Abstract
Background
Proteinuria is a well-known risk factor for progression of renal dysfunction in a variety of chronic kidney diseases. In adult-onset Systemic Lupus Erytematosus (SLE) patients with lupus nephritis (LN), proteinuria takes a significant period of time to normalise, with proteinuric remission being associated with improved renal survival and reductions in mortality. The length of time required to attain proteinuric remission has not previously been investigated in Juvenile-onset SLE (JSLE). The aim of this study was to elucidate when proteinuric remission occurs, and whether clinical/demographic factors at LN onset bear influence on the time to proteinuric remission.
Methods
Participants of the UK JSLE Cohort Study and Repository were included if they had active LN (renal biopsy and/or renal British Isles Lupus Assessment Grade (BILAG) score defined active LN) and proteinuria. Univariate Cox proportional hazard regression modelling was used to explore factors associated with time to proteinuric recovery. Covariates with p-value < 0.2 were included in a multivariable Cox regression model, and backward stepwise variable selection applied.
Result
64/350 (18%) of UK JSLE Cohort Study patients fulfilled the study inclusion criteria. 25 (39%) achieved proteinuric remission within a median of 17 months (min 2.4, max 78). Within a multivariate Cox proportional hazard regression model, age at time of LN flare (p = 0.007, HR 1.384, CI 1.095–1.750), eGFR (p = 0.035, HR 1.016, CI 1.001–1.030) and haematological involvement (p = 0.016, HR 0.324, CI 0.129–0.812) at the time of LN onset were found to be significantly associated with time to proteinuric recovery.
Conclusions
A significant proportion of children with LN have on-going proteinuria approximately two years after their initial flare. Poor prognostic factors all at time of LN onset include younger age, low eGFR, and concomitant haematological involvement.
Keywords: Juvenile-onset systemic lupus erythematosus, JSLE, Lupus nephritis, Active LN, Proteinuria, Prognosis
Background
Following a lupus nephritis (LN) flare, proteinuria has been shown to take a significant period of time to normalise in adults with systemic lupus erythematosus (SLE), with 53% of patients requiring up to 2 years to recover and only 74% recovering by 5 years [1]. Several adult SLE studies have shown that an early reduction in proteinuria following initiation of immunosuppressive therapy is associated with improved longer term renal outcomes [2–5]. Whilst proteinuria is on-going, differentiating between proteinuria due to on-going LN flare or chronic renal damage can be problematic. This leads the clinician to consider repetition of the renal biopsy, despite a lack of agreement as to the appropriate timing and indications for a repeat renal biopsy, particularly in children [6, 7].
Time to recovery from proteinuria in children with active LN receiving standard treatment has not been described to date. It is therefore of great interest to explore this within the national UK JSLE Cohort Study, to appreciate how long proteinuria persists for within a real world clinical setting (in contrast for example to a clinical trial setting). Identification of clinical and demographic factors at the onset of LN which are predictive of longer duration to resolution of proteinuria would be useful for stratifying patients as having high or low risk for achieving proteinuric remission, and helping to modify the intensity and duration of early immunosuppressive therapy.
Methods
Aim
The main aim of this study was to use data arising from the UK JSLE Cohort Study (a UK-wide multi-centre longitudinal cohort study collecting data as part of routine care) [8] between 1995 and 2015 to assess whether clinical and demographic factors can be used to predict time to proteinuric remission following an LN flare.
Patients
Participants of the UK JSLE Cohort Study [8], aged < 16 years at the time of diagnosis and with ≥4 American College of Rheumatology (ACR) SLE classification criteria, were included in the current study if they had the following:
Active LN - defined in terms of having either renal biopsy defined active LN (International Society of Nephrology / Renal Pathology Society 2003 score, ISN/RPS classification score [9]), or renal British Isles Lupus Assessment Grade (BILAG) domain score of A or B [10].
Significant proteinuria - defined as a urine protein creatinine ratio (UPCR) or urine albumin creatinine ratio (UACR) of > 50 mg/mmol or a 24-h urine protein of ≥0.5 g.
At least two consecutive follow-up visits with significant proteinuria following its initial proteinuria onset.
Patients were therefore excluded if they did not have BILAG (renal domain score of C-E) or renal biopsy defined active LN, if their UPCR was ≤50 mg/mmol or 24-h urine protein was < 0.5 g, or if they had < two follow-up visits following the onset of proteinuria (as this precluded the ability for longitudinal follow-up). The proteinuria cutoff was chosen on the basis of the renal BILAG score, where a UPCR or UACR ratio of > 50 mg/mmol or a 24-h urine protein of ≥0.5 g is required for at least a score of B to be achieved, signifying moderate LN activity [10]. Further practical information on the BILAG score is detailed within a review by Lattanzi et al., comparing the BILAG score to other scores such as the SLEDAI score [11].
Potential predictors of proteinuric recovery
Clinical and demographic factors (at the time of LN onset) were assessed as predictors within the analyses. Disease activity data was collected using the BILAG disease activity score at each routine clinic visit (approximately 3 monthly) [10]. Demographic details were collected using a standardized UK JSLE Cohort study case report form [8]. The demographic factors included gender, age at LN onset, ethnicity (caucasian/non-caucasian) and length of disease since diagnosis. Clinical renal factors consisted of proteinuria (spot UPCR or UACR), severe hypertension (blood pressure rising to > 170/110 mmHg within 1 month with grade 3 or 4 Keith-Wagener-Barker retinal changes), nephrotic syndrome (heavy proteinuria ≥3.5 g/day or protein-creatinine ratio of ≥350 mg/mmol or albumin-creatinine ratio of ≥350 mg/mmol, with hypoalbuminaemia and oedema), serum creatinine, presence of active urine sediment and estimated glomerular filtration rate (eGFR). Haematological features included haemoglobin, white cell count, neutrophils, lymphocytes and platelets. Immunological features consisted of complement factors 3 and 4 (C3/C4), anti-double-stranded DNA antibodies (anti-dsDNA), immunoglobulins (IgG, IgA, IgM), erythrocyte sedimentation rate (ESR) and c-reactive protein (CRP). Data on Coomb’s positivity was not included as the dataset was incomplete.
The physician global assessment, total numerical BILAG score and concomitant active involvement of different BILAG domains were also considered (score of A or B for a given organ domain) in the analyses, including constitutional, mucocutaneous, neuropsychiatric, musculoskeletal, cardiorespiratory, gastrointestinal, opthalmological and haematological domain involvement. Medication use at the time of LN onset was also recorded (hydroxychloroquine, azathioprine, mycophenelate mofetil, prednisolone, intra-venous immunoglobulin (IVIG), angiotensin inhibitor or angiotensin receptor blocker, previous rituximab or cyclophosphamide use) for the analyses.
Study outcomes
Patients were categorised as having attained proteinuric recovery if their spot UPCR or UACR ratio was < 25 mg/mmol at two consecutive visits, or not recovered if the spot UPCR or UACR ratio was > 25 mg/mmol. The proteinuria cut off levels were chosen on the basis of the renal BILAG score as a spot UPCR or UACR ratio of < 25 mg/mmol would lead to a renal BILAG score of D, signifying inactive LN but previous renal involvement.
Statistical analysis
The study was undertaken using retrospective data from a longitudinal patient cohort. The outcome for each patient was defined as time to the date of proteinuric recovery, or to the date of the last visit date if they had not achieved proteinuric recovery and were censored. Cox proportional hazard regression modelling was used to univariately test the association between each clinical/demographic variable of interest and outcome. Variables with missing data were tested with complete cases only univariately. Covariates with p < 0.2 on univariate analysis were included in a multiple Cox regression model. Where > 10% of the data was missing for an included covariate, ‘MICE’ package in R version 3.2.0 was used to undertake multiple imputation [12]. Covariates to be retained in the final model were chosen by using a backward stepwise model selection procedure (threshold p < 0.05). Hazard ratios (HRs), 95% confidence intervals (CIs) and p-values were summarised for covariates present in the final model. The results were displayed graphically with Kaplan-Meier curves and risk tables. All analysis was undertaken with R version 3.2.0 [13].
Results
The study cohort consisted of 350 UK JSLE Cohort Study patients, of which 64/350 (18%) met the study’s inclusion criteria and were therefore considered within these analyses. 43/64 (67%) had renal biopsy defined LN (class II n = 4, class III n = 10, class IV n = 28, class V n = 1) and 21/64 (33%) had renal BILAG defined LN (A = 3, B = 18). 55/350 patients met some but not all of the inclusion criteria and were therefore excluded (see Fig. 1 for further details). 54/64 patients had LN at the time of their initial diagnosis and 10/64 had a subsequent recurrent episode of LN. During the study follow-up period, proteinuric remission was achieved in 25/64 (39%) patients, within a median of 17 months (interquartile range, IQR 3.5–49.2).
Fig. 1.
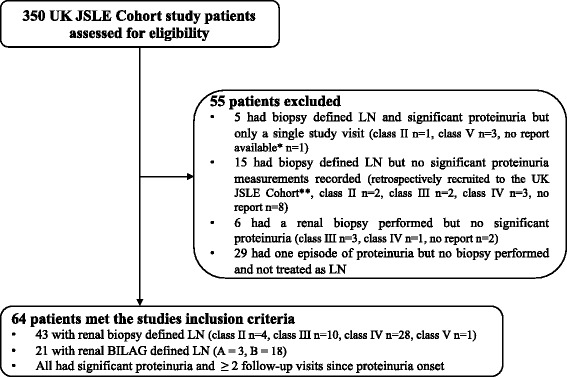
Participant flow diagram. ISN/RPS classification reported for renal biopsies. *Box ticked on the BILAG case report form that the patient had ‘histological evidence of active nephritis within 3 months of the clinic visit’ but the full biopsy report was not provided. **The UK JSLE Cohort Study largely recruits and prospectively collects clinical data from participants across the UK. At the time of initial study set up, patients seen between 1995-2006 were retrospectively recruited and limited retrospective clinical data collected
Clinical / demographic factors influencing time to proteinuric recovery?
Using univariate Cox proportional hazard regression modelling, age at LN onset, serum creatinine, eGFR, neutrophil count, physician global assessment and BILAG defined haematological involvement all gave p values of < 0.2 (see Table 1), and therefore were considered within a multiple Cox regression model. There was no statistical difference in the distribution of renal biopsy subclasses, or whether patients had LN at diagnosis or recurrent LN, amongst the recovered and not-recovered groups (p = 0.388 and p = 0.0784 respectively).
Table 1.
Univariate Cox proportional hazard regression modelling looking at time to proteinuric remission
| Clinical and demographic factors at LN onset | Not recovered (n = 39)a | Recovered (n = 25)b | Hazard Ratio (95% CI) | p-value | |
|---|---|---|---|---|---|
| Factors included within the multivariate model | Age at LN onset (yrs) | 13.9 (6.4, 17.7) | 13.6 (8.1, 17.9) | 1.0007 (1.0001, 1.0013) | 0.013 |
| S creatinine (micromol/l, NA = 8) | 61 (34, 234) | 50 (36, 177) | 0.991 (0.976, 1.005) | 0.184 | |
| eGFR (ml/min/m2, NA = 2) | 104 (29, 159) | 121 (29,153) | 1.014 (0.999, 1.028) | 0.060 | |
| Neutrophil count (× 109/L, NA = 2) | 3.4 (1.1, 17.8) | 3.44 (0.4, 12.33) | 0.932 (0.837, 1.037) | 0.197 | |
| Physicians global assessment | 23 (0, 75) | 41 (1, 71) | 1.027 (0.994, 1.061) | 0.107 | |
| Haematological involvementc | Y: 33, N: 6 | Y: 13, N: 12 | 0.421 (0.187, 0.952) | 0.038 | |
| Demographics, clinical features and laboratory investigations | LN ISN/RPS renal biopsy classd | Class II: 1 Class III: 5 Class IV: 15 Class V: 2 |
Class II: 3 Class III: 5 Class IV: 10 Class V: 2 |
0.769 (0.429, 1.378) | 0.388 |
| LN at diagnosis or recurrent LN | Diagnosis: 33 Recurrent: 6 |
Diagnosis: 21 Recurrent: 4 |
0.320 (0.090, 1.138) | 0.078 | |
| Female gender | 32/39 (82%) | 18/25 (72%) | 1.572 (0.642, 3.852) | 0.322 | |
| Caucasian ethnicitye | 13/39 (33%) | 13/25 (52%) | 1.404 (0.637, 3.091) | 0.421 | |
| Length of disease at LN onset (days) | 225 (0, 4857) | 27 (0, 2679) | 0.9999 (0.9994, 1.0005) | 0.934 | |
| Baseline Proteinuriaf | 149 (50, 2772) | 252 (51, 1418) | 0.9999 (0.9993, 1.0006) | 0.989 | |
| Severe hypertension (NA = 3)g | Y: 6, N: 31 | Y: 3, N: 21 | 0.482 (0.140, 1.671) | 0.253 | |
| Nephrotic syndrome (NA = 3)h | Y: 7, N: 30 | Y: 5, N: 19 | 0.853 (0.318, 2.342) | 0.765 | |
| Active urinary sediment (NA = 40)i | Y: 5, N: 9 | Y: 3, N: 7 | 1.722 (0.402, 7.423) | 0.466 | |
| Haemoglobin (g/dl) | 10.8 (5.6, 96) | 11.3 (7.1, 14.9) | 0.999 (0.949, 1.052) | 0.979 | |
| WCC (×109/L) | 4.8 (2.5, 22.4) | 6.4 (0.5, 9.1) | 0.965 (0.885, 1.053) | 0.426 | |
| Lymphocytes (×109/L, NA = 2) | 1.40 (0.1, 5.0) | 1.53 (0.1, 5.42) | 0.970 (0.670, 1.410) | 0.879 | |
| Platelets (×109/L) | 245 (77, 589) | 225 (82, 522) | 0.998 (0.995, 1.002) | 0.342 | |
| ESR (mm/h, NA = 11) | 40 (2, 170) | 37.5 (4, 102) | 0.994 (0.982, 1.006) | 0.353 | |
| CRP (mg/L, NA = 27) | 5 (1, 19) | 5 (1, 295) | 0.998 (0.991, 1.005) | 0.601 | |
| C3 (g/L, NA = 7) | 0.51 (0.18, 1.61) | 0.71 (0.22, 1.31) | 1.011 (0.301, 3.400) | 0.986 | |
| C4 (g/L, NA = 7) | 0.06 (0.01, 0.90) | 0.07 (0.02, 0.21) | 0.282 (0.005, 15.171) | 0.537 | |
| Anti-dsDNA titres (IU/L, NA = 22) | 119 (0, 3503) | 220 (42, 3770) | 0.999 (0.999, 1.000) | 0.577 | |
| IgG (g/L, NA = 27) | 14.6 (0.9, 70.2) | 11.8 (2.8, 33.1) | 0.957 (0.895, 1.022) | 0.296 | |
| IgA (g/L, NA = 28) | 2.06 (0.8, 4.9) | 2.36 (0.3, 3.7) | 0.870 (0.493, 1.542) | 0.636 | |
| IgM (g/L, NA = 28) | 1.11 (0.4, 9.6) | (0.07, 2.5) | 0.614 (0.271, 1.412) | 0.252 | |
| Medications at LN onset | Hydroxychloroquinej | Y: 21, N: 18 | Y: 12, N: 13 | 1.514 (0.663, 3.411) | 0.328 |
| Azathioprine | Y: 8, N: 31 | Y: 2, N: 23 | 0.520 (0.123, 2.222) | 0.377 | |
| Mycophenolate Mofetil | Y: 10, N: 29 | Y: 6, N: 19 | 1.400 (0.550, 3.590) | 0.475 | |
| Prednisolone | Y: 24, N: 15 | Y: 14, N: 11 | 1.260 (0.560, 2.860) | 0.581 | |
| Intravenous immunoglobulin | Y: 2, N: 37 | Y: 2, N: 23 | 0.790 (0.170, 3.640) | 0.762 | |
| Rituximab ever | Y: 2, N: 37 | Y: 1, N: 14 | 0.373 (0.046, 2.982) | 0.351 | |
| Cyclophosphamide ever | Y: 3, N: 36 | Y: 2, N: 23 | 0.574 (0.132, 2.563) | 0.463 | |
| ACEi or AT2ik | Y: 11, N: 28 | Y: 4, N: 21 | 0.753 (0.263, 2.222) | 0.607 | |
| Concomitant BILAG defined organ involvement | Constitutional involvement | Y: 15, N: 24 | Y: 15, N: 10 | 1.410 (0.621, 3.183) | 0.411 |
| Mucocutaneous involvement | Y: 23, N: 16 | Y: 15, N: 10 | 0.971 (0.431, 2.182) | 0.936 | |
| Neuropsychiatric involvement | Y: 3, N:36 | Y: 3, N: 22 | 0.920 (0.261, 3.231) | 0.901 | |
| Musculoskeletal involvement | Y: 19, N: 20 | Y: 14, N: 11 | 0.612 (0.251, 1.473) | 0.272 | |
| Cardiorespiratory involvement | Y: 3, N: 36 | Y: 5, N: 20 | 1.253 (0.462, 3.422) | 0.663 | |
| Gastrointestinal involvement | Y: 0, N: 39 | Y: 3, N: 22 | 1.311 (0.361, 4.761) | 0.680 | |
| Ophthalmological involvement | Y: 0, N: 39 | Y: 2, N: 23 | 1.082 (0.221, 5.300) | 0.919 | |
| Total numerical BILAG score | 11 (3, 27) | 11 (1, 53) | 0.980 (0.951, 1.021) | 0.424 |
Summary statistics used for continuous variables in the recovered and not recovered columns (median, min, max), whereas number count was detailed for discrete variables. Missing data shown in brackets with NA. p-values are from univariate Cox Proportion hazard models. aNot recovered = not attained proteinuric recovery during the follow up period. bRecovered = attained proteinuric recovery defined as if their spot UPCR or UACR ratio being <25mg/mmol at two consecutive visits. cBILAG defined active organ domain involvement (score of A or B). dActive LN defined in terms of having either renal biopsy defined active LN (ISN/RPS score), 32 or renal BILAG domain score of A or B. 43/64 patients had renal biopsy defined LN and 21/64 had renal BILAG defined LN. ePatients grouped as caucasian / non-caucasian for the purposes of the analysis. fBaseline Proteinuria = spot UPAC or UAUC measurements depending on hospital laboratory. gBILAG defined severe hypertension. hNephrotic syndrome = heavy proteinuria (> 50 mg/kg/day or > 3.5 g/day or protein-creatinine ratio > 350 mg/mmol or albumin-creatinine ratio > 350mg/mmol) + hypoalbuminaemia + oedema. iActive urine sediment = pyuria (> 5 WCC/hpf), haematuria (> 5 RBC/hpf) or red cell casts in absence of other causes. jMedication use (yes) or non-use (no) considered rather than absolute drug dose. kACEi or AT2i = Angiotensin inhibitor or angiotensin receptor blocker
After applying stepwise backward variable selection to the multivariable Cox regression model, it was identified that those who were older, or had higher eGFR values at the time of LN onset, were more likely to achieve proteinuric recovery. Further, those with haematological involvement at the time of LN onset were less likely to achieve proteinuric recovery (see Table 2).
Table 2.
Multivariable–Cox regression model displaying factors associated with time to proteinuric recovery
| Clinical and demographic factors at LN onset | HR (95% CI) | p value |
|---|---|---|
| Age at LN onset (years) | 1.384 (1.095, 1.750) | 0.007 |
| eGFR | 1.016 (1.001, 1.030) | 0.035 |
| Haematological involvement | 0.324 (0.129, 0.812) | 0.016 |
eGFR estimated glomerular filtration rate, HR hazard ration, CI confidence interval
Multivariable Cox regression model after applying variable selection
Kaplan Meier plots for age, eGFR and BILAG defined haematological involvement are shown in Fig. 2. Figure 2a demonstrates that at a given time, patients who are older at the time of LN onset (> 14 years vs < 14 years) are more likely to achieve proteinuric recovery. The median patient age across the whole patient group was 14 years, therefore, that is why this cut-off was used to divide patients into younger or older groups. Median time to recovery was 4.95 years (95% CI: 0.33–9.09) in the younger age group and 0.65 years (95% CI: 0.29–5.31) in the older age group (p = 0.021). The Kaplan Meier plot for eGFR (Fig. 2b) divides patients into two clinically relevant sub-groups with an eGFR of less or greater that 80 mls/min, demonstrating that at a given time, patients with a eGFR of > 80 mls/min at LN onset are more likely to recover. Those with eGFR levels < 80 mls/min had a median time to recovery of 2.00 years (95% CI: 0.43–8.85) whilst those above 80 mls/min had median time to recovery of 1.82 years (95% CI: 0.28–7.95, p = 0.170). Figure 2c illustrates that at a given time, patients without haematological involvement at LN onset are more likely to attain proteinuric recovery at each time point. Median time to recovery was 1.73 years (95% CI: 0.28–8.34) in those without involvement and 2.14 years (95% CI: 0.30–8.13) in those with haematological involvement (p = 0.038).
Fig. 2.
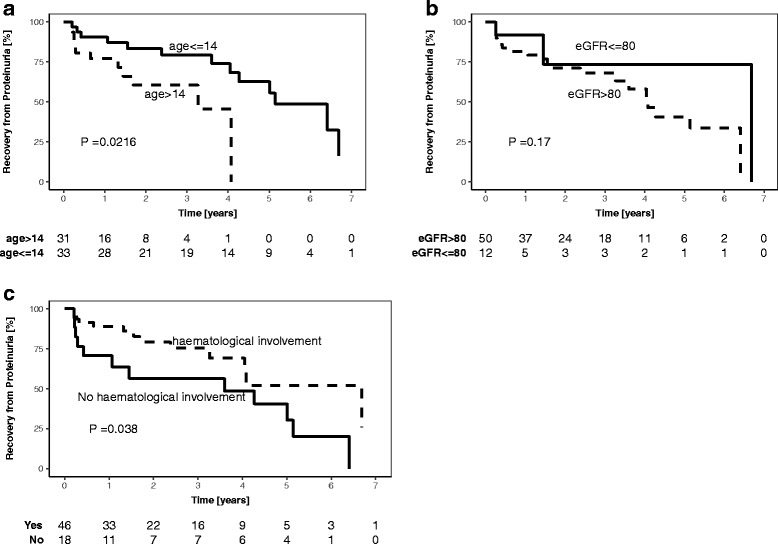
legend: Kaplan-Meier plot for age, eGFR and haematological involvement. a Patients aged <=14 years, n = 33. Patients aged > 14 years, n = 31. b Patients with eGFR of >80mls/min, n = 50. Patients with eGFR<=80mls/min, n = 12. The Kaplan-Meier plot for eGFR appeared to marginally deviate from the assumption of proportional hazards, however this could be due to the very small number of patients in the <80mls/min group after the second time-point. c Patients with BILAG defined haematological involvement, n = 46. Patients without haematological involvement, n = 18. Non-imputed data used for development of Kaplan-Meier plots, therefore n = 64 for age plot, 62 for eGFR and 64 for BILAG defined haematological involvement plot. The table below each plot shows the number of patients who continue to be at risk of developing LN at each time point. P-values on the Kaplan-Meier plots are from log-rank tests of dichotomised variables, and therefore differ from the regression model p-values shown in Table 2
Discussion
Using data from a large national cohort of patients recruited to the UK JSLE Cohort Study, this study has demonstrated that proteinuria can be persistent following a LN flare. Clinical and demographic data have been used within this study to characterise patients who are at increased risk of having a prolonged period of proteinuria following an LN flare. Early reduction in proteinuria following initiation of immunosuppressive therapy has been shown to be associated with improved longer term renal outcomes [1, 14] and patient survival [4] in adult SLE, therefore, appreciation of those who are likely to need longer to achieve proteinuric recovery may influence monitoring and treatment decisions. Out-with a LN setting, levels of albuminuria have been shown to relate to all cause and cardiovascular mortality [15, 16], with proteinuria reduction being the main target for halting the progression of diabetic [17] and many non-diabetic kidney diseases [18].
A total of 39% of patients were shown to reach proteinuric remission following a LN flare during the study period, within a median of 17 months (IQR 3.5–49.2). This observation provides useful information on the length of time necessary to achieve proteinuric recovery following a LN flare within real world clinical practice, as opposed to a tightly regulated clinical trial setting. A similar study in adult SLE showed proteinuric recovery to occur in 53% of patients by 2 years [1], suggest that proteinuria may take longer to normalize in children than adults with SLE. In a Korean study including 193 adults with SLE and severe proliferative LN, proteinuric remission was attained in 8% of patients within 12 months, 19% between 12 and 60 months, and 16% after more than 60 months post renal biopsy, suggesting that those with the most severe LN histological sub-types may need even longer to achieve proteinuric remission [14].
Younger age at the time of LN onset was shown to be the strongest predictor of having a prolonged time to proteinuric recovery, likely reflecting the more severe disease phenotype and potential genetic predisposition to JSLE/LN seen in younger patients [8, 19–24]. This study did not collect information on pubertal status, but in future work it would be useful to assess the influence of pubertal status on time to proteinuric recovery. Reductions in eGFR usually occur following significant renal damage and may be preceded by a period of hyper-filtration [25–27]. It is therefore not unexpected that low eGFR at the time of LN onset was associated with longer time to proteinuric recovery in the current study.
Haematological involvement at the time of LN onset was associated with a longer time to proteinuric recovery. It is of interest that individual haematological measures (e.g. haemoglobin, white count, platelets) were not individually associated with time to proteinuric recovery, but as reflected by the haematological domain of the BILAG score, combinations of abnormalities were found to be important. It can be speculated that concomitant haematological involvement may also affect the ability to intensify immunosuppressant treatment due to concerns about treatment toxicity, thereby influencing time to proteinuric recovery. Awareness of these factors is important for stratification of patients at the time of LN onset, and considering the intensity and duration of early immunosuppressive therapy. In future work it would also be of interest to look at whether patients were Coombs’ positive and their reticulocyte counts.
The strengths of this study lie in the large real-world patient cohort receiving a multitude of treatment regimens, as opposed to a tightly regulated clinical trial. Certain limitations of this study do however warrant recognition and should be addressed in future work. The study did not include all LN patients; with five biopsy proven LN patients excluded as they only had a single follow-up visit, and a further 15 patients excluded who were retrospectively recruited to the cohort and had insufficient proteinuria measurements recorded for meaningful analysis to be undertaken. The analysis looked at treatment at baseline and did not look at the influence of on-going treatment (steroids and DMARDs) due to the number and complexity of the different treatment plans used, precluding meaningful analysis in this area. Similarly, these analyses did not consider serially collected disease activity and damage data as the aim of this work was to look at factors at the time of LN onset which predict time to proteinuric remission. Further work involving sophisticated longitudinal modelling of all data would be of interest in future work.
The definition of proteinuric remission used in this study was based on the renal BILAG score. Other cut-offs could have been chosen based on other scoring systems, e.g. the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) or the European Community Lupus Activity Measure (ECLAM), that both define proteinuric remission as < 0.5 g/24 h, and the ACR score defines it as persistently < 0.5 g/24 h or <+++ on protein dipstix if quantification not performed [28–30]. The BILAG score provides the most comprehensive assessment of renal disease activity of these disease activity scores and includes UPCR or UACR measurements which are more practical in children, less dependent on patient compliance and yield more complete longitudinal data.
The urine samples were collected at different times of the day and in future work it would be useful to standardize the time of sample collection (e.g. all early morning urine samples) to minimize the influence of orthostatic proteinuria. Within the inclusion criteria it was specified that patients must have at least two consecutive follow-up visits with significant proteinuria following its initial proteinuria onset, to minimize the inclusion of patients with one off episodes of orthostatic proteinuria. The study also did not include patients with mild LN within the active LN group (renal BILAG domain score of C) as such a BILAG score can easily be obtained by having 1+ of urine dipstick proteinuria, which may be due to orthostatic proteinuria. This study looked at medication use at the time of LN onset but could not sub-divide patients according to the LN treatment regimen received in view of the multitude of different treatment regimens used in clinical practice over time. The cohort study data is collected alongside routine clinical practice leading to missing data and the need for multiple imputation within the analyses, therefore further analysis in more complete datasets would be of interest in future work.
Conclusions
A high proportion of JSLE patients develop LN [8, 31, 32]. This study has demonstrated that proteinuria can be persistent within a real world clinical setting. This study has elucidated basic characteristics of patients who are at increased risk of having prolonged proteinuria following an LN flare, namely patients who are younger (< 14 years), have an abnormal eGFR (< 80 mls/min) and concomitant haematological involvement at the time of LN onset. These data cannot guide the clinician as to when to repeat the renal biopsy and/or intensify immunosuppressive treatment, however they do highlight at an early stage, patients who need closer surveillance as they are at increased risk of having a more prolonged period to proteinuric remission. Ideally there would be a specific biomarker or urinary biomarker panel that clinicians could easily test to highlight patients who will display persistence of proteinuria following LN flare [33]. Whilst awaiting this development, it is important to be aware of such clinical and demographic factors. Early reduction in proteinuria following initiation of immunosuppressive therapy has been shown to be associated with improved longer term renal outcomes and survival in adult SLE. Therefore, appreciation of those at risk of prolonged proteinuria may help the clinician to change or fine-tune the intensity and duration of early immunosuppressive therapy.
Acknowledgements
The authors would like to acknowledge all patients and their families for participating in this Study, as well as all the support given by the entire multi-disciplinary team within each of the paediatric centres. The study was supported by the National Institute of Health Research (NIHR) Clinical Research Network (CRN): Children and CRN Research Nurses and staff in all centres. Special acknowledgement is given to the principle investigators in each of the participating centres and lead nurse(s) for the Study, and all those who have supported the work of the UK JSLE Study Group to date including Dr. Eslam Al Abadi, Dr. Jane Tizard, Dr. Janet Gardiner Medwin, Dr. Joyce Davidson, Dr. Clarissa Pilkington, Dr. Valentina Leone, Dr. Flora McErlane, Dr. Satyapal Rangaraj, Dr. Phil Riley, Dr. Nick Wilkinson, Dr. Kate Armon, Dr. John Ioannou, Dr. Devesh Mewar, Dr. Gita Modgil, Dr. Alice Leahy, Dr. Arani Sridhar, Dr. Dan Hawley and Dr. Kirsty Haslam. We acknowledge the important work of the NIHR and other funded Clinical Research Facilities that have supported this Study, including specifically the NIHR Alder Hey Clinical Research Facility. Particular thanks to the UK’s ‘Experimental Arthritis Treatment Centre for Children’ funded by Arthritis Research UK, the University of Liverpool, Alder Hey Children’s NHS Foundation Trust and the Alder Hey Charity. Specific acknowledgement goes to Duncan Appleby for database and information technology support and Carla Roberts for co-ordination of the Cohort study.
Ethics approval, consent to participate and permissions
Written patient assent / consent and parental consent was obtained, and the study had full ethical approvals in place from the National Research Ethics Service North West, Liverpool East (REC reference 06/Q1502/77).
Funding
This work was supported by the Alder Hey Children’s Kidney Fund through a training fellowship [UOG10065 to ES]. Lupus UK also provides financial support for co-ordination of the UK JSLE Cohort Study. The funders have not played a role in the design of the study and collection, analysis, interpretation of data or writing of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACR
American College of Rheumatology
- anti-dsDNA
anti-double-stranded DNA antibody
- BILAG
British Isles Lupus Assessment Grade
- C3
complement factor 3
- C4
complement factor 4
- CI
confidence interval
- CRP
c-reactive protein
- ECLAM
European Community Lupus Activity Measure
- eGFR
estimated glomerular filtration rate
- ESR
erythrocyte sedimentation rate
- HR
hazard ratio
- Ig
immunoglobulin
- IVIG
intra-venous immunoglobulin
- JSLE
Juvenile Systemic Lupus Erythematosus
- LN
lupus nephritis
- MMF
mycophenolate mofetil
- SLEDAI
Systemic Lupus Erythematosus Disease Activity Index
- UACR
urine albumin creatinine ratio
- UK
United Kingdom
- UPCR
urine protein creatinine ratio
Authors’ contributions
All authors participated in conception, design of the study and interpretation of the data. ES and MWB participated in the acquisition of data. PY, AJ and ES performed the statistical analysis. MWB is Chief Investigator of the UK JSLE Cohort Study. All authors were involved in drafting the manuscript and revising it critically for important intellectual content. They have also all read and given final approval of the version to be published.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eve M. D. Smith, Email: esmith8@liverpool.ac.uk
Peng Yin, Email: peng.yin@siat.ac.cn.
Andrea L. Jorgensen, Email: aljorgen@liverpool.ac.uk
Michael W. Beresford, Email: m.w.beresford@liverpool.ac.uk
References
- 1.Touma Z, Urowitz MB, Ibanez D, Gladman DD. Time to recovery from proteinuria in patients with lupus nephritis receiving standard treatment. J Rheumatol. 2014;41:688–697. doi: 10.3899/jrheum.130005. [DOI] [PubMed] [Google Scholar]
- 2.Houssiau FA, Vasconcelos C, D'Cruz D, Sebastiani GD, de Ramon Garrido E, Danieli MG, Abramovicz D, Blockmans D, Mathieu A, Direskeneli H, et al. Early response to immunosuppressive therapy predicts good renal outcome in lupus nephritis: lessons from long-term followup of patients in the euro-lupus nephritis trial. Arthritis Rheum. 2004;50:3934–3940. doi: 10.1002/art.20666. [DOI] [PubMed] [Google Scholar]
- 3.Houssiau FA, Vasconcelos C, D'Cruz D, Sebastiani GD, de Ramon Garrido E, Danieli MG, Abramovicz D, Blockmans D, Cauli A, Direskeneli H, et al. The 10-year follow-up data of the euro-lupus nephritis trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis. 2010;69:61–64. doi: 10.1136/ard.2008.102533. [DOI] [PubMed] [Google Scholar]
- 4.Korbet SM, Lewis EJ, Collaborative Study G. Severe lupus nephritis: the predictive value of a >/= 50% reduction in proteinuria at 6 months. Nephrol Dial Transplant. 2013;28:2313–2318. doi: 10.1093/ndt/gft201. [DOI] [PubMed] [Google Scholar]
- 5.Dall'Era M, Cisternas MG, Smilek DE, Straub L, Houssiau FA, Cervera R, Rovin BH, Mackay M. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the euro-lupus nephritis cohort. Arthritis Rheumatol. 2015;67:1305–1313. doi: 10.1002/art.39026. [DOI] [PubMed] [Google Scholar]
- 6.Greloni G, Scolnik M, Marin J, Lancioni E, Quiroz C, Zacariaz J, De la Iglesia Niveyro P, Christiansen S, Pierangelo MA, Varela CF, et al. Value of repeat biopsy in lupus nephritis flares. Lupus Sci Med. 2014;1:e7099. doi: 10.1136/lupus-2013-000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subils G, Alba P, Gobbi C, Astesana P, Babini A, Albiero E. The repeated biopsy in patients with lupus nephritis. Rev Fac Cien Med Univ Nac Cordoba. 2014;71:165–170. [PubMed] [Google Scholar]
- 8.Watson L, Leone V, Pilkington C, Tullus K, Rangaraj S, McDonagh JE, Gardner-Medwin J, Wilkinson N, Riley P, Tizard J, et al. Disease activity, severity, and damage in the UK juvenile-onset systemic lupus erythematosus cohort. Arthritis Rheum. 2012;64:2356–2365. doi: 10.1002/art.34410. [DOI] [PubMed] [Google Scholar]
- 9.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–250. doi: 10.1097/01.ASN.0000108969.21691.5D. [DOI] [PubMed] [Google Scholar]
- 10.Isenberg DA, Rahman A, Allen E, Farewell V, Akil M, Bruce IN, D'Cruz D, Griffiths B, Khamashta M, Maddison P, et al. BILAG 2004. Development and initial validation of an updated version of the British isles lupus assessment Group's disease activity index for patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005;44:902–906. doi: 10.1093/rheumatology/keh624. [DOI] [PubMed] [Google Scholar]
- 11.Lattanzi B, Consolaro A, Solari N, Ruperto N, Martini A, Ravelli A. Measures of disease activity and damage in pediatric systemic lupus erythematosus: British isles lupus assessment group (BILAG), European consensus lupus activity measurement (ECLAM), systemic lupus activity measure (SLAM), systemic lupus erythematosus disease activity index (SLEDAI), Physician's global assessment of disease activity (MD global), and systemic lupus international collaborating clinics/American College of Rheumatology Damage Index (SLICC/ACR DI; SDI) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S112–S117. doi: 10.1002/acr.20623. [DOI] [PubMed] [Google Scholar]
- 12.MICE package in R [https://cran.r-project.org/web/packages/mice/index.html]. Accessed 19 Feb 2018.
- 13.R: A language and environment for statistical computing version 3.3.3 [https://www.r-project.org//]. Accessed 19 Feb 2018.
- 14.Koo HS, Kim S, Chin HJ. Remission of proteinuria indicates good prognosis in patients with diffuse proliferative lupus nephritis. Lupus. 2016;25:3–11. doi: 10.1177/0961203315595130. [DOI] [PubMed] [Google Scholar]
- 15.Oh SW, Baek SH, Kim YC, Goo HS, Heo NJ, Na KY, Chae DW, Kim S, Chin HJ. Mild decrease in estimated glomerular filtration rate and proteinuria are associated with all-cause and cardiovascular mortality in the general population. Nephrol Dial Transplant. 2012;27:2284–2290. doi: 10.1093/ndt/gfr622. [DOI] [PubMed] [Google Scholar]
- 16.Oh SW, Kim S, Na KY, Kim KW, Chae DW, Chin HJ. Glomerular filtration rate and proteinuria: association with mortality and renal progression in a prospective cohort of a community-based elderly population. PLoS One. 2014;9:e94120. doi: 10.1371/journal.pone.0094120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strippoli GF, Bonifati C, Craig M, Navaneethan SD, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev. 2006:CD006257. [DOI] [PMC free article] [PubMed]
- 18.Remuzzi G1, Chiurchiu C, Ruggenenti P. Proteinuria predicting outcome in renal disease: nondiabetic nephropathies (REIN). Kidney Int Suppl. 2004;(92):S90-6. https://www.ncbi.nlm.nih.gov/pubmed/15485427. [DOI] [PubMed]
- 19.Brunner HI, Gladman DD, Ibañez D, Urowitz MD, Silverman ED. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum. 2008;58:556–62. [DOI] [PubMed]
- 20.Hoffman IE, Lauwerys BR, De Keyser F, Huizinga TW, Isenberg D, Cebecauer L, Dehoorne J, Joos R, Hendrickx G, Houssiau F, Elewaut D. Juvenile-onset systemic lupus erythematosus: different clinical and serological pattern than adult-onset systemic lupus erythematosus. Ann Rheum Dis. 2009;68:412–415. doi: 10.1136/ard.2008.094813. [DOI] [PubMed] [Google Scholar]
- 21.Stegert M, Bock M, Trendelenburg M. Clinical presentation of human C1q deficiency: how much of a lupus? Mol Immunol. 2015;67:3–11. doi: 10.1016/j.molimm.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Gargiulo Mde L, Gomez G, Khoury M, Collado MV, Suarez L, Alvarez C, Sarano J. Association between the presence of anti-C1q antibodies and active nephritis in patients with systemic lupus erythematosus. Medicina (B Aires) 2015;75:23–28. [PubMed] [Google Scholar]
- 23.van Schaarenburg RA, Schejbel L, Truedsson L, Topaloglu R, Al-Mayouf SM, Riordan A, Simon A, Kallel-Sellami M, Arkwright PD, Ahlin A, et al. Marked variability in clinical presentation and outcome of patients with C1q immunodeficiency. J Autoimmun. 2015;62:39–44. doi: 10.1016/j.jaut.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Orbai AM, Truedsson L, Sturfelt G, Nived O, Fang H, Alarcon GS, Gordon C, Merrill J, Fortin PR, Bruce IN, et al. Anti-C1q antibodies in systemic lupus erythematosus. Lupus. 2015;24:42–49. doi: 10.1177/0961203314547791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cottiero RA, Madaio MP, Levey AS. Glomerular filtration rate and urinary albumin excretion rate in systemic lupus erythematosus. Nephron. 1995;69:140–146. doi: 10.1159/000188429. [DOI] [PubMed] [Google Scholar]
- 26.Park M, Yoon E, Lim YH, Kim H, Choi J, Yoon HJ. Renal hyperfiltration as a novel marker of all-cause mortality. J Am Soc Nephrol. 2015;26:1426–1433. doi: 10.1681/ASN.2014010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altay S, Onat A, Ozpamuk-Karadeniz F, Karadeniz Y, Kemaloglu-Oz T, Can G. Renal "hyperfiltrators" are at elevated risk of death and chronic diseases. BMC Nephrol. 2014;15:160. doi: 10.1186/1471-2369-15-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bencivelli W, Vitali C, Isenberg DA, Smolen JS, Snaith ML, Sciuto M, Bombardieri S. Disease activity in systemic lupus erythematosus: report of the consensus study Group of the European Workshop for rheumatology research. III. Development of a computerised clinical chart and its application to the comparison of different indices of disease activity. The European consensus study Group for Disease Activity in SLE. Clin Exp Rheumatol. 1992;10:549–554. [PubMed] [Google Scholar]
- 29.Vitali C, Bencivelli W, Isenberg DA, Smolen JS, Snaith ML, Sciuto M, d'Ascanio A, Bombardieri S. Disease activity in systemic lupus erythematosus: report of the consensus study Group of the European Workshop for rheumatology research. I. A descriptive analysis of 704 European lupus patients. European consensus study Group for Disease Activity in SLE. Clin Exp Rheumatol. 1992;10:527–539. [PubMed] [Google Scholar]
- 30.Vitali C, Bencivelli W, Isenberg DA, Smolen JS, Snaith ML, Sciuto M, Neri R, Bombardieri S. Disease activity in systemic lupus erythematosus: report of the consensus study Group of the European Workshop for rheumatology research. II. Identification of the variables indicative of disease activity and their use in the development of an activity score. The European consensus study Group for Disease Activity in SLE. Clin Exp Rheumatol. 1992;10:541–547. [PubMed] [Google Scholar]
- 31.Tucker LB, Menon S, Schaller JG, Isenberg DA. Adult- and childhood-onset systemic lupus erythematosus: a comparison of onset, clinical features, serology, and outcome. Br J Rheumatol. 1995;34:866–872. doi: 10.1093/rheumatology/34.9.866. [DOI] [PubMed] [Google Scholar]
- 32.Hiraki LT, Benseler SM, Tyrrell PN, Hebert D, Harvey E, Silverman ED. Clinical and laboratory characteristics and long-term outcome of pediatric systemic lupus erythematosus: a longitudinal study. J Pediatr. 2008;152:550–556. doi: 10.1016/j.jpeds.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Smith EMD, Beresford MW. Urinary biomarkers in childhood lupus nephritis. Clin Immunol. 2017;185:21–31. doi: 10.1016/j.clim.2016.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


