Abstract
Background:
Breast cancer (BC), because of its invasive characteristics, is one of the most common and deadliest cancers among the female population around the world. Research has demonstrated that AhR signaling also plays a vital role in BC initiation and development as well. Therefore, blocking this pathway to natural interferences paves a new channel for the prevention of BC. Several natural compounds such as flavonoids possess the anticancer activities against different cancers.
Objective:
The present study has been designed to estimate the chemotherapeutic potential of taxifolin (TAX) against 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary carcinoma in Sprague-Dawley rats.
Materials and Methods:
Initially, the molecular docking analysis of AhR and cytochrome P450s (CYPs) (CYP1A1 and CYP1B1) was performed using MAESTRO tool, in an attempt to rationalize the activity of TAX, based on their CYP1-binding potential. The in vitro CYP1A1 activity was determined by luciferase assay with CYP1A1 substrate luciferin CEE. The in vivo analysis was performed by administrating TAX at 10, 20, 40 mg/kg BW for 28 days intragastrically in DMBA induced (25 mg/animal dose) at 55 days of age Sprague-Dawley (SD) rats. BC initiates after 90 days of tumor induction phase. The molecular mechanism of TAX on Ahr and CYPs was also examined through the mRNA and protein expressions using reverse transcription-quantitative polymerase chain reaction and Western blotting analysis.
Results:
Furthermore, TAX altered the energy regulation on DMBA-induced BC in SD rats by considerably restoring the cancer-induced modulations in tumor growth. Our results showed that TAX reduced the expressions of CYP1A1 and CYP1B1 in DMBA-induced mammary carcinoma by downregulating the AhR signaling pathway.
Conclusion:
This study revealed that TAX might be able to act as a chemotherapeutic agent against CYP1A1- and CYP1B1-mediated cancer and the inhibition of the DMBA-induced mammary carcinogenesis in a rat model.
Abbreviations used: CYPs: Cytochrome P450s; PAH: polycyclic aromatic hydrocarbons; HRP- Horseradish peroxidase; BSA: Bovine serum albumin; DTTP: Deoxythymidine Triphosphate (nucleotide); RT-qPCR: Real Time quantitative polymerase chain reaction; CADD: Computer Aided Drug Drafting.
Keywords: 7,12-dimethylbenz[a] anthracene; AhR; breast cancer; cytochrome P450s; molecular modeling; taxifolin
INTRODUCTION
Cancer is a metastasizing tumorous growth efficiency to invade adjacent tissues and it is an important health concern with increased toxic exposures which ultimately leads to cancer threat. Among, breast cancer (BC) is one the most important deadly disease on global health menace. It is a leading cause of morbidity and mortality among women globally with greater prevalence in the developed countries. In both developed and developing countries, the majority of the cancer death among women is due to BC, as commonly diagnosed malignancy. BC is characterized by physiological and cellular alterations through the growth of a tumor.[1] Various enzymes, signaling proteins are involved in energy regulation in normal and cancerous cell physiology. Cancer cell alters the expression of different elements to achieve their basic needs for proliferation.
Cancer chemoprevention is described as the use of synthetic, biological, or natural chemical agents that suppress the development of neoplastic cell to a tumor in the early stage of carcinogenesis. There are several classes of cancer chemopreventive agents and some of them are blocking agents; they inhibit the cancer growth at the initial phase of chemically induced carcinogenesis.[2] Cytochrome P450s (CYPs) are hemeproteins containing enzymes that play a vital role in the activation, metabolism, and detoxification of xenobiotics, including many drugs, toxins, endogenous and exogenous substances, and chemical carcinogens.[3] The human CYP1 subfamily member mainly consists of CYP1A1, CYP1B1, and CYP1A2. All these CYP1A1 enzymes mainly take part in the metabolism of potential procarcinogens such as polycyclic aromatic hydrocarbons (PAH). CYP1A1 and CYP1B1are the two extrahepatic enzymes, which are involved in cancer initiation and progression of carcinogens while CYP1A2 mainly expressed in the liver.[4,5,6] The majority of mammary tumors constitutively express CYP1A1 and CYP1B1,[7] and the expression of CYP1A1 mainly controls mammary cancerous cell proliferation and survival as reported earlier.[8]
The expression of CYP1A1 and CYP1B1 is mainly controlled through AhR, which is the member of basic Helix–Loop–Helix (bHLH)/Per–Arnt–Sim (bHLH/PAS) of a protein family.[9] As AhR is a ligand-dependent transcription factor, after binding it moves to the nucleus and dimerizes in nuclear translocator. Then, it binds at the xenobiotic responsive elements to the DNA and it inhibits the transcription of AhR. In liver, mammary as well as lung cancer AhR, CYP1A1, and CYP1B1were earlier reported as a cancer promoter.[8,10,11] Activated AhR promotes the invasiveness of tumor cells.[12] On the other hand, various studies showed that AhR has also played a pivotal role in the normal physiology and growth of the various cell.[13,14,15,16] Earlier studies showed that the level of expression of AhR was upregulated in a mammary cancer cell as compared to control mammary cell.[17]
Flavonoids are the class of natural compounds, possessing potential anticancer property.[18,19] Taxifolin (TAX) is a flavanonol subclass of flavonoid present abundantly in Pinus Roxburghii, Taxus Chinensis, and also in citrus fruits.[20,21] It has enormous biological activities such as anti-inflammatory, antiapoptotic, antidiabetic, and antioxidant activities.[22,23,24] It has been reported to inhibit the proliferation of BC cells and cervical cancer cells in vitro in a dose-dependent manner due to its high antioxidant property.[25,26] The effect of TAX on the AhR pathway has not yet been explored in detail. This study was designed to investigate whether treatment of TAX downregulates the expression of AhR and so as CYP1A1 and CYP1B1 in in vivo BC model.
Initially, molecular docking studies were performed using Schrödinger Maestro 8.5 software package for evaluating the efficacy of TAX to inhibit AhR and cytochrome p450 metabolism (AhR and CYP antagonist activity). Further, we assessed the inhibitory activity of TAX on CYP1A1 and CYP1B1 in in vitro inhibition studies. Then, we investigated the chemotherapeutic effect of TAX against 7,12-dimethylbenz(a) anthracene (DMBA)-induced tumor initiation and progression in mammary glands of female Sprague-Dawley rats. Furthermore, the activities of AhR were assessed, which is a key regulator in controlling CYP1 expressions.
MATERIALS AND METHODS
Materials
The carcinogen 7, 12- DMBA and TAX were procured from Sigma-Aldrich (St. Louis, MO, USA). All other reagents used were of high purity analytical grade and purchased from the local commercial supplier.
Molecular docking of taxifolin on AhR, CYP1A1, and CYP1B1
The X-ray crystal structures of AhR which are a dimer (chain A, B) have tert-butyl benzoate analog (4KQ) as a co-crystallized ligand (PDB entry: 4GHI), CYP1A1 (PDB entry: 418V), and CYP1BI (PDB entry: 3PM0). Their co-crystallized ligands were downloaded from RCSB protein database (http://www.rcsb.org). Ligand preparation was done using Ligprep module (Schrodinger, Maestro 8.5). After the software validation by the re-docking method, TAX was docked into the active site of the AhR, CYP1A1, and CYP1B1 within the radius of 6.5A°. Maestro software is based on the constructive incremental algorithm. The docking scores and suitable binding patterns were reported compared with the reference ligands.
In vitro enzyme inhibition assay
CYP1A1 activity was determined by a luciferase assay with CYP1A1 substrate luciferin-CEE (Promega, CA). The formed product from the reaction was determined by measuring the luminescence using MITHRAS luminometer equipment. In this, 100 mM potassium phosphate buffer (pH 7.4) was present in the incubation mixture. The stock solution of the TAX was of 1 mM (of pH 6.7). The final reaction volume was 100 μL. The inhibition assays were performed using eight different concentrations of TAX (0, 5, 10, 20, 40, 60, 80, 100 μM). Through luciferin-CEE luminogenic substrate, HCC microsomes with 22 pmol and the total CYP were preincubated with luciferin-CEE (60 μM) and with the respective TAX (different concentrations mentioned above) at 37°C for 30 min; then, NADPH generating system was added (5.8 mM isocitrate, 0.8 mM NADP+, 8 mM MgCl2, and 0.3unit/mL of isocitrate dehydrogenase), and the mixture was incubated for another 10 min. Then, the detection reagent was mixed with it and the reaction mixture was again re-incubated for another 20 min. Two different experiments were performed with triplicates which did not reveal any difference by more than 10%. The inhibitory activity of CYP1A1 was assessed by plotting the remaining activity against inhibitor concentrations.
Experimental animals, carcinogenic induction, and study design
Female Sprague-Dawley (SD) rats (50–55 days old) were taken from our central animal facility, Birla Institute of Technology, Mesra, Ranchi, Jharkhand, India (Reg. No. 621/02/ac/CPCSEA). All the studies were carried out on the animals after approval by the Institutional Animal Ethics Committee (IAEC) with protocol approval no. BIT/PH/IAEC/13/2013. All the experimental rats were accommodated in separate cages, maintained with standard laboratory conditions (relative humidity 60 ± 5%, temperature 25 ± 1°C, and12 h dark and light cycle) throughout the experimental period. These animals were given to eat with pellet diet and water ad libitum. Before starting the experiment, all the experimental animals were acclimatized for a week.
Tumor induction and treatment protocol
Mammary tumor induction was carried out by the carcinogen DMBA using “air pouch technique” method,[27,28] with slight modification as required. A volume of 2–3 ml of sterile air was inserted subcutaneously, just below the second and third mammary glands, so that it produces an air pouch containing sterilized air. After 24 h, a single dose of DMBA (20 mg) in olive oil (0.5 ml) was vortexed in a stirrer, to get a uniform suspension and was carefully inserted into the air pouch.
All the experimental animals were divided into five different groups, and in each group, ten animals were included in the study. Group I: Control animals (normal), these animals received only (0.5 ml) olive oil in air pouch. Group II: Induced control animals, they were administered with DMBA (20 mg) with olive oil (0.5 ml) in the air pouch only. Group III, IV, and V: These animals were treated with TAX at the dose level of 10, 20, and 40 mg/kg b. w/day (i. p.), respectively, for 28 days, along with Group II of DMBA induction.
Tumorigenesis assessment
On a weekly basis, all rats were palpated to observe the beginning of tumourigenesis and the location of the tumor was monitored and checked after the process of induction. At the end of 90 days of DMBA-inducted duration, the tumor growth was excessively grown. Then, the animals were treated with TAX in different doses for 28 days. Each group of the experimental animals was weighing every week. The changes in the weight of the body were also recorded.
After the 118 days (90 + 28) of the experimental period, all animals were starved overnight. Afterward, animals were sacrificed by cervical decapitation and perfuse. Breast tumors of all the groups of animals isolated and washed with ice-cold normal PBS (phosphate-buffered saline, PH 7) to remove the blood stains and homogenized in PBS at 4°C. Fractions of mammary tumor sections were preserved instantly for mRNA and protein analysis using a standard protocol.
Effect of taxifolin on AhR, CYP1A1, and CYP1B1 mRNA expression through reverse transcription-quantitative polymerase chain reaction
To measure the level of mRNA, a reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assay was used. In all groups of the animals, total cellular RNA was isolated from the breast tissue cells using TRIzol reagent® (Sigma-Aldrich, Bengaluru). After this, they were purified by the addition of a mixture which consists of the following reagents: DDW (150 μL), 3M sodium acetate (20 μL), glycogen (8 μL), and absolute ethanol (600 μL). For cDNA synthesis, the mixture of the RNA (1 μg) was keeping it aside at-80°C for 2 h. After this it was centrifugated it at 4°C for 15 min at 13000 rpm, to get a purified RNA. We have to follow the conditions that were recommended by the manufacturer's protocol; initially, strand cDNA was synthesized with 1 μg of oligo-DTTP and total RNA sample through cDNA Synthesis Kit (Bio-Rad RT-PCR Kit). The similar quantity of cDNA was subsequently amplified in a 20 μl reaction, by particular primers. PCR reaction was carried out with 2x SYBR qPCR Master Mix (Kapa-Biosystems). The qPCR was carried out using the subsequent sense and antisense, i. e., forward and backward primer sequence: Sense 5’-GCCAGGACCAGTGTAGAGC-3’ (Location: 287-305) and antisense 5’-ATTCAGCGCCTGTAACAAGAA-3’ (Location: 363-343) for AhR and sense 5’-GACCCTTACAAGTATTTGGTCGT-3’ (Location: 565-587) and antisense 5’-GGTATCCAGAGCCAGTAACCT-3’ ((Location: 709-689)) for CYP1A1 and sense 5’-ACGACGATGCGGAGTTCCTA-3’ (Location: 647-666) and antisense 5’-CGGGTTGGGAAATAGCTGC-3’ (Location: 759-741) for CYP1B1 and sense 5’-CTGGAATTATGAGTG CCCCAAA-3’ (Location: 135-156) and antisense 5’-ACGACTGTACT GAAGACAAAGC-3’ (Location: 329-308) for GAPDH. The cycling conditions of each PCR cycle are as follows for Ahr, CYP1A1, CYP1B1, and GAPDH. One cycle for 3 min at 95°C, this is followed by 39 cycles for 10 s at 95°C and 58°C for 30 s for denaturation. The amplification of PCR was confirmed by the presence of a single peak (60.0-95°C, increment 0.5°C for 0.05). Every time RT-qPCR experiment was repeated more than two times. For this, GAPDH was used as internal control.
Western blot analysis of AhR, CYP1A1, and CYP1B1 in mammary tissues
Breast tissue sections from all the experimental groups of animals were stored in liquid nitrogen at −80°C and they were rinsed with PBS (phosphate-buffered saline) at least three times and lysed for 10 min in ice cold (500 μL) with buffer which consist of sucrose (0.5 M), NaCl (50 mM), ethylenediaminetetraacetic acid (EDTA; 0.1 mM), 10 mM HEPES (pH 7.9), 1 mM dithiothreitol (DTT), triton (0.5%), 1 mM phenylmethylsulfonyl fluoride, phosphatase inhibitor (5 μL), and protease inhibitor (5 μL) cocktails. The extract of cytoplasmic cell lysate was separated through centrifugating the cell lysate for 10 min at 4°C in 1000 rpm to get a clear cytoplasmic fraction of protein and then it was stored at −80°C. Afterward, the saline buffer (500 μL) which consists of NaCl (500 mM), EDTA (0.1 mM), NP40 (0.1%), DTT (1 mM), HEPES (10 mM) at PH 7.9 and protease inhibitor (5 μL), and the cocktail of the mixture was added and it was vortexed at 4°C for almost 15 min; then, the nuclear extract was again centrifuged for 10 min at 14,000 g (4°C) and stored it at −80°C. The estimation of protein concentration was carried out through Bradford protein assay. Afterward, an equal quantity of samples of protein was subjected to separate through sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Then, the separated proteins were transferred to a nitrocellulose membrane (Millipore) electrophoretically. The nonspecific binding sites of the blots were then blocked with blocking buffer which consists of nonfat dry milk (5% w/v) in Tris-buffered saline-Tween-20 (TBST) for 1 h at room temperature. Afterward, blots were incubated over a night with a specific primary antibody at 4°C; then, the mixture of a solution was further washed three times in TBST at 5 min interval each. After this, the blots were again re-incubated with secondary antibody (HRP-conjugated) at room temperature for 1 h. By the help of chemiluminescent system (Thermo Fisher Scientific) membranes were developed and obtained a film-like strip (CL-Xposure, 8 × 10 in). The following primary antibodies were used (at the indicated dilutions): AhR, CYP1A1, CYP1B1, and β-actin (Cell Signaling Technology, USA, and dilution 1:500) in BSA (5%w/v) at TBST. The optical density, i. e., densitometry analysis of the protein band was measured through Image-J software (NIH, Washington, USA). β-actin was used as internal controls for AhR and CYPs assessment.
Statistical analysis
Statistical comparisons of the data in between control, induced (control), and treatment group of animals were carried out with GraphPad Prism software by one-way ANOVA, followed by Bonferroni's multiple comparison tests with similar no of sample size. The level of significance was measured at P < 0.05. Data were represented as mean ± standard error of the mean.
RESULTS
Molecular docking and binding pattern analysis
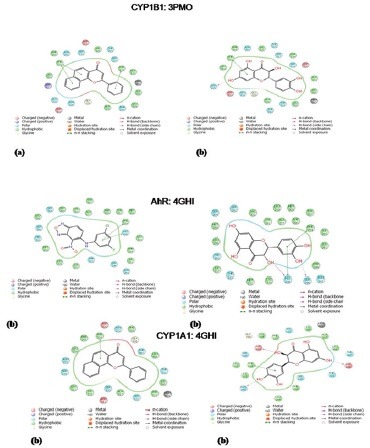
The apparent action of the drug to activate or inhibit a particular target of protein in CADD was mainly based on the docking score. Before the docking with TAX, the co-crystallized ligand of the receptor AhR (PDB: 4GHI) was re-docked to authenticate the reliability of the method. A docking score of -11.35 on AhR, -11.31 on CYP1A1, and -9.90 on CYP1B1 was observed with TAX. Molecular scaffold and binding pattern of TAX with AhR (PDB: 4GHI), CYP1A1 (PDB: 418V), and CYP1B1 (PDB: 3PM0) was represented in [Figure 1a–f], respectively. The interacting amino acids, which are present at the active sites of protein structures, are as follows. Results indicated that TAX showed a potentiated binding interaction with AhR forming hydrogen bond with Ser246, His248, and hydrophobic interaction with Phe254, Tyr281, Met309, Phe280, Tyr307, and with CYP1A1 forming hydrogen bond with Asp313, Asn255, Asp320 and hydrophobic interaction (Phe224, Phe123, Phe258, Leu254), and with CYP1B1 forming hydrogen bond with Asn228 and hydrophobic interactions with Phe231, Leu264, Phe134, Ala133, Phe268 amino acids, respectively, on the active site. These all interactions proposed strong binding of TAX to the respective receptors and suggested its activity on these selected receptors.
Figure 1.
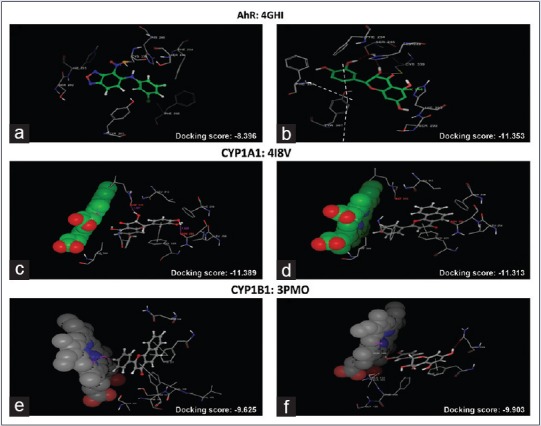
Molecular docking analysis of reference ligand and taxifolin where (a) interaction of AhR with reference ligand (molecular scaffold), (b) interaction of AhR with taxifolin (molecular scaffold). (c) Interaction of CYP1A1 with reference ligand (molecular scaffold) and (d) interaction of CYP1A1 with taxifolin (molecular scaffold). (e) Interaction of CYP1B1 with reference ligand (molecular scaffold); (f) interaction of CYP1B1 with TAX (molecular scaffold)
Taxifolin-inhibited CYP1A1 In vitro
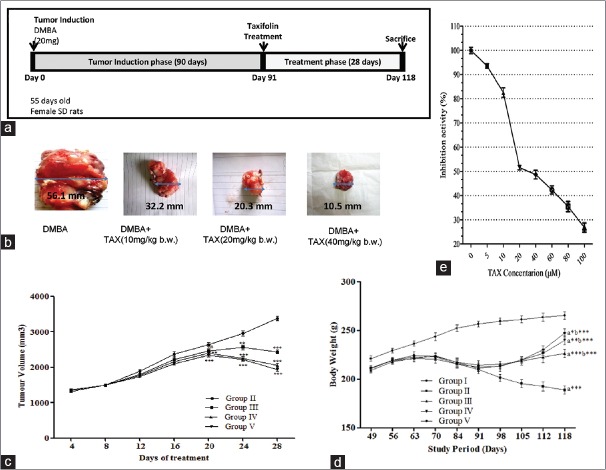
CYP1A1 is the enzyme which is actively involved in the activation of DMBA-induced carcinogenesis in BC. We know that DMBA itself may enhance the expression of these CYPs through transcription. Hence, we tested whether the TAX could affect the inhibition of CYP1A1. The activity was determined by a luciferase assay with CYP1A1 substrate luciferin-CEE (Promega, CA), as shown in Figure 2e. The treatment with TAX inhibited the CYP enzyme activity with an IC50 value of 20 μM.
Figure 2.
(a) Treatment protocol for in vivo studies. (b) Tumorigenesis assessment in control and taxifolin-treated experimental animals. Isolated tumor tissue from all cancer bearing treatment groups; (c) tumor volume in all treatment groups. (d) Effect of taxifolin treatment on body weight in cancer-inducing animals. Data represented as mean standard error of the mean (n = 10); where ***P < 0.001; **P < 0.01; *P < 0.05; nsp > 0.05; Group II - 7,12-dimethylbenz(a) anthracene (20 mg); Group III - 7,12-dimethylbenz (a) anthracene (20 mg) + taxifolin (10 mg/kg b. w.); Group IV - 7,12-dimethylbenz (a) anthracene (20 mg) + taxifolin (20 mg/kg b. w.); Group V - 7,12-dimethylbenz(a) anthracene (20 mg) + taxifolin (40 mg/kg b. w.). (e) Effect of TAX on CYP1A1 inhibition in vitro. CYP1A1 activity was determined by a luciferase assay with CYP1A1 substrate. All experiments were performed in triplicate. Bars indicate mean ± standard deviation * P < 0.05 versus the control
Taxifolin reduced the tumor volume and body weight of 7,12-dimethylbenz(a) anthracene-induced mammary cancer
As the administration of DMBA in SD rats starts, it consistently and gradually produced a tumor. The tumors become measurably more or less 2 weeks after induction. Consequently, the size of the tumor doubled weekly, it is represented in Figure 2a. Tumor promotional phase is a reversible phase in the multistage carcinogenesis so that it is one of the most appropriate phases to slow down or reverse the carcinogenesis process. There was a significant tumor growth phase in the Group II BC-induced rats up to 90 days of tumor induction when it was compared with normal control groups. In the TAX-treated rats (Group III, IV, and V), the size of the tumors gradually declined in size as the doses of the TAX increase when it was compared with Group II cancer-induced group. However, there was a significant (P < 0.01) regression of tumor volume in all the treated groups, as shown in Figure 2b and c. However, analysis of tumor growth has revealed that TAX-treated rats significantly inhibited the tumor size in DMBA-induced BC.
The alteration in body weight of control, DMBA, and TAX-treated groups during the study period was represented in Figure 2d. The control (Group I) animals showed an increase in body weight during (90 + 28) 118 days of study, whereas cancer-induced untreated animals showed a decrease in body weight gradually and a significant (P < 0.001) decrease was recorded as compared to control animals (Group I). When treated with TAX (Group III, IV, and V), animals revealed an increase in body weight as compared to Group II animals in a dose-dependent fashion.
Taxifolin changed the mRNA expressions of AhR, CYP1A1, and CYP1B1 in cancer-bearing Sprague-Dawley rats
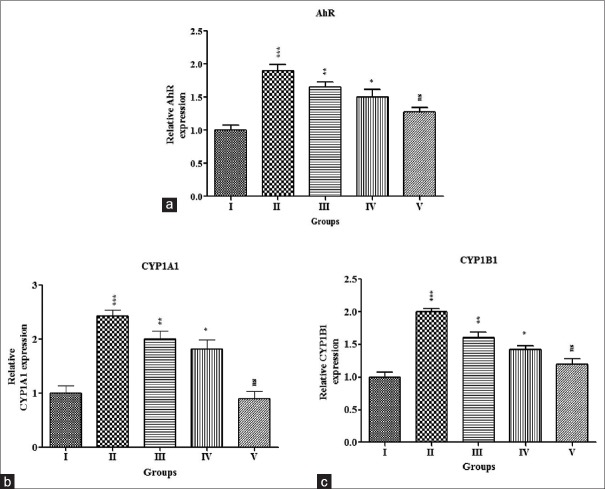
We examined the effectiveness of TAX on AhR, CYP1A1, and CYP1B1 mRNA expressions in mammary tumor cells, which were assessed through RT-qPCR analysis and were represented in Figure 3a–c, respectively. There was a significant (P < 0.001) increase in the AhR and CYPs-mRNA expressions in the cancer-inducing animals (Group-II) as compared with normal control (Group I). The mRNA expressions were reduced significantly in the treatment group of animals (Group III, IV, and V) as compared to induced control animals, i.e., TAX efficiently restored the changes toward normal in a dose-dependent manner.
Figure 3.
Effect of TAX on mRNA expressions of AhR, CYP1A1, and CYP1B1 in mammary carcinogenesis animals. RNA was extracted from breast tissue and the analysis of reverse transcription-quantitative polymerase chain reaction was carried out with specific primer sequences for AhR (a), CYP1A1 (b), CYP1B1 (c), and GAPDH (control). Relative expressions were expressed as fold induction above control after normalization; each column represents the mean standard error of the mean (n = 3), ***P < 0.001; *P < 0.05 when compared with control. Group I - Control; Group II - DMBA (20 mg); Group III - DMBA (20 mg) + TAX (10 mg/kg b. w.); Group IV – DMBA (20 mg) + TAX (20 mg/kg b. w.); Group V - DMBA (20 mg) + TAX (40 mg/kg b. w.). TAX: Taxifolin, DMBA: 7,12-dimethylbenz(a) anthracene
Taxifolin altered the protein expressions of AhR, CYP1A1, and CYP1B1 in carcinogenic tissue
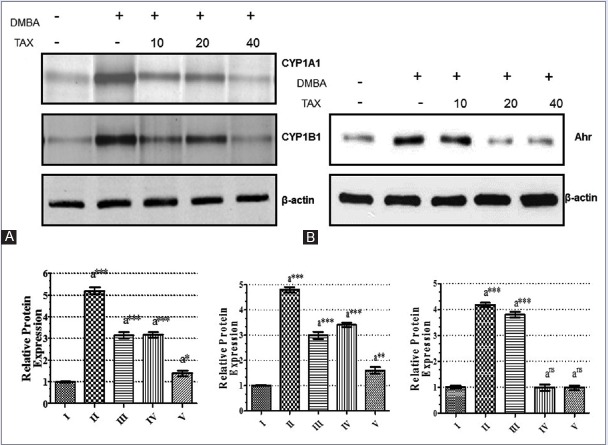
To examine the changes of protein expressions of CYP1A1 and CYP1B1 with its liable protein AhR, Western blot analysis was carried out in the samples of mammary tissue of all groups of animals. The results were depicted in Figure 4a and b. A significant (P < 0.001) elevation in the expression of AhR protein with simultaneously upregulation of CYP1A1 and CYP1B1 expressions which were observed in the DMBA-induced tumor animals (Group II) as compared with normal control (Group I) animals. The expressions of AhR protein were downregulated considerably in all treated groups of animals (Group III to V) in a dose-dependent manner when it was compared with cancer-induced (Group II) animals.
Figure 4.
Effect of TAX on protein expression of AhR, CYP1A1, and CYP1B1. Western blot analysis of AhR (B), CYP1A1 and CYP1B1 (A) expression in cytoplasmic fraction of the DMBA-induced mammary cell extracts with or without TAX; the relative phosphorylation levels normalized with the ß-actin and converted to fold induction above the control value as shown in adjacent bar diagrams. Each column represents the mean standard error of the mean (n = 3); ***P < 0.001; **P < 0.01; *P < 0.05 as compared to control. Group I - Control; Group II - DMBA (20 mg); Group III - DMBA (20 mg) + TAX (10 mg/kg b. w.); Group IV - DMBA (20 mg) + TAX (20 mg/kg b. w.); Group V - DMBA (20 mg) + TAX (40 mg/kg b. w.). TAX: Taxifolin, DMBA: 7,12-dimethylbenz(a)anthracene
DISCUSSION
The several hormone-mediated cancers such as breast, prostate, endometrial, and ovarian cancers have prominent prevalence rates worldwide. BC is one of the most deadly disorders among women worldwide. Studies reported that several factors are responsible for this disease such as environmental toxic xenobiotics, endogenous and exogenous estrogen-induced expression of CYP1A1, CYP1B1, and CYP1A2 enzymes. DMBA is reported to be as procarcinogen and required its metabolic conversion into carcinogen metabolite (DMBA-3, 4-dihydrodiol-1, 2-epoxide) through CYP1A1 or CYP1B1.[29,30] DMBA may stimulate these CYPs by AhR-mediated transcriptional activation into its reactive metabolites. Our findings revealed that TAX inhibited the induction of CYP1A1 activity through luciferase activity in vitro. Similar CYP inhibition was reported earlier, with other flavonoids such as curcumin[31] and eugenol.[32]
After the introduction of carcinogenesis, cancer cells gradually divide and grow in number resulting in massive tumor burden. Rapid growing neoplastic cells modify different metabolic pathways which were mainly involved in cytochrome p450 enzyme and other energy regulation to fulfill the basic requirements for proliferation. Among, the various experimental animal models used for examining the various aspects of breast carcinogenesis and chemical carcinogen, i.e., DMBA-induced BC in SD rats is one of the important models mainly utilized, which closely resembles with human BC.[33]
In carcinogenesis, abnormality in various intermediate pathways brings molecular changes, activation and overexpression of several enzymes, and proteins involved in CYPs synthesis. Recently, inhibition of CYPs in tumor cells has gained a research interest. DMBA can activate the CYPs by AhR-mediated transcription activation into respective reactive metabolites responsible for carcinogenesis.
In this study, we evaluated the inhibitory effect of TAX on AhR and tried to correlate the antitumor or chemotherapeutic potential for TAX with the regulation CYPs in DMBA-induced BC in SD rats. It was verified from our docking studies that TAX can efficiently bind to AhR receptor on the same pocket as that of tamoxifen, which might predict its inhibitory activity on AhR.
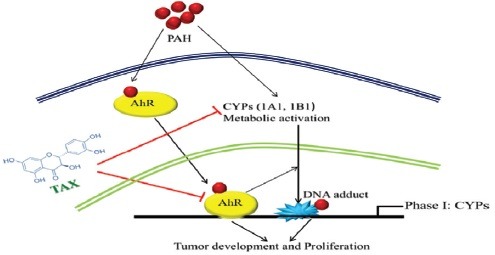
The docking results showed a high chance of TAX to inhibit the AhR receptor and CYPs, which were further justified on the basis of in vitro inhibition, in vivo mRNA, and protein expressions of CYPs and Western blot analysis of AhR, respectively. Thus, we proposed to find the efficiency of TAX on CYPs in an in vivo BC model. Furthermore, it was shown from the results that TAX-induced inhibition of CYPs in BC inducing animals slows down the cancer progression by downregulating the expression of AhR.
AhR transferred to the nucleus on ligand binding followed with dimerization with the AhR nuclear translocator and subsequent binding to the DNA at the xenobiotic responsive cascades to inhibit AhR transcription target genes.[34] Moreover, quercetin and curcumin as natural polyphenol macromolecules exhibited the similar interaction with AhR[35] as that of TAX. The influence of TAX on the AhR pathway has not been well established at molecular level comprehensively. The study revealed that TAX mediated inhibition of AhR, CYP1A1, and CYP1B1 expressions in BC tissue.
The previous study revealed that treatment with the AhR-ligand, the carcinogen (DMBA) enhanced CYP1A1, CYP1B1, and also the levels of protein in BC cells. CYP1A1 and CYP1B1 enzymes were extremely expressed in mammary cancer.[7] Our findings also revealed that treatment of TAX remarkably suppressed the CYP1A1 and CYP1B1expression in mammary tumors. Hence, the reduced expression of CYP1A1 and CYP1B1 by TAX can also play an important role to prevent breast tumor initiation and development. Due to unusual AhR activation either through endogenous or exogenous stimulation, it can disturb the different biological and pathological processes such as metabolism, differentiation, proliferation, apoptosis, and angiogenesis, and these can cause the initiation and progression of cancer.[11] Due to the toxic exposure, PAH is involved in different types of cancer in the humans as well as animals and it was mediated mainly by AhR.[11] Our study revealed that TAX has the ability to downregulate the expression of AhR and so as to suppress the CYP1A1 and CYP1B1 mRNA expressions. Conclusively, AhR played a vital role in the regulation of CYPs. Our results also indicated that TAX can bring substantial inhibition on AhR protein expressions which are a prerequisite for CYP expressions.
CONCLUSION
This study reports that the chemotherapeutic potential of TAX, a flavonoid, strongly suppresses inducible CYP1A1 and CYP1B1 expressions. The reduced expression of these enzymes by TAX was mediated by the downregulation of AhR expressions of DMBA-induced mammary gland carcinogenesis in SD rats. In the pursuit of positive contributions toward safe and less toxic chemotherapy, this study focuses primarily on the chemotherapeutic aspects of TAX in treating BC. The secondary purpose of this study is to establish and show through experiment that TAX has a definite potential to act as a chemopreventive agent and may be used against CYP1A1- and CYP1B1-mediated progression of invasive BC.
Financial support and sponsorship
We are grateful to the University Grant Commission (Basic Scientific Research), Government of India for giving financial support of this work [UGC Grant number: No. F.7-36/2007(BSR)].
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to thank the Birla Institute of Technology for providing the necessary facilities for this work.
REFERENCES
- 1.Nowsheen S, Wukovich RL, Aziz K, Kalogerinis PT, Richardson CC, Panayiotidis MI, et al. Accumulation of oxidatively induced clustered DNA lesions in human tumor tissues. Mutat Res. 2009;674:131–6. doi: 10.1016/j.mrgentox.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wattenberg LW. An overview of chemoprevention: Current status and future prospects. Proc Soc Exp Biol Med. 1997;216:133–41. doi: 10.3181/00379727-216-44163. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz D, Roots I. In vitro assessment of inhibition by natural polyphenols of metabolic activation of procarcinogens by human CYP1A1. Biochem Biophys Res Commun. 2003;303:902–7. doi: 10.1016/s0006-291x(03)00435-2. [DOI] [PubMed] [Google Scholar]
- 4.Wang JF, Zhang CC, Chou KC, Wei DQ. Structure of cytochrome p450s and personalized drug. Curr Med Chem. 2009;16:232–44. doi: 10.2174/092986709787002727. [DOI] [PubMed] [Google Scholar]
- 5.Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Androutsopoulos VP, Papakyriakou A, Vourloumis D, Spandidos DA. Comparative CYP1A1 and CYP1B1 substrate and inhibitor profile of dietary flavonoids. Bioorg Med Chem. 2011;19:2842–9. doi: 10.1016/j.bmc.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Vinothini G, Nagini S. Correlation of xenobiotic-metabolizing enzymes, oxidative stress and NFkappaB signaling with histological grade and menopausal status in patients with adenocarcinoma of the breast. Clin Chim Acta. 2010;411:368–74. doi: 10.1016/j.cca.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez M, Potter DA. CYP1A1 regulates breast cancer proliferation and survival. Mol Cancer Res. 2013;11:780–92. doi: 10.1158/1541-7786.MCR-12-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–40. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 10.Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Cytochrome P450 CYP1A1: Wider roles in cancer progression and prevention. BMC Cancer. 2009;9:187. doi: 10.1186/1471-2407-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng S, Cao Z, Wang X. Role of aryl hydrocarbon receptor in cancer. Biochim Biophys Acta. 2013;1836:197–210. doi: 10.1016/j.bbcan.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Gramatzki D, Pantazis G, Schittenhelm J, Tabatabai G, Köhle C, Wick W, et al. Aryl hydrocarbon receptor inhibition downregulates the TGF-beta/Smad pathway in human glioblastoma cells. Oncogene. 2009;28:2593–605. doi: 10.1038/onc.2009.104. [DOI] [PubMed] [Google Scholar]
- 13.Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30:447–54. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Lindén J, Lensu S, Tuomisto J, Pohjanvirta R. Dioxins, the aryl hydrocarbon receptor and the central regulation of energy balance. Front Neuroendocrinol. 2010;31:452–78. doi: 10.1016/j.yfrne.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 15.McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor sans xenobiotics: Endogenous function in genetic model systems. Mol Pharmacol. 2007;72:487–98. doi: 10.1124/mol.107.037259. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eltom SE, Gasmelseed AA, Saoudi-Guentri D. The aryl hydrocarbon receptor is overexpressed and constitutively activated in advanced breast carcinoma: AACR. 2006:47. [Google Scholar]
- 18.Yu EA, Kim GS, Jeong SW, Park S, Lee SJ, Kim JH, et al. Flavonoid profile and biological activity of Korean citrus varieties (II): Pyunkyul (Citrus tangerina Hort.ex Tanaka) and overall contribution of its flavonoids to antioxidant effect. Journal of Functional Foods. 2014;6:637–42. [Google Scholar]
- 19.Tsuchiya H, Iinuma M. Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua. Phytomedicine. 2000;7:161–5. doi: 10.1016/S0944-7113(00)80089-6. [DOI] [PubMed] [Google Scholar]
- 20.Li C, Huo C, Zhang M, Shi Q. Chemistry of Chinese yew, Taxus chinensis var. mairei. Biochem Syst Ecol. 2008;36:266–82. [Google Scholar]
- 21.Willför S, Ali M, Karonen M, Reunanen M, Arfan M, Harlamow R. Extractives in bark of different conifer species growing in Pakistan. Holzforschung. 2009;63:551–8. [Google Scholar]
- 22.Sun X, Chen RC, Yang ZH, Sun GB, Wang M, Ma XJ, et al. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem Toxicol. 2014;63:221–32. doi: 10.1016/j.fct.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Gupta MB, Bhalla TN, Gupta GP, Mitra CR, Bhargava KP. Anti-inflammatory activity of taxifolin. Jpn J Pharmacol. 1971;21:377–82. doi: 10.1254/jjp.21.377. [DOI] [PubMed] [Google Scholar]
- 24.Manigandan K, Jayaraj RL, Elangovan N. Taxifolin ameliorates 1, 2-dimethylhydrazine induced cell proliferation and redox avulsions in mice colon carcinogenesis. Biomed Prev Nutr. 2014;4:499–509. [Google Scholar]
- 25.Alshatwi AA. Catechin hydrate suppresses MCF-7 proliferation through TP53/Caspase-mediated apoptosis. J Exp Clin Cancer Res. 2010;29:167. doi: 10.1186/1756-9966-29-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Hazzani AA, Alshatwi AA. Catechin hydrate inhibits proliferation and mediates apoptosis of SiHa human cervical cancer cells. Food Chem Toxicol. 2011;49:3281–6. doi: 10.1016/j.fct.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Arun B, Udayachander M, Meenakshi A. 12-dimethylbenzanthracene induced mammary tumours in wistar rats by ‘air pouch’ technique – A new approach. Cancer Lett. 1984;25:187–94. doi: 10.1016/s0304-3835(84)80044-0. [DOI] [PubMed] [Google Scholar]
- 28.Pattanayak SP, Mazumder PM. Histopathological approach to rat liver tissue, Animal techniques, Animal Histology and Pathology, Lab's reference book. 2009. Protocol Online (http://www.protocol-online.org .
- 29.Christou M, Moore CJ, Gould MN, Jefcoate CR. Induction of mammary cytochromes P-450: An essential first step in the metabolism of 7,12-dimethylbenz[a]anthracene by rat mammary epithelial cells. Carcinogenesis. 1987;8:73–80. doi: 10.1093/carcin/8.1.73. [DOI] [PubMed] [Google Scholar]
- 30.Kleiner HE, Vulimiri SV, Reed MJ, Uberecken A, DiGiovanni J. Role of cytochrome P450 1a1 and 1b1 in the metabolic activation of 7,12-dimethylbenz[a]anthracene and the effects of naturally occurring furanocoumarins on skin tumor initiation. Chem Res Toxicol. 2002;15:226–35. doi: 10.1021/tx010151v. [DOI] [PubMed] [Google Scholar]
- 31.MacDonald CJ, Ciolino HP, Yeh GC. Dibenzoylmethane modulates aryl hydrocarbon receptor function and expression of cytochromes P50 1A1, 1A2, and 1B1. Cancer Res. 2001;61:3919–24. [PubMed] [Google Scholar]
- 32.Han EH, Hwang YP, Jeong TC, Lee SS, Shin JG, Jeong HG, et al. Eugenol inhibit 7,12-dimethylbenz[a]anthracene-induced genotoxicity in MCF-7 cells: Bifunctional effects on CYP1 and NAD (P) H: quinone oxidoreductase. FEBS Lett. 2007;581:749–56. doi: 10.1016/j.febslet.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 33.Costa I, Solanas M, Escrich E. Histopathologic characterization of mammary neoplastic lesions induced with 7,12 dimethylbenz(alpha)anthracene in the rat: A comparative analysis with human breast tumors. Arch Pathol Lab Med. 2002;126:915–27. doi: 10.5858/2002-126-0915-HCOMNL. [DOI] [PubMed] [Google Scholar]
- 34.Delescluse C, Lemaire G, de Sousa G, Rahmani R. Is CYP1A1 induction always related to AHR signaling pathway? Toxicology. 2000;153:73–82. doi: 10.1016/s0300-483x(00)00305-x. [DOI] [PubMed] [Google Scholar]
- 35.Ciolino HP, Daschner PJ, Wang TT, Yeh GC. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Biochem Pharmacol. 1998;56:197–206. doi: 10.1016/s0006-2952(98)00143-9. [DOI] [PubMed] [Google Scholar]