Abstract
Background:
Meyna spinosa (M.S) (Roxb.) ex Link and Oroxylum indicum (O.I) (Linn.) Vent, widely used traditional Northeast Indian medicinal plant used for various purposes, have not yet explored for safety profile.
Objective:
To investigate the safety profile of M.S (Roxb.) ex Link leaves and O.I (Linn.) Vent stem bark extracts collected from Northeast region of India.
Materials and Methods:
In this study, mutagenic, cytotoxic, and genotoxic and/or nontoxic potential of these two plant extracts using various toxicological investigations, as per the regulatory test guidelines, were evaluated. The mutagenic, cytotoxic, and genotoxic potential of these two plants were assayed using Ames test, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, comet assay, and micronucleus test in the bone marrow cells.
Results:
The results demonstrated that the tested doses of M.S (Roxb.) ex Link leaves extract showed mutagenic, cytotoxic, and genotoxic effects, whereas O.I (Linn.) Vent stem bark extracts showed nonmutagenic, noncytotoxic, and nongenotoxic effects.
Conclusion:
The stem bark extracts of O.I (Linn.) Vent has no mutagenic, cytotoxic, and genotoxic or clastogenic effects in our experimental conditions. However, M.S (Roxb.) ex Link leaves extract caused a significant increase in DNA damage as compared with the positive control, i.e., cyclophosphamide. Thus, the present study revealed that M.S (Roxb.) ex Link leaves extract is toxic, while O.I (Linn.) Vent stem bark extract was found to be safe.
SUMMARY
For the first time, we reported the safety performance of these two plants.
The absence of toxicity in Oroxylum indicum (O.I) plant extracts was observed at various doses in animals.
Interestingly, our result indicated that Meyna spinosa (M.S) extract shows toxicological effect.
Therefore, O.I plant extracts was considered as safer plant extract as compared to M.S.
Abbreviations used: MS: Meyna spinosa; OI: Oroxylum indicum.
Keywords: Cytotoxicity, genotoxicity, Meyna spinosa, mutagenicity, Oroxylum indicum
INTRODUCTION
Oroxylum indicum (O.I) (Linn.) Vent, belonging to the family Bignoniaceae, is a medium-sized deciduous evergreen tree up to 12 m high with light grayish brown, soft, spongy bark; large pinnate, bipinnate or tripinnate, ovate or elliptic leaves; lurid, purple, fleshy, flowers, and large, flat, sword-shaped capsules full of many flat and papery thin seeds with broad silvery wings.[1] It is distributed throughout the areas of Bangladesh, India, Malacca, Sri Lanka, Malay Islands, and China.[2] The plant is known as Sona, Sonpatti, Shoyanka, Sonpatha Kanak, or Midnight Horror in native language, which has been reported for its medicinal properties. Phytochemical studies reported that O.I bark contains large amount of flavonoids, alkaloids, glycosides, sitosterol, p-coumaric acid, and naphthalene compounds.[3] Reports indicate the plant being used as an important constituent of several Ayurvedic and tribal medicines.[4] Traditionally, the plant is used as diaphoretic, astringent, carminative, diuretic, stomachic, aphrodisiac and has high potential for stimulating digestion, curing fevers, coughs, and other respiratory disorders.[5] The plant is also reported to possess anti-inflammatory, antibacterial, antiarthritic, antifungal, antiulcer, antioxidant, diuretic, hepatoprotective, and immunomodulatory activities.[4,5,6,7,8] The medicinal uses of O.I and the components of the tree which encourage these uses are impressively set out in the introduction to genetic diversity in O.I.[9] The antimicrobial activity of the petroleum ether, ethyl acetate, and methanol extracts has been reported.[10] Numerous Ayurvedic formulations with this plant are being used as antihelminthic, antibronchitic, antirheumatic, antianorexic and for treatment of leprosy and tuberculosis.[11]
Meyna spinosa (M.S) (Roxb.) ex Link, Rubiaceae, is a spiny, usually a shrub or armed small tree, which can grow up to 8 m. Branches are busy, and spines are axillary, straight, sharp, 5–40 mm. Leaves are membranous, ovate or elliptic-oblong, while flowers crowded into fascicales and have shorter pedicels and petioles. Fruits are yellowish, subglobose drupe, smooth with persistent calyx lobes. The plant is used in traditional folk medicines and widely distributed in the Northeastern, Eastern, and Southern parts of India.[12] Fruits and the bark of the plant are used to treat headache,[13] while the fruits and leaves are beneficial in diabetes, jaundice, and other gastrointestinal disorders.[14,15] Tender leaves, ripe fruits, and seeds are useful to cure skin infections and pimples,[15,16,17] the leaf is also prescribed in indigestion and to treat dyspepsia,[18] Fruits are a good source of nutrient and are used to cure cough and as a refrigerant traditionally.[13,18] The plant is also important for its abortifacient activity; seeds and fruits are used by several ethnic groups in India to induce abortion.[19] Recently, two compounds were isolated from the fruits of M.S which possess antimicrobial activity against Bacillus subtilis, Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus and Candida. One was identified as oleanolic acid which possesses the highest antimicrobial activity. Buragohain and Goswami et al.[16,20] have also reported the antifungal activity of the methanol extract of M.S. Thus, the interest in herbal medicines used for the prevention and therapeutic treatment of diseases has increased due to the increasing resistance of microorganisms to synthetic antibiotics, raising costs, and side effects of synthetic drugs for the maintenance of personal health. Similarly, natural products have been traditionally used as conventional remedies due to popular belief that they possess negligible side effects.[21] However, several natural compounds have been reported to act as mutagens and/or carcinogens.[22,23,24] These genotoxic insults often lead to cancer,[25] Alzheimer's disease,[26] and other chronic degenerative diseases, such as atherosclerosis and other cardiovascular diseases, which are the leading cause of death in human and animal populations.[27] As such, genotoxicity studies of both naturally occurring and synthetic substances are of great interest because of the widespread and often chronic use of specific or fictitious herbal remedies, modern medicinal products, and food ingredients, as well as other household and environmental chemicals.[28] Moreover, governmental regulatory policies worldwide are now making it vital that all newly produced natural or synthetic substances, with or without antitumor properties, be subjected to genotoxic/mutagenic screening.[29]
Hence, recent concerns have been raised over the lack of quality control and scientific evidence for the safety of herbal medicine.[30] However, finger count scientific investigations have explored the safety and toxicity of herbal medicines.[31,32,33] However, to the best of our knowledge, no investigations have been performed till date based on these two plants in terms of their safety and toxic profile. Considering the applauded medicinal significance of these two plants and the need for practical data on safety, we assessed the mutagenicity, cytotoxicity, and genotoxicity studies employing in vitro and in vivo experimental models. Thus, our research objectives directed us toward the initiation of this study for the first time to explore the possible toxic effect of these two plants in accordance with the Organization for Economic Cooperation and Development (OECD) test guidelines.
MATERIALS AND METHODS
Collection of plant materials
The fresh, mature whole leaves of M.S and stem bark of O.I were collected from Tezpur, Assam, during April 2012. The plant was identified by its vernacular name and later validated by Prof. (Dr.) S. K. Borthakur, Department of Botany, Gauhati University, Guwahati, Assam, India. The identified voucher specimens of O.I (GUBH 3964) and M.S (GUBH 3965) were deposited at the Gauhati University Botany Herbarium, Department of Botany, Gauhati University, Guwahati, Assam, India, for future reference.
Preparation of the extracts
The plant parts were washed thoroughly under running water, cut into smaller pieces, and then air-dried. The air-dried parts were grinded with a mechanical grinder into coarse powder. The powdered materials were extracted with methanol using a Soxhlet apparatus for solvent extraction, which was concentrated to dryness under reduced pressure to yield hydroalcoholic mixture (50:50% v/v). The mixtures were then filtered by filter paper. The extracts were then kept in Petri dish under water bath at a temperature of 50°C –55°C until evaporated to dryness. The dried extracts, herein referred to as M.S and O.I extracts, were then collected into a glass container and stored in a desicator.
Mutagenicity
Ames test
All the plant extracts were examined for its mutagenic potency in four histidine-requiring Salmonella typhimurium-mutant strains. S. typhimurium strains were obtained from Institute of Microbial Technology, Chandigarh, India. The strains used were TA98 and TA1538 which detect frameshift mutations, TA100 and TA1535 which detect basepair substitutions.[22,34] This assay was performed according to the plate incorporation procedure described by the OECD test guideline 471 recommendations.[35,36] Tester bacteria were exposed to four different concentrations ranging from 2.5 up to 5 μg/plate, with and without metabolic activation. Three parallel plates were tested in each concentration. Negative and positive controls were run simultaneously with the test.
Animals and dosing
Healthy, adult Balb/c albino mice (weighing 20–25 g, 5–6 week age, female) were obtained from Central Animal Resources, Defence Research Laboratory, Tezpur, Assam, India. The animals were placed in polypropylene cages, with free access to standard laboratory diet (Pranav Agro Industries Limited, Maharashtra, India) and provided water ad libitum. Animals were housed in an environmentally controlled room with temperature of 22°C ± 3°C and 40%–70% relative humidity with a 12-h light/dark cycle for an acclimation period of 7 days to laboratory conditions before the beginning of the experiment. All the experimental procedures described were performed according to the “Principles of Laboratory Animal Care” and approved by the Institutional Animal's Ethical Committee. The Balb/c albino mice were divided into four groups, i.e., control (C), positive control (cyclophosphamide [CP]; 50 mg/kg bw), M.S-treated group (400, 800 and 1600 mg/kg) and O.I-treated group (500, 1000, and 2000 mg/kg) by gavage for 14 days. All the mice were then sacrificed after the treatments by cervical dislocation and the cells were prepared for further analysis.
Cytotoxicity study
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
The effect of M.S and O.I on hepatocyte primary cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.[37] The hepatocytes were seeded in 96-well plates. Each treated cells was added to the medium. A vehicle control was included in which cells were incubated only with solvent (0.1 M NaHCO3). After 12 h treatment, cells were washed with PBS and a fixed concentration of MTT in PBS was added to each microwell. After incubation for 4 h at 37°C, the supernatant was removed, the insoluble crystals were dissolved in dimethyl sulfoxide (DMSO), and absorbance was measured at 570 nm by using a Microplate Reader (SpectraMax Plus384; Molecular Devices, Sunnyvale, CA, USA).
Measurement of apoptosis by flow cytometer
Cell apoptosis was evaluated by a flow cytometer (Guava easyCyte 6HT; Merck Millipore, Darmstadt, Germany). The cells from each treated groups were centrifuged at 50 ×g at 4°C for 5 min, the supernatant was discarded, and the pellets were washed with 1× phosphate-buffered saline (PBS) three times and finally resuspended in 50 μl of 1× PBS. From this suspension, cells per ml were mixed with the Guava Nexin reagent (Merck Millipore) following the manufacturer's instruction. The cells were analyzed using Guava software version 2.2.[38]
Genotoxicity study
Comet assay
The comet assay was performed for in vivo genotoxicity evaluations.[39] The blood samples were collected from the retro-orbital plexus after treatment and before euthanasia and treated with 1× red blood cell lysis buffer for 10 min at 25°C and leukocytes were isolated and suspended in 50 μl of PBS (pH 7.5). Cells were mixed with 100 mL of 0.5% low melting point agarose at 37°C and rapidly spread onto microscope slides, precoated with 1% normal melting point agarose. The slides were coverslipped and allowed to polymerize at 4°C for 20 min. The coverslips were gently removed and the slides were then immersed in cold, freshly prepared lysing solution for consisting of 44.5 mL of a stock solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, pH set to 10.0 with ~8 g solid NaOH, 890 mL of distilled water, and 1% sodium lauryl sarcosine), plus 0.5 mL of Triton X-100 and 5.0 mL of DMSO. The slides were allowed to stand at 4°C for 1 h and then placed in a high pH (>13) electrophorosis buffer (300 mM NaOH, 1 mM Na2-EDTA, pH 13.0) at 4°C for 20 min before electrophoresis, to allow DNA unwinding. The electrophoresis run was performed at 4°C under dim light at 300 mA and 25 V for 30 min. The slides were then submerged in a neutralization buffer (0.4 M Tris–HCl, pH 7.5) for 15 min and stained with 20 μg/mL 4’,6-diamidino-2-phenylindole for 10 min and cover slipped. The material was evaluated immediately at a fixed magnification, using a fluorescence microscope (Coslab HL-23; Cos Lab, Haryana, India). A total of 100 nonoverlapping comets per sample on each randomly coded slide were scored using TriTek Comet Score™ (Sumerduck, VA, USA). The cells were classified into four different comet score classes: class 0 - undamaged cells; Class I - tail shorter than the diameter of the head (nucleus); Class II - tail length 1–2 times the diameter of the head; and Class III - tail length more than twice the diameter of the head. The cells were blindly scored using light microscope at a higher magnification.
Micronucleus assay
The micronucleus (MN) test was conducted in accordance with the OECD guideline 474[35] and the protocols were followed as recommended.[40,41] The bone marrow from one femur was flushed out using 2 mL of saline (0.9% NaCl) and centrifuged for 10 min. The supernatant was discarded and smears were made on slides. The slides were coded for a blind analysis, fixed with methanol, and stained with 5% Giemsa[42] For the analysis of the micronucleated polychromatic erythrocytes (MNPCE) per treatment group observed in bone marrow cells of mice were scored to determine the clastogenic property of the extract. The cells were blindly scored using a light microscope at higher magnification. The mean number of MNPCE in individual mice was used as the experimental unit, with variability (standard deviation) based on differences within the same group.[43,44]
Statistical analysis
Data were expressed as mean ± standard error of the mean. The data obtained from the comet assay were subjected to analysis of variance (ANOVA) and analyzed by Tukey's Test (significant level at P < 0.05). For genotoxicity study, the distribution of the comet figures did not follow a Gaussian distribution. The data obtained from the MN assay were submitted to the ANOVA test with linear regression, both using the GraphPad InStat software (version 3.01, California corporation USA). The results were considered statistically significant at P < 0.05.
RESULTS
Mutagenicity
Ames test
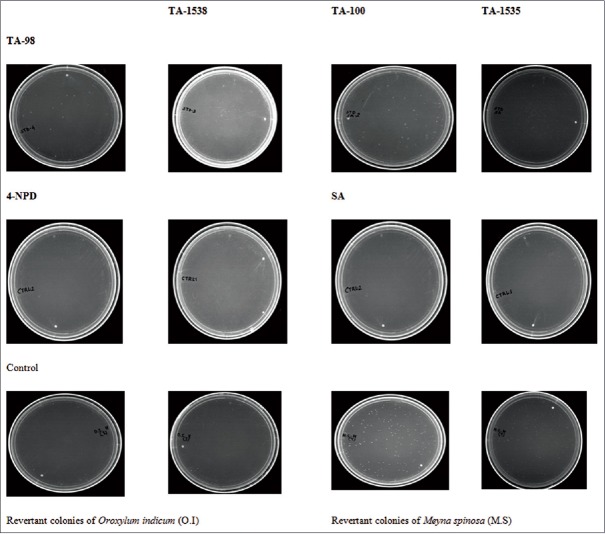
A serious concentration-dependent increase in M.S and no increase in the number of revertant colonies occurred in the four test strains (TA-98, 100, 1538, and 1535) at any concentrations of O.I, either in the presence or absence of S9 mixture [Table 1], and similar findings were obtained from the spot test results [Figure 1]. No obvious concentration-depended relationship had been found only in the case of O.I extract; therefore, the Ames test result of O.I extract was negative. However, for M.S extract, the test result was shown to be positive.
Table 1.
Ames test results of Oroxylum indicum and Meyna spinosa extracts in four strains of Salmonella typhimurium
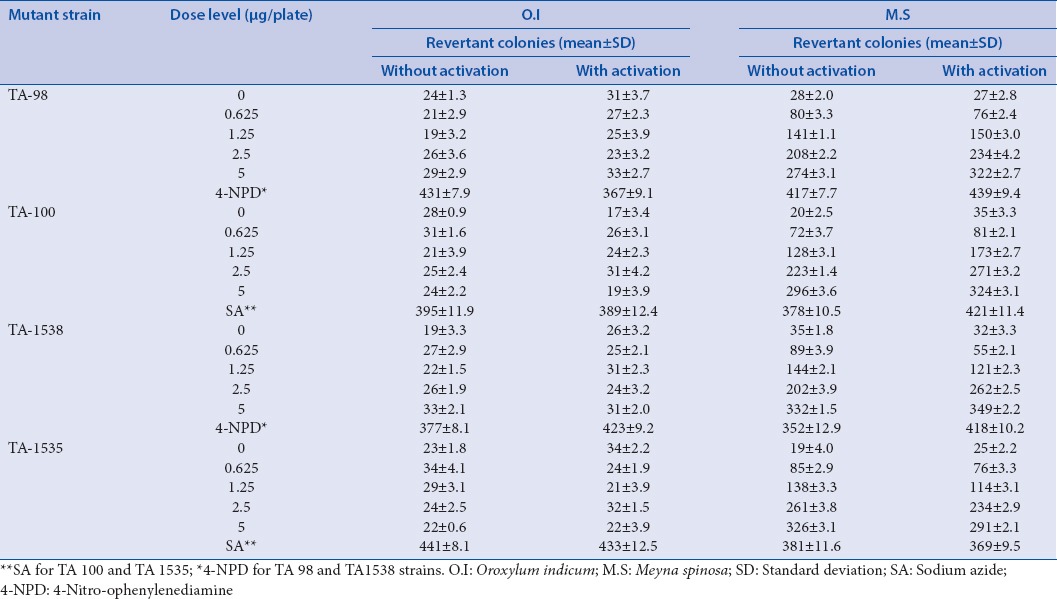
Figure 1.
Spot test with different Salmonella typhimurium strain
Cytotoxicity study
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
MTT assay was performed to determine cell viability by measuring the conversion of tetrazolium salts to formazan, the amount of which is proportional to the number of living cells [Figure 2]. The viability of cells was not affected by the control, vehicle, and O.I-treated group (>90%). However, a significant decreased on cell viability was observed in M.S-treated group in a dose-dependent manner (<65%; P < 0.05).
Figure 2.
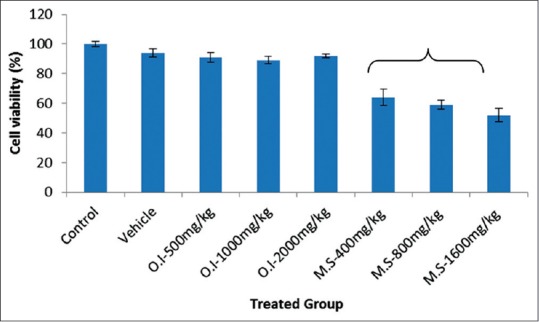
Results of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay on percentage cell viability. Data are expressed as mean ± standard error of the mean (n = 6). Asterisk (*) indicates statistical significance (P < 0.05)
Measurement of apoptosis by flow cytometer
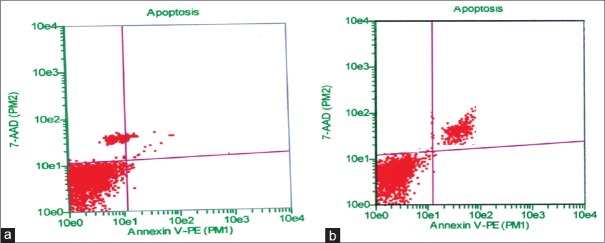
Flow cytometric analysis of hepatocyte cells using Guava Nexin reagent (stained with Annexin V-PE and 7-AAD) indicates M.S at a concentration of 400 mg/kg body weight induced cellular apoptotic events in hepatocyte primary cell culture. The results indicated that control, vehicle-treated, and O.I at a concentration of 2000 mg/kg body weight treated cells contained approximately 94%, 89%, and 85% viable cells, 4%, 6%, and 8% early apoptosis, 2%, 3%, and 4% late apoptosis, and 0%, 2%, and 3% necrosis, respectively. On the other hand, M.S treatment at different concentrations resulted in approximately 51%–61% viable cells, 13%–16% early apoptosis, 14%–19% late apoptosis, and 9%–17% necrotic cells [Figure 3]. These findings clearly indicated that cell viability is decreased and apoptotic events (both early and late stages) are increased with M.S-treated group in a concentration-dependent manner which ultimately revealed the cytotoxic nature of M.S at concentration of 400 mg/kg body weight and noncytotoxic nature of O.I extracts at concentration of 2000 mg/kg body weight [Figure 4].
Figure 3.
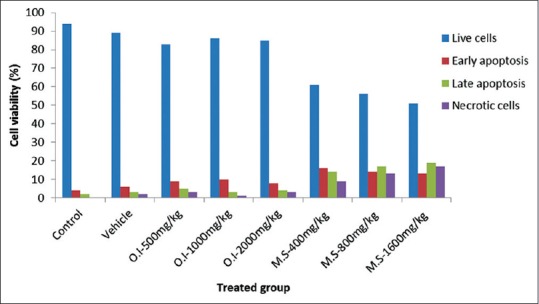
Flowcytometric evaluation of Oroxylum indicum and Meyna spinosa extracts at different concentrations in apoptotic events on cell viability (%) by primary cell culture using Guava Nexin assay kit
Figure 4.
Flowcytometric analysis of apoptosis in blood cells by staining with Annexin V-PE (PM1) and 7-AAD (PM2): (a) Oroxylum indicum (2000 mg/kg body weight) treated lower left quadrant, viable cells (86.09%), lower right quadrant, early apoptosis (7.93%) and upper right, late apoptosis (3.70%) and (b) Meyna spinosa (400 mg/kg body weight) treated lower left quadrant, viable cells (61.23%), lower right quadrant, early apoptosis (16.11%) and upper right, late apoptosis (13.88%)
Genotoxicity study
Comet assay
The comet assay indicated that DNA damage did not occur in the control, vehicle, and at all concentrations of O.I-treated groups. On the other hand, significant DNA damage was observed in M.S-treated group in a concentration-dependent manner in comparison to an untreated control group, as evidenced from the comet assay results and score, i.e., tail length, %DNA in tail, and olive moment [Table 2]. Furthermore, the cells treated with positive control (50 μg/ml, CP) exhibited a higher DNA damage index (P < 0.05) as compared to the others except M.S-treated group [Figure 5].
Table 2.
Comet assay for the assessment of genotoxicity of Oroxylum indicum and Meyna spinosa extracts in hepatocyte primary cell culture
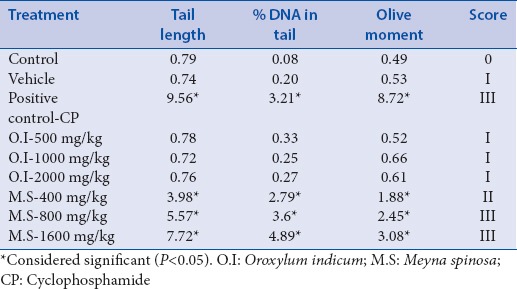
Figure 5.
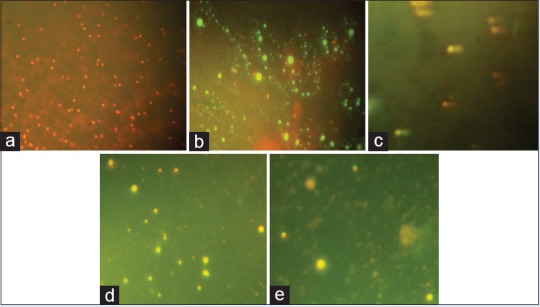
Comet assay images of (a) control, (b) vehicle, (c) positive control (50 μg/ml cyclophosphamide), (d) Oroxylum indicum (2000 mg/kg body weight) treated and (e) Meyna spinosa (400 mg/kg body weight) treatment for the assessment of genotoxicity in hepatocyte primary cell culture (×100)
Micronucleus assay
The MN test is based on the evaluation of an increase in the frequency of polychromatic erythrocytes with micronuclei. In our study, the results of clastogenic and anticlastogenic analysis obtained for the treatment are provided in Table 3. Statistically significant differences (P < 0.05) were exhibited in the frequency of MNPCEs between the control and M.S-treated group, indicating a presence of clastogenic/aneugenic effects induced by plant extracts [Figure 6]. However, animals treated with positive control (CP) showed a high frequency of MNPCE in the bone marrow cells when compared to the control (P < 0.05). However, the frequencies of MNPCE in the MN test in mice were not significantly statistically different between O.I-treated group and control group.
Table 3.
Percent of micronucleated polychromatic erythrocytes observed in bone marrow cells of mice for the assessment of genotoxicity of Oroxylum indicum and Meyna spinosa extracts
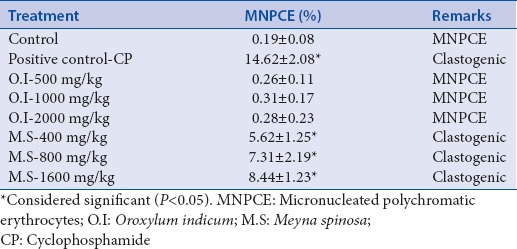
Figure 6.
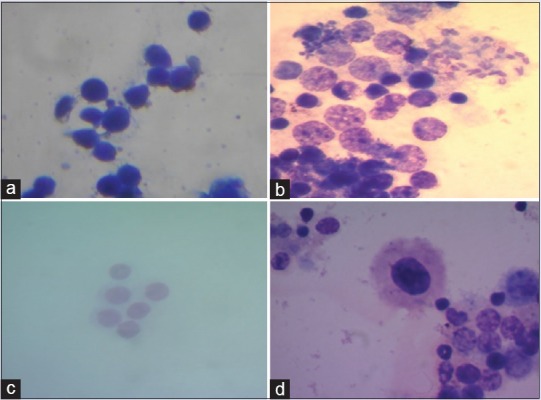
Micronucleus images of (a) control, (b) positive control (50 μg/ml cyclophosphamide), (c) Oroxylum indicum. (2000 mg/kg body weight) treated and (d) Meyna spinosa (400 mg/kg body weight) treatment for the assessment of genotoxicity in bone marrow cells of mice (×100)
In this study, the results showed that O.I-treated group did not induced any toxicological signs, thus reveals its safety nature. However, surprisingly, M.S altered the toxicological profiles and revealed toxic in nature. As expected, the positive controls (CP) showed significant increases in the frequency of necrotic cells when compared with the control.
DISCUSSION
A series of safety studies were performed systematically to investigate the safety of the stem bark extract of O.I and M.S leaves extract in Northeast region. The results of the current study are consistent by using the tester strains TA98, TA100, TA1535, and TA1538 to evaluate the mutagenicity of extracts by the Ames assay. Mutagenicity was induced only by M.S extracts in a concentration-dependent manner with and without metabolic activation in S. typhimurium tester strains. In the present study, tester strains TA98 and TA1538 were used to detect frameshift mutations, whereas tester strains TA100 and TA1535 were used to detect basepair substitution mutations. Different investigators have used different tester strains to determine the mutagenicity of test materials.[45] It is noted that some biomaterials are mutagenic to one tester strain while it is not mutagenic to another. Even though many investigators have sometimes used just two strains to determine the mutagenic potential of materials, it is felt that the use of at least four tester strains as recommended by Mortelmans and Zeiger[46] gives a more definite result.
Cytotoxicity is an important parameter for assessing chemical agents for toxicity and health risks. In genotoxicity testing, cytotoxicity analysis is a prior step because the cytotoxic effect of chemicals may lead to false interpretation of genotoxicity. In the present study, we observed that hepatocytes in the control, vehicle, and O.I-treated groups at a various dose showed no significant cytotoxic effect (>90% viable cells). However, it was also interesting to note that M.S-treated groups showed a potent cytotoxic effect in a concentration-dependent fashion (<65%; P < 0.05) in hepatocytes. Again, the results of flow cytometric analysis of apoptosis correlated with the morphological studies showed more precisely that the frequency of early apoptotic cells reached a peak level. The frequency of late apoptotic cells gradually increased and reached a maximum level after a certain period of M.S treatment at highest dose (1600 mg/kg body weight). We compared the comet assay results (the significantly increased level of DNA single strand breaks) with flow cytometric data (the significantly increased frequency of late apoptotic cells, a possible confounding factor) and observed similar correlation. Hence, it may be expected that M.S-treated group leads to break DNA single strand that are related to genotoxicity and cytotoxicity as well. However, the molecular mechanism behind cellular apoptosis is still not clear and needs to be investigated further.
Since M.S extract reveals cytotoxic which is highly toxic in nature, thus, the aim of the present study was to investigate the DNA damaging properties of M.S. Therefore, the present study evaluated the genotoxic effect of M.S extract (cytotoxic and noncytotoxic) to draw conclusions about its relevance in cellular toxicity using the established comet assay. The comet assay has become one of the standard methods for assessing DNA damage due to its simplicity, sensitivity, versatility, speed, and economy. The alkaline (pH >13) assay detects single-strand breaks, cross-links, incomplete excision repair sites, as well as apurinic or apyrimidinic sites, which are alkali labile.[46] Finally, in our study, no significant increase in the total comet score was detected between the control, vehicle, and O.I-treated group, but M.S elevated the comet score at elevated doses. This might be due to the excessive production of reactive oxygen species, which induce oxidative stress and might lead to lipid peroxidation. Therefore, the present findings suggested an indirect genotoxic effect in which the oxidative stress preceded cytotoxicity and DNA damage, leading to carcinogenicity.
CONCLUSION
Herbal medicine is widely popular as a primary therapeutics or supplements for improving health related problems. However, scientific evidence for the safety of the herbal products has become an important concern and requires regulatory clearance for wide acceptance. Although many studies have been reported in context to the extensive investigations of these two plants properties based on their efficacy evaluations, in molecular level toxicological investigations in terms of mutagenicity, cytotoxicity and genotoxicity and/or antigenotoxicity have not been investigated properly so far. Therefore, for the first time, we evaluated the safety performance of these two plants using standard toxicological tests recommended as per the OECD test guidelines and our test result indicated the absence of toxicity in O.I plant extracts at various doses. Interestingly, our result indicated that M.S extract shows toxicological effect which is completely concentration dependent in comparison to O.I extract. Thus, our research objective motivated us just to drop this plant extract for further efficacy assessment study but directed us toward the exploration of O.I plant extract for efficacy assessment against mycotoxin-induced toxicity in experimental animal model. Moreover, further investigations are also needed to clarify the protective mechanism of O.I extract against mycotoxin-induced toxicity, which may be of great pharmacological importance, and might be beneficial for cancer prevention.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Shweta Singh is grateful to Defence Research Laboratory, Tezpur, Assam, India, for providing necessary instrumental facilities for this research work and as well as to the administration of the Gauhati University, Guwahati, Assam, India, for providing necessary administrative support for carrying out her Ph. D work.
REFERENCES
- 1.Tiwari S, Singh K, Shah P. In vitro propagation of Oroxylum indicum-An endangered medicinal tree. Biotechnology. 2007;6:299–301. [Google Scholar]
- 2.Gokhale M, Bansal YK. Avowal of importance of endangered tree Oroxylum indicum (Linn.) Vent. Nat Prod Rad. 2006;5:112–4. [Google Scholar]
- 3.Ghani A. Medicinal Plants of Bangladesh with Chemical Constituents and Uses. 2nd ed. Asiatic Dhaka: Society of Bangladesh; 2003. pp. 329–30. [Google Scholar]
- 4.Warrier PK, Nambiar VP, Ramankutty C. A Compendium of 500 Species, Indian Medicinal Plants. Madras, India: Orient Longman Ltd; 1995. Oroxylum indicum; pp. 186–90. [Google Scholar]
- 5.John AP. Bignoniaceae, Oroxylum indicum. Wallingford, UK: CABI Publishing; 2001. Healing plants of peninsular India; pp. 169–71. [Google Scholar]
- 6.Hong T, Jin GB, Cho S, Cyong JC. Evaluation of the anti-inflammatory effect of Baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med. 2002;68:268–71. doi: 10.1055/s-2002-23143. [DOI] [PubMed] [Google Scholar]
- 7.Chiang LC, Ng LT, Chiang W, Chang MY, Lin CC. Immunomodulatory activities of flavonoids, monoterpenoids, triterpenoids, iridoid glycosides and Phenolic compounds of Plantago species. Planta Med. 2003;69:600–4. doi: 10.1055/s-2003-41113. [DOI] [PubMed] [Google Scholar]
- 8.Ng TB, Liu F, Wang ZT. Antioxidative activity of natural products from plants. Life Sci. 2000;66:709–23. doi: 10.1016/s0024-3205(99)00642-6. [DOI] [PubMed] [Google Scholar]
- 9.Jayaram K, Prasad MN. Genetic diversity in Oroxylum indicum (L.) Vent. (Bignoniaceae), a vulnerable medicinal plant by random amplified polymorphic DNA marker. Afr J Biotechnol. 2008;7:254–62. [Google Scholar]
- 10.Uddin K, Sayeed A, Islam A, Rahman AA, Khatun S, Khan G, et al. Biological activities of extracts and two flavonoids from Oroxylum indicum Vent. (Bignoniaceae) J Biol Sci. 2003;3:371–5. [Google Scholar]
- 11.Zaveria M, Jain S. Phytopharmacognostical studies on root bark of Oroxylum indicum, Vent. Int J Pharm Sci Rev Res. 2010;4:132–5. [Google Scholar]
- 12.Bora PJ, Kumar Y. Floristic Diversity of Assam: Study of Pabitora Wild Life Sanctuary. Delhi: Daya Publishing House; 2003. pp. 183–4. [Google Scholar]
- 13.Doley B, Gajurel PR, Rethy P, Singh B, Buragohain R, Potsangbam S. Lesser known ethnomedicinal plants used by the Nyshi community of Papumpare District, Arunachal Pradesh. J Bio Sci Res. 2010;1:34–6. [Google Scholar]
- 14.Chakraborty N. Tripurar Bheshaja Udvid (In Bengali) Agartala, India: Gyan Bichitra Prakashani; 2009. pp. 109–10. [Google Scholar]
- 15.Sen S, Chakraborty R, De B, Devanna N. An ethnobotanical survey of medicinal plants used by ethnic people in West and South district of Tripura, India. J Forestry Res. 2011;22:417–26. [Google Scholar]
- 16.Buragohain J. Phytochemical and antimicrobial investigation on the fruits of Meyna spinosa. Res J Biotechnol. 2008;3:372–4. [Google Scholar]
- 17.Das HB, Majumdar K, Dutta BK, Roy D. Ethnobotanical uses of some plants of Tripuri and Reang tribes of Tripura. Nat Prod Rad. 2009;8:172–80. [Google Scholar]
- 18.Nadkarni AK. Indian Materia Medica. Delhi, India: Popular Prakashan; 2005. [Google Scholar]
- 19.Mitra S, Mukherjee SK. Some arbortifacient plants used by the tribal people of West Bengal. Nat Prod Rad. 2009;8:167–71. [Google Scholar]
- 20.Goswami S, Bora L, Das J, Begam M. In vitro evaluation of some medicinal plants against Candida albicans. J Cell Tissue Res. 2006;6:837–9. [Google Scholar]
- 21.Bellini MF, Angeli JP, Matuo R, Terezan AP, Ribeiro LR, Mantovani MS, et al. Antigenotoxicity of Agaricus blazei mushroom organic and aqueous extracts in chromosomal aberration and cytokinesis block micronucleus assays in CHO-K1 and HTC cells. Toxicol In Vitro. 2006;20:355–60. doi: 10.1016/j.tiv.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Ames BN. Dietary carcinogens and anti-carcinogens. J Toxicol Clin Toxicol. 1984;22:291–301. doi: 10.3109/15563658408992561. [DOI] [PubMed] [Google Scholar]
- 23.Ames BN, Profet M, Gold LS. Nature's chemicals and synthetic chemicals: Comparative toxicology. Proc Natl Acad Sci U S A. 1990;87:7782–6. doi: 10.1073/pnas.87.19.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demma J, Engidawork E, Hellman B. Potential genotoxicity of plant extracts used in ethiopian traditional medicine. J Ethnopharmacol. 2009;122:136–42. doi: 10.1016/j.jep.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Akiyama M, Umeki S, Kusunoki Y, Kyoizumi S, Nakamura N, Mori T, et al. Somatic-cell mutations as a possible predictor of cancer risk. Health Phys. 1995;68:643–9. doi: 10.1097/00004032-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Gajdusek DC. The transmissible amyloidoses: Genetical control of spontaneous generation of infectious amyloid proteins by nucleation of configurational change in host precursors: Kuru-CJD-GSS-scrapie-BSE. Eur J Epidemiol. 1991;7:567–77. doi: 10.1007/BF00143141. [DOI] [PubMed] [Google Scholar]
- 27.De Flora S, Izzotti A, Randerath K, Randerath E, Bartsch H, Nair J, et al. DNA adducts and chronic degenerative disease. Pathogenetic relevance and implications in preventive medicine. Mutat Res. 1996;366:197–238. [PubMed] [Google Scholar]
- 28.Dearfield KL, Cimino MC, McCarroll NE, Mauer I, Valcovic LR, et al. US Environmental Protection Agency. Genotoxicity risk assessment: A proposed classification strategy. Mutat Res. 2002;521:121–35. doi: 10.1016/s1383-5718(02)00236-x. [DOI] [PubMed] [Google Scholar]
- 29.Sen S, De B, Devanna N, Chakraborty R. Hypoglycemic and hypolipidemic effect of Meyna spinosa leaves in high fat diet-alloxan induced type 2 diabetic rats. Bangladesh J Pharmacol. 2013;8:181–5. [Google Scholar]
- 30.Umar-Tsafe N, Mohamed-Said MS, Rosli R, Din LB, Lai LC. Genotoxicity of goniothalamin in CHO cell line. Mutat Res. 2004;562:91–102. doi: 10.1016/j.mrgentox.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Firenzuoli F, Gori L. Herbal medicine today: Clinical and research issues. Evid Based Complement Alternat Med. 2007;4:37–40. doi: 10.1093/ecam/nem096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seeff LB. Herbal hepatotoxicity. Clin Liver Dis. 2007;11:577–96. doi: 10.1016/j.cld.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Tang JL, Liu BY, Ma KW. Traditional chinese medicine. Lancet. 2008;372:1938–40. doi: 10.1016/S0140-6736(08)61354-9. [DOI] [PubMed] [Google Scholar]
- 34.Shin IS, Seo CS, Lee MY, Ha HK, Huh JI, Shin HK, et al. In vitro and in vivo evaluation of the genotoxicity of gumiganghwal-tang, a traditional herbal prescription. J Ethnopharmacol. 2012;141:350–6. doi: 10.1016/j.jep.2012.02.045. [DOI] [PubMed] [Google Scholar]
- 35.Ames BN, Mccann J, Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975;31:347–64. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- 36.Paris: OECD; 1997. OECD. Guideline for Testing of Chemicals, Mammalian Erythrocyte Micronucleus Test. 474. [Google Scholar]
- 37.Ames BN, Whitfield HJ. Frame shift mutagenesis in Salmonella. Quant Biol. 1966;23:221–5. doi: 10.1101/sqb.1966.031.01.030. [DOI] [PubMed] [Google Scholar]
- 38.Yeap SK, Alitheen NB, Ali AM, Omar AR, Raha AR, Suraini AA, et al. Effect of rhaphidophora korthalsii methanol extract on human peripheral blood mononuclear cell (PBMC) proliferation and cytolytic activity toward HepG2. J Ethnopharmacol. 2007;114:406–11. doi: 10.1016/j.jep.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira MV, Jahnen-Dechent W, Neuss S. Standardization of automated cell-based protocols for toxicity testing of biomaterials. J Biomol Screen. 2011;16:647–54. doi: 10.1177/1087057111405380. [DOI] [PubMed] [Google Scholar]
- 40.Singh NP. Microgels for estimation of DNA strand breaks, DNA protein crosslinks and apoptosis. Mutat Res. 2000;455:111–27. doi: 10.1016/s0027-5107(00)00075-0. [DOI] [PubMed] [Google Scholar]
- 41.Krishna G, Hayashi M. In vivo rodent micronucleus assay: Protocol, conduct and data interpretation. Mutat Res. 2000;455:155–66. doi: 10.1016/s0027-5107(00)00117-2. [DOI] [PubMed] [Google Scholar]
- 42.Schmid W. The micronucleus test. Mutat Res. 1976;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 43.Gollapudi B, Karma OP. An application of a simple giemsa staining method in the micronucleus test. Mutat Res. 1979;64:45–6. doi: 10.1016/0165-1161(79)90135-3. [DOI] [PubMed] [Google Scholar]
- 44.Alimba CG, Bakare AA, Latunji CA. Municipal land fill leachates induced chromosome aberrations in rat bone marrow. Afr J Biotechnol. 2006;5:2053–7. [Google Scholar]
- 45.Banerjee S, Singh S, Policegoudra R, Chattopadhyay P, Ghosh A, Veer V. Evaluation of the mutagenic potential of a prophylactic transdermal patch by Ames test. Immuno Anal Biol Spec. 2013;28:322–6. [Google Scholar]
- 46.Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;455:29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]