---|
|
| |||||
| N (%) | Median 12-CK GES (IQR) | N (%) | Median 12-CK GES (IQR) | ||
| Esophageal SCCa | 1 (16.7) | 1.959 (1.959, 1.959) | 5 (83.3) | −0.492 (−0.696, 0.217) | - |
| Breast Medullary Carcinoma | 7 (87.5) | 1.656 (1.517, 1.963) | 1 (12.5) | 1.328 (1.328, 1.328) | - |
| Thyroid Papillary Carcinoma | 3 (10.7) | 1.478 (1.336, 1.779) | 25 (89.3) | −0.860 (−1.266, −0.127) | - |
| Vulva squamous | 1 (25.0) | 1.453 (1.453, 1.453) | 3 (75.0) | 0.759 (0.556, 0.895) | - |
| Lung Large Cell Carcinoma | 28 (66.7) | 1.394 (1.014, 1.605) | 14 (33.3) | 0.657 (−0.293, 1.352) | 0.017 |
| Desmoplastic Melanoma | 1 (20.0) | 1.370 (1.370, 1.370) | 4 (80.0) | −0.378 (−0.674, −0.051) | - |
| Cervix SCCa | 22 (68.8) | 1.215 (0.854, 1.475) | 10 (31.2) | 0.136 (−0.136, 0.464) | - |
| Lung Small Cell Carcinoma | 11 (31.4) | 1.129 (0.690, 1.428) | 24 (68.6) | 0.017 (−0.490, 0.576) | 0.002 |
| Pancreas Adenosquamous | 3 (60.0) | 1.059 (0.292, 1.362) | 2 (40.0) | −1.067 (−1.256, −0.877) | - |
| Oropharynx SCCa | 9 (90.0) | 1.051 (0.919, 1.612) | 1 (10.0) | 0.059 (0.059, 0.059) | - |
| Anorectal SCCa | 7 (35.0) | 1.024 (0.212, 1.274) | 13 (65.0) | 0.454 (−0.614, 0.758) | - |
| Lung Adenocarcinoma | 584 (50.1) | 1.005 (0.627, 1.358) | 581 (49.9) | 0.279 (−0.187, 0.716) | <0.001 |
| Cutaneous Melanoma | 25 (21.7) | 0.983 (0.584, 1.326) | 90 (78.3) | −0.030 (−0.611, 0.641) | <0.001 |
| Non-Oropharynx Head and Neck SCCa | 43 (45.3) | 0.939 (0.546, 1.383) | 52 (54.7) | −0.036 (−0.388, 0.548) | <0.001 |
| Lung SCCa | 311 (53.4) | 0.930 (0.575, 1.272) | 271 (46.6) | 0.305 (−0.161, 0.716) | <0.001 |
| Sarcoma | 23 (22.5) | 0.922 (0.510, 1.523) | 79 (77.5) | −0.274 (−0.917, 0.596) | <0.001 |
| Cutaneous BCCa and SCCa | 11 (35.5) | 0.919 (0.423, 1.503) | 20 (64.5) | −0.228 (−0.731, 0.009) | <0.001 |
| Ovary Dysgerminoma | 1 (50.0) | 0.916 (0.916, 0.916) | 1 (50.0) | 0.815 (0.815, 0.815) | - |
| Endometrial SCCa | 7 (58.3) | 0.833 (0.201, 1.070) | 5 (41.7) | 0.121 (0.034, 0.161) | - |
| Small Intestine Adenocarcinoma | 7 (87.5) | 0.831 (0.588, 0.913) | 1 (12.5) | −0.695 (−0.695, −0.695) | - |
| Lung Mesothelioma | 2 (16.7) | 0.828 (0.496, 1.160) | 10 (83.3) | 0.146 (−0.111, 0.608) | - |
| Lung Bronchoalveolar Carcinoma | 45 (44.1) | 0.803 (0.428, 1.130) | 57 (55.9) | 0.244 (−0.141, 0.497) | <0.001 |
| Stomach Signet ring | 3 (33.3) | 0.801 (0.451, 1.177) | 6 (66.7) | 0.210 (0.103, 0.267) | - |
| Other | 143 (32.4) | 0.796 (0.090, 1.271) | 298 (67.6) | −0.345 (−1.086, 0.291) | <0.001 |
| Lung Neuroendocrine Carcinoma | 22 (34.9) | 0.787 (0.444, 1.103) | 41 (65.1) | −0.838 (−1.884, 0.074) | <0.001 |
| Bladder SCCa | 5 (55.6) | 0.727 (−0.104, 0.916) | 4 (44.4) | 0.339 (−0.006, 0.507) | - |
| Bladder TCC | 69 (35.8) | 0.540 (−0.261, 1.266) | 124 (64.2) | −0.128 (−0.775, 0.526) | <0.001 |
| Rectal Adenocarcinoma | 37 (32.7) | 0.531 (−0.324, 0.810) | 76 (67.3) | −0.063 (−0.753, 0.306) | <0.001 |
| Large Bowel Adenocarcinoma | 600 (46.0) | 0.449 (−0.100, 0.972) | 704 (54.0) | −0.291 (−0.811, 0.184) | <0.001 |
| Pancreas Adenocarcinoma | 66 (23.7) | 0.424 (−0.086, 0.884) | 212 (76.3) | −0.092 (−0.556, 0.328) | <0.001 |
| Stomach Adenocarcinoma | 25 (47.2) | 0.348 (−0.227, 0.816) | 28 (52.8) | 0.048 (−0.508, 0.551) | 0.192 |
| Breast IDC | 1147 (46.1) | 0.333 (−0.335, 1.076) | 1340 (53.9) | 0.111 (−0.491, 0.626) | <0.001 |
| Kidney RCC | 291 (40.6) | 0.317 (−0.125, 0.798) | 425 (59.4) | −0.321 (−0.833, 0.267) | <0.001 |
| Liver HCC | 41 (85.4) | 0.161 (−0.512, 0.416) | 7 (14.6) | −0.842 (−1.289, −0.527) | - |
| Esophageal Adenocarcinoma | 28 (52.8) | 0.145 (−0.422, 0.682) | 25 (47.2) | 0.090 (−0.167, 0.506) | 0.951 |
| Stomach GIST | 7 (22.6) | 0.119 (−0.253, 0.870) | 24 (77.4) | −0.452 (−1.235, 0.047) | - |
| Endometrial Carcinosarcoma | 4 (14.8) | 0.096 (−0.217, 0.524) | 23 (85.2) | −1.145 (−1.449, −0.754) | - |
| Ovary Adenocarcinoma | 108 (42.7) | 0.070 (−0.646, 0.599) | 145 (57.3) | −0.845 (−1.565, −0.164) | <0.001 |
| Ampulla Of Vater Adenocarcinoma | 10 (62.5) | 0.065 (−0.142, 0.220) | 6 (37.5) | −0.715 (−1.297, 0.032) | - |
| Bladder Adenocarcinoma | 3 (42.9) | 0.054 (−0.778, 0.966) | 4 (57.1) | −1.029 (−1.275, −0.458) | - |
| Kidney Urothelial Carcinoma | 23 (67.6) | −1.574 (−1.792, −0.851) | 11 (32.4) | −1.134 (−2.411, −0.369) | 0.913 |
| Brain Glioma | 3 (1.7) | −1.056 (−1.244, −0.548) | 171 (98.3) | −1.355 (−1.827, −0.901) | - |
| Pancreas Neuroendocrine Carcinoma | 6 (11.3) | −0.692 (−0.846, −0.564) | 47 (88.7) | −0.999 (−1.585, −0.695) | - |
| Endometrial Sarcoma | 4 (28.6) | −0.601 (−0.859, −0.056) | 10 (71.4) | −1.168 (−1.685, −0.517) | - |
| Lung Carcinoid Tumor | 7 (8.9) | −0.581 (−1.233, 0.279) | 72 (91.1) | −1.790 (−2.105, −1.218) | - |
| Large Bowel Neuroendocrine Carcinoma | 7 (46.7) | −0.569 (−1.284, −0.277) | 8 (53.3) | −1.220 (−1.404, −0.984) | - |
| Endometrial Adenosquamous | 7 (23.3) | −0.252 (−0.789, 0.532) | 23 (76.7) | −0.590 (−1.355, −0.289) | - |
| Prostate Adenocarcinoma | 73 (39.2) | −0.226 (−1.007, 0.158) | 113 (60.8) | −0.661 (−1.065, −0.282) | <0.001 |
| Breast Mucinous/Tubular/Papillary Carcinoma | 35 (42.7) | −0.173 (−0.669, 0.310) | 47 (57.3) | −0.160 (−0.772, 0.450) | 0.723 |
| Endometrial Adenocarcinoma | 180 (32.5) | −0.116 (−0.760, 0.496) | 374 (67.5) | −0.756 (−1.196, −0.197) | <0.001 |
| Cervix Adenocarcinoma | 10 (58.8) | −0.114 (−0.436, 0.517) | 7 (41.2) | −0.873 (−1.109, −0.742) | - |
| Large Bowel Signet ring | 3 (27.3) | −0.045 (−0.169, 0.197) | 8 (72.7) | 0.220 (−0.095, 0.522) | - |
| Breast ILC | 172 (40.3) | −0.020 (−0.605, 0.540) | 255 (59.7) | −0.025 (−0.468, 0.424) | 0.547 |
| Ovary Granulosa Cell Carcinoma | 0 (0.0) | NA | 10 (100.0) | −1.603 (−1.911, −1.076) | - |
Figure 2.
Tumor radiosensitivity and immune activation are associated across human tumor samples.
The 12-CK and RSI GES were then tested by Spearman correlation (Supplemental figure 1 and Supplemental Table 1). Across all tumors types, there was a negative correlation between RSI and 12-CK GES (R=−0.355, p<0.001), indicating that tumors with a high relative radiosensitivity also often have a high level of immune activation, while radioresistant tumors tend to have a lower level of immune activation. When assessed by unique tumor site and histology, this negative correlation was consistent across the majority of tumors. The degree of the negative correlation varied between tumor types.
An exploratory combined model including both RSI and 12-CK phenotype predicts clinical outcome in the Erasmus Dataset
The prognostic value of the RSI and 12-CK GES were then explored in an independent dataset of 282 breast cancer patients treated with breast conservation therapy. No differences were observed in the RSI score when compared between patient clinicopathologic and tumor characteristics (Supplemental Table 2). Patients with a high 12-CK score (immune activated) were significantly younger (median 51 vs. 57 years, respectively; p=0.002), more often pre-menopausal (60.8% vs. 42.9%, respectively; p=0.008) and more frequently had ER-/PR- tumors (34.3% vs. 9.1%, respectively; p<0.001) compared to patients whose tumors had low 12 CK scores. Similar to the TCC analysis, the dichotomized RSI and 12-CK GES in the Erasmus dataset were associated with each other (p=0.002, data not shown).
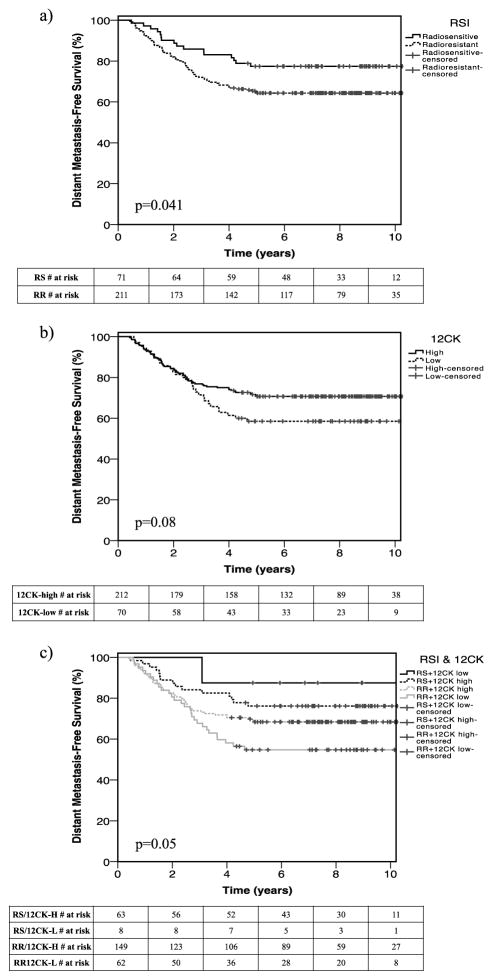
When each GES was assessed separately with patient outcomes, both an RSI-low (more radiosensitive tumor) status (Figure 3, Table 2; HR 0.58 [95% CI 0.34, 1.00]; p=0.05) and a 12-CK-high immune-active status (HR 0.61 [95% CI 0.39, 0.96]; p=0.03) were independently associated with improved distant metastasis-free survival. In addition, a composite model including all three variables (p=0.02) outperformed both age-adjusted individual models for RSI and 12-CK (AIC for composite model one unit lower than the best individual model).
Figure 3.
Kaplan Meier plots demonstrating distant metastasis free survival among 282 patients with breast cancer treated with radiotherapy compared by (a) radiosensitive (bottom 25% RSI) and less radiosensitive (upper 75% RSI) gene expression profiles, (b) 12-chemokine (12-CK) high and low gene expression profiles, and (c) by combined RSI and 12-CK expression profiles.
Table 2.
Age-adjusted risk of distant metastasis free survival in the Erasmus breast cancer cohort compared using Cox multivariate modeling with clinicopathologic and treatment variables and a) RSI gene expression signature (GES) alone, b) 12-CK GES alone, and (c) RSI and 12-CK GES combined.
| Variable | HR | CI (low) | CI (high) | P | |
|---|---|---|---|---|---|
| RSI | Age (years) | 0.99 | 0.97 | 1 | 0.11 |
| RSI-S (ref: RSI-R) | 0.58 | 0.34 | 1 | 0.05 | |
|
| |||||
| 12-CK | Age (years) | 0.98 | 0.96 | 1.00 | 0.04 |
| 12-CK- high (ref: 12-CK- low) | 0.61 | 0.39 | 0.96 | 0.03 | |
|
| |||||
| RSI and 12-CK | Age (years) | 0.98 | 0.97 | 1.00 | 0.05 |
| RSI-S (ref: RSI-R) | 0.63 | 0.36 | 1.09 | 0.10 | |
| 12-CK- high (ref: 12-CK- low) | 0.66 | 0.41 | 1.04 | 0.07 | |
Discussion
We demonstrate a clear association between tumor radiosensitivity and immune activation among a large cohort of patients using two separate microarray GES. Each signature was designed for a unique purpose and previously studied in different patient populations. RSI was designed to detect intrinsic tumor radiosensitivity using 10 genes that play a role in DNA damage response, histone deacetylation, cell-cycle regulation, apoptosis, and proliferation.(12,28,29) RSI has been shown to predict outcomes among patients treated with radiation therapy with breast cancer(13,30), head and neck cancer, rectal cancer, esophageal cancer(12) pancreatic cancer,(15), glioblastoma(16), and metastatic colorectal cancer(14). In contrast, 12-CK was designed using 12 immune-related and inflammation-related genes with the purpose of detecting intra-tumoral lymphoid cell aggregates as a marker of immune activation.(17,19) Twelve CK was previously found to be associated with the presence of TL-ELNs and was able to predict patient outcomes among patients with both colorectal cancer(17) and metastatic melanoma.(18,19) By combining the two gene signatures, we provide evidence for the presence of a clinical interplay between radiosensitivity and immune activation across a wide-variety of tumor types that has not previously been shown in the clinical setting.
There are clinical implications to the observed association between radiosensitivity and immune-activation in solid tumors. We hypothesize that this association could have clinical implications. In one scenario it is possible that the immune system primes the tumor for improved response to radiation. Conversely it could be that radiation therapy primes the tumor for increased immune activation, or potentially both processes take place in a synergistic manner. Understanding the clinical impact of this association is critical given the emerging immunotherapy options that could be utilized in combination with RT.
In the absence of radiation therapy, it is now well-established that tumors have a variety of innate mechanisms by which they can suppress the body’s natural immune response directed towards them.(31) Fortunately, many of these mechanisms provide therapeutic targets for immune modulation.(32) Two more recently studied targets of tumor-directed immune modulation include the CTLA4 axis, which causes immune tolerization, and the PD1-PDL1 axis, which causes T-cell exhaustion.(4) Immune modulators including the anti-CTLA4 antibody Ipilimumab (Yervoy, Bristol-Myers Squibb), and the anti-PD1 antibodies Nivolumab (Opdivo, Bristol-Myers Squibb) and Pembrolizumab (Keytruda, Merck), effectively remove the tumor brake-pedal, increasing the tumor-directed immune activation. These targeted immunotherapies have been shown to positively affect tumor response and survival initially among melanoma patients(33–39), and subsequently among many other tumors including squamous cell and non-squamous non-small-cell carcinoma of the lung, renal cell carcinoma, and lymphoma.(40–43) Interestingly, cutaneous melanoma (r=−0.56), lung adenocarcinoma (r=−0.50) and renal cell carcinoma (r=−0.56) each had a relatively high degree of correlation between immune activation and radiosensitivity in the current study (Supplemental Table 1), suggesting that combined immunotherapy and radiation therapy treatment might also be beneficial for these tumors. Results from immunotherapy trials thus far have been compelling and exciting, yet there remains a lack of biomarkers to more appropriately select patients for immunotherapy trials(44). Additionally, it remains unclear as to whether immunotherapy-specific biomarkers, if successfully developed and implemented, would also predict for response to combined modality treatment if radiation is added as an immune-stimulatory mechanism. (45)
The ability of radiation therapy to influence tumor-directed immune responses in the pre-clinical setting has been supported by many disease site-specific studies.(5) Radiation has been shown to stimulate the immune system by upregulating MHC class I molecules(46), enhancing FAS surface expression(47), activating dendritic cells(48), and by increasing the concentration of tumor infiltrating lymphocytes(49). Radiation also modulates the expression of immune checkpoint molecules in both favorable and unfavorable ways(5), which are less predictable and less well understood. Additionally, radiation has been shown to increase the concentration of regulatory T-cells in tumors(50–52), which acts to suppress the benefits of tumor-directed immune activation. Because of the multifactorial influences of radiation therapy on the immune system, an abundance of clinical trials are underway in an attempt to harness the potential synergy of immunomodulators and radiation therapy of various disease sites.(4,53,54) In support of the combined radiation and immunotherapy trials, Twyman-Saint Victor et al(55) demonstrated in a preclinical model that radiation therapy and immune checkpoint blockade activated non-redundant immune mechanisms in cancer. However, there remains a dearth of pre-treatment and tumor-specific biomarkers to identify patients who might, and more importantly might not, benefit from combined immunotherapy and radiation therapy. Since the composite model outperforms both individual RSI and 12-CK models, we hypothesize that these signatures may serve as biomarkers to identify patients that may benefit from combined RT and immunotherapy.
Moving forward, we hypothesize that the combined 12-CK/RSI phenotype could provide a pre-treatment biomarker to identify patients who will have increased response and outcomes to combined immunotherapy and radiation therapy treatment. For instance, patients with immune active/radiosensitive tumors could be selected for combined immunotherapy and radiation therapy trials, while those with immune inactive/less radiosensitive tumors could either be treated without radiation therapy, or selected for dose-escalated radiation trials with or without immunotherapy. Recently, we have developed the genomic-adjusted radiation dose (GARD) a genomic-based model that provides for the first time the opportunity to adjust the dose of radiation to match the individual radiosensitivity of the tumor. It is possible that the immune active/radiosensitive GES phenotype from the present study could be used to more accurately identify patients who experience the seemingly elusive abscopal effect.(9) To date, the abscopal effect remains a clinically rare and relatively unpredictable event(56,57), where radiation of a single metastatic site causes systemic regression of unirradiated distant metastases. When the abscopal effect does take place, it can render patients with a high metastatic disease burden, free of disease.(9) If a GES could improve the identification of these patients, even by a few percent, it would likely be deemed a success and as such, we believe that this is worth pursuing. A likely first-step to evaluate this would be to assess the 12-CK/RSI phenotype among a cohort of patients treated with combined modality treatment.
In conclusion, tumor radiosensitivity is associated with immune activation across solid tumors as measured by two unique gene expression signatures. Radiosensitive tumors are more frequently present in patients with a phenotype of immune-activation and the combined RSI and 12-CK GES has the potential to improve the prognostic ability of patients undergoing combined radiation therapy and immunotherapy.
Supplementary Material
Acknowledgments
This work has been supported in part by the DeBartolo Family’s contributions to the Total Cancer Care® Initiative at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center.
Shared Resource Acknowledgement: This work has been supported in part by the Biomedical Informatics Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center.
Footnotes
Conflict of Interest Statement: SAE and JTR hold several awarded and pending patents regarding the technology herein. They are also shareholders and officers of Cvergenx, Inc., which holds an exclusive license for the commercial application of RSI.
References
- 1.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. The New England journal of medicine. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. International journal of cancer Journal international du cancer. 2007;121(8):1813–20. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 3.Tang C, Fuller CD, Garden AS, Awan MJ, Colen RR, Morrison WH, et al. Characteristics and kinetics of cervical lymph node regression after radiation therapy for human papillomavirus-associated oropharyngeal carcinoma: quantitative image analysis of post-radiotherapy response. Oral oncology. 2015;51(2):195–201. doi: 10.1016/j.oraloncology.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nature reviews Cancer. 2015;15(7):409–25. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. The lancet oncology. 2015;16(13):e498–509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 6.Order SE. The effects of therapeutic irradiation on lymphocytes and immunity. Cancer. 1977;39(2 Suppl):737–43. doi: 10.1002/1097-0142(197702)39:2+<737::aid-cncr2820390708>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. International journal of radiation oncology, biology, physics. 2013;85(2):293–5. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. The New England journal of medicine. 2012;366(10):925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41(6):503–10. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein MB, Garnett CT, Zhang H, Velcich A, Wattenberg MM, Gameiro SR, et al. Radiation-induced modulation of costimulatory and coinhibitory T-cell signaling molecules on human prostate carcinoma cells promotes productive antitumor immune interactions. Cancer Biother Radiopharm. 2014;29(4):153–61. doi: 10.1089/cbr.2013.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer research. 2014;74(19):5458–68. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 12.Eschrich SA, Pramana J, Zhang H, Zhao H, Boulware D, Lee JH, et al. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. International journal of radiation oncology, biology, physics. 2009;75(2):489–96. doi: 10.1016/j.ijrobp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eschrich SA, Fulp WJ, Pawitan Y, Foekens JA, Smid M, Martens JW, et al. Validation of a radiosensitivity molecular signature in breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18(18):5134–43. doi: 10.1158/1078-0432.CCR-12-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed KA, Fulp WJ, Berglund AE, Hoffe SE, Dilling TJ, Eschrich SA, et al. Differences Between Colon Cancer Primaries and Metastases Using a Molecular Assay for Tumor Radiation Sensitivity Suggest Implications for Potential Oligometastatic SBRT Patient Selection. International journal of radiation oncology, biology, physics. 2015 doi: 10.1016/j.ijrobp.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strom T, Hoffe SE, Fulp W, Frakes J, Coppola D, Springett GM, et al. Radiosensitivity index predicts for survival with adjuvant radiation in resectable pancreatic cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2015;117(1):159–64. doi: 10.1016/j.radonc.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed KA, Chinnaiyan P, Fulp WJ, Eschrich S, Torres-Roca JF, Caudell JJ. The radiosensitivity index predicts for overall survival in glioblastoma. Oncotarget. 2015;6(33):34414–22. doi: 10.18632/oncotarget.5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, et al. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. The American journal of pathology. 2011;179(1):37–45. doi: 10.1016/j.ajpath.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Scientific reports. 2012;2:765. doi: 10.1038/srep00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mihm MC, Jr, Mule JJ. Reflections on the Histopathology of Tumor-Infiltrating Lymphocytes in Melanoma and the Host Immune Response. Cancer Immunol Res. 2015;3(8):827–35. doi: 10.1158/2326-6066.CIR-15-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenstermacher DA, Wenham RM, Rollison DE, Dalton WS. Implementing personalized medicine in a cancer center. Cancer journal. 2011;17(6):528–36. doi: 10.1097/PPO.0b013e318238216e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welsh EA, Eschrich SA, Berglund AE, Fenstermacher DA. Iterative rank-order normalization of gene expression microarray data. BMC bioinformatics. 2013;14:153. doi: 10.1186/1471-2105-14-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, et al. Subtypes of breast cancer show preferential site of relapse. Cancer research. 2008;68(9):3108–14. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365(9460):671–9. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 24.Pawitan Y, Bjohle J, Amler L, Borg AL, Egyhazi S, Hall P, et al. Gene expression profiling spares early breast cancer patients from adjuvant therapy: derived and validated in two population-based cohorts. Breast cancer research: BCR. 2005;7(6):R953–64. doi: 10.1186/bcr1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic acids research. 2003;31(4):e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 27.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 28.Torres-Roca JF, Eschrich S, Zhao H, Bloom G, Sung J, McCarthy S, et al. Prediction of radiation sensitivity using a gene expression classifier. Cancer research. 2005;65(16):7169–76. doi: 10.1158/0008-5472.CAN-05-0656. [DOI] [PubMed] [Google Scholar]
- 29.Eschrich S, Zhang H, Zhao H, Boulware D, Lee JH, Bloom G, et al. Systems biology modeling of the radiation sensitivity network: a biomarker discovery platform. International journal of radiation oncology, biology, physics. 2009;75(2):497–505. doi: 10.1016/j.ijrobp.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torres-Roca JF, Fulp WJ, Caudell JJ, Servant N, Bollet MA, van de Vijver M, et al. Integration of a Radiosensitivity Molecular Signature Into the Assessment of Local Recurrence Risk in Breast Cancer. International journal of radiation oncology, biology, physics. 2015;93(3):631–8. doi: 10.1016/j.ijrobp.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finn OJ. Cancer immunology. The New England journal of medicine. 2008;358(25):2704–15. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 32.Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14(8):561–84. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- 33.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. The lancet oncology. 2015;16(8):908–18. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. The New England journal of medicine. 2015;372(21):2006–17. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. The New England journal of medicine. 2015;372(26):2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 37.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. The lancet oncology. 2015;16(5):522–30. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 38.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. The lancet oncology. 2015;16(4):375–84. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 39.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. The New England journal of medicine. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 40.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373(17):1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2015;373(19):1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. The New England journal of medicine. 2015;373(2):123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. The New England journal of medicine. 2015;372(4):311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng X, Huang Z, Teng F, Xing L, Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015;41(10):868–76. doi: 10.1016/j.ctrv.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Mahoney KM, Atkins MB. Prognostic and predictive markers for the new immunotherapies. Oncology (Williston Park) 2014;28(Suppl 3):39–48. [PubMed] [Google Scholar]
- 46.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer research. 2004;64(21):7985–94. doi: 10.1158/0008-5472.CAN-04-1525. [DOI] [PubMed] [Google Scholar]
- 48.Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189(2):558–66. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 49.Hallahan D, Kuchibhotla J, Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer research. 1996;56(22):5150–5. [PubMed] [Google Scholar]
- 50.Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. 2015;3(4):345–55. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wirsdorfer F, Cappuccini F, Niazman M, de Leve S, Westendorf AM, Ludemann L, et al. Thorax irradiation triggers a local and systemic accumulation of immunosuppressive CD4+ FoxP3+ regulatory T cells. Radiation oncology. 2014;9:98. doi: 10.1186/1748-717X-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kachikwu EL, Iwamoto KS, Liao YP, DeMarco JJ, Agazaryan N, Economou JS, et al. Radiation enhances regulatory T cell representation. International journal of radiation oncology, biology, physics. 2011;81(4):1128–35. doi: 10.1016/j.ijrobp.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Formenti SC. Seminars in Radiation Oncology. Introduction Seminars in radiation oncology. 2015;25(1):1–3. doi: 10.1016/j.semradonc.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Crittenden M, Kohrt H, Levy R, Jones J, Camphausen K, Dicker A, et al. Current clinical trials testing combinations of immunotherapy and radiation. Seminars in radiation oncology. 2015;25(1):54–64. doi: 10.1016/j.semradonc.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15(17):5379–88. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. International journal of radiation oncology, biology, physics. 2004;58(3):862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.