Abstract
Background:
The production of triterpenes from plants for pharmacological purposes varies in concentration, due to genetic and environmental factors. In vitro culture enables the control and increase of these bioactive molecules.
Objective:
To evaluate the effect of plant growth regulators and elicitors in the induction of calli and the production of ursolic acid (UA) and oleanolic acid (OA) in Lepechinia caulescens.
Materials and Methods:
Leaf explants were exposed for the induction of calli at different concentrations and combinations of 2,4-dichlorophenoxyacetic acid (2,4-D) and 6-benzylaminopurine (BAP). Methyl jasmonate (MJ) and salicylic acid were used as elicitors. High-performance liquid chromatography method was used to quantify UA and OA content in each treatment.
Results:
Treatment with 3.0 mg/L of 2,4-D and 0.1 mg/L of BAP produced the best results for calli induction and production of UA (1.57 mg/g dry weight [DW]) and OA (1.13 mg/g DW). Both elicitors facilitated the accumulation of triterpenes.
Conclusion:
The combination of auxins and cytokinins showed favorable results for the induction of calli. Variation concerning the accumulation of UA and OA was observed between treatments. MJ increased the production of triterpenes five times after 8 h of exposure, compared to control treatment. There is a greater accumulation of UA (16.58 mg/g DW) and OA (1.94 mg/g DW) in leaves of wild plants.
SUMMARY
Callus cultures of Lepechinia caulescens were obtained from leaf explants treated with 2,4-dichlorophenoxyacetic acid and 6-bencylaminopurine
Resulting cultures were elicited with methyl jasmonate (MJ) and salicylic acid to increase the production of the triterpenes, ursolic acid (UA), and oleanolic acid (OA)
The cultures elicited with MJ increased the production of UA and OA five times, as compared to the control.
Abbreviations used: 2,4-D: 2,4-dichlorophenoxyacetic acid, BAP: 6-benzylaminopurine, DW: Dry weight, MJ: Methyl jasmonate, OA: Oleanolic acid, PGRs: Plant growth regulators, UA: Ursolic acid, SA: Salicylic acid.
Keywords: Elicitor, Lepechinia caulescens, plant growth regulators, triterpenes
INTRODUCTION
Lepechinia caulescens (Ortega) Epling (Lamiaceae) is commonly known as “bretónica” and has been used in traditional Mexican medicine for the treatment of diabetes, diarrhea, vomit, and hypertension.[1] This plant is rich in substances with biological potential, including terpenoids and sterols.[2,3] Among the triterpenoids reported in L. caulescens, ursolic acid (UA, 3ß-hydroxy-urs-12-en-28-oic acid) and its isomer oleanolic acid (OA) stand out. These triterpenoids are believed to be responsible for many of its medicinal properties.[4,5] Many studies have demonstrated that OA, UA, and their derivatives possess low toxicity,[6] as well as strong antioxidative, anti-inflammatory, anti-cancer, hepatoprotective, antimicrobial, and anti-HIV properties.[7,8] They also possess a potential application for the treatment of leishmaniasis and Chagas disease and type II diabetes.[9,10,11] There are many pharmaceutical and biological properties that make these triterpenes an interesting component for pharmaceuticals, foods, and applications in the cosmetic industry. These compounds are widely found in the plant kingdom. They are present in low and variable quantities in the plant itself depending on its genetic composition and ecological, environmental, and physiological conditions.[12,13] For this reason, plant cell, tissues, and organ culture technology represent an alternative for the production of high-value bioactive compounds, such as UA and OA. The use of plant cell culture techniques has several advantages over traditional cultivation, for example, control of production conditions, weather independency, and continuous production.[14] Moreover, these methods allow amendments for enhanced production of specific secondary metabolites. Several strategies have been investigated to try and enhance the production of UA and OA using plant tissue cultures from different species of the Lamiaceae family.[15,16] However, the potential utilization of these strategies by the pharmaceutical industry has been limited by the low yield of in vitro culture. So far, in vitro cultures of L. caulescens have not been studied in this context and may provide a reliable source of UA and OA. Correspondingly, the purpose of this study was to evaluate the effect of plant growth regulators (PGRs) on the establishment of L. caulescens cell cultures and triterpenes production. This work also aimed to determine the eliciting effect of methyl jasmonate (MJ) and salicylic acid (SA) on accumulation of UA and OA, to validate biotechnological strategies that produce both triterpenes, using in vitro cultures of L. caulescens. To the best of our knowledge, this is the first report on the induction of triterpenes production by MJ and SA, in in vitro cultures of L. caulescens.
MATERIALS AND METHODS
Seed scarification and disinfection protocol
Plants and seeds of L. caulescens were collected from a wild, naturally occurring population in Morelos state, Mexico, at above 2823 m altitude in November 2014. A voucher specimen (32444) was authenticated and deposited in Herbarium of the University of Morelos, Mexico. Mature seeds were surface-sterilized with 70% (v/v) ethanol solution for 1 min. Subsequently, seeds were rinsed three times with sterile distilled water followed by a solution of 50% (v/v) commercial bleach (Clorox®) for 15 min, and finally rinsed three times in sterile, distilled water. Mechanical scarification was performed by nicking the testa of each seed using a scalpel. Afterward, 10 seeds were placed in 60 ml glass jars containing 20 ml of MS solid medium without plant growth regulators (PGRs).[17] All cultures were incubated at 25°C ± 2°C for a 16-h photoperiod (55 μmol m2/s), using a LED lamp. Emergence of the radicle was considered as positive germination.
Callus induction
For callus induction, five glass jars each one with a five-leaf explant segment from a 4 weeks old seedling were inoculated into MS/B5 medium, containing MS salts and B5 vitamins supplemented with 3% sucrose,[17,18] myo-inositol (100 mg/L), and various concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D) (1.0, 2.0, 3.0, 4.0, 5.0, and 6.0 mg/L), alone or in combination with 0.1 mg/L of 6-benzylaminopurine (BAP). Medium without PGRs was used as a control. Medium pH was adjusted to 5.7 ± 0.1 before the addition of Phytagel™ (0.3%). Then, medium was autoclaved at 121°C for 20 min. Cultures were grown under the same conditions as previously described. The callus was subcultured at 4-week intervals on the same media. After 8 weeks of culture, calli were air-dried at room temperature, and their growth was assessed in terms of dry weight (DW).
Elicitor treatment for ursolic and oleanolic acid induction
We studied the effect of MJ and SA on the stimulation of UA and OA in the callus cultures of L. caulescens. After two subcultures, calli initiated on media MS/B5 with 2,4-D (3.0 mg/L) and BAP (0.1 mg/L) were transferred to the same media, supplemented with MJ (50 μM) or SA (360 μM) but without gelling. Calli subcultured in the same liquid medium without inducers were used as control. Cultures were maintained on a rotary shaker at 100 rpm in the dark at 25°C. The calli responses were measured 8 and 24 h after adding the MJ and SA and compared with control cultures. The content of UA and OA in the calli was determined by the high-performance liquid chromatography (HPLC) method and compared with the standard. Experiments were repeated three times and mean values were considered.
Extract preparation
The collected air-dried wild plant material (leaves and stems) and calli were ground into powder and extracted exhaustively by successive maceration (three times) at room temperature with hexane and dichloromethane for 72 h. Following filtration, the extracts were concentrated in vacuum at 40°C using a Rotavapor (Heidolph®) until complete removal of the solvent.
Identification and quantification of ursolic acid and oleanolic acid in dichloromethane extracts
For quantification, we established a calibration curve using UA and OA standard commercial from Sigma-Aldrich (≥97%). Standard stock solution was prepared in methanol to afford 1.56, 3.12, 6.25, 12.5, 25, 50, and 100 μg/mL, which were injected into the HPLC system in triplicate, in 10 μl portions. Standard curve was plotted between peak area (y-axis) and concentration (x-axis), revealing good linearity. Chromatographic analysis was performed using an HPLC-Waters 996 photodiode array detector. Control of the equipment, data acquisition, processing, and management of chromatographic information were performed by Empower 2002 software program. A Waters XBridge™ C18 column (4.6 mm × 75 mm) was used. The mobile phase was composed of methanol: acid water (H3 PO4) 83:17 (v/v) and pumped with a flow rate of 0.9 ml/min. The sample volume injected was set at 10 μL. Each sample was analyzed in triplicate. Eluting compounds were detected by monitoring at 205 nm.
Data collection and statistical analysis
For calculation of the relative change of UA and OA content after elicitor treatment, the average relative concentration values were expressed as the ratio of treated to untreated callus. In each case, total error was calculated. All data obtained were analyzed with the statistical program Statistic 7 using a completely randomized design. All data were subjected to a one-way analysis of variance. The significance of difference between control and each treatment was analyzed using Tukey test with P < 0.05.
RESULTS AND DISCUSSION
Effect of 2,4-dichlorophenoxyacetic acid and 6-benzylaminopurine on the establishment of Lepechinia caulescens cell cultures and triterpene production
Seventy percent of mature scarified seeds of L. caulescens were germinated on day 20. Maximum callusing response (100%) was recorded at 2.0 and 3.0 mg/L of 2,4-D with 0.1 mg/L of BAP. This showed highest cell growth and green compact callus that spread to become full explant at day 28. Calli with brown and necrotic areas were observed in callus inducing medium, supplemented with 1.0, 4.0, 5.0, or 6.0 mg/L 2,4-D and 0.1 mg/L BAP, after 2–3 weeks of culture. No callus formation or growth was observed on explants inoculated on MS media without PGRs or that supplemented only with 2,4-D or BAP. This inhibition of cell growth may be due to low concentration of BAP or to the overexpression of auxin response regulator genes promoted by the presence of 2,4-D. This can attenuate cytokinin signaling to a sufficiently low level, resulting in reduced sensitivity to cytokinin and affecting cell division, as previously reported.[19,20]
To quantify UA and OA, we constructed the calibration curves using the HPLC method. Retention time Rt of UA was equal to 10.9 ± 0.2 min and correlation coefficient (r) was 0.99, and for OA, its Rt was 10.1 ± 0.2 min and r was 0.99. UA and OA were successfully identified and quantified in all calli cultures. Leaves from wild-growing plants showed the highest content of UA and OA at a concentration of 16.58 ± 0.05 and 1.94 ± 0.05 mg/g of dry plant material (DW) respectively. Nevertheless, callus concentration was lowest and influenced by the PGR in the culture medium [Figure 1 and Table 1]. The results based on DW showed mean values for UA and OA contents for calli cultures range from 0.37 to 1.57 mg/g DW and 0.38 to 1.13 mg/g DW, respectively. The highest yield of UA (1.57 ± 0.01 mg/g DW) and OA (1.13 ± 0.01 mg/g DW) was detected in callus grown in MS/B5 medium containing 3.0 mg/L 2,4-D and 0.1 mg/L BAP. Previous studies have demonstrated that the presence of PGRs in culture media significantly influenced the morphogenetic potential and secondary metabolite production in medicinal plant species.[21,22] In other species of Lamiaceae, similar results were observed by Pandey et al.,[23] who found variation in the content of betulinic acid (pentacyclic triterpenoid) depending upon the auxin sources used in the calli of three Ocimum species. Similarly, the UA and OA contents in calli and suspension cells from several Salvia species varied depending on growth conditions.[15,16,24,25] The results clearly indicate that calli cultures have low concentration of bioactive compound compared to leaves or stems from plants growing in the wild. However, in L. caulescens, the concentration of UA and OA was high when compared to cell cultures from other species.[16,25] Organization and cell differentiation may explain the differences in concentration observed between tissues evaluated; this involves differential mechanisms of gene expression regulation and therefore differences in the biosynthesis and accumulation of bioactive compound in callus and parts of plant.
Figure 1.
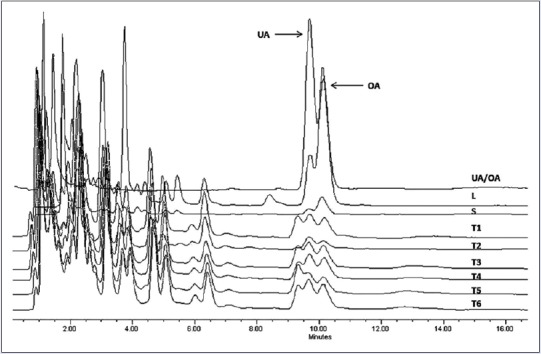
High-performance liquid chromatography profiling of ursolic acid and oleanolic acid presented in the crude extracts of Lepechinia caulescens wild plant and callus tissues. Chromatographic conditions: C18 column (4.6 mm × 75 mm), methanol: acid water (H3PO4) 83:17 (v/v), flow rate of 0.9 mL/min, and 205 nm detection. L: Natural leaves; S: Natural stems
Table 1.
Influence of plant growth regulators on growth of callus and ursolic and oleanolic acid content in Lepechinia caulescens
Effect of methyl jasmonate and salicylic acid on accumulation of triterpenes
The efficiency of UA and OA production using calli cultures can be increased with an elicitation. Several studies have demonstrated that MJ and SA act as elicitors of secondary metabolite production throughout the plant kingdom.[26,27] Exogenous application of MJ and SA stimulates the activation of gene expression[28] and accumulation of secondary metabolites, such as phenylpropanoids, alkaloids, and terpenoids.[29] As elicitation manifests a time-dependent response, the influence of MJ and SA on L. caulescens callus was performed with incubation periods of 8 and 24 h. The elicitor, similar concentrations, and exposure time evaluated in this paper resemble those used for others species of Lamiaceae.[15,30] Our experiment shows that both elicitors increase the content of triterpenes. Treatments with MJ (50 μM) and SA (360 μM) resulted in an approximately fivefold increase in the content of both triterpenes, compared to the control after 8 h of culture exposure. However, 24 h after elicitation, the color of calli turned from light green to brown, which was accompanied by response suppression to MJ treatments, as reflected by a drastic fall in the accumulation of both triterpenes [Figure 2]. This was probably due to significant biological toxicity caused by long exposure to elicitors; this often leads to the generation of oxidative damage that can result in cell death.[31] Negative effects on cell growth and viability were observed to occur in Calendula officinalis, especially after 48 h treatment of the suspension cultures with application of jasmonic acid.[32]
Figure 2.
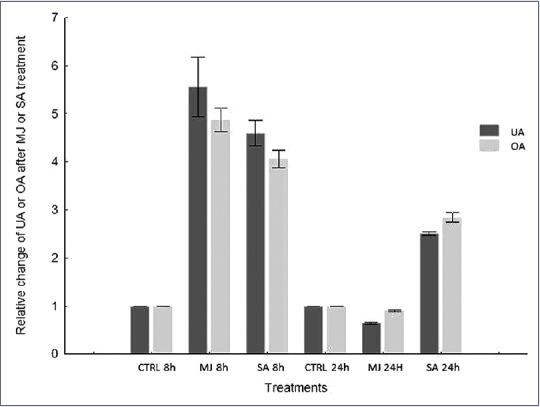
Effect of methyl jasmonate and salicylic acid on ursolic and oleanolic acid accumulation in calli cultures of Lepechinia caulescens. h: Hours
CONCLUSION
UA and OA concentration in L. caulescens callus cultures proved to be strongly affected by the amount of PGRs applied. Out of the treatments tested, the highest UA and OA content was achieved using the combination 3.0 mg/L 2,4-D and 0.1 mg/L BAP. Likewise, the elicitation of callus triggers the production of these important compounds. MJ treatment augmented the production of UA and OA compared to SA treatment, producing a 5.6-fold increase of UA and 4.7-fold increase of OA after 8 h of elicitation compared to the control. A more complete understanding of the activity of genes involved in the biosynthesis pathway of triterpenes will help us to achieve a highly sustained rate of UA and OA. The results indicate that callus cultures of L. caulescens have the potential to produce important triterpenoids for use in cosmetic and health products.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Victor Vergara-Martínez acknowledges fellowship 559379/296765 from CONACYT. We are also grateful to Centro de Investigación en Biotecnología of UAEM for providing necessary facilities with which to conduct this research. We are indebted to M.I. Ariadna Zenil Rodríguez Cisneros for technical assistance.
REFERENCES
- 1.Monroy-Ortiz C, Castillo-España P. Plantas Medicinales Utilizadas en el Estado de Morelos. 2nd ed. México: CONABIO; 2007. [Google Scholar]
- 2.Delgado G, Hernández J, Chávez MI, Alvarez L, Gonzaga V, Martínez E. Di-and triterpenoid acids from Lepechinia caulescens. Phytochemistry. 1994;37:1119–21. [Google Scholar]
- 3.Delgado G, Sánchez E, Hernández J, Chávez MI, Alvarez L, Martínez E. Abietanoid acids from Lepechinia caulescens. Phytochemistry. 1992;31:3159–61. [Google Scholar]
- 4.Aguirre-Crespo F, Vergara-Galicia J, Villalobos-Molina R, Javier López-Guerrero J, Navarrete-Vázquez G, Estrada-Soto S, et al. Ursolic acid mediates the vasorelaxant activity of Lepechinia caulescens via NO release in isolated rat thoracic aorta. Life Sci. 2006;79:1062–8. doi: 10.1016/j.lfs.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Estrada-Soto S, Rodríguez-Avilez A, Castañeda-Avila C, Castillo-España P, Navarrete-Vázquez G, Hernández L, et al. Spasmolytic action of Lepechinia caulescens is through calcium channel blockade and NO release. J Ethnopharmacol. 2007;114:364–70. doi: 10.1016/j.jep.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Liu J. Oleanolic acid and ursolic acid: Research perspectives. J Ethnopharmacol. 2005;100:92–4. doi: 10.1016/j.jep.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 7.Pollier J, Goossens A. Oleanolic acid. Phytochemistry. 2012;77:10–5. doi: 10.1016/j.phytochem.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Kashyap D, Tuli HS, Sharma AK. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016;146:201–13. doi: 10.1016/j.lfs.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 9.da Silva Ferreira D, Esperandim VR, Toldo MP, Kuehn CC, do Prado Júnior JC, Cunha WR, et al. In vivo activity of ursolic and oleanolic acids during the acute phase of Trypanosoma cruzi infection. Exp Parasitol. 2013;134:455–9. doi: 10.1016/j.exppara.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Labib RM, Ebada SS, Youssef FS, Ashour ML, Ross SA. Ursolic acid, a natural pentacylcic triterpene from Ochrosia elliptica and its role in the management of certain neglected tropical diseases. Pharmacogn Mag. 2016;12:319–25. doi: 10.4103/0973-1296.192207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camer D, Yu Y, Szabo A, Huang XF. The molecular mechanisms underpinning the therapeutic properties of oleanolic acid, its isomer and derivatives for type 2 diabetes and associated complications. Mol Nutr Food Res. 2014;58:1750–9. doi: 10.1002/mnfr.201300861. [DOI] [PubMed] [Google Scholar]
- 12.Jäger S, Trojan H, Kopp T, Laszczyk MN, Scheffler A. Pentacyclic triterpene distribution in various plants-rich sources for a new group of multi-potent plant extracts. Molecules. 2009;14:2016–31. doi: 10.3390/molecules14062016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv Y, Tahir II, Olsson ME. Factors affecting the content of the ursolic and oleanolic acid in apple peel: Influence of cultivars, sun exposure, storage conditions, bruising and Penicillium expansum infection. J Sci Food Agric. 2016;96:2161–9. doi: 10.1002/jsfa.7332. [DOI] [PubMed] [Google Scholar]
- 14.Smetanska I. Production of secondary metabolites using plant cell cultures. Adv Biochem Eng Biotechnol. 2008;111:187–228. doi: 10.1007/10_2008_103. [DOI] [PubMed] [Google Scholar]
- 15.Haas C, Hengelhaupt KC, Kümmritz S, Bley T, Pavlov A, Steingroewer J. Salvia suspension cultures as production systems for oleanolic and ursolic acid. Acta Physiol Plant. 2014;36:2137–47. [Google Scholar]
- 16.Georgiev V, Marchev A, Haas C, Weber J, Nikolova M, Bley T, et al. Production of oleanolic and ursolic acids by callus cultures of Salvia Tomentosa Mill. Biotechnol Equip. 2011;25(Supp1):S34–8. [Google Scholar]
- 17.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–97. [Google Scholar]
- 18.Gamborg OL, Miller RA, Ojima K. Nutrient requirement of suspension cultures of soybean root cells. Exp Cell Res. 1968;50:151–8. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- 19.Müller B, Sheen J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature. 2008;453:1094–7. doi: 10.1038/nature06943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandler JW, Werr W. Cytokinin-auxin crosstalk in cell type specification. Trends Plant Sci. 2015;20:291–300. doi: 10.1016/j.tplants.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad N, Abbasi BH, ur Rahman I, Fazal H. Piper nigrum: Micropropagation, antioxidative enzyme activities, and chromatographic fingerprint analysis for quality control. Appl Biochem Biotechnol. 2013;169:2004–15. doi: 10.1007/s12010-013-0104-7. [DOI] [PubMed] [Google Scholar]
- 22.Raj D, Kokotkiewicz A, Drys A, Luczkiewicz M. Effect of plant growth regulators on the accumulation of indolizidine alkaloids in Securinega suffruticosa callus cultures. Plant Cell Tissue Organ. 2015;123:39–45. [Google Scholar]
- 23.Pandey H, Pandey P, Singh S, Gupta R, Banerjee S. Production of anti-cancer triterpene (betulinic acid) from callus cultures of different Ocimum species and its elicitation. Protoplasma. 2015;252:647–55. doi: 10.1007/s00709-014-0711-3. [DOI] [PubMed] [Google Scholar]
- 24.Bolta Ž, Baričevič D, Bohanec B, Andrenšek S. A preliminary investigation of ursolic acid in cell suspension culture of Salvia officinalis. Plant Cell Tissue Organ. 2000;62:57–63. [Google Scholar]
- 25.Feria-Romero I, Lazo E, Ponce-Noyola T, Cerda-García-Rojas CM, Ramos-Valdivia AC. Induced accumulation of oleanolic acid and ursolic acid in cell suspension cultures of Uncaria tomentosa. Biotechnol Lett. 2005;27:839–43. doi: 10.1007/s10529-005-6215-7. [DOI] [PubMed] [Google Scholar]
- 26.De Geyter N, Gholami A, Goormachtig S, Goossens A. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 2012;17:349–59. doi: 10.1016/j.tplants.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Hayat S, Irfan M, Wani A, Nasser A, Ahmad A. Salicylic acids: Local, systemic or inter-systemic regulators? Plant Signal Behav. 2012;7:93–102. doi: 10.4161/psb.7.1.18620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shabani L, Ehsanpour AA, Esmaeili A. Assessment of squalene synthase and beta-amyrin synthase gene expression in licorice roots treated with methyl jasmonate and salicylic acid using real-time qPCR. Russ J Plant Physiol. 2010;57:480–4. [Google Scholar]
- 29.Giri CC, Zaheer M. Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: Recent trends and a sky eye view appraisal. Plant Cell Tissue Organ. 2016;1:1–18. [Google Scholar]
- 30.Misra RC, Maiti P, Chanotiya CS, Shanker K, Ghosh S. Methyl jasmonate-elicited transcriptional responses and pentacyclic triterpene biosynthesis in sweet basil. Plant Physiol. 2014;164:1028–44. doi: 10.1104/pp.113.232884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Qian J, Yao L, Lu Y. Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresour Bioprocess. 2015;2:1–9. [Google Scholar]
- 32.Wiktorowska E, Długosz M, Janiszowska W. Significant enhancement of oleanolic acid accumulation by biotic elicitors in cell suspension cultures of Calendula officinalis L. Enzyme Microb Technol. 2010;46:14–20. [Google Scholar]


