Abstract
Background:
Quercetin (QR) and thymoquinone (TQ) are herbal remedies that are currently extensively used by the general population to prevent and treat various chronic conditions. Therefore, investigating the potential of pharmacokinetic interactions caused by the concomitant use of these herbal remedies and conventional medicine is warranted to ensure patient safety.
Purpose of the Study:
This study was conducted to determine the inhibitory effect of QR and TQ, two commonly used remedies, on the activities of selected cytochrome P450 (CYP) enzymes that play an important role in drug metabolism and/or toxicology.
Materials and Methods:
The in vitro studies were conducted using fluorescence-based high throughput assays using human c-DNA baculovirus expressed CYP enzymes. For measuring CYP2E1 activity, a validated High-performance liquid chromatography (HPLC) assay was utilized to measure the formation of 6-hydroxychlorzoxazone.
Results:
The obtained half-maximum inhibitory concentration values with known positive control inhibitors of this study were comparable to the published values indicating accurate experimental techniques. Although QR did not show any significant effect on CYP1A2 and CYP2E1, it exhibited a strong inhibitory effect against CYP2D6 and a moderate effect against CYP2C19 and CYP3A4. On the other hand, TQ demonstrated a strong and a moderate inhibitory effect against CYP3A4 and CYP2C19, respectively.
Conclusions:
The findings of this study may indicate that consumption of QR or TQ, in the form of food or dietary supplements, with drugs that are metabolized by CYP2C19, CYP2D6, or CYP3A4 may cause significant herb-drug interactions.
SUMMARY
Neither QR nor TQ has any significant inhibitory effect on the activity of CYP1A2 or CYP2E1 enzymes
Both QR and TQ have a moderate to strong inhibitory effect on CYP3A4 activity
QR has a moderate inhibitory effect on CYP2C19 and a strong inhibitory effect on CYP2D6
Both QR and TQ are moderate inhibitors of the CYP2C9 activity.
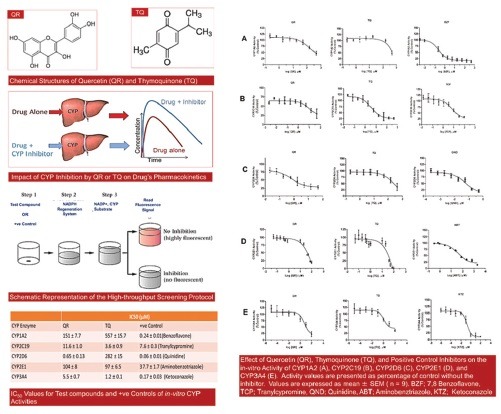
Abbreviations used: ABT: Aminobenztriazole, BZF: 7,8 Benzoflavone, CYP: Cytochrome P450, GB: Gingko Biloba, IC50: Half-maximum inhibitory concentration, KTZ: Ketoconazole, QND: Quinidine, QR: Quercetin, TCP: Tranylcypromine, TQ: Thymoquinone.
Keywords: Cytochrome P450, half-maximum inhibitory concentration, herb-drug interactions, quercetin, thymoquinone
INTRODUCTION
Herbal remedies have been used for the treatment of diseases for more than 2000 years, and their use is being increased. Contrary to public perception, the use of herbal remedies is not risk free. Several and severe herb-drug interactions have been reported in patients who often combine herbal and dietary supplements with their medications.[1] Components of herbal extracts were found to affect metabolism/elimination of conventional pharmaceuticals through modulation of the activity of cytochrome P450 (CYP) enzymes. Such modulation of CYP enzymes could potentially affect the duration of drug action and its therapeutic outcome.[2] CYP enzymes are heme-containing enzymes that play an important role in catalyzing the oxidative biotransformation of drugs to generate more polar and readily excreted metabolites. Human CYP isoforms that are extensively involved in drug biotransformation include CYP1A2, CYP2C19, CYP2D6, CYP2E1, and CYP3A4.[3] These enzymes are vital in the process of drug discovery as the US Food and Drug Administration and other national and international regulatory agencies request a classification of each new drug with respect to CYP enzymes, i.e., substrate, inducer, or inhibitor.[4]
Quercetin (QR) is a strong antioxidant and a major bioflavonoid found in a variety of foods including apples, berries, brassica vegetables, grapes, onions, and tomatoes, as well as medicinal botanicals including Ginkgo Biloba (GB).[5] Although the average daily intake of QR from food sources ranges from 5 to 40 mg, it is also available as a dietary supplement in oral formulations with a recommended daily dose of 1000 mg or more.[6] Recently, QR has gained great scientific and clinical interests since it can improve mental/physical performance and decrease infection risk added to potential benefits to overall health, such as anticarcinogenic, anti-inflammatory, antioxidant, inhibition of lipid peroxidation, platelet aggregation, and capillary permeability.[6] For example, daily consumption of 500 mg QR for 8 weeks by 50 women with rheumatoid arthritis resulted in significant improvements in clinical symptoms, disease activity, high-sensitivity tumor necrosis factor-α, and erythrocyte sedimentation rate.[7] Despite the several clinical trials and animal experimentations suggesting that QR may be a promising natural treatment for and prophylaxis against several chronic illnesses, the impact of QR on human drug metabolizing enzymes is underreported.
The seeds of Nigella sativa, also known as the black seeds, is a herb that is believed to have healing powers in the Islamic tradition and have long been used in folk medicine in the middle and far east as a traditional medicine to treat a wide range of illnesses including allergy, bronchial asthma, headache, infections, obesity, back pain, hypertension, and gastrointestinal problems.[8] Thymoquinone (TQ), the most active ingredient in N. sativa seeds, has demonstrated effects in reducing oxidative stress and the probability of developing cancer.[1] Its safety measured by normal liver enzymes was reported, while its effect on human CYP enzymes has not been documented to our knowledge.
If there is widespread consumption of QR and TQ because of their health-promoting effects, then there should be some understanding of how these herbal remedies may affect drug-metabolizing enzymes. The importance of this is emphasized by the findings that certain phytochemicals in vegetables and fruits have the capacity to modulate drug-metabolizing enzymes and significantly affect pharmacokinetics, and hence pharmacodynamics, of concomitantly administered medications.
Therefore, this study was undertaken to investigate the potential inhibitory effect of QR and TQ on CYP enzymes using recombinant human CYP isoforms by investigating the in vitro effect of QR and TQ on the activities of human CYPs 1A2, 2C19, 2D6, 2E1, and 3A4 enzymes. In our studies, we measured the half maximal inhibitory concentration (IC50) which is a quantitative measurement of the concentration of needed to cause 50% inhibition of the CYP activity.
MATERIALS AND METHODS
These assays were carried out using high throughput inhibitor screening assay kits from BD Gentest™ (Woburn, Suite Tewksbury, MA) and performed according to the provided protocol. The kits contained cDNA-expressed recombinant human CYP enzymes (CYP1A2, CYP2C19, CYP2D6, or CYP3A4) produced by baculovirus-infected insect cells, buffer solution (0.5–1 M potassium phosphate buffer, pH 7.4), stop reagent (Tris base), cofactors (nicotinamide adenine dinucleotide phosphate, MgCl2, and glucose 6-phosphate), glucose 6-phosphate dehydrogenase, and CYP-selective fluorogenic substrates and metabolites [Table 1]. CYP-selective positive control inhibitors were either provided with the kit or purchased from Sigma-Aldrich (St. Louis, USA). Fluorimetric assays were performed using flat-bottom black 96-well plates (Greiner Bio-one, Germany), and the fluorescence intensity was measured using a BioTek Synergy microplate reader (Winooski, VT).
Table 1.
Experimental parameters involved in the cytochrome P450 inhibition screening assays

Screening for CYP2E1 inhibition was conducted using chlorzoxazone as a selective substrate and measuring the formation of 6-hydroxyxhlorzoaxazone as described previously.[9]
QR (purity ≥99%, high-performance liquid chromatography [HPLC]), TQ (purity ≥95%, HPLC), chlorzoxazone (purity ≥98%, HPLC), (6 OH chlorzoxazone) (purity ≥98%, HPLC), and 1-aminobenzotriazole (purity ≥95%, HPLC), were purchased from Sigma-Aldrich (St. Louis, USA). All solvents used for sample preparation were either HPLC or analytical grade.
Results were expressed as mean ± standard error of mean of three replicates with three independent experiments (n = 9). The IC50 values were calculated using the GraphPad Prism 5® (GraphPad Software Inc., Suite 230 La Jolla, CA, USA) by plotting the log concentration of QR, TQ, or positive control inhibitors versus percentage activity of CYP enzymes.
RESULTS AND DISCUSSION
The last two decades have witnessed a significant increase in the number of people who use herbal remedies as complementary and/or alternative to conventional medicine. Although considered “natural,” herbal remedies contain several ingredients that may have the potential to modulate the activity of drug-metabolizing enzymes and therefore alter the pharmacokinetics and pharmacodynamics of the coadministered drugs. To ensure patient safety, research is warranted to assess the possible effect of constituents of herbal products on the major drug-metabolizing enzymes. In this study, we investigated the inhibitory effect of QR and TQ, two active ingredients of commonly used herbal remedies and dietary supplements, on the activity of the five principal human CYP isoforms. These enzymes were selected because they metabolize more than 90% of available drugs in the market. To achieve this goal, we have utilized high throughput CYP inhibition screening assay kits and validated HPLC assays.
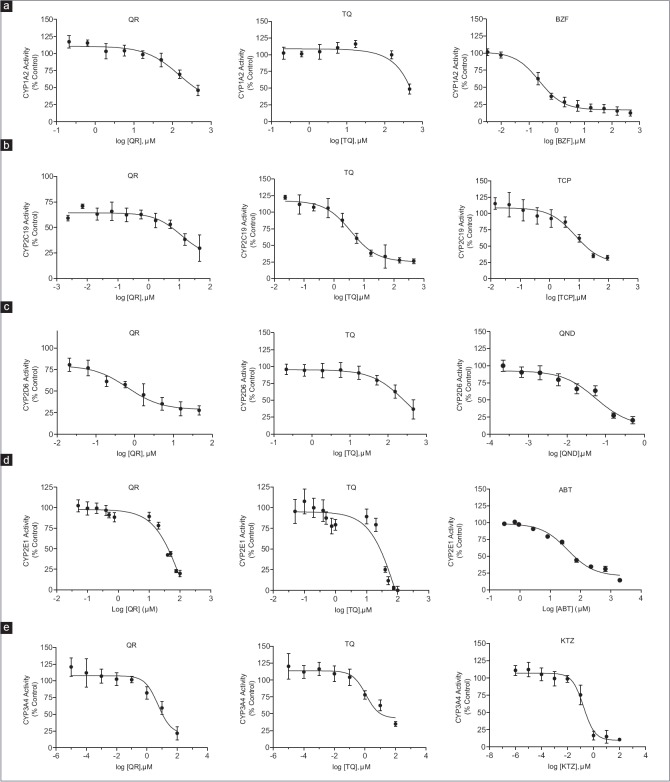
Figure 1 shows the inhibitory effect of QR, TQ, and positive control inhibitors on the activity of the five examined CYP enzymes. The inhibitory effects, reported as IC50 values, of positive controls (7,8 benzoflavone, tranylcypromine, quinidine, ketoconazole, and aminobenztriazole) on the CYP activities were similar to previous reports,[10] which validates the effectiveness of our assays. Our results show that QR and TQ have variable inhibitory effects on the examined CYP isoforms with an order of CYP2D6 > CYP3A4 > CYP2C19 > CYP2E1 > CYP1A2 for QR and CYP3A4 > CYP2C19 > CYP2E1 > CYP2D6 > CYP1A2 for TQ [Table 2].
Figure 1.
Effect of quercetin, thymoquinone, and positive control inhibitors on the in vitro activity of cytochrome P450 1A2 (CYP1A2) (a), cytochrome P450 2C19 (b), cytochrome P450 2D6 (c), cytochrome P450 2E1 (d), and cytochrome P450 3A4 (e). Activity values are presented as percentage of control without the inhibitor. Values are expressed as mean ± standard error of mean (n = 9). BZF: 7,8 Benzoflavone, TCP: Tranylcypromine, QND: Quinidine, ABT: Aminobenztriazole, KTZ: Ketoconazole
Table 2.
Half-maximum inhibitory concentration values (μM) of quercetin, thymoquinone, and positive control inhibitors in inhibiting activities of selected human cytochrome P450enzymes
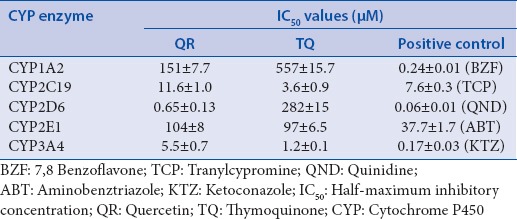
Based on the inhibition evaluation standards[10] (IC50<1 μM, potent inhibition, IC50≥1≤10 μM, moderate inhibition, and IC50≥10 μM, weak inhibition), neither QR nor TQ has any significant inhibitory effect on the activity of CYP1A2 or CYP2E1 enzymes [Figure 1a and d], and TQ has no inhibitory effect on CYP2D6 [Figure 1c]. Therefore, clinically relevant herb-drug interactions of QR or TQ with substrates of these isoforms are considered unlikely. Inconsistent with our results, oral administration of 1 ml/kg N. sativa oil everyday for 1 week resulted in a drastic suppression of mRNA and protein expression of CYP1A2 and CYP2E1 in rats.[11] Although this study did not measure enzyme activity, contribution of active components of the N. sativa oil other than TQ on the mRNA and protein expression should not be ignored.
Our results also indicate that both QR and TQ have a moderate to strong inhibitory effect on CYP3A4 activity [Figure 1e]. Inhibition of CYP3A4 activity by QR and TQ has been demonstrated in earlier in vitro and animal studies. Oral administration of Ginkgo leaf tablet and QR to male Sprague Dawley rats resulted in suppression of CYP3A activity as reflected by a significant increase in maximum plasma concentration (Cmax), area under the concentration-time curve (area under the curve [AUC]), and elimination half-life (t½), of amlodipine, a CYP3A4 substrate.[12] Similarly, alcoholic extract of Centella asiatica, relatively rich in QR, showed potent noncompetitive inhibition of CYP3A4 enzyme in human liver microsomes (HLM).[13] Diabetic rats treated with a single dose TQ demonstrated a marked decrease in hepatic protein expressions of CYP3A2 enzymes as reflected by 32.0% and 17.4% increase in the AUC and t½ of glibenclamide, respectively.[14] Using human subjects and N-demethylation of dextromethorphan to 3-methoxymorphinan as a marker for CYP3A4 activity, Al-Jenoobi et al. reported a 1.6-fold decrease in CYP3A4 activity after consumption of black seed extract rich in TQ.[15]
As CYP3A4 isoform is responsible for the metabolism of 50% of all pharmaceutical agents, modulation of its activity by QR and TQ may result in a high risk of herb-drug interactions especially for CYP3A4 substrates with a narrow therapeutic index such as cyclosporine, tacrolimus, and carbamazepine.
Our study showed that QR has a moderate inhibitory effect on CYP2C19 (IC50 =11.6 μM) and a strong inhibitory effect on CYP2D6 (IC50 =0.65 μM) [Figure 1b and c]. These results are in agreement with an in vitro study using HLMs where QR was found to be a potent competitive inhibitor of CYP2C19 and a moderate competitive inhibitor of CYP2D6.[16] Hyperoside, QR-3-O-galactoside, strongly inhibited CYP2D6-catalyzed dextromethorphan O-demethylation, with IC50 value of 0.81 μM in HLM.[17] However, a number of small clinical studies reported inconsistent effect of GB on the activity of CYP2C19. For example, Yin et al. examined the effect of GB extract on CYP2C19 in 18 healthy Chinese controls using omeprazole as a probe substrate and concluded that the inhibitory effect of GB on CYP2C19 is rather weak and may have only limited clinical significance.[18] Other studies reported induction or no effect on CYP2C19 activity. Similarly, although our study did not show any inhibitory effect of TQ on the activity of CYP2D6, administration of 2.5 g N. sativa seed twice daily for 7 days resulted in inhibition of the metabolic activity of CYP2D, using dextromethorphan as specific substrate, in four healthy human subjects (Al-Jenoobi, Korashy, Ahad, Raish, Al-Mohizea, Alam, Al-Suwayeh, and Alkharfy, 2014). These discrepancies may be explained by differences in study design, QR and TQ contents of the GB and N. sativa extract, genetic variations, and duration of treatment.
As CYP2D6 is the primary CYP isoform that catalyzes biotransformation of the antidepressants and antipsychotics, an extreme caution should be exercised when herbal remedies containing QR or TQ are coadministered with these agents.
The moderate inhibitory effect of TQ on CYP2C19 activity in our study is supported by several in vitro reports. Ahmad et al. reported that chronic administration of TQ increases t½ of glibenclamide by 92% which could be explained by the reduction of CYP450 activity, especially CYP2C19 that is expected to have the more dominant effect on glibenclamide metabolic pathway in vitro.[14] In addition, the aqueous thyme extract (common spices containing TQ) was found to be a potent inhibitor of CYP2C19 in vitro.[19] On the other hand, Alkharfy et al. reported that AUC and t½ of phenytoin, a CYP2C19 substrate, were reduced by 87% and 77%, respectively with N. sativa ingestion in dogs.[20] It has been estimated that CYP2C9 is responsible for the metabolic clearance of up to 10% of all drugs including antiplatelet, anticonvulsants, antidepressants, antineoplastic drugs, antiretroviral/antifungal drugs, antimalarial drugs, beta blockers, and proton pump inhibitors. Because our study shows that both QR and TQ are moderate inhibitors of the CYP2C9 activity, patients who concomitantly administer herbal remedies containing these agents with any of the aforementioned CYP2C19 substrates may be advised to consult with their physicians or pharmacists to avoid drug toxicity.
CONCLUSIONS
The present study demonstrates that while QR and TQ have no significant effect on the activity of CYP1A2 and CYP2E1, they have a moderate to potent inhibitory effect on CYP2C19, CYP2D6, and CYP3A4. Although further investigations are necessary to establish if these in vitro effects would be reflected in vivo, concomitant administration of these herbal remedies with drug substrates of these CYP enzymes may result in undesirable therapeutic outcomes, especially with narrow therapeutic window drugs.
Financial support and sponsorship
This work was supported in part by the Faculty Development Grant and Research Incentive Grant from Pacific University, Oregon.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Elbarbry F, Ragheb A, Marfleet T, Shoker A. Modulation of hepatic drug metabolizing enzymes by dietary doses of thymoquinone in female New Zealand white rabbits. Phytother Res. 2012;26:1726–30. doi: 10.1002/ptr.4628. [DOI] [PubMed] [Google Scholar]
- 2.Nakajima M, Yoshida R, Shimada N, Yamazaki H, Yokoi T. Inhibition and inactivation of human cytochrome P450 isoforms by phenethyl isothiocyanate. Drug Metab Dispos. 2001;29:1110–3. [PubMed] [Google Scholar]
- 3.Elbarbry FA, McNamara PJ, Alcorn J. Ontogeny of hepatic CYP1A2 and CYP2E1 expression in rat. J Biochem Mol Toxicol. 2007;21:41–50. doi: 10.1002/jbt.20156. [DOI] [PubMed] [Google Scholar]
- 4.Hermann R, von Richter O. Clinical evidence of herbal drugs as perpetrators of pharmacokinetic drug interactions. Planta Med. 2012;78:1458–77. doi: 10.1055/s-0032-1315117. [DOI] [PubMed] [Google Scholar]
- 5.Parvaresh A, Razavi R, Rafie N, Ghiasvand R, Pourmasoumi M, Miraghajani M. Quercetin and ovarian cancer: An evaluation based on a systematic review. J Res Med Sci. 2016;21:34. doi: 10.4103/1735-1995.181994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M. Anticancer efficacy of polyphenols and their combinations. Nutrients. 2016:8. doi: 10.3390/nu8090552. pii:E552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javadi F, Ahmadzadeh A, Eghtesadi S, Aryaeian N, Zabihiyeganeh M, Rahimi Foroushani A, et al. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: A double-blind, randomized controlled trial. J Am Coll Nutr. 2017;36:9–15. doi: 10.1080/07315724.2016.1140093. [DOI] [PubMed] [Google Scholar]
- 8.Mohtashami A, Entezari MH. Effects of Nigella sativa supplementation on blood parameters and anthropometric indices in adults: A systematic review on clinical trials. J Res Med Sci. 2016;21:3. doi: 10.4103/1735-1995.175154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J, Oshiro T, Thomas S, Higa A, Black S, Todorovic A, et al. Inactivation of CYP2A6 by the dietary phenylpropanoid trans-cinnamic aldehyde (cinnamaldehyde) and estimation of interactions with nicotine and letrozole. Drug Metab Dispos. 2016;44:534–43. doi: 10.1124/dmd.115.067942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krippendorff BF, Lienau P, Reichel A, Huisinga W. Optimizing classification of drug-drug interaction potential for CYP450 isoenzyme inhibition assays in early drug discovery. J Biomol Screen. 2007;12:92–9. doi: 10.1177/1087057106295897. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim ZS, Ishizuka M, Soliman M, ElBohi K, Sobhy W, Muzandu K, et al. Protection by Nigella sativa against carbon tetrachloride-induced downregulation of hepatic cytochrome P450 isozymes in rats. Jpn J Vet Res. 2008;56:119–28. [PubMed] [Google Scholar]
- 12.Wang R, Zhang H, Sun S, Wang Y, Chai Y, Yuan Y. Effect of Ginkgo leaf tablets on the pharmacokinetics of amlodipine in rats. Eur J Drug Metab Pharmacokinet. 2016;41:825–33. doi: 10.1007/s13318-015-0312-3. [DOI] [PubMed] [Google Scholar]
- 13.Savai J, Varghese A, Pandita N, Chintamaneni M. Investigation of CYP3A4 and CYP2D6 interactions of Withania somnifera and Centella asiatica in human liver microsomes. Phytother Res. 2015;29:785–90. doi: 10.1002/ptr.5308. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad A, Khan RM, Alkharfy KM, Raish M, Al-Jenoobi FI, Al-Mohizea AM. Effects of thymoquinone on the pharmacokinetics and pharmacodynamics of glibenclamide in a rat model. Nat Prod Commun. 2015;10:1395–8. [PubMed] [Google Scholar]
- 15.Al-Jenoobi FI, Al-Thukair AA, Abbas FA, Ansari MJ, Alkharfy KM, Al-Mohizea AM, et al. Effect of black seed on dextromethorphan O- and N-demethylation in human liver microsomes and healthy human subjects. Drug Metab Lett. 2010;4:51–5. doi: 10.2174/187231210790980435. [DOI] [PubMed] [Google Scholar]
- 16.Rastogi H, Jana S. Evaluation of inhibitory effects of caffeic acid and quercetin on human liver cytochrome p450 activities. Phytother Res. 2014;28:1873–8. doi: 10.1002/ptr.5220. [DOI] [PubMed] [Google Scholar]
- 17.Song M, Hong M, Lee MY, Jee JG, Lee YM, Bae JS, et al. Selective inhibition of the cytochrome P450 isoform by hyperoside and its potent inhibition of CYP2D6. Food Chem Toxicol. 2013;59:549–53. doi: 10.1016/j.fct.2013.06.055. [DOI] [PubMed] [Google Scholar]
- 18.Yin OQ, Tomlinson B, Waye MM, Chow AH, Chow MS. Pharmacogenetics and herb-drug interactions: Experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841–50. doi: 10.1097/00008571-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Foster BC, Vandenhoek S, Hana J, Krantis A, Akhtar MH, Bryan M, et al. In vitro inhibition of human cytochrome P450-mediated metabolism of marker substrates by natural products. Phytomedicine. 2003;10:334–42. doi: 10.1078/094471103322004839. [DOI] [PubMed] [Google Scholar]
- 20.Alkharfy KM, Al-Jenoobi FI, Al-Mohizea AM, Al-Suwayeh SA, Khan RM, Ahmad A. Effects of lepidium sativum, Nigella sativa and Trigonella foenum-graceum on phenytoin pharmacokinetics in beagle dogs. Phytother Res. 2013;27:1800–4. doi: 10.1002/ptr.4947. [DOI] [PubMed] [Google Scholar]