Abstract
Background:
Asparagus adscendens Roxb. (Asparagaceae), is native to the Himalayas. This plant has been used in the prevention and effective treatment of various forms of cancers.
Objective:
This paper reports, for the first time, on the cytotoxicity of the methanol (MeOH) extract of the roots of A. adscendens and its solid-phase extraction (SPE) fractions against four human carcinoma cell lines and LC-ESI-QTOF-MS analysis of the SPE fractions.
Materials and Methods:
Finely powdered roots of A. adscendens were macerated in methanol and extracted through SPE using gradient solvent system (water: methanol) proceeded for analysis on LC-ESI-QTOF-MS and cytotoxicity against four human carcinoma cell lines: breast (MCF7), liver (HEPG2), lung (A549), and urinary bladder (EJ138), using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide assay.
Results:
The MeOH extract and four SPE fractions exhibited cytotoxicity against all cell lines with the IC50 values ranging from 6 to 79 μg/mL. As observed in other Asparagus species, the presence of saponins and sapogenins in the SPE fractions was evident in the liquid chromatography-mass spectrometry data.
Conclusion:
It is reasonable to assume that the cytotoxicity of the MeOH extract of the roots of A. adscendens and its SPE fractions, at least partly, due to the presence of saponins and their aglycones. This suggests that A. adscendens could be exploited as a potential source of cytotoxic compounds with putative anticancer potential.
SUMMARY
The MeOH extract and all solid-phase extraction (SPE) fractions exhibited various levels of cytotoxicity against all cell lines with the IC50 values ranging from 6 to 79 μg/mL
The presence of saponins and sapogenins in the SPE fractions was evident in the Liquid chromatography-mass spectrometry data
Due to the presence of saponins and their aglycones, suggest that A. adscendens could be exploited as a potential source of cytotoxic compounds with putative anticancer potential.
Abbreviation used: SPE: Solid-phase extraction, MCF7: Breast cancer cell line, HEPG2: Liver cancer cell line, A549: Lung liver cancer cell line, EJ138: Urinary bladder cancer cell line, MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide, LC-MS: Liquid chromatography-mass spectrometry.
Keywords: 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazoliumbromide, asparagaceae, Asparagus adscendens, cancer, cytotoxicity, liquid chromatography-mass spectrometry, sapogenins, saponin
INTRODUCTION
Asparagus adscendens Roxb.(Asparagaceae), commonly known as “safed musli” in Pakistan and “Shatawari,” “Shatavar,” “Shatamuli,” “Sahasrapal,” or “Sainsarbuti” in India, is native to the Himalayas.[1] This plant grows 1–2 m tall and prefers to take root in gravelly, rocky soils high up in piedmont plains, at 1300–1500 m above the sea level.[2] This plant that has been used for a long time as a component of various complementary and alternative medicinal preparations in India and Pakistan.[3] Conventionally, A. adscendens is considered to be a general health promoting tonic and has been used to treat various sexual disorders in men.[4]
The genus Asparagus comprises of about 300 species, and most of the European species are used as vegetables.[5] Of the species that grow in the Himalayan region of Pakistan, A. adscendens and A. racemose, are the most commonly used species in traditional medicines. Aliphatic, nitrogenous and phenolic compounds, saponins, steroids, and triterpenoids have been reported from A. adscendens of Indian origin;[1] β-sitosterol glucoside, spirostanol glycosides (asparanin C and asparanin D), and furostanol glycosides (asparoside C and asparoside D) were isolated;[6] steroidal saponins and glycosides, and various lipophilic compounds were found in the tuberous roots and leaves;[2] sarsasapogenin, diosgenin, b-sitosterol glucoside, spirostanol glycosides (asparanin A and B), and furostanol glycosides (asparoside A and B) [Figure 1], were isolated from this plant.[7,8,9]
Figure 1.
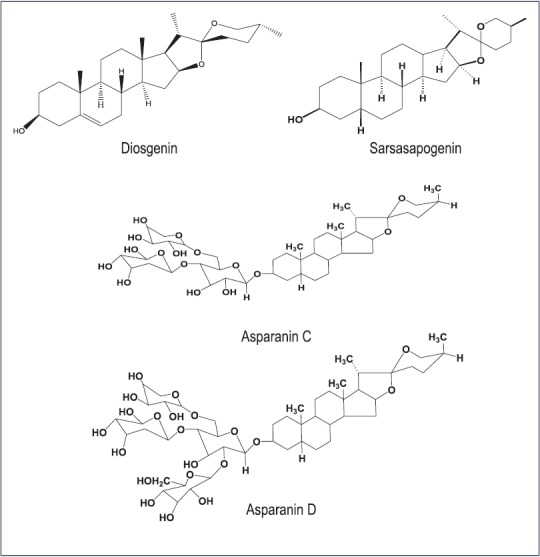
Major saponins and sapogenins previously isolated from Asparagus adscendens
Due to its aphrodisiac and immunomodulatory properties, A. adscendens has become one of the most commercially exploited species in India and Pakistan.[10] Nowadays, A. adscendens has been used in the prevention and effective treatment of various forms of cancers.[11] In addition, several pharmacological properties of the plants of the genus Asparagus have been well-documented, including antimicrobial,[12] anti-inflammatory, antitussive,[13] hepatoprotective,[14] immunomodulatory activity,[10] antistress,[3] anti-secretory, and antiulcer activity.[5,15] A study of ancient classical Ayurvedic literature claimed several therapeutic attributes for the root of Asparagus and has been especially recommended in cases of threatened abortion and as a galactogogue.[16] However, with the only exception of the report on the isolation and identification of conypododiol from A. adscendens,[17] and significant acetylcholinesterase-and butyrylcholinesterase-inhibitory activity (and inactivity against monkey kidney epithelial cell (LCMK-2) and mice hepatocytes),[1] to the best of our knowledge, there has been no systematic pharmacological and phytochemical work performed on A. adscendens native to Pakistan. Therefore, this study was undertaken to explore potential cytotoxicity of the MeOH extract of the roots of A. adscendens and its solid-phase extraction (SPE) fractions against four human carcinoma cell lines: breast (MCF7), liver (HepG2), lung (A549), and urinary bladder (EJ138) using the in vitro 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) cytotoxicity/viability assay and to carry out LC-ESI-QTOF-MS analysis of the SPE fractions.
MATERIALS AND METHODS
Reagents and chemicals
All chemicals were purchased from Sigma-Aldrich (Dorset, UK). Solvents were obtained from Fischer Scientific (Loughborough, UK). All cell culture reagents were purchased from Biosera (Nauaille, France).
Plant materials
Plant sample was collected from Nathia Gali, region of Khyber Pakhtunkhwa, Pakistan and identified as A. adscendens Roxb. by Dr. Muhammad Zafar, Herbarium Botanist, Department of Plant Sciences, Quaid-I-Azam University, Islamabad, Pakistan. A herbarium specimen (voucher number: Acc no. PAC1001) has been deposited and retained in the above herbarium.
Extraction and preparation of plant samples
Shade-dried and finely powdered roots (2.5 kg) of A. adscendens were macerated in MeOH (5 L) for 10 days at room temperature, filtered, and the solvent was evaporated under vacuum using a rotatory evaporator (<45°C) to get a concentrated gummy crude extract.
Solid-phase extraction and sample purification
The procedure was similar to that described.[18] In brief, a portion of the dried MeOH extract (2 g) was suspended in 20 mL of HPLC grade water and loaded onto a Strata C-18 cartridge (20 g), initially washed with MeOH (50 mL) followed by equilibration with water (100 mL). The cartridge was eluted with MeOH-water mixture of decreasing polarity to generate four fractions: 20, 50, 80, and 100% MeOH in water (250 mL each), coded respectively, as AAMF1, AAMF2, AAMF3, and AAMF4, evaporated to dryness using a rotary evaporator and a freeze-dryer, re-dissolved in MeOH (10 mg/mL), centrifuged at 12,000 rpm for 3 min, filtered through 0.20 μm sterile syringe filter for injection (10 μL) into the Liquid chromatography-mass spectrometry (LC-MS) system.
Liquid chromatography-mass spectrometry
An Alliance HPLC System 2695 (Waters) was used. Reversed-phase chromatography was performed on a Phenomenex Gemini-NX 5 mm C18 column (250 mm × 4.6 mm). The column temperature was set at 25°C. A variable wavelength UV-Vis detector was set at 220 nm. An elution gradient was applied with solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in MeOH). The initial mobile phase composition was 70% of A and 30% B at 0 min, then linear gradient to 100% of B over 30 min and held at that composition for 5 min (flow rate of 1 mL/min).
The LC system was connected to a quadrupole time-of-flight (TOF) mass spectrometer (Waters Micromass LCT) having an electrospray ion source. The response was recorded in real time by the mass spectrometer data system (Waters MassLynx version 4.1). The tuning parameters were set as follows: electrospray interface 3000 V, rangefinder lens 250 V, extraction cone 3 V, desolvation temperature 20°C, source temperature 100°C, nebulizer gas flow 20 L/h, desolvation gas flow 760 L/h, and TOF tube 4687 V. Data acquisition method was set as follows: cycle time 1 s, scan duration 0.9 s, inter-scan delay 0.1 s, mass range 100–1600, and centroid mode. Positive ion mode was operated with many cone voltage settings of 40V.
Cell lines, cell cultures, and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide assay
The potential cytotoxicity of the MeOH extract of the roots of A. adscendens and its SPE fractions was studied against four human carcinoma cell lines: breast (MCF7), liver (HepG2), lung (A549), and urinary bladder (EJ138) using the MTT assay.[18,19,20] The cells were washed by phosphate buffer saline and harvested by trypsinization. All cell lines were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum. All cells were cultured at 37°C in 95% air and 5% CO2. For the MTT assay, cells were seeded into 24 well plates at density 1.2 × 104 cells/well in a working volume of 1 mL/well and allowed to grow for 24 h before the commencement of each experiment.
The cells were treated for 24 h with different concentrations of test samples (the MeOH extract and SPE fractions; 0, 0.8, 4, 20, 100, and 500 μg/mL). Dilution of stock solutions was made in culture medium yielding final sample concentrations with a final dimethyl sulfoxide concentration of 0.1%, including the control. Each sample was used to treat four wells of cells in each 24-well plate. After 24-h treatment period, the cytotoxicity of the samples on each carcinoma cell line was quantified using MS Excel. To achieve this, the medium in each well was replaced by MTT solution (500 μg/mL) and incubated for 2 h. Toxicity was assessed by the ability of the cells to reduce the yellow dye MTT to a blue formazan product.[21] MTT reagent was removed, and the formazan crystals produced by viable cells were dissolved in isopropanol and absorbance (560 nm) was determined with the microplate reader (CLARIO Star Microplate reader, BMG Labtech, UK). The average absorbance obtained from all the control wells (without test sample) on each plate was arbitrarily set at 100% and the absorbance value for the average of wells of cells treated with each test samples was expressed as a percentage of this control. Each assay was performed on a minimum of three separate occasions, and the IC50 values were for each sample on each cell line were calculated using Microsoft Excel version 2013 (Redmond, WA, USA).
Statistical analysis
The data were expressed as mean values ± standard error of the mean (SEM) of three parallel replicates. The graph was plotted using nonlinear regression with the use of Microsoft Excel version 2013. The means were separated at confidence level P ≤ 0.05 using analysis of variance with Tukey's range test.
RESULTS AND DISCUSSION
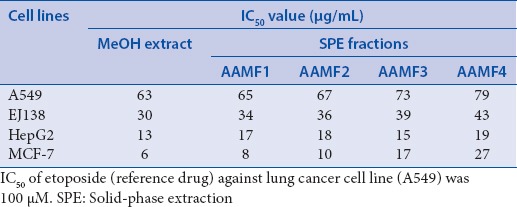
The MeOH extract of the roots of A. adscendens and its SPE fractions (AAMF1, AAMF2, AAMF3 and AAMF4) displayed different levels of cytotoxicity against four human carcinoma cell lines, breast (MCF7), liver (HepG2), lung (A549) and urinary bladder (EJ138) in the in vitro MTT cytotoxicity/viability assay [Table 1]. The MeOH extract exhibited the highest level of cytotoxicity against the breast cancer cell line (MCF7; IC50 =6 μg/mL), but it was also active against three other cell lines, HepG2, EJ138, and A549 (IC50 =13, 30 and 63 μg/mL, respectively) [Table 1]. The SPE fraction AAMF1, which had the most polar components of the parent MeOH extract, showed the most significant cytotoxicity against MCF7 (IC50 =8 μg/mL), and considerable cytotoxicity against HepG2, EJ138, and A549 with the IC50 values of 17, 34, and 65 μg/mL, respectively. This SPE fraction was almost as potent as its parent MeOH extract in terms of cytotoxicity against the urinary bladder cell line EJ138. The SPE fraction AAMF2 showed most prominent cytotoxicity against the breast cancer cell line MCF7 (IC50 =10 μg/mL), and also activity against other three cell lines, HepG2, EJ138, and A549 (IC50 =18, 36 and 67 μg/mL, respectively). The cytotoxicity pattern of the SPE fraction AAMF3 was quite similar to that of the AAMF1 and the MeOH extract; it showed most significant cytotoxicity against the HepG2 cell line (IC50 =15 μg/mL). This fraction was also cytotoxic to MCF7, EJ138, and A549 cell lines with the IC50 values of 17, 39, and 73 μg/mL, respectively. The SPE fraction AAMF4, which contained the least polar components of the parent MeOH extract, exhibited notable cytotoxicity against the HepG2 cell line (IC50 =19 μg/mL), and was also active against the MCF7, EJ138, and A549 cell lines (IC50 =27, 43 and 79 μg/mL, respectively). With the exception of AAMF3 and AAMF4, two other SPE fractions showed the highest level of cytotoxicity against the MCF7 cell line, as was observed with their parent MeOH extract.
Table 1.
The IC50 values of the MeOH extract of the roots of Asparagus adscendens and its solid-phase extraction fractions against four carcinoma cell lines
This is the first report on cytotoxicity of the MeOH extract of the roots of A. adscendens and its SPE fractions against any carcinoma cell lines. The current finding is in line with the findings of a few other previous studies on cytotoxicity of some other species of the genus Asparagus.[22,23,24,25,26] The bioactive components of the genus Asparagus belong predominantly to the chemical classes of sapogenins and saponins [Figure 1], which are well-known to exhibit cytotoxicity. Most of the compounds isolated previously from the genus Asparagus as described in the literature are steroidal sapogenins and saponins. Sarsasapogenin, diosgenin, β-sitosterol and its glucoside, spirostanol glycoside, and furostanol glycoside were reported from A. adscendens.[2,7,9] In another study, the steroidal saponins from the roots of A. filicinus showed significant cytotoxic activities against human lung carcinoma (A549) and breast adenocarcinoma (MCF7) tumor cell lines,[22] compounds isolated from this species were cytotoxic to human breast adenocarcinoma cell line MDA-MB-231 (IC50 3.4–6.6 μM).[23] A. officinalis displayed significant activity against HeLa and BEL-7404 cells in a dose-dependent manner in vitro at 10 mg/mL.[25] The alkaloid, aspastiluine, isolated from A. stipularis was shown to be active against MCF7 (IC50 =47.7 mm).[26] A series of sarsasapogenin and diosgenin derived steroidal constituents, isolated from A. racemosus, were screened for their ability to induce cell death and apoptosis against HCT-116 human colon carcinoma cell line and the carbohydrates moieties linked to the steroid backbone were found to strongly influence cytotoxic activity and cell death mode.[24]
LC-MS studies on the SPE fractions of the MeOH extract of the roots of A. adscendens were carried out to get an insight into the possible chemical composition of the fractions, particularly, to have an indication whether they contain saponins and sapogenins as possible contributors to the significant cytotoxicity of the extract and its fractions. The chromatographic conditions were optimized by method development. A linear gradient elution with water and MeOH containing 0.1% formic acid as the mobile phase offered the best resolution. Typical chromatograms of fractions with mass spectrometric detection in positive ion mode exhibited quite complex patterns of peaks [Table 2], and only the possible presence of saponins (e.g., Paris saponins VII at tR 18.64 [Figure 2], [M + 1] + m/z 1032) and spirostanol (e.g., Type VI daucosterol at tR 34.82, [M + 1] + m/z 576) in all SPE fractions could be suggested from the retention times and the MS spectral data of the separated peaks.[27,28,29,30] The presence of saponins and their aglycones was in agreement with that of other Asparagus species.
Table 2.
tR and corresponding pseudo molecular ions [M + H]+ of the peaks separated by liquid chromatography and electrospray ionization quadrupole time-of-flight mass spectrometry of four solid-phase extraction fractions of the MeOH extract of the roots of Asparagus adscendens
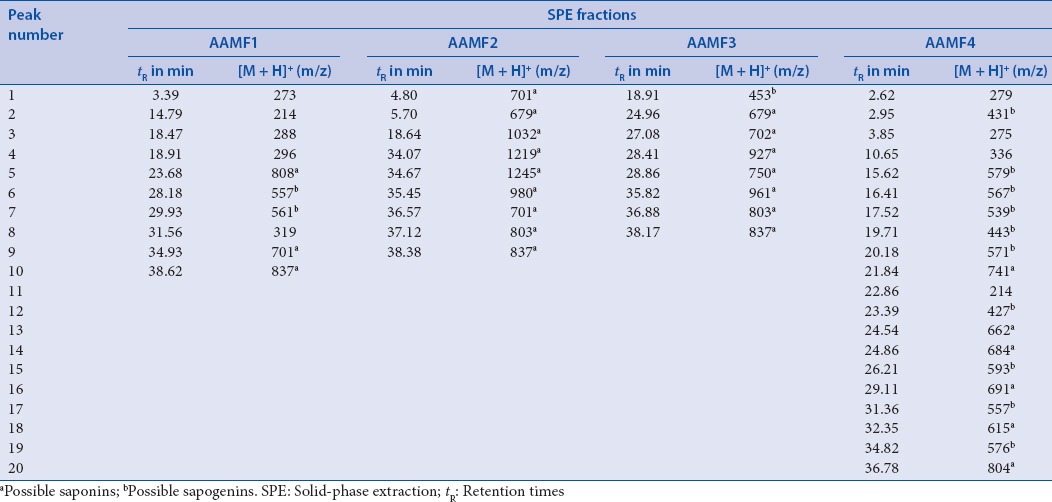
Figure 2.
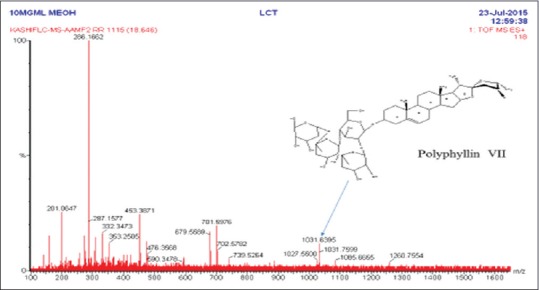
Electrospray ionisation mass spectra of AAMF2 in positive mode. Arrow indicates the proposed structure of compound reported in literature
CONCLUSION
It is reasonable to assume that the cytotoxicity of the MeOH extract and its SPE fractions of the roots of A. adscendens might be, at least partly, due to the presence of saponins and their aglycones, as implicated in several previously published studies outlined earlier. This is the first report, on the preliminary LC-MS analysis on A. adscendens. The significant cytotoxicity observed against four carcinoma cell lines in the current study, and the previously published data on the antitumor/anticancer potential of the genus Asparagus as well as the presence of saponins in A. adscendens, suggest that A. adscendens could be exploited as a potential source of cytotoxic compounds with putative anticancer potential.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mannan A, Khan KM, Arfan M, Ihsan ul H, Hussain I. Phytochemical and biological investigations of Asparagus adscendens growing in Himalayas Region of Pakistan. In Vitro Cell Dev Biol Anim. 2015;51:S58. [Google Scholar]
- 2.Thakur S, Sharma D. Review on medicinal plant: Asparagus adscendens Roxb. Int J Pharm Sci Heath Care. 2015;3:122–37. [Google Scholar]
- 3.Alok S, Jain SK, Verma A, Kumar M, Mahor A, Sabharwal M. Plant profile, phytochemistry and pharmacology of Asparagus racemosus (Shatavari): A review. Asian Pac J Trop Dis. 2013;3:242–51. [Google Scholar]
- 4.Thakur M, Chauhan NS, Bhargava S, Dixit VK. A comparative study on aphrodisiac activity of some ayurvedic herbs in male albino rats. Arch Sex Behav. 2009;38:1009–15. doi: 10.1007/s10508-008-9444-8. [DOI] [PubMed] [Google Scholar]
- 5.Goyal RK, Singh J, Lal H. Asparagus racemosus – An update. Indian J Med Sci. 2003;57:408–14. [PubMed] [Google Scholar]
- 6.Sharma SC, Chand R, Bhatti BS, Sati OP. New oligospirostanosides and oligofurostanosides from Asparagus adscendens roots. Planta Med. 1982;46:48–51. doi: 10.1055/s-2007-970018. [DOI] [PubMed] [Google Scholar]
- 7.Tandon M, Shukla YN. Sapogenins from Asparagus adscendens and Chlorophytum arundinaceum. J Indian Chem Soc. 1992;69:893. [Google Scholar]
- 8.Jadhav A, Bhutani K. Steroidal saponins from the roots of Asparagus adscendens Roxb and Asparagus racemosus Willd. Indian J Chem Sect B. 2006;45:1515–24. [Google Scholar]
- 9.Sharma SC, Chand R, Sati OP. Steroidal sapogenins from the fruits of Asparagus adscendens Roxb. Pharmazie. 1980;35:711–2. [Google Scholar]
- 10.Gautam M, Saha S, Bani S, Kaul A, Mishra S, Patil D, et al. Immunomodulatory activity of Asparagus Racemosus on systemic Th1/Th2 immunity: Implications for immunoadjuvant potential. J Ethnopharmacol. 2009;121:241–7. doi: 10.1016/j.jep.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Jawaid T, Dubey SD. Therapeutic plants of Ayurveda; A review on anticancer. J Phcog. 2011;3:1–11. [Google Scholar]
- 12.Mandal SC, Nandy A, Pal M, Saha BP. Evaluation of antibacterial activity of Asparagus racemosus Willd. root. Phytother Res. 2000;14:118–9. doi: 10.1002/(sici)1099-1573(200003)14:2<118::aid-ptr493>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Jian R, Zeng KW, Li J, Li N, Jiang Y, Tu P, et al. Anti-neuroinflammatory constituents from Asparagus cochinchinensis. Fitoterapia. 2013;84:80–4. doi: 10.1016/j.fitote.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Zhu X, Zhang W, Zhao J, Wang J, Qu W. Hypolipidaemic and hepatoprotective effects of ethanolic and aqueous extracts from Asparagus officinalis L. By-products in mice fed a high-fat diet. J Sci Food Agric. 2010;90:1129–35. doi: 10.1002/jsfa.3923. [DOI] [PubMed] [Google Scholar]
- 15.Bhatnagar M, Sisodia SS. Antisecretory and antiulcer activity of Asparagus racemosus willd. Against indomethacin plus phyloric ligation-induced gastric ulcer in rats. J Herb Pharmacother. 2006;6:13–20. [PubMed] [Google Scholar]
- 16.Umashanker M, Shruti S. Traditional Indian herbal medicine used as antipyretic, antiulcer, anti-diabetic and anticancer: A review. Int J Res Pharm Chem. 2011;1:1152–9. [Google Scholar]
- 17.Khan I, Nisar M, Khan N, Saeed M, Nadeem S, Fazal-ur-Rehman, et al. Structural insights to investigate conypododiol as a dual cholinesterase inhibitor from Asparagus adscendens. Fitoterapia. 2010;81:1020–5. doi: 10.1016/j.fitote.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Khan KM, Nahar L, Al-Groshi A, Zavoianu AG, Evans A, Dempster NM, et al. Cytotoxicity of the roots of Trillium govanianum against breast (MCF7), liver (HepG2), lung (A549) and urinary bladder (EJ138) carcinoma cells. Phytother Res. 2016;30:1716–20. doi: 10.1002/ptr.5672. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Basar N, Oridupa OA, Ritchie KJ, Nahar L, Osman NM, Stafford A, et al. Comparative cytotoxicity of Glycyrrhiza glabra roots from different geographical origins against immortal human keratinocyte (HaCaT), lung adenocarcinoma (A549) and liver carcinoma (HepG2) cells. Phytother Res. 2015;29:944–8. doi: 10.1002/ptr.5329. [DOI] [PubMed] [Google Scholar]
- 21.Popescu T, Lupu AR, Raditoiu V, Purcar V, Teodorescu VS. On the photocatalytic reduction of MTT tetrazolium salt on the surface of TiO2 nanoparticles: Formazan production kinetics and mechanism. J Colloid Interface Sci. 2015;457:108–20. doi: 10.1016/j.jcis.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Zhou LB, Chen TH, Bastow KF, Shibano M, Lee KH, Chen DF, et al. Filiasparosides AD, cytotoxic steroidal saponins from the roots of Asparagus filicinus. J Nat Prod. 2007;70:1263–7. doi: 10.1021/np070138w. [DOI] [PubMed] [Google Scholar]
- 23.Wu JJ, Cheng KW, Zuo XF, Wang MF, Li P, Zhang LY, et al. Steroidal saponins and ecdysterone from Asparagus filicinus and their cytotoxic activities. Steroids. 2010;75:734–9. doi: 10.1016/j.steroids.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Bhutani KK, Paul AT, Fayad W, Linder S. Apoptosis inducing activity of steroidal constituents from Solanum xanthocarpum and Asparagus racemosus. Phytomedicine. 2010;17:789–93. doi: 10.1016/j.phymed.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Q, Xie B, Yan J, Zhao F, Xiao J, Yao L, et al. In vitro antioxidant and antitumor activities of polysaccharides extracted from Asparagus officinalis. Carbohydr Ploym. 2012;87:392–6. doi: 10.1016/j.carbpol.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 26.Galala AA, Ahmad KF, Zaghloul MG, Mansour ES. Two new alkaloids from Asparagus stipularis Forssk roots. Phytochem Lett. 2015;12:220–3. [Google Scholar]
- 27.Woldemichael GM, Wink M. Identification and biological activities of triterpenoid saponins from Chenopodium quinoa. J Agric Food Chem. 2001;49:2327–32. doi: 10.1021/jf0013499. [DOI] [PubMed] [Google Scholar]
- 28.Madl T, Sterk H, Mittelbach M, Rechberger GN. Tandem mass spectrometric analysis of a complex triterpene saponin mixture of Chenopodium quinoa. J Am Soc Mass Spectrom. 2006;17:795–806. doi: 10.1016/j.jasms.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Liang J, Liu J, Zhao Y, Gao J, Sun W, et al. Quality control and identification of steroid saponins from Dioscorea zingiberensis CH. Wright by fingerprint with HPLC-ELSD and HPLC-ESI-quadrupole/Time-of-fight tandem mass spectrometry. J Pharm Biomed Anal. 2014;91:46–59. doi: 10.1016/j.jpba.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao X, Sun W, Fu Q, Niu X. Rapid identification of steroidal saponins in Trillium tschonoskii maxim by ultraperformance liquid chromatography coupled to electrospray ionisation quadrupole time-of-flight tandem mass spectrometry. Phytochem Anal. 2015;26:269–78. doi: 10.1002/pca.2560. [DOI] [PubMed] [Google Scholar]


