Abstract
Background:
Exacum lawii (Gentianaceae), a bitter herb conventionally used in kidney diseases and eye problems, endemic to the Western coast and Southern part of India.
Aim:
Folklore reports encourage the author to explore the nephroprotective effect of the standardized ethanolic extract of E. lawii against cisplatin-induced renal toxicity in the rat to scientifically validate its traditional use.
Materials and Methods:
Ethanolic extract of the whole plant of E. lawii was standardized with swertiamarin (secoiridoid glycoside) using high-performance liquid chromatography and tested for subacute toxicity according to the OECD guidelines. Nephroprotective potential at different doses of extract was evaluated against cisplatin (6 mg/kg, intraperitoneal) in experimental rats. The changes in serum renal toxicity markers, renal tissue oxidative stress biomarkers, and proinflammatory cytokines level were measured. To estimate the change in oxidative status of renal tissues, DNA and single viable cells were isolated from treated rat kidney, DNA fragmentation assay and flow cytometric analysis of reactive oxygen species (ROS) were performed. Histopathology of renal tissues was also examined.
Results:
Swertiamerin was found to be 119.59 mg/g of extract. Administration of E. lawii extract (ELE) restored the biochemical parameters. It also decreases the elevated proinflammatory cytokines level in kidney tissues and protected rat kidneys from oxidative stress in rats. Nephroprotective activity was validated by estimating ROS production in kidney live cells and DNA damage in kidney tissue. The histological architecture was also conserved.
Conclusion:
ELE showed significant renal protection against cisplatin through reducing oxidative stress and inflammation.
SUMMARY
High-performance liquid chromatography standardisation of Exacum lawii extract with Swertiamerin and its subacute toxicity study
Nephroprotective activity of Exacum lawii extract and Swertiamerin was evaluated in cisplatin-induced model and justified by various biochemical parameters and histopathological study
Role of oxidative stress in cisplatin-induced nephrotoxicity was confirmed by measuring levels of antioxidant markers and proinflammatory cytokines in rat renal tissues, ROS estimation by flow cytometry and DNA fragmentation assay by gel electrophoresis in renal cells.

Abbreviations used: ELE: Exacum lawii ethanolic extract; WHO: World Health Organization; SOD: Superoxide dismutase; CAT: Catalase; MDA: Malondialdehyde; HPTLC: High performance thin layer chromatography; p.o.: Per oral; i.p.: Intraperitoneal; TNF-α: Tumor necrosis factor alpha; IL-1β: Interleukin 1 beta; IL-6: Interleukin 6; ROS: Reactive oxygen species.
Keywords: Cisplatin, DNA fragmentation assay, Exacum lawii, flow-cytometric analysis, nephroprotective, swertiamerin
INTRODUCTION
The name Exacum was first used by Linnaeus.[1,2] The genus Exacum (Gentianaceae, tribe Exaceae) consists of 64 species distributed across paleotropical regions including Africa, Madagascar, Socotra, Himalayas, Arabian Peninsula, India, Sri Lanka, Southern China and Malaysia, and Northern Australia.[3] Exacum lawii C.B. Clarke in Hook species of genus Exacum is small herb commonly distributed in the Western peninsula, Western coast region of India, Mysore and Coimbatore and Southern part India. It is endemic to Jarandeshwar hill from Satara district, Maharashtra and Western Ghat of Karnataka. The common English name is Law's Persian violet. It is locally known as Lahan chirayata in Maharashtra, Manali in Malayalam, Marukozhunthu in Tamil. The plant is an annual, glabrous, small erect herb rarely reaching 15 cm tall. Flowers are bluish-purple. The whole plant possesses the medicinal property and has been used as folk remedy for the treatment of kidney disorders, eye diseases and also used as a laxative.[4,5,6,7,8,9] The presence of alkaloids, flavonoids, and steroids was reported in E. lawii.[10] However, extensive literature survey results in less or no information for the ethnopharmacology of E. lawii, the plant was selected for the present study to scientifically validate the nephroprotective effect of E. lawii.
The prevalence of cancer is constantly not only increasing with radiotherapy and chemotherapy are established therapeutic interventions but also associated with several side effects. Kidneys are the major targets for the toxic effects of various chemical agents. Nephrotoxicity is a very common toxic effect when the body is exposed to drugs or some toxins. It results in failure to filter excess urea and excess of nitrogenous compounds and creatinine that leads to uremia. There is no specific therapy for acute renal failure, only the supportive care is necessary for renal function restoration. It can only be prevented by avoidance of nephrotoxic substances, maintenance of adequate hydration and perfusion.[11,12,13]
Cisplatin (cis-diamminedichloroplatinum-II, CP) is a platinum-containing alkylating-like agent and most widely used to treat a variety of malignancies. However, cisplatin at its high dose is restricted by various significant side effects such as bone marrow suppression, peripheral neuropathy, ototoxicity, anaphylaxis, and nephrotoxicity. Approximately one-third of the patients develop nephrotoxicity after a single dose of cisplatin (50–100 mg/m2).[14,15]
Cisplatin is taken up through passive diffusion and also through organic cation transporter-2 and get accumulated in highest concentrations in S3 segment of proximal tubular cells of the outer medulla and inner cortex.[16,17] Direct tubular toxicity, vascular factors, inflammation, and oxidative stress are the resultant outcome of cisplatin-induced nephrotoxicity. Inflammatory cytokines and reactive oxygen species (ROS) play an important role in pathogenesis of cisplatin-related nephrotoxicity. Reportedly, cisplatin-induced renal toxicity has been correlated with proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-1beta (IL-1β).[18,19,20,21,22]
Within the cell, cisplatin is converted into a highly reactive form rapidly reacting with thiol-containing antioxidant molecules such as glutathione (GSH). Generation of ROS, accumulation of lipid peroxidation (LPO) products in kidneys, and suppressed antioxidant systems are thought to be major mechanisms of cisplatin-induced kidney toxicity. ROS affect cell function by directly acting on cell components, including lipids, protein, and DNA, and destroying their structure. Therefore, it may be etiologically involved in renal disorder and many tissue disorders. In cells, ROS are produced through the xanthine–xanthine oxidase system, mitochondria, or NADPH oxidase.[14,23,24,25,26,27,28,29,30]
The proinflammatory character of cisplatin-induced acute kidney injury (AKI) and secretion of various cytokines such as IL-1β and IL-6 has been documented.[31] Furthermore, TNF-α knockout mice were resistant to cisplatin nephrotoxicity and TNF-α inhibition, have also been reported to be protective against cisplatin-induced AKI.[19,32] In aerobic cells, ROS are generated as an outcome of the normal mitochondrial activity. During altered cellular redox state, ROS production is not controlled, it can cause severe damage to cellular macromolecules, especially the DNA. Nucleosomal fragmentation of DNA is a distinctive feature of apoptosis (programmed cell death).[33,34]
The aim of the present study is to evaluate the nephroprotective activity of E. lawii and scientifically validate its traditional use by measuring proinflammatory cytokines level and antioxidant parameters, ROS analysis through flow cytometry, DNA fragmentation assay through gel electrophoresis and histopathological examination.
MATERIALS AND METHODS
Plant authentication and extraction process
The fresh plant samples were collected in the year of 2015 in the month of August–October from Mahabaleshwar, Maharashtra, India. The plant was identified and authenticated by Dr. N. M. Dongarwar, Assistant Professor, Department of Botany, Rashtrasant Tukadoji Maharaj Nagpur University, India. A voucher specimen (Cog/EL/2014-15) was deposited for future reference in Pharmacognosy laboratory of Department of Pharmaceutics, Indian Institute of Technology (Banaras Hindu University), Varanasi, India. The plants were freshly collected, thoroughly washed and shade dried for 1 week at temperature not exceeding 60°C to prevent the deactivation of thermo labile phytoconstituents. The dried plant was coarsely powdered using mechanical grinder, passed through sixty mesh sieve size, and stored at room temperature until extraction. The powdered drug (1000 g) was defatted with petroleum ether (60–80°C) and exhaustively extracted by cold maceration using 95% ethanol (3 L v/v) as solvent. The E. lawii extract (ELE) was filtered and concentrated to 50 ml under vacuum rotary evaporator (IKA Germany) and stored in desiccator until use.
Animals
The certified pathogen-free healthy Charles Foster albino male and female rats (150–250 g) were procured from the Central Animal House (Reg. No. 542/02/ab/CPCSEA), Institute of Medical Sciences, Banaras Hindu University, Varanasi, India. Animals were housed in polypropylene cages and maintained under standard conditions (12 h light and dark cycles at an ambient temperature of 25°C and 45%–55% relative humidity). Rats were permitted for free access to water and standard feed. The animals were allowed to acclimatize to the laboratory environment for 7 days before the commencement of experiments. All experimental protocols were approved by the Central Animal Ethical Committee of Banaras Hindu University (No. Dean/2015/CAEC/1132) and were conducted in accordance with accepted standard guidelines of National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication number 85-23, revised 1985).
Preliminary phytochemical screening
The ethanolic extract of E. lawii was screened for various phytoconstituents using phytochemical tests and thin layer chromatography (TLC). The total phenolic content and flavonoid content were measured.[35,36] The amount of vitamins (C and E) was determined using association of official analytical chemists methods.[37] The presence of swertiamerin in the extract was ensured by TLC along with the standard of swertiamarin (Natural remedies Pvt. Ltd.,) using solvent system ethyl acetate:methanol:water (7.7:1.5:0.5).[38]
Standardization of extract using high-performance liquid chromatography
The ELE was standardized with Swertiamerin as standard. High-performance liquid chromatography (HPLC) analysis was carried out using Waters 1500-series pump (Milford, MA, USA) attached to Waters 2998 photodiode array detector and data were analyzed by waters Breeze software (Waters, USA). The mobile phase was filtered through a 0.45 μm membrane filter using solvent filtration apparatus (Millipore, USA). Samples for HPLC analysis were also filtered through a 0.45 μm membrane filter (Waters, USA). Analysis of the samples was carried out in Column LiChroCART® 250-4 C18 column and methanol-water (1:1) were used to get better separation, peak shape, and resolution at UV 238 nm and thus used for further analysis. The chromatographic conditions were as follows: flow gradient started with flow rate of 0.5 mL/min. At 4.9 min, the flow rate was increased to 0.8 mL/min the flow rate was then increased to 1.0 ml/min at 5.1 min. The flow rate was kept constant up to 5.4 min and then finally decreased to 0.5 ml/min, restoring the initial conditions at 8 min. Detector's wavelength was 238 nm and the injection volume was 10 μl. The best baseline separation (peak purity >95%) for swertiamarin was achieved at 238 nm also reported earlier. Swertiamarin was found to show maximum absorption at λmax 238 nm in three-dimensional ultraviolet absorption spectra using photodiode array detector.[39]
Repeated dose 28-day oral toxicity study
Repeated dose 28-day oral toxicity study in experimental rats was performed according to OECD test guideline 407. Nulliparous and nonpregnant five female and five healthy male rats were taken in each group. The first group was taken as control group, given with 0.5 ml normal saline. Second, third and fourth group were administered with ELE at dose of 1000 mg/kg, per oral (p.o.), 2000 mg/kg, p.o. and 4000 mg/kg, p.o. to overnight fasted rats. The observation was conducted individually for any neurological and behavioral changes such as tremors, convulsions, diarrhea, salivation, sleep, feeding, and lacrimation behavior as a sign of acute toxicity for 48 h. Mortality and morbidity were observed daily for 28 days. Delayed occurrences and reversibility, of signs of toxicity, were distinguished, for at least 14 days post-treatment. Preclinical signs, body weight, and organ weight of different groups were compared with control. Further blood was collected by retro-orbital bleeding under anesthesia to estimate hematological parameters by mythic 18 hematology analyzer and biochemical parameters using span diagnostic Ltd.,
Dose standardisation of cisplatin
To standardize the dose of cisplatin for induction of nephrotoxicity in rats, three groups having six rats in each group were taken. Cisplatin in sodium chloride 0.9% w/v (Cytoplatin-10, Cipla) at a dose of 4, 6, and 8 mg/kg was administered intraperitoneal to rats in each group. After 72 h of cisplatin administration, blood was collected through retro-orbital venous plexus under light anesthesia. Serum urea and creatinine level were determined using standard kits (Erba Diagnostics Mannheim, Germany).
Drug treatment protocol
To evaluate the nephroprotective effect of ELE on cisplatin-induced nephrotoxicity, rats were randomly divided into six groups of six animals each. The ELE was suspended in 0.5% carboxymethyl cellulose (CMC) and given by oral administration (p.o.) to rats. Cisplatin was freshly prepared in normal saline (0.9% NaCl) and administered by intraperitoneal (i.p.) injection. The doses of ELE were selected as one-tenth of the safe dose from acute toxicity study. Different doses of ELE and swertiamerin (20 mg/kg, p.o.)[40] were given for total 7 days to respective groups and cisplatin (6 mg/kg, i.p.) was administered on the 4th day of the treatment. Finally on 7th day, rats were euthanized by method of cervical dislocation to collect blood and harvest kidneys for measuring various parameters (renal function test, liver function test, antioxidant parameters, and proinflammatory cytokines level), ROS analysis in single viable cells of treated kidney, DNA fragmentation assay to assess the degree of DNA damage in kidney cell and histological studies.
Group I (normal control): Vehicle (aqueous solution of 0.5% CMC) for 7 consecutive days and 0.9% NaCl on the 4th day
Group II (toxic control): Vehicle (aqueous solution of 0.5% CMC) for 7 consecutive days and cisplatin (6 mg/kg, i.p.) on the 4th day
Group III: ELE (100 mg/kg, p.o.) for 7 consecutive days and cisplatin (6 mg/kg, i.p.) on the 4th day
Group IV: ELE (200 mg/kg, p.o.) for 7 consecutive days and cisplatin (6 mg/kg, i.p.) on the 4th day
Group V: ELE (400 mg/kg, p.o.) for 7 consecutive days and cisplatin (6 mg/kg, i.p.) on the 4th day
Group VI: Swertiamerin (20 mg/kg, p.o.) for 7 consecutive days and cisplatin (6 mg/kg, i.p.) on the 4th day.
Determination of biochemical parameters in serum
Enzyme assay kits were purchased from span diagnostic Ltd., Serum urea[41] and serum creatinine[42] had been measured using blood urea nitrogen method and modified rate Jaffe's kinetic method by autoanalyzer. Serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase,[43] total protein,[44] total bilirubin and direct bilirubin,[45] and alkaline phosphate[46] had been also analyzed according to standard methods using the auto analyzer.
Determination of antioxidant parameters in renal tissue homogenate
Minced kidney tissues were homogenized in 0.1 M potassium phosphate buffer (pH-7) with the protease inhibitor. Obtained homogenates (10% w/v) were centrifuged (10,000 ×g) at 4°C for 20 min and used for detection of antioxidant parameters. LPO levels had been determined in terms of thiobarbituric acid reacting substance and expressed as equivalent to malondialdehyde (MDA) using 1’1’3’3’-tetramethoxypropane as standard MDA.[47] Superoxide dismutase (SOD) had been estimated in terms of the capacity to inhibit the reduction of nitro blue tetrazolium by superoxide, generated in the presence of riboflavin in reaction system through a photosensitive reaction,[48] Catalase (CAT) activity had been expressed from the rate of decomposition of H2O2 at 240 nm with the addition of tissue homogenate[49] and reduced GSH level was calculated in terms of protein-free sulfhydryl content using 5,5-dithiobis-2-nitrobenzoic acid.[50]
Determination of proinflammatory in renal tissue homogenate
Cisplatin accumulation in kidney tissues led to the generation of proinflammatory cytokines due to toxicity induced and had been investigated by measuring levels of TNF-α, IL-6 and IL-1β on homogenized renal tissues using ELISA kit (Komabiotech, Korea). Standard and detection antibodies provided were reconstituted in sterile water. Serial dilutions of standard and samples were prepared. Selected wells in microplate were washed with washing solution. A volume of 100 ml of samples and standard were added to wells followed by addition of diluted detection antibody after incubation. Color was developed with the addition of color development enzyme and color development solution. Finally, absorbance was measured using microplate reader (BioTek Instruments Inc., USA).
Renal single cell preparation
Rats were anesthetized with diethyl ether and sacrificed. Kidneys were isolated washed with 1X phosphate-buffered saline (PBS) and minced into small pieces with fine scissor. The minced tissues were transferred in 20 ml of 0.1% Type IV collagenase in 1X PBS solution and placed in a 37°C CO2 incubator for 2 h. The cell suspension was passed through cell strainer (40 μm mesh size). The cells were centrifuged at 3000 rpm for 10 min, 3 times. The supernatant was discarded, and the pellet was suspended in FAC sheath to perform flow cytometric analysis.
Reactive oxygen species measurement by flow cytometry
FACS Calibur flow cytometer (BD Bioscience) in conjunction Cell-Quest Pro software was used for flow cytometry. 20 μM of 2’,7’-dichlorofluorescin diacetate (Sigma) was added to the renal single cell suspension and incubated in dark for 15 min at 37°C. Fluorescence intensity was acquired at FL1 channel with BD FACS calibur (BD Biosciences). 10,000 events were taken and Cell Quest Pro software (Becton Dickinson, Franklin Lakes, New Jersey, USA) was used for analysis.
Qualitative DNA fragmentation assay
Rat kidneys were washed with 1X PBS, minced tissues were homogenized with 1X PBS buffer. Obtained homogenate were centrifuged 5 times at 3000 rpm for 10 min at room temperature, and the supernatant was discarded to remove cell debris and red blood cells. The cells were lysed in 50 μl of lysis buffer containing 50 mmol/l of Tris–HCl (pH 8.0) and 0.5% sodium dodecyl sulfate and incubated for 30 min at 37°C. The cell pellet was stirred with a wide-bore pipette tip to ensure uniform mixing of cells, followed by the addition of 2 μl of 10 mg/ml DNAase free RNase (Thermo scientific™) and incubated for 2 h at 37°C. Samples were further appended with 5 μl of Proteinase K (Thermo scientific™) solution and incubated at 50°C for 90 min. The precipitated DNA was dissolved in a 20 μl of Tris-EDTA buffer (1X) and quantified spectrophotometrically as described previously. An equal concentration of DNA was resolved on 1% agarose gel stained with ethidium bromide. The gel was viewed under UV light, followed by documentation using the Alpha Innotech system (San Leandro, California, USA).
Histopathological study
Isolated kidneys were washed with isotonic saline were fixed in 10% neutral buffered formalin for 48 h and embedded in paraffin wax. Sections (5–6 μm thickness) had been made from paraffin blocks by microtome and stained with Periodic acid-Schiff reagents and subjected to microscopic and imaging system (Nikon, Japan).[51]
Statistical analysis
The experimental data were expressed as mean ± standard error of the mean., with six animals in each group. Analysis of variance (ANOVA) was performed using one-way ANOVA followed by Newman–Keuls multiple comparison test for determining the statistical significance between different groups. A difference in the mean values of P < 0.05 was considered to be statistically significant. Graph Pad Prism 5.0 software (GraphPad software, Inc., La Jolla, CA, USA) was used for all statistical analysis.
RESULTS
Preliminary phytochemical screening
The ELE was found to be rich in phenolic compounds, flavonoids, and terpenoids. The total phenolic content (12.21 ± 0.58 mg GAE/g), total flavonoid content (21.86 ± 0.22 mg rutin/g) and total flavonol content (15.53 ± 0.05 mg rutin/g). Ascorbic acid was found to be 6.11 mg/100 g of air-dried E. lawii powder. The Vitamin E content was 5.06 mg/100 g air-dried E. lawii powder. The swertiamerin was detected at Rf= 0.35.
Standardization of extract using high-performance liquid chromatography
Concentration of swertiamarin in the extract was calculated using standard curve obtained from standard swertiamerin compound. Mean retention time was of 1.3 min. The concentration of swertiamarin in ELE was found to be 119.59 mg/gm. Chromatogram of swertiamerin and extract is shown in Figure 1.
Figure 1.
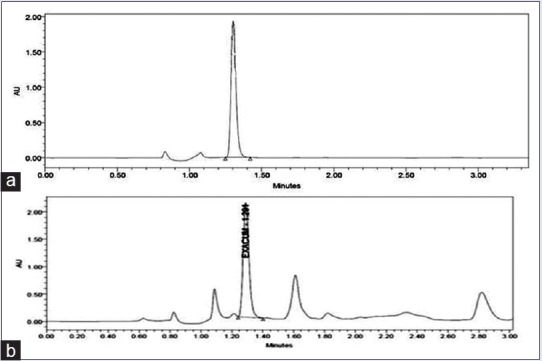
(a) High-performance liquid chromatography chromatogram showing peak of standard Swertiamerin, (b) high-performance liquid chromatography chromatogram of ethanolic extract of Exacum lawii showing peak of swertiamerin
Repeated dose 28-days oral toxicity study
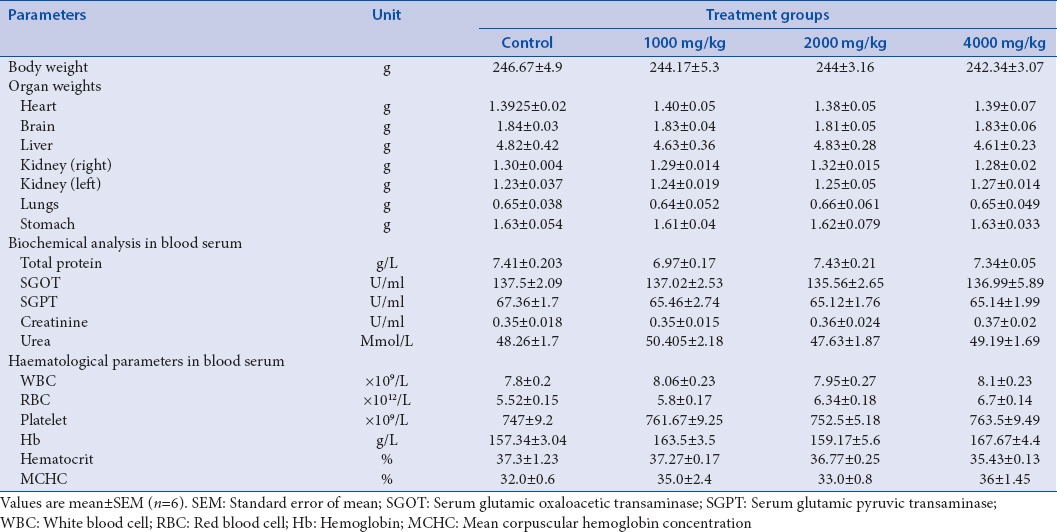
Animals were observed throughout the period of 28 days of study, there were no signs abnormal preclinical conditions such as loss of consciousness, tremors, convulsions, irregular breathing, lethargy, aggression, diaphragmatic breathing, gait, licking, and piloerection were observed. It was observed that rats in each group were survived with a normal weight gain pattern until the termination of experiment. ELE (1000 mg/kg, p.o., 2000 mg/kg, p.o., and 4000 mg/kg, p.o. per day) administration for 28 days causes no significant difference body weight, vital organ weight, biochemical parameters, and hematological when compared with the control group [Table 1]. The study established that the ELE is safe up to 4000 mg/kg, p.o. Therefore according to the OECD guidelines, one-tenth of the safe dose of the extract was selected as a median pharmacological dose for further study, while the dose level of 100 mg/kg and 200 mg/kg were taken as to be lower and higher limits.
Table 1.
Various parameters studied in repeated dose 28-days oral toxicity study of Exacum lawii extract
Dose standardization of cisplatin
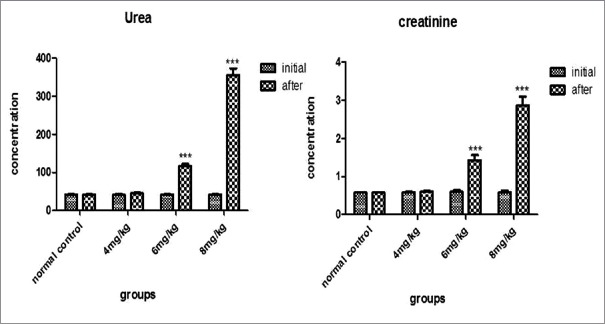
Cisplatin (4 mg/kg, i.p.) had not shown any significant elevation (P > 0.05) in urea and creatinine level after 72 h of cisplatin administration. Cisplatin at dose of 6 mg/kg, i.p. and 8 mg/kg, i.p. caused significant elevation (P < 0.05) in urea and creatinine concentration. Administration of cisplatin at the dose of 8 mg/kg, i.p., causes mortality (one-half) in a group of tested rats. Therefore, nephroprotective activity of ELE had been evaluated in rats treated with 6 mg/kg, i.p of cisplatin [Figure 2].
Figure 2.
Effect of different doses of cisplatin on blood urea and creatinine concentration
Effect of Exacum lawii extract on cisplatin-induced nephrotoxicity
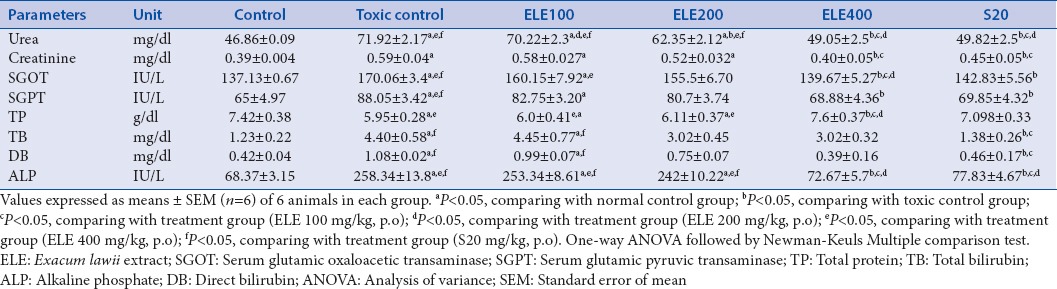
Cisplatin induces kidney injury markers such as serum urea and creatinine and also raises some biochemical markers. Pre- and post-treatment with ELE (400 mg/kg, p.o.) and Swertiamerin (20 mg/kg) for 7 days was found to lower these markers significantly (P < 0.05) and balanced up to normal level as compared to control [Table 2].
Table 2.
Effect of different doses of Exacum lawii extract and swertiamerin on serum biochemical parameters in rats treated with cisplatin (6 mg/kg, i.p.)
Effect of Exacum lawii extract on antioxidant markers
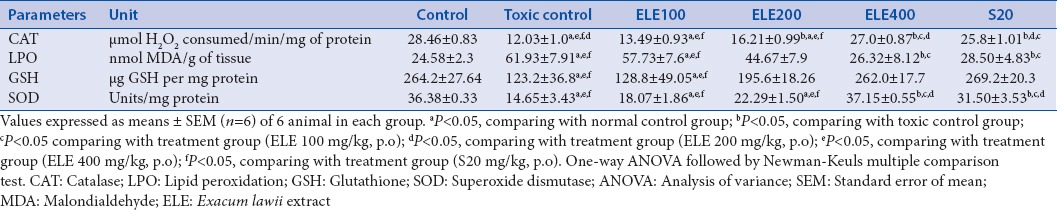
The activity of SOD, CAT, and GSH content had decreased, whereas MDA level in cisplatin (6 mg/kg, i.p.) treated rat was found be elevated might be due to increase in ROS in renal tissues. ELE (400 mg/kg, p.o.) and swertiamerin (20 mg/kg) treatment significantly inverted the changes in antioxidant enzymes in dose-dependent manner (P < 0.05) [Table 3].
Table 3.
Effect of different doses of Exacum lawii extract and swertiamerin on renal tissue antioxidant enzyme levels in rats treated with cisplatin (6 mg/kg, i.p.)
Effect of Exacum lawii extract on proinflammatory cytokines
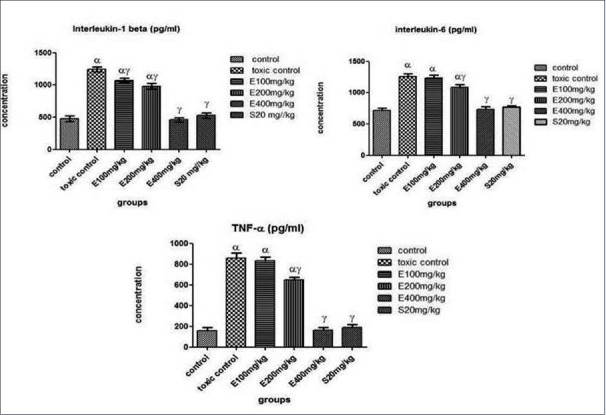
The proinflammatory cytokines in rat kidney tissues were investigated in vitro enzyme-linked immunosorbent assay by measuring cytokine levels of TNF-α, IL-6, and IL-1β on homogenized renal tissues using specific antibodies coated 96-well plates. The level of TNF-α, IL-6, and IL-1β in serum was found to be elevated in cisplatin-treated rats. However, ELE (400 mg/kg, p.o.) and swertiamerin (20 mg/kg) treatment had reduced the expression of these proinflammatory molecules in the kidney tissues [Figure 3].
Figure 3.
Effect of different doses of Exacum lawii extract and Swertiamerin treatment on proinflammatory cytokines, interlukin 1β, interlukin 6 and tumor necrosis factor alpha in renal tissue of rats treated with cisplatin (6 mg/kg, i. p.). Values expressed as means ± SEM (n=6) of 6 rats in each group. αP<0.05, comparing with normal control group; γP<0.05, comparing with toxic control group.
Flow cytometric measurement of reactive oxygen species
Flow cytometry analysis of intracellular ROS production by kidney cells of experimental rats after exposure to cisplatin. H2O2 treated kidney cells were taken as external control. Data for geometrical mean (Geo mean ± standard deviation) values of fluorescence intensities for each group from two independent experiments are represented in the Bar graph [Figure 4]. Overlay histogram showed the fluorescent intensities of ELE (400 mg/kg, p.o.), Swertiamerin (20 mg/kg, p.o.), cisplatin (6 mg/kg, i.p.) treated, control, and unstained cells [Figure 5].
Figure 4.
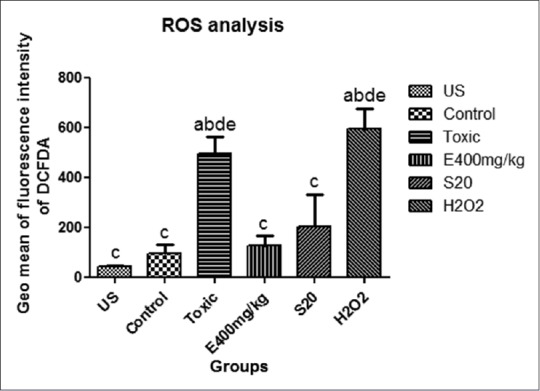
Geometrical mean values of the fluorescence intensities of respective groups. Values expressed as means ± SEM (n=3). aP<0.05, comparing with unstained, bP<0.05, comparing with control group; cP<0.05, comparing with toxic control group; dP<0.05, comparing with treatment group (ELE 400 mg/kg, p.o); eP<0.05, comparing with S20mg/kg
Figure 5.
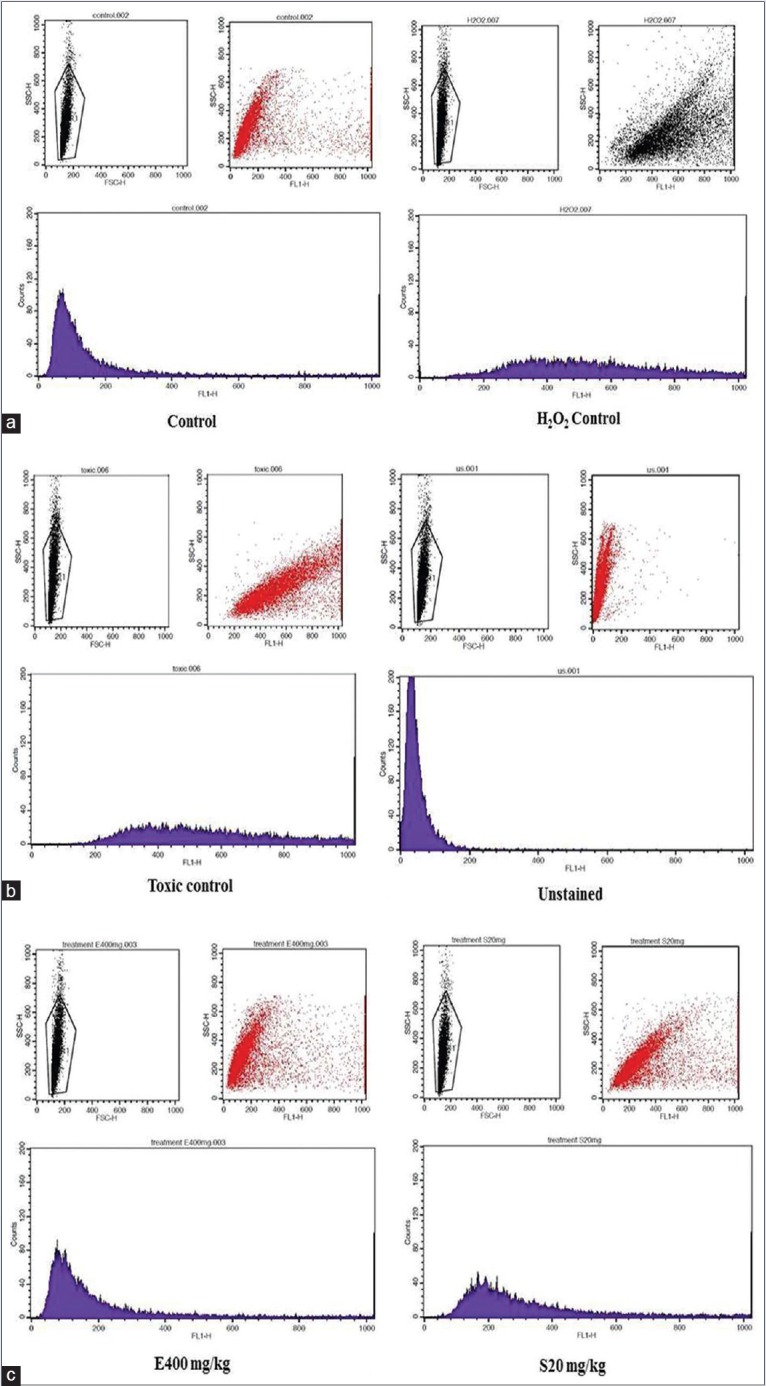
Histogram showing flow cytometric analysis of reactive oxygen species production in kidney cells of respective treatment group. Along with dot plot of flow cytometric analysis represents live cells (a) control group and external control (H2O2) group. (b) Toxic control (cisplatin treatment) and unstained group. (c) Treatment of Exacum lawii extract (E400 mg/kg) and Swertiamerin (S20 mg/kg)
Qualitative DNA fragmentation assay
To further define the mechanism involved in cisplatin-induced nephrotoxicity the DNA fragmentation assay against marker 1 kb ladder was performed. Cisplatin administration led to DNA fragmentation. Cisplatin-induced DNA breakage at multiple positions across chromosomal DNA may leads to apoptosis. Rats treated with ELE (400 mg/kg, p.o.) and Swertiamerin (20 mg/kg, p.o.) showed very less or no DNA fragmentation [Figure 6].
Figure 6.
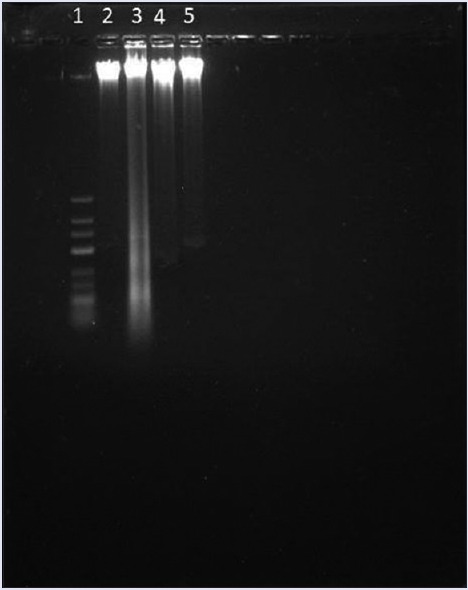
DNA fragmentation of renal cells exposed to cisplatin. Each lane reflecting the presence of DNA fragments was viewed on an ethidium bromide-stained gel. Lane 1: Marker, Lane 2: Control group, Lane 3: Cisplatin treated group, Lane 4: E. lawii extract 400 mg/kg treated, Lane 5: Swertiamerin 20 mg/kg treated
Histopathological study
Cisplatin treatment showed prominent alteration and loss in normal architecture of renal tissues. The renal histology of toxic group was found with distorted histology with atrophied glomerulus, collecting tubules with necrosis. Rats treated with ELE (100 mg/kg, p.o. and 200 mg/kg, p.o.) showed no prominent protective effect on cisplatin-induced nephrotoxicity. Treatment with ELE (400 mg/kg, p.o.) and swertiamerin (20 mg/kg, p.o.) justified the protective action by minimizing renal vein congestion, tubular necrosis, inflammation, and vacuolization in renal histology and cortex was observed with normal histology of convoluted tubules and medulla with collecting tubule and pars recta [Figure 7].
Figure 7.

Photomicrographs of Periodic acid-Schiff reagents stained kidney tissue sections showing the protective effect of E. lawii extract on cisplatin induced renal injury in experimental rats. a: Collecting tubule; b: Convoluted tubule; g: Glomerulus; p: Collagen deposition around parietal layer of the Bowman's capsule; d: DISTAL convoluted tubules showed variable degrees of dilatation; v: Cytoplasmic vacuolization lb: Lobulated and shrunken glomeruli; h & n: Hemolysis and necrosis i: Inflammation; h: Hyaline cast
DISCUSSION
Medicinal plants play a very important role in primary health-care system, from the ancient time. The World Health Organization also suggested that about 80% of the world's population depends on developing countries on traditional remedies and medicinal plants for prevention, treatment, and cure of diseases.[52]
In the present study, swertiamerin was selected as biomarker as it is widely distributed in plants of family Gentianaceae. E. lawii contains Swertiamerin as one of the potent phytoconstituent and is also reported to possess the hepatoprotective, antioxidant activity,[53] anti-inflammatory activity,[54] and improve diabetic nephropathy.[55]
Cisplatin is the potent platinum-containing anticancer drug.[56] Nephrotoxicity is the major dose-limiting side effect of cisplatin because it accumulates in tubules epithelial cells five times more than in serum.[18,57] Its mechanism of renal toxicity is different from killing tumour cells in the kidney.[58] The disproportionate accumulation of cisplatin in kidney tissues may contribute to cisplatin-induced nephrotoxicity. Cisplatin forms crosslink with DNA of tumor cells produces cytotoxic lesions in tumors and other dividing cells. The quiescent proximal tubule cells are also selectively damaged by cisplatin. The in vivo pathogenesis of cisplatin-induced nephrotoxicity is complex and involves inflammation, oxidative stress and apoptosis.[59,60]
A single dose of cisplatin (6 mg/kg, i.p.) administration causes a marked elevation in serum urea, creatinine, and other biochemical parameters. The ELE (400 mg/kg, p.o.) and swertiamerin (20 mg/kg, p.o.) treatment significantly (P < 0.05) balanced the serum urea and creatinine concentration along with other biochemical parameters. It showed the protective effect of ELE and swertiamerin against cisplatin-induced toxicity.
Abnormal elevation in intracellular ROS level in renal cells leads to the oxidative stress phenomenon, which further leads to a disturbance in the normal redox level of the renal cell.[61] ROS produced through the pathways mainly xanthine–xanthine oxidase system, mitochondria, and NADPH oxidase in renal cells and are implicated in the pathogenesis of acute cisplatin-induced renal injury. ROS directly act on cellular components, including lipids, proteins, and DNA, and destroy their structure. The effect of ROS on cellular processes depends on its duration and strength on cells. The continued or high levels of ROS exposure triggered to apoptosis mechanisms.[62]
These free radicals damage the lipid components of the cell membrane by denaturation of proteins and peroxidation, which leads to enzymatic inactivation such as SOD and CAT.[63] GSH (non-protein thiol) is the primary antioxidant defense system in xenobiotic metabolism and protects tissue from damage and reportedly decreased in kidney mitochondria.[64] It was observed that SOD, CAT, and GSH got depleted while LPO level elevated in cisplatin-treated renal tissues. These antioxidant parameters were restored significantly by administration of ELE (400 mg/kg, p.o.) and swertiamerin (20 mg/kg, p.o.).
Recent studies suggested that inflammatory mechanism mediates pathogenesis of cisplatin-induced nephrotoxicity. Cisplatin-induced kidney toxicity extensively depends on TNF-α, as TNF-α-deficient mice and TNF-α antibody-treated wild-type mice reported to be resistant to cisplatin-induced kidney damage. It has been reported to increase the renal expression of proinflammatory cytokines such as IL-6 and IL-1β. TNF-α in cisplatin-treated rats which orchestrate the inflammatory response.[19,65,66] Treatment with ELE (400 mg/kg, p.o.) and S (20 mg/kg, p.o) decreases the proinflammatory markers.
ROS produced in renal cells as result of cisplatin treatment had been explored using the flow cytometric technique. The flow cytometric measurement of the redox state of single cells using fluorescent and chemiluminescent probes 2’-7’-dichlorodihydrofluorescein diacetate is one of the most widely used techniques.[67] Geo mean values of the fluorescence intensities were found to be higher in cisplatin-treated renal cells compared with control group due to ROS production. ROS production in renal cells treated with ELE (400 mg/kg, p.o.) and Swertiamerin (20 mg/kg, p.o.) showed reduced ROS production. These findings indicated that ROS production plays an important role in cisplatin-induced renal toxicity.
DNA fragmentation assay was performed to determine the DNA damage as cellular response to oxidative stress produced by cisplatin accumulation. ELE (400 mg/kg, p.o.) and Swertiamerin (20 mg/kg, p.o.) treatment prevented the oxidative DNA damage or repaired the damaged DNA. The histological architecture of kidney tissues exposed to cisplatin revealed the glomerular changes, collagen deposition around renal glomeruli, dilations in distal convoluted tubules, hemolysis, and necrosis in tissues and cytoplasmic vacuolization in their lining epithelial cells. Treatment with ELE (400 mg/kg, p.o.) and Swertiamerin (20 mg/kg, p.o.) showed normal histological architecture reappearance comparable to tissue histology control group.
ELE (400 mg/kg, p.o.) treatment showed the significant nephroprotective effect as compared to swertiamerin by lowering urea, creatinine along with other biochemical markers and significantly maintained oxidant markers and proinflammatory cytokines level in cisplatin-treated kidney tissues. Intracellular ROS production, DNA damage, and impaired histology of kidney tissues in cisplatin-treated rats were brought to normal by ELE (400 mg/kg, p.o.) treatment. ELE was found to be a rich source of bioactive constituents along with Swertiamerin mainly phenols, flavonoids (quercetin), terpenoids (ursolic acid), Vitamin C, and Vitamin E. The phenolic compounds are reported to possess potent antioxidant activity and capable to cure nephrotoxicity.[68] Quercetin,[69] ascorbic acid (Vitamin C),[70] and Vitamin E[71] scientifically reported to have nephroprotective potential. It has been reported that whole extract produces a better effect than single active ingredient due to the cumulative effect of other bioactive phytoconstituents.[72]
CONCLUSION
Based on observed results and the described mechanism, it may be concluded that E. lawii possess nephroprotective activity and potential to attenuate cisplatin-induced renal toxicity by reducing inflammatory markers and ROS production and also protects cisplatin administered kidneys from DNA fragmentation and altered tissue architecture.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Interdisciplinary School of Life Sciences and UGC-UPE, B.H.U for equipment facility and Department of Pharmaceutics, IIT (B.H.U) Varanasi is highly acknowledged for providing financial support to Ms Sonam Sharma as Teaching Assistantship.
REFERENCES
- 1.Linnaeus C. Dissertatio Dassow. Sweden: C M Nova Plantarum Genera Stockholm; 1747a. [Google Scholar]
- 2.Linnaeus C. Flora Zeylanica. Stockholmiae: Sumtu & literis Laurentii Salvii; 1747b. [Google Scholar]
- 3.Struwe L, Hagen KB, Kadereit JW, Klackenberg J, Nilsson JS, Thiv M, et al. Systematics, character evolution and biogeography of Gentianaceae, including a new tribal and subtribal classification. In: Struwe L, Albert VA, editors. Gentianaceae-Systematics and Natural History. United Kingdom: Cambridge University Press; 2002. [Google Scholar]
- 4.Clarke CB. Gentianaceae. In: Hooker JD, editor. The Flora of British India. London: L Reeve & Company; 1883. [Google Scholar]
- 5.Hooker JD. The Flora of British India. L. London: Reeve & Company; 1885. [Google Scholar]
- 6.Gamble JS. The Flora of Presidency of Madras. London: Adlard and Son Ltd; 1923. [Google Scholar]
- 7.Kirtikar KR, Basu BD. Indian Medicinal Plants. Allahabad: Bishen Singh Mahendra Pal Singh; 1935. [Google Scholar]
- 8.Chopra RN, Nayar SL, Chopra IC, Asolkar LV, Kakkar KK. Glossary of Indian Medicinal Plants. New Delhi: Council of Scientific and Industrial Research; 1956. [Google Scholar]
- 9.Damaji B, Khandekar VP. Endemic plants of Maharashtra state at Jarandeshwar hill of Satara District (Maharashtra) Indian Stream Res J. 2011;1:1–3. [Google Scholar]
- 10.Vinayaka KS, Ashwini HS, Prashith KT, Krishanamurthy YL. Traditional utilization and phytochemical analysis of medicinal herb Exacum Linn. From central Western Ghats. Asian Pac J Health Sci. 2016;3:161–4. [Google Scholar]
- 11.Cornelison TL, Reed E. Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecol Oncol. 1993;50:147–58. doi: 10.1006/gyno.1993.1184. [DOI] [PubMed] [Google Scholar]
- 12.Kang DG, Lee AS, Mun YJ, Woo WH, Kim YC, Sohn EJ, et al. Butein ameliorates renal concentrating ability in cisplatin-induced acute renal failure in rats. Biol Pharm Bull. 2004;27:366–70. doi: 10.1248/bpb.27.366. [DOI] [PubMed] [Google Scholar]
- 13.Hausberg M, Schaefer RM. Management of acute renal failure in intensive care patients. Med Klin (Munich) 2006;101(Suppl 1):90–4. [PubMed] [Google Scholar]
- 14.Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis. 1986;8:368–79. doi: 10.1016/s0272-6386(86)80112-3. [DOI] [PubMed] [Google Scholar]
- 15.Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: An historical perspective and an update. Eur J Cancer. 1998;34:1522–34. doi: 10.1016/s0959-8049(98)00224-x. [DOI] [PubMed] [Google Scholar]
- 16.Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, Madsen K, et al. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol. 2000;278:F726–36. doi: 10.1152/ajprenal.2000.278.5.F726. [DOI] [PubMed] [Google Scholar]
- 17.Gately DP, Howell SB. Cellular accumulation of the anticancer agent cisplatin: A review. Br J Cancer. 1993;67:1171–6. doi: 10.1038/bjc.1993.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhlmann MK, Burkhardt G, Köhler H. Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol Dial Transplant. 1997;12:2478–80. doi: 10.1093/ndt/12.12.2478. [DOI] [PubMed] [Google Scholar]
- 19.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–42. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates Cisplatin-induced renal injury: Importance of superoxide. J Am Soc Nephrol. 2001;12:2683–90. doi: 10.1681/ASN.V12122683. [DOI] [PubMed] [Google Scholar]
- 21.Ramesh G, Reeves WB. Inflammatory cytokines in acute renal failure. Kidney Int Suppl. 2004;66:S56–61. doi: 10.1111/j.1523-1755.2004.09109.x. [DOI] [PubMed] [Google Scholar]
- 22.Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther. 2007;322:8–15. doi: 10.1124/jpet.107.119792. [DOI] [PubMed] [Google Scholar]
- 23.Siddik ZH. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 24.Aydinoz S, Uzun G, Cermik H, Atasoyu EM, Yildiz S, Karagoz B, et al. Effects of different doses of hyperbaric oxygen on cisplatin-induced nephrotoxicity. Ren Fail. 2007;29:257–63. doi: 10.1080/08860220601166487. [DOI] [PubMed] [Google Scholar]
- 25.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–55. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Drug Metab Rev. 1999;31:971–97. doi: 10.1081/dmr-100101947. [DOI] [PubMed] [Google Scholar]
- 27.Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8:132–40. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- 28.Halliwell B. Oxidants and human disease: Some new concepts. FASEB J. 1987;1:358–64. [PubMed] [Google Scholar]
- 29.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, et al. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285:H2290–7. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 30.Young TA, Cunningham CC, Bailey SM. Reactive oxygen species production by the mitochondrial respiratory chain in isolated rat hepatocytes and liver mitochondria: Studies using myxothiazol. Arch Biochem Biophys. 2002;405:65–72. doi: 10.1016/s0003-9861(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 31.Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int 2014. 2014 doi: 10.1155/2014/967826. 967826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramesh G, Reeves WB. Salicylate reduces cisplatin nephrotoxicity by inhibition of tumor necrosis factor-alpha. Kidney Int. 2004;65:490–9. doi: 10.1111/j.1523-1755.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 33.Barzilai A, Yamamoto K. DNA damage responses to oxidative stress. DNA Repair (Amst) 2004;3:1109–15. doi: 10.1016/j.dnarep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–9. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 35.Grubesic RJ, Vukovic J, Kremer D, Vladimir-Knezevic S. Spectrophotometric method for polyphenols analysis: Prevalidation and application on Plantago L. species. J Pharm Biomed Anal. 2005;39:837–42. doi: 10.1016/j.jpba.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Kumaran A, Karunakaran J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. Food Sci Technol. 2006;40:344–52. [Google Scholar]
- 37.Horwitz W. Official Methods of Analysis of AOAC International. 17th ed. USA: Association of Official Analytical Communities; 2003. [Google Scholar]
- 38.Wagner H, Bladt S. Plant Drug Analysis: A Thin Layer Chromatography Atlas. 2nd ed. New York: Springer-Verlag Berlin Heidelberg; 1984. [Google Scholar]
- 39.Rana VS. Separation and identification of swertiamarin from Enicostema axillare Lam. Raynal by centrifugal partition chromatography and nuclear magnetic resonance-Mass Spectrometry. J Pharm Sci Emerg Drugs. 2013;2:1. [Google Scholar]
- 40.Xu GL, Li HL, He JC, Feng EF, Shi PP, Liu YQ, et al. Comparative pharmacokinetics of swertiamarin in rats after oral administration of swertiamarin alone, Qing Ye Dan tablets and co-administration of swertiamarin and oleanolic acid. J Ethnopharmacol. 2013;149:49–54. doi: 10.1016/j.jep.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13:156–9. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsen K. Creatinine assay by a reaction-kinetic principle. Clin Chim Acta. 1972;41:209–17. doi: 10.1016/0009-8981(72)90513-x. [DOI] [PubMed] [Google Scholar]
- 43.Schumann G, Bonora R, Ceriotti F, Férard G, Ferrero CA, Franck PF, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 5. Reference procedure for the measurement of catalytic concentration of aspartate aminotransferase. Clin Chem Lab Med. 2002;40:725–33. doi: 10.1515/CCLM.2002.125. [DOI] [PubMed] [Google Scholar]
- 44.Doumas BT, Waston WA, Biggs AG. Biuret method for quantitative estimation of total protein in serum or plasma. Clin Chem Acta. 1971;31:87–91. [Google Scholar]
- 45.Garber CC. Jendrassik – Grof analysis for total and direct bilirubin in serum with a centrifugal analyzer. Clin Chem. 1981;27:1410–6. [PubMed] [Google Scholar]
- 46.Kind PR, King D. In vitro determination of serum alkaline phosphatase. J Clin Pathol. 1972;7:322. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 48.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;25(244):6049–55. [PubMed] [Google Scholar]
- 49.Aebi HE. Catalase. In: Burgmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 3. New York: Academic Press; 1983. pp. 273–286. [Google Scholar]
- 50.Boyne AF, Ellman GL. A methodology for analysis of tissue sulfhydryl components. Anal Biochem. 1972;46:639–53. doi: 10.1016/0003-2697(72)90335-1. [DOI] [PubMed] [Google Scholar]
- 51.Tan PV, Mezui C, Enow-Orock G, Njikam N, Dimo T, Bitolog P. Teratogenic effects, acute and sub chronic toxicity of the leaf aqueous extract of Ocimum suave Wild (Lamiaceae) in rats. J Ethnopharmacol. 2008;17(115):232–7. doi: 10.1016/j.jep.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 52.Demiray S, Pintado ME, Castro PM. Evaluation of phenolic profiles and antioxidant activities of Turkish medicinal plant: Tilia argentea, Crataegi folium leaves and Polygonum bistorta roots. World Acad Sci Eng Technol. 2009;54:312–7. [Google Scholar]
- 53.Jaishree V, Badami S. Antioxidant and hepatoprotective effect of swertiamarin from Enicostemma axillare against D-galactosamine induced acute liver damage in rats. J Ethnopharmacol. 2010;130:103–6. doi: 10.1016/j.jep.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 54.Jiang M, Han YQ, Zhou MG, Zhao HZ, Xiao X, Hou YY, et al. The screening research of anti-inflammatory bioactive markers from different flowering phases of Flos Lonicerae Japonicae. PLoS One. 2014;9:e96214. doi: 10.1371/journal.pone.0096214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sonawane RD, Vishwakarma SL, Lakshmi S, Rajani M, Padh H, Goyal RK. Amelioration of STZ-induced type 1 diabetic nephropathy by aqueous extract of Enicostemma littorale Blume and swertiamarin in rats. Mol Cell Biochem. 2010;340:1–6. doi: 10.1007/s11010-010-0393-x. [DOI] [PubMed] [Google Scholar]
- 56.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 57.Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol. 2009;61:223–42. doi: 10.1016/j.etp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Hanigan MH, Devarajan P. Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther. 2003;1:47–61. [PMC free article] [PubMed] [Google Scholar]
- 59.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: A review. Am J Med Sci. 2007;334:115–24. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 60.Pabla N, Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 61.Das J, Ghosh J, Manna P, Sil PC. Taurine protects rat testes against doxorubicin-induced oxidative stress as well as p53, Fas and caspase 12-mediated apoptosis. Amino Acids. 2012;42:1839–55. doi: 10.1007/s00726-011-0904-4. [DOI] [PubMed] [Google Scholar]
- 62.Kawai Y, Nakao T, Kunimura N, Kohda Y, Gemba M. Relationship of intracellular calcium and oxygen radicals to Cisplatin-related renal cell injury. J Pharmacol Sci. 2006;100:65–72. doi: 10.1254/jphs.fp0050661. [DOI] [PubMed] [Google Scholar]
- 63.Yilmaz HR, Iraz M, Sogut S, Ozyurt H, Yildirim Z, Akyol O, et al. The effects of erdosteine on the activities of some metabolic enzymes during cisplatin-induced nephrotoxicity in rats. Pharmacol Res. 2004;50:287–90. doi: 10.1016/j.phrs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Sugiyama S, Hayakawa M, Kato T, Hanaki Y, Shimizu K, Ozawa T. Adverse effects of anti-tumor drug, cisplatin, on rat kidney mitochondria: Disturbances in glutathione peroxidase activity. Biochem Biophys Res Commun. 1989;159:1121–7. doi: 10.1016/0006-291x(89)92225-0. [DOI] [PubMed] [Google Scholar]
- 65.Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol. 2005;289:F166–74. doi: 10.1152/ajprenal.00401.2004. [DOI] [PubMed] [Google Scholar]
- 66.Zhang B, Ramesh G, Norbury CC, Reeves WB. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-alpha produced by renal parenchymal cells. Kidney Int. 2007;72:37–44. doi: 10.1038/sj.ki.5002242. [DOI] [PubMed] [Google Scholar]
- 67.Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol. 2010;594:57–72. doi: 10.1007/978-1-60761-411-1_4. [DOI] [PubMed] [Google Scholar]
- 68.Kumar AR, Sivasudha T, Jeyadevi R, Sangeetha B, Bell GS, Maheshwari M. Profiling of phenolic compounds using UPLC–Q-TOF-MS/MS and nephroprotective activity of Indian green leafy vegetable Merremia emarginata. Food Res Int. 2013;50:94–101. [Google Scholar]
- 69.Annie S, Rajagopal PL, Malini S. Effect of Cassia auriculata Linn. Root extract on cisplatin and gentamicin-induced renal injury. Phytomedicine. 2005;12:555–60. doi: 10.1016/j.phymed.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 70.Huang A, Vita JA, Venema RC, Keaney JF., Jr Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem. 2000;275:17399–406. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- 71.Bursell SE, Clermont AC, Aiello LP, Aiello LM, Schlossman DK, Feener EP, et al. High-dose Vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care. 1999;22:1245–51. doi: 10.2337/diacare.22.8.1245. [DOI] [PubMed] [Google Scholar]
- 72.Weerapreeyakul N, Machana S, Barusrux S. Synergistic effects of melphalan and Pinus kesiya Royle ex Gordon (Simaosong) extracts on apoptosis induction in human cancer cells. Chin Med. 2016;11:29. doi: 10.1186/s13020-016-0103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]