Abstract
Background
Based on in vitro and in vivo rat experiments, the newly developed acetylcholinesterase (AChE) reactivator, K203, appears to be much more effective in the treatment of tabun poisonings than currently fielded oximes.
Methods
To determine if this reactivating efficacy would extend to humans, studies were conducted in vitro using human brain homogenate as the source of AChE. The efficacy of K203 was compared with commercially available oximes; pralidoxime, obidoxime and asoxime (HI-6).
Results
Reactivation studies showed that K203 was the most effective reactivator with a second order kinetic constant (kr) of 2142 min− 1. M− 1, which was 51 times higher than that obtained for obidoxime (kr = 42 min− 1. M− 1). Both pralidoxime and asoxime (HI-6) failed to significantly reactivate tabun-inhibited human AChE.
Discussion
According to these results and previous studies, using K203, it appears that oxime K203 is the most effective reactivator of tabun-inhibited cholinesterase in several species including humans and should be considered as a possible medical countermeasure to tabun exposure.
Keywords: Antidotes, Chemical warfare agents, Poisoning, Treatment, Reactivator, Oxime
Background
Organophosphorus nerve agents comprise a group of the most toxic chemicals ever synthesized. [1] There is currently a lack of sufficiently effective antidotes against the entire spectrum of these compounds [2, 3]. Tabun (Fig. 1), is considered one of the most toxic compounds within this group; especially considering that there are very few fielded cholinesterase-reactivators able to treat tabun intoxications [4–6]. Tabun’s extraordinary toxicity and resistance to current medical countermeasures appear to be the result of inhibition of acetylcholinesterase (AChE), which is difficult to reactivate due to the presence of a lone electron pair on the amidic group [7]. This novel enzyme inhibition mechanism was presented by Ekstrom et al. using a theoretical binding site model [8]. However, from a treatment point of view, if the efficacy of cholinesterase-reactivation directly correlates to the effectiveness of treatment, there are several reactivators with low to moderate potency to treat tabun intoxications.
Fig. 1.
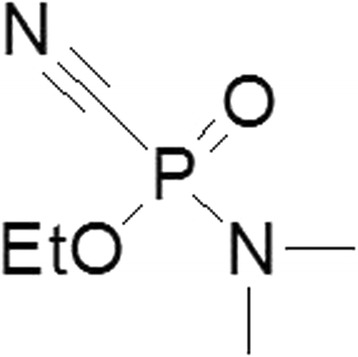
Chemical structure of tabun
Among these reactivators trimedoxime and obidoxime, symmetrical bispyridinium bis-oximes, are considered the best of the commercially available acetylcholinesterase (AChE) reactivators [4, 9, 10]. Unfortunately their use has been associated with adverse effects [11, 12]. Consequently novel reactivators with higher reactivation activity and lower toxicity should be developed [13]. In 2003, two new oximes aimed at the reactivation of tabun-intoxications, K027 and K048 were developed [14, 15]. They differ from the chemical structure of trimedoxime and obidoxime, which are symmetric compounds, and a carbamoyl group was introduced into their structure to decrease their toxicity. Their in vitro and in vivo properties were shown to be promising [5, 16–21]. Two additional oximes were synthesized – K074 and K075 – differing from trimedoxime and obidoxime by the linker between the two heteroarenium rings [22, 23]. Although these oximes achieved higher reactivation potency, they were more toxic than the original compounds [24, 25].
Recently a new low-toxic oxime with high reactivation potency was developed, K203 [26, 27] (Fig. 2). When compared with other oximes K203 has been shown to have the best reactivation activity and toxicity. All previous studies with K203 have been conducted in rodents and therefore we were interested in determining its in vitro reactivation of human brain AChE. As is known, differences in reactivation are observed depending on the species used [22, 23, 28, 29]. This raises the intriguing question of how to improve the action of drugs. Firstly, one way is to identify structural and electronic determinants of drug sensitivity by mean of experimental data as well as theoretical calculations, which in turn provide important clues about the active site of the molecular targets and its interaction with inhibitors. Thus, in the current work, to rationalize the electronic and steric features that modulate the oxime action, molecular modeling techniques were employed. In this line, the the current study demonstrates the efficacy of K203 reactivation of tabun-inhibited AChE in human brain homogenate. Commercially available pralidoxime, obidoxime, asoxime (HI-6) were used in this study as the standards for comparison.
Fig. 2.
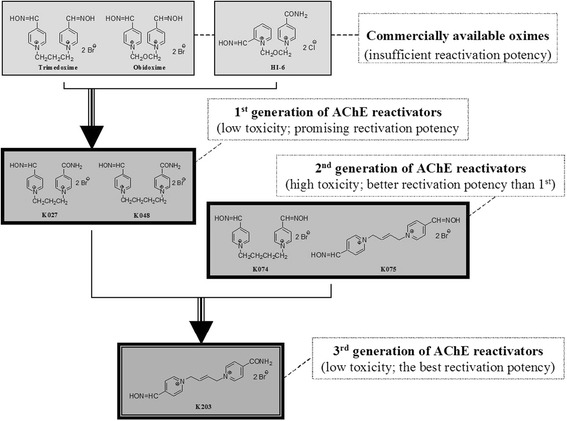
Developmental strategy of the AChE reactivator K203
Methods
Chemicals
K203 ((E)-1-(4-carbamoylpyridinium)-4-(4-hydroxyiminomethylpyridinium)-but-2-ene dibromide) was prepared at the Toxicology Department according to the published method [26]. The structure of K203 and structure of oximes tested for comparison (pralidoxime, obidoxime, asoxime (HI-6)) are shown in Fig. 3. Purity of all tested reactivators was measured using TLC (DC-Alufolien Cellulose F; Merck, Germany; mobile phase BuOH-CH3COOH-H2O 5–1-2; detection by solution of Dragendorff reagent), HPLC (P200 gradient pump Spectra-Physics Analytical, Fremont, USA; a 7125 injection valve – 10 ul loop, Rheodyne, Cotati, USA; an UV1000 detector, Spectra-Physics Analytical, Fremont, USA) and melting point determination (Micro heating stage PHMK 05; VEB Kombinat Nagema, Radebeul, Germany) [30, 31].
Fig. 3.
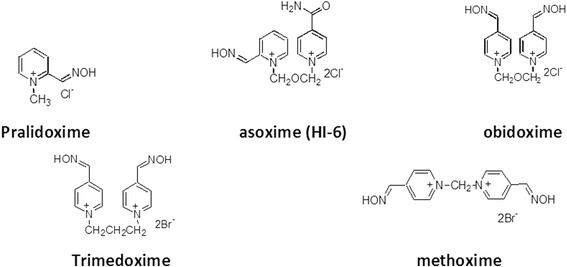
Currently commercially available oximes
Tabun (GA; O-ethyl-N,N-dimethyl phosphoramidocyanidate) was obtained from the Military Facility Brno and was shown to have a purity of at least 97% purity. All other chemicals used were of reagent grade (Sigma-Aldrich, Czech Republic).
Source of human brain cholinesterase
The nucleus caudatus was obtained from autopsy patient material (male) aged 68 and 70, who died by accident (Department of Forensic Medicine, Faculty of Medicine in Hradec Kralove, Charles University In Prague). The brain parts were directly frozen. Ex tempore, 1–2 g of defrosted human brain tissue macroscopically free from white matter was taken and homogenized by Ultra-Turrax apparatus in saline solution (1/10, wet weight/volume of 0.9% saline solution). Experiments were approved in compliance with relevant laws and institutional guidelines and were approved by the Ethics Committee of the Faculty of Military Health Sciences in Hradec Kralove (Czech Republic).
Cholinesterase assay
Cholinesterase activity was measured using the procedures previously published by Kuca and Kassa [16]. Briefly, reactivation efficacy of the oximes was evaluated in an in vitro model of brain cholinesterases inhibited by tabun using the standard reactivation method with electrometric instrumentation. The human brain homogenate (0.5 ml) was mixed with 0.01 μM isopropanol tabun solution (0.5 ml) and then incubated for 30 min at room temperature to reach approximately 95% AChE inhibition. Tabun-inhibited AChE was incubated for 10 min with an oxime solution (1 ml) at various oxime concentrations. Following incubation, 3 M sodium chloride solution (2.5 ml) was added, along with distilled water, to a constant volume (23 ml). Finally, 0.02 M acetylcholine iodide (2 ml) was added and the enzyme activity was measured titrimetrically at pH 7.6 and 25 °C on an Autotitrator RTS 822 (Radiometer, Denmark).
Computational details
Docking procedure
Firstly, the molecular docking technique was performed using the reactivators K203 and Obidoxime to perform the docking methodology with the non-aged form of Mus musculus Acetylcholinesterase (MmAChE) inhibited by tabun (PDB code 3DL4; resolution = 2.50 Å) [32], and with the human enzyme, also inhibited by this nerve agent, being a model of HssAChE already used in other works [33–35]. The oximes structures were constructed by using the PC Spartan Pro® software [36], with a subsequent optimization at the DFT level, with the Gaussian 09 package [37], using B3LYP density functional and 6-31 g (d, p) basis set. The partial charges of the atoms were elucidated via the Chelpg method [34]. The ligands were docked within the crystallographic structure of both AChE species by employing the Molegro Virtual Docker software (MVD®) [38], considering the same procedures previously employed [39–41]. For the docking procedure, within a radius of 8 Å, water molecules and amino acid residues were considered as flexible. Due to the essence of the molecular docking methodology, the calculations were performed giving rise to 50 different conformations (poses) for the reactivators. The most significant conformation for each ligand was chosen taking into account the most promising interaction energy within the enzyme active site, always searching for appropriate accommodations in the site, beyond the potent conformations for the reactivation mechanism.
The MolDock scoring function employed in the MVD software has its origin on the piecewise linear potential (PLP), a reduced potential whose settings are adjusted to enzyme-ligand complexes [42]. The docking scoring function values, Escore, are defined by Eq. 1:
| 1 |
Where:
| 2 |
EPLP stands for “piecewise linear potential”, which consists in the use of two different parameter sets, as described as follows: one employed for approach of the steric term among atoms (Van der Waals), and the other for the hydrogen bonding. The second term is associated to electrostatic interactions in relation to overloaded atoms. It consists of a Coulomb potential, presenting a dielectric constant dependent on the range (D(r) = 4r). The numeric amount of 332.0 is important, given that the electrostatic energy unit can be provided in kilocalories per molecule [38]. Eintra is associated to the inner energy of the ligand:
| 3 |
The first portion of the equation (double addition) is related to all pairs of atoms in the ligand, ruling out those that are linked by two bonds. The second term qualifies the torsional energy, where θ is the torsional angle of the bond. If several torsions are capable of being resolved, each torsional energy is important and an average in relation to them is employed. The last measure, Eclash, attributes a penalty equals to 1000 in the situation of distances less than 2.0 Å among heavy atoms, not considering impracticable ligand conformations [38]. The search algorithm in MVD combines the differential evolution optimization method with a cavity forecast algorithm, throughout the search process, thus allowing a rapid and precise recognition of potential binding modes (poses) [38, 43, 44].
Reaction mechanism and QM/MM methodology
From the structures selected with the docking methodology, the hybrid QM/MM was carried out to evaluate the energetic barrier (activation energy) for the mechanism in the reactivation pathway of the rat and human AChE inhibited by tabun. QM/MM techniques allow the modeling of larger systems, like reactions within enzymes, by combining the electronic degrees of a quantum chemical approach with the MM methods, increasing performance and decreasing computational demand [45]. Thus, in this study, hybrid quantum and molecular mechanics (QM/MM) associated with molecular docking methods have been carried out to determine the reactivation reaction pathway for the inhibited AChE, using different reactivators. Actually, this theoretical strategy has been previously applied in other occasions [35, 46–48]. With the purpose of getting more accurate results, capturing electronic effects, QM calculations were performed at the density functional theory (DFT) level with the Gaussian 09 package [37]. DFT techniques have shown a significant performance for large systems, such as biomolecules [49–51]. This relationship between functional and basis sets has been tested for similar systems. [44, 52]
The QM systems were constituted of amino acid residues, water molecules, reactivators and Ser203-tabun complex, for both AChE species. In the reaction mechanism simulation, all transition states were calculated and characterized identifying imaginary frequencies [53, 54]. Each system was fully optimized at DFT level, with conjugate gradient and quasi-Newton-Raphson algorithms. The final geometries were obtained with B3LYP functional density [43], using 6-31 g (d, p) basis set.
Results
In vitro results obtained in this study are summarized in Table 1 and Fig. 4. The reactivation process is characterized by several constants. Constant KR represents the affinity of the AChE reactivator to the inhibited enzyme. kR is the first order kinetic constant characterizing the splitting of the bond between enzyme and inhibitor. And finally, kr is the second order kinetic constant characterizing the entire reactivation process.
Table 1.
Tabun-inhibited acetylcholinesterase reactivation constants
| HUMAN | RAT | ||||
|---|---|---|---|---|---|
| Constants |
K
R
[μM] |
k
R
[min−1] |
k
r
[min− 1. M− 1] |
k
r
c
[min− 1. M− 1] |
Species Difference ratio |
| Oxime | |||||
| Pralidoxime | - a | - a | - a | 10 | - b |
| Obidoxime | 1412 | 0.060 | 42 | 6250 | 149 |
| Asoxime (HI-6) | - a | - a | - a | 1111 | - b |
| K203 | 56 | 0.120 | 2142 | 16,000 | 7.5 |
a, bUnable to estimate the appropriate constants due to lack of reactivated AChE by this oxime
cRat brain homogenate results published by Musilek et al. J. Med. Chem. (2007)
Fig. 4.
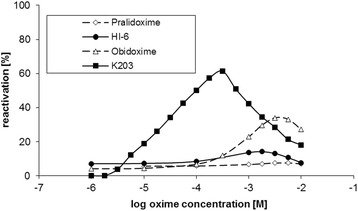
The effectiveness of oximes at varying concentrations to reactivate tabun-inhibited human cholinesterase. Cholinesterase activity measured in human brain homogenate is shown as a percentage of control activity. Tabun was incubated with brain homogenate for 30 min prior to the addition of the oxime. Reactivation was carried out for 10 min. All experiments were carried out at pH 7.6 and 25 °C. Each point represents the mean±SEM for 2 measurements
From the results obtained, only obidoxime and K203 were able to reactivate tabun-inhibited human brain cholinesterases. When they were directly compared, K203 surpassed obidoxime in all parameters including a 25-times higher affinity towards the inhibited enzyme. K203 was also able to disrupt the inhibitor-enzyme complex at twice the rate of obidoxime. Based on these two parameters the overall second order kinetic constant characterizing the whole reactivation process (kr) favored K203 for reactivation of tabun-inhibited AChE. If the reactivation curves were compared, K203 surpasses all other reactivators tested in this study. K203 reactivated over 60% of cholinesterase activity at human attainable concentrations. Obidoxime was the second most efficient oxime with 33% reactivation, but at concentrations between 10− 2 and 10− 3 M that are supposed to be not attainable in plasma after i.v. or i.m. administration. Other oximes did not appreciably reactivate tabun-inhibited human AChE.
Discussion
This study is the first to compare the effectiveness of four oximes in reactivating tabun-inhibited acetylcholinesterase (AChE) in human brain homogenate. Only five oximes are currently clinicaly available worldwide for the treatment of organophosphorus nerve agent or pesticide poisoning, pralidoxime, trimedoxime, obidoxime, methoxime and asoxime (HI-6). Asoxime (HI-6) has been the most extensively investigated as a broad-spectrum antidote but has been shown to be a very weak reactivator of tabun-inhibited AChE in several rodent species [9, 10, 22, 23, 55, 56]. The ineffectiveness of asoxime (HI-6) has also been reported in in vivo and in vitro studies conducted in rats [26, 57]. Of the commercially available oximes, only trimedoxime and obidoxime are able to reactivate tabun-inhibited AChE, however, their reactivation potency is less than ideal [4, 5]. K203 has been previously shown to be the most effective reactivator of tabun-inhibited AChE in both in vitro and in vivo rodent studies [57]. This study was the first to evaluate the efficacy of K203 on real human brain AChE and as was observed in the rodent study, K203 is more effective in reactivating tabun-inhibited enzyme than obidoxime. At therapeutic concentrations, K203 was able to reactivate over 60% of tabun-inhibited AChE, while obidoxime reactivated only 33% of its activity at supra-pharmacological doses (Fig. 4). When the overall reaction rate constant (kr) of human tissue is compared (Table 1), the constant for K203 was 51-times higher than that of obidoxime but only 2.5-times higher when the rat tissue was tested [26]. However when kr ratios are compared between human and rat enzymes, obidoxime results decrease 149-times while K203 decreases only 7.5-times. These results suggest that a direct correlation in oxime-mediated reactivation between species does not exist and that comparative studies in one species may not truly reflect the reactivation effects in humans.
The better reactivation efficacy of K203 was expected based on structure-activity relationship requirements [4, 58]. K203 meets four main requirements; two quaternary pyridinium rings, one nucleophilic oxime group, oxime at position four on the pyridinium ring and four carbon linkages between pyridinium rings (Fig. 5). Hundreds of compounds have been synthesized over the last decade to develop an effective reactivator of tabun-inhibited AChE. Of these compounds, K027, K048, K074 and K075 have yielded promising results against other organophosphorus compounds and are currently being evaluated by several laboratories worldwide [5, 19, 21, 59–64]. However, K203 appears to be the most effective reactivator following tabun exposure and it has also been reported to have a lower acute toxicity than trimedoxime and obidoxime [26]. The efficacy and toxicity profile of K203 supports the continued efforts in developing this compound as a medical countermeasure against.
Fig. 5.
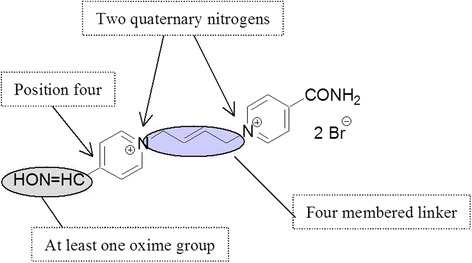
Structural requirements for reactivators of tabun-inhibited AChE
Molecular affinity: Docking studies
At the first scenario, molecular docking calculations have been used in order to look into the association/affinity between oxime and inhibited AChE. A cavity forecast algorithm, which is based on a 3D box was employed to bring forth binding sites in the enzymes, and for this, the Molegro Virtual Docker program was quite helpful. [38] The oximes were docked in a cavity whose volume was 115,200 Å [3] in MmAChE and 282,112 Å [3] in HssAChE. The respective energy values acquired from the oximes-AChE interactions were determined to have a better understanding the binding modes of each ligand, thus investigating the structural aspects that modulate the biological activity in the enzyme reactivation. Table 2 shows the intermolecular interaction energy values of the most appropriate pose of K203, selected for the subsequent mechanistic studies in both enzymes. By observing Table 2, it is easy to notice that K203 interacted better with the human enzyme, HssAChE, with an intermolecular interaction energy value of − 101.94 kcal.mol− 1, and an energy difference of 12.16 kcal.mol− 1 in relation to the rat enzyme, MmAChE. K203 has shown itself to have a high stability in the MmAChE, experimenting hydrogen bonds with two water molecules as well as two amino acid residues, Thr238 and Gly234. Interestingly the fact that this oxime presented much more hydrogen bonds in the HssAChE active site, where it also presented a good intermolecular interaction energy, − 89.78 kcal.mol− 1. In the human enzyme, K203 showed several interactions with amino acid residues and water, more precisely, six water molecules and two amino acid residues, Tyr120 and Gly117. Besides those interactions in the HssAChE active site, K203 showed a better energy value when docked in the MmAChE active site, and with this event, one can suppose that there are other important features that assist the good stabilization of this oxime in the enzyme active site, such as structural aspects, i.e., MmAChE interacts better with K203 in relation to HssAChE.
Table 2.
Intermolecular interaction energy (ΔE) values and hydrogen bonds between K203 and both AChE species
| AChE | Interaction Energy (kcal.mol− 1) |
H-Bonds | H-bond Strength (kcal.mol− 1) |
|---|---|---|---|
| Human | −89.78 | H2O | − 4.92 |
| H2O | −2.50 | ||
| H2O | −1.47 | ||
| H2O | −1.39 | ||
| H2O | −4.73 | ||
| H2O | −4.79 | ||
| Tyr120 | −0.66 | ||
| Gly117 | −1.34 | ||
| Rat | −101.94 | H2O | −3.89 |
| H2O | −2.41 | ||
| Thr238 | −6.12 | ||
| Gly234 | −5.00 |
The docking methodology provides a good start to understand the performance of these oximes in the inhibited AChE active site. It is important to keep in mind that diverse interactions are responsible for the better accommodation and affinity of these reactivators within the active site. Among these interactions, the hydrogen bonds are the most common (H-bonds), but it is noteworthy to cite electrostatic interactions as well as hydrophobic interactions, which could even have a great contribution to the conformation and orientation adopted by the reactivators in the active site. The H-bonds performed by this oxime, in both enzymes, are shown in Fig. 6.
Fig. 6.
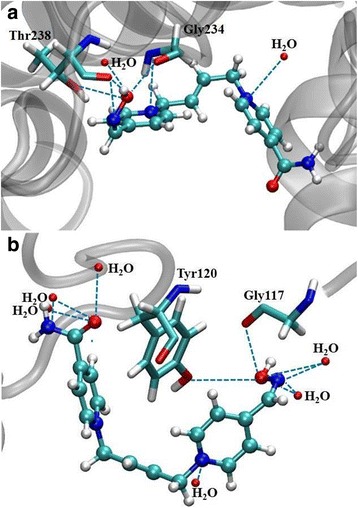
Interactions performed by K203 in the MmAChE (a) and HssAChE (b) active site
Both enzymes (MmAChE and HssAChE) were evaluated in order to have a deeper understanding of their main aspects and hydrophobic and electrostatic parameters. All these features, including the flexibility effect on the residues in a significant radius of the active site, contributed to simulate the interaction of these reactivators with the inhibited enzymes, producing a good starting point for the reaction mechanism calculations. The theoretical methodologies employed here are quite important in the design of new drugs. Other docking calculations were also carried out with Obidoxime. These results are presented in Table 3.
Table 3.
Intermolecular interaction energy (ΔE) values and hydrogen bonds between Obidoxime and both AChE species
| AChE | Interaction Energy (kcal.mol−1) | H-Bonds | H-bond Strength (kcal.mol− 1) |
|---|---|---|---|
| Human | −107.98 | H2O | −2.43 |
| H2O | −4.17 | ||
| H2O | −2.50 | ||
| H2O | −5.00 | ||
| Ser294 | −2.24 | ||
| Glu281 | −2.50 | ||
| Gly117 | −1.69 | ||
| Rat | −91.88 | H2O | −1.18 |
| H2O | −3.46 | ||
| H2O | −5.00 | ||
| Thr238 | −7.52 | ||
| Gly234 | −1.18 | ||
| Glu313 | −2.24 | ||
| Asn533 | −0.72 | ||
| Gln369 | −0.96 |
From the theoretical calculations, the docking results for Obidoxime are shown in Table 3. Those findings indicate that Obidoxime presented higher stability when docked in the HssAChE active site, with an intermolecular interaction energy of − 107.98 kcal.mol− 1. On the other hand, this oxime also interacted very favorably with the rat enzyme, with an energy value of − 91.88 kcal.mol− 1. For Obidoxime, the energy difference was 16.10 kcal.mol− 1 between both enzymes. Obidoxime performed several hydrogen bonds in the HssAChE active site, being four water molecules and three amino acid residues, Ser294, Glu281 and Gly117, which are important for the stabilization of this reactivator in the site. Meanwhile, this oxime also presented many interactions in the MmAChE active site, being three water molecules and many amino acid residues (Thr238, Gly234, Glu313, Asn533 and Gln369), thus showing the great affinity of the rat enzyme with this antidote. These interactions are shown in Fig. 7. Our docking calculations are in good agreement to KR values, which represent the affinity of the AChE reactivator to the inhibited enzyme.
Fig. 7.
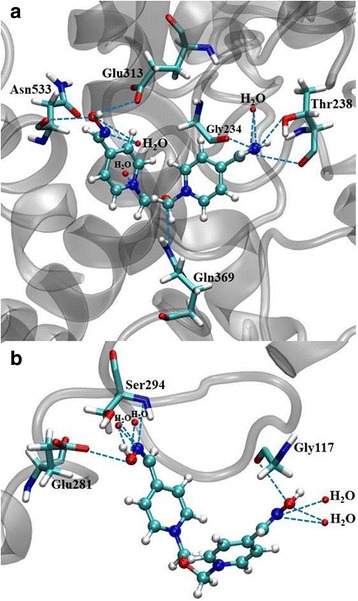
Interactions performed by Obidoxime in the MmAChE (a) and HssAChE (b) active site
Chemical reactivity: Quantum studies in the rat and human AChE active site
For the reactivation process, it is necessary to take into account the steric and electronic effects in the reaction as well as the preferential binding modes of the reactivators. In this context, the QM/MM techniques could be employed for the understanding of the binding modes between the oximes K203 and Obidoxime in the AChE active site, for both human and rat species. During the reactivation mechanism simulation, the energetic barriers for the reaction process (activation energy) were obtained and the values described in Table 4.
Table 4.
Relative activation energy values (∆∆E#) obtained for the reaction mechanism in the reactivation process and experimental results
| Oxime/Enzyme | ΔΔE# | kr [min−1. M−1] |
|---|---|---|
| K203 | ||
| Rat | 0.00 | 16,000 |
| Human | 38.81 | 2142 |
| Obidoxime | ||
| Rat | 0.00 | 6250 |
| Human | 54.42 | 42 |
According to Table 4, the oximes K203 and Obidoxime are most reactive in the rat enzyme than in the human AChE. These quantum theoretical results corroborate our experimental rate constant (kr) values very well. K203 has shown itself to be very efficient in reactivating the inhibited MmAChE, revealing an energetic barrier of 38.81 kcal.mol− 1 lower than the value obtained for the human enzyme, which is in a good agreement with the experimental results, wherein kr is 16,000 min− 1.M− 1 for the rat enzyme. This fact leads us to believe that the transition state is better stabilized in the reactivation pathway of MmAChE than HssAChE, allowing the oxime to interact stronger with the nerve agent.
This trend also corroborates to the intermolecular interaction energies from molecular docking calculations (Table 2), which have shown the best interaction and stability of K203 in MmAChE. From the experimental side (Table 1), there is good agreement between binding constant (KR) and ΔE values. It should be kept in mind, however, that the mechanistic studies with Obidoxime are also in a good agreement with the experimental results. By observing the kr values for the oxime mediated reactivation, the difference between the rat and human enzyme is significant, and as expected, the reaction mechanism simulation has also shown this result. Obidoxime interacted much more in the rat enzyme, with a large difference between the values found for the energetic barrier in the reaction pathway, being 54.42 kcal.mol− 1 in relation to HssAChE. From the docking results, in contrast to K203, Obidoxime showed a slightly better interaction energy value for HssAChE, but during the simulation, there must be intermediate species which are better stabilized, thus leading to lower global activation energy during the full process.
Conclusions
We have applied computational chemistry methods in order to evaluate the high reactivity of the oximes K203 and Obidoxime for different AChE species. Our theoretical results showed that these oximes are more effective towards the rat enzyme. The reactivation process for MmAChE takes place more easily by both oximes investigated. This result was confirmed by DFT calculations, being the lowest activation energy barrier for the rat enzyme, corroborating our experimental results. From molecular docking results, both reactivators are well stabilized in both AChE species, mainly due to the hydrogen bonds with the amino acid residues Ser, Glu and Gly performed in the active site.
Acknowledgements
No.
Funding
Authors are grateful to the Grant agency of the Czech Republic for the financial support (No. 15-16701S). This work was also supported by the long term development plan UHHK and UHK and the Brazilian agencies FAPEMIG, CAPES, and CNPq.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request. Oxime K203 is available by authors for non-commercial use.
Abbreviations
- AChE
Acetylcholinesterase
- HPLC
High-performance liquid chromatography
- QM/MM
Quantum and molecular mechanics
- TLC
Thin-layer chromatography
Authors’ contributions
Writing of the manuscript (KK, KM. DJ, JM, TF, MV, TR), synthesis of compounds (EN, KM, KK, MH), purity evaluation (JZK, DJ, OS), in vitro evaluation (KK, MH, OS, DJ), computational chemistry (TF, EDC, ADC, TR). All authors read and approved the final manuscript.
Ethics approval and consent to participate
Experiments were approved in compliance with relevant laws and institutional guidelines and were approved by the Ethics Committee of the Faculty of Military Health Sciences in Hradec Kralove (Czech Republic).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marrs TC. Organophosphate poisoning. Pharmacol. Ther. 1993;58:51–66. doi: 10.1016/0163-7258(93)90066-M. [DOI] [PubMed] [Google Scholar]
- 2.Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem. 2004;38:151–216. doi: 10.1016/S0065-2423(04)38006-6. [DOI] [PubMed] [Google Scholar]
- 3.Jokanovic M, Stojiljkovic MP. Current understanding of the application of pyridinium oximes ascholinesterase reactivators in treatment of organophosphate poisoning. Eur J Pharmacol. 2006;553:10–17. doi: 10.1016/j.ejphar.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 4.Cabal J, Kuca K, Kassa J. Specification of the structure of oximes able to reactivate tabun inhibited acetylcholinesterase. Basic Clin Pharmacol Toxicol. 2004;95(2):81–86. doi: 10.1111/j.1742-7843.2004.950207.x. [DOI] [PubMed] [Google Scholar]
- 5.Calic M, Lucic VA, Radic B, Jelic D, Jun D, Kuca K, Kovarik Z. In vitro and in vivo evaluation of pyridinium oximes: mode of interaction with acetylcholinesterase, effect on tabun- and soman-poisoned mice and their cytotoxicity. Toxicol. 2006;219(1-3):85–96. doi: 10.1016/j.tox.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Koplovitz I, Stewart JR. A comparison of the efficacy of HI-6 and 2-PAM against soman, tabun, sarin and VX in the rabbit. Toxicol Lett. 1994;70:169–179. doi: 10.1016/0378-4274(94)90121-X. [DOI] [PubMed] [Google Scholar]
- 7.Wilson IB, Sondheimer F. A specific antidote against lethal alkyl phosphate intoxication. V. Antidotal properties. Arch. Biochem. Biophys. 1957;69:468–474. doi: 10.1016/0003-9861(57)90511-8. [DOI] [PubMed] [Google Scholar]
- 8.Ekstrom F, Akfur C, Tunemalm AK, Lundberg S. Structural changes of phenylalanine 338 and histidine 447 revealed by the crystal structures of tabun-inhibited murine acetylcholinesterase. Biochem. 2006;45:74–81. doi: 10.1021/bi051286t. [DOI] [PubMed] [Google Scholar]
- 9.Puu G, Artursson E, Bucht G. Reactivation of nerve agent inhibited acetylcholinesterases by HI-6 and obidoxime. Biochem Pharmacol. 1986;35:1505–1510. doi: 10.1016/0006-2952(86)90116-4. [DOI] [PubMed] [Google Scholar]
- 10.Clement JG, Shiloff JD, Gennings C. Efficacy of a combination of acetylcholinesterase reactivators, HI-6 and obidoxime, against tabun and soman poisoning in mice. Arch Toxicol. 1987;61:70–75. doi: 10.1007/BF00324551. [DOI] [PubMed] [Google Scholar]
- 11.Jun D, Kuca K, Hronek M, Opletal L. Effect of some acetylcholinesterase reactivators on human platelet aggregation in vitro. J App Toxicol. 2006;26(3):262–268. doi: 10.1002/jat.1126. [DOI] [PubMed] [Google Scholar]
- 12.Balali-Mood M, Shariat M. Treatment of organophosphate poisoning. Experience of nerve agents and acute pesticide poisoning on the effects of oximes. J Physiol (Paris) 1998;92:375–378. doi: 10.1016/S0928-4257(99)80008-4. [DOI] [PubMed] [Google Scholar]
- 13.Musilek K, Kuca K, Jun D, Dohnal V, Dolezal M. Synthesis of the novel series of bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against chlorpyrifos-inhibited acetylcholinesterase. Bioorg Med Chem Lett. 2006;16(3):622–627. doi: 10.1016/j.bmcl.2005.10.059. [DOI] [PubMed] [Google Scholar]
- 14.Kuca K, Bielavský J, Cabal J, Bielavská M. Synthesis of a potential reactivator of acetylcholinesterase 1-(4-hydroxyiminomethylpyridinium)-3-(carbamoylpyridinium)-propane dibromide. Tetrahedron Lett. 2003;44:3123–3125. doi: 10.1016/S0040-4039(03)00538-0. [DOI] [Google Scholar]
- 15.Kuca K, Bielavský J, Cabal J, Kassa J. Synthesis of a new reactivator of tabun inhibited acetylcholinesterase. Bioorg Med Chem Lett. 2003;13:3545–3547. doi: 10.1016/S0960-894X(03)00751-0. [DOI] [PubMed] [Google Scholar]
- 16.Kuca K, Kassa J. A Comparison of the Ability of a New Bispyridinium Oxime--1-(4-hydroxyiminomethylpyridinium)-4-(4-carbamoylpyridinium)butane Dibromide and Currently used Oximes to Reactivate Nerve Agent-inhibited Rat Brain Acetylcholinesterase by In Vitro Methods. J Enzym Inhib Med Chem. 2003;18:529–535. doi: 10.1080/14756360310001605552. [DOI] [PubMed] [Google Scholar]
- 17.Kuca K, Kassa J. In vitro reactivation of acetylcholinesterase using of the oxime K027. Veterinary and Human Toxicology. 2004;46:15–18. [PubMed] [Google Scholar]
- 18.Kuca K, Kassa J. Oximes-induced reactivation of rat brain acetylcholinesterase inhibited by VX agent. Hum Exp Toxicol. 2004;23(4):167–171. doi: 10.1191/0960327104ht434oa. [DOI] [PubMed] [Google Scholar]
- 19.da Silva AP, Farina M, Franco JL, Dafre AL, Kassa J, Kuca K. Temporal effects of newly developed oximes (K027, K048) on malathion-induced acetylcholinesterase inhibition and lipid peroxidation in mouse prefrontal cortex. NeuroToxicology. 2008;29(1):184–189. doi: 10.1016/j.neuro.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Kassa J, Kuca K, Cabal J, Paar M. A comparison of the efficacy of new asymmetric bispyridinium oximes (K027, K048) with currently available oximes against tabun by in vitro and in vivo methods. J Toxicol Environ Health. 2006;69(20):1875–1882. doi: 10.1080/15287390600631730. [DOI] [PubMed] [Google Scholar]
- 21.Lucić-Vrdoljak A, Čalić M, Radić B, Berend S, Kuca K, Kovarik Z. Pre-treatment with pyridinium oximes improves antidotal therapy against tabun poisoning. Toxicology. 2006;228(1):41–50. doi: 10.1016/j.tox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Kuca K, Cabal J, Kassa J. In vitro reactivation of sarin-inhibited brain acetylcholinesterase from various species by various oximes. J Enzym Inhib Med Chem. 2005;20(3):227–232. doi: 10.1080/14756360500043208. [DOI] [PubMed] [Google Scholar]
- 23.Kuca K, Cabal J, Musilek K, Jun D, Bajgar J. Effective bisquaternary reactivators of tabun-inhibited AChE. J Appl Toxicol. 2005;25(6):491–495. doi: 10.1002/jat.1084. [DOI] [PubMed] [Google Scholar]
- 24.Kassa J, Jun D, Kuca K. A comparison of reactivating efficacy of newly developed oximes (K074, K075) and currently available oximes (obidoxime, HI-6) in cyclosarin and tabun-poisoned rats. J Enzym Inhib Med Chem. 2007;22(3):297–300. doi: 10.1080/14756360601114361. [DOI] [PubMed] [Google Scholar]
- 25.Kuca K, Cabal J, Jun D, Musilek K. In vitro reactivation potency of acetylcholinesterase reactivators – K074 and K075 – to reactivate tabun inhibited human brain cholinesterases. Neurotoxicity Res. 2007;11(2):101–106. doi: 10.1007/BF03033389. [DOI] [PubMed] [Google Scholar]
- 26.Musilek K, Jun D, Cabal J, Kassa J, Gunn-Moore F, Kuca K. Design of a Potent Reactivator of Tabun-Inhibited Acetylcholinesterase - Synthesis and evaluation of (E)-1-(4-carbamoylpyridinium)-4-(4-hydroxyiminomethylpyridinium)-but-2-ene Dibromide (K203) J Med Chem. 2007;50(22):5514–5518. doi: 10.1021/jm070653r. [DOI] [PubMed] [Google Scholar]
- 27.Kassa J, Karasova J, Musilek K, Kuca K. An evaluation of therapeutic and reactivating effects of newly developed oximes (K156, K203) and commonly used oximes (obidoxime, trimedoxime, HI-6) in tabun-poisoned rats and mice. Toxicology. 2008;243(3):311–316. doi: 10.1016/j.tox.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Worek F, Reiter G, Eyer P, Szinicz L. Reactivation kinetics of acetylcholinesterase from different species inhibited by highly toxic organophosphates. Arch Toxicol. 2002;76:523–529. doi: 10.1007/s00204-002-0375-1. [DOI] [PubMed] [Google Scholar]
- 29.Wiesner J, Kriz Z, Kuca K, Jun D, Koca J. Acetylcholinesterases – the structural similarities and differences. J Enzym Inhib Med Chem. 2007;22(4):417–424. doi: 10.1080/14756360701421294. [DOI] [PubMed] [Google Scholar]
- 30.Jun D, Stodulka P, Kuca K, Koleckar V, Dolezal B, Simon P, Veverka M. HPLC analysis of HI-6 dichloride and dimethanesulfonate – antidotes against nerve agents and organophosphorus pesticides. Anal Lett. 2007;40(14):2783–2787. doi: 10.1080/00032710701588531. [DOI] [Google Scholar]
- 31.Jun D, Stodulka P, Kuca K, Koleckar V, Dolezal B, Simon P, Veverka M. TLC analysis of intermediates arising during the preparation of oxime HI-6 dimethanesulfonate. J Chromatogr Sci. 2008;46(4):316–319. doi: 10.1093/chromsci/46.4.316. [DOI] [PubMed] [Google Scholar]
- 32.Carletti E, Li H, Li B, Ekstroem F, Nicolet Y, Loiodice M, Gillon E, Froment MT, Lockridge O, Schopfer LM, Masson P, Nachon F. Aging of cholinesterases phosphylated by tabun proceeds through O-dealkylation. J Am Chem Soc. 2008;130:16011–16020. doi: 10.1021/ja804941z. [DOI] [PubMed] [Google Scholar]
- 33.Goncalves AS, Franca TCC, Wilter A, Figueroa-Villar JD. Molecular Dynamics of the Interaction of Pralidoxime and Deazapralidoxime with Acetylcholinesterase Inhibited by the Neurotoxic Agent Tabun. J Braz Chem Soc. 2006;17:968–975. doi: 10.1590/S0103-50532006000500022. [DOI] [Google Scholar]
- 34.Gonçalves AS, França TCC, Figueroa-Villar JD, Pascutti PG. Molecular Dynamics Simulations and QM/MM Studies of the Reactivation by 2-Pam of Tabun Inhibited Human Acethylcolinesterase. J Braz Chem Soc. 2011;22:155–165. doi: 10.1590/S0103-50532011000100021. [DOI] [Google Scholar]
- 35.Hehre WJ, Deppmeier BJ, Klunzinger PE. PC SPARTAN Plus TUTORIAL version 2.0. Irvine, CA: Wavefunction, Inc.; 1999. [Google Scholar]
- 36.Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A. Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., Pople, J.A.: Gaussian 03, Revision C.02. Gaussian, Inc., Wallingford, Connecticut (2004)
- 37.Thomsen R, Christensen MH. MolDock: A new technique for high-accuracy molecular docking. J Med Chem. 2006;49:3315–3321. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]
- 38.Silva MC, Torres JA, Castro AA, da Cunha EF. Alves de Oliveira LC., Corrêa AD., Ramalho TC. Combined experimental and theoretical study on the removal of pollutant compounds by peroxidases: affinity and reactivity toward a bioremediation catalyst. J Biomol Struct Dyn. 2016;34(9):1839–1848. doi: 10.1080/07391102.2015.1063456. [DOI] [PubMed] [Google Scholar]
- 39.Guimarães AP, França TCC, Ramalho TC, Rennó MN, Ferreira da Cunha EF, Matos KS, Mancini DT, Kuča K. Docking studies and effects of. syn-anti isomery of oximes derived from pyridine. imidazol bicycled systems as potential human. acetylcholinesterase reactivators. J Appl Biomed. 2011;9:163–171. doi: 10.2478/v10136-009-0037-1. [DOI] [Google Scholar]
- 40.Matos KS, Mancini DT, da Cunha EF, Kuca K, França TC, Ramalho TC. Molecular aspects of the reactivation process of acetylcholinesterase inhibited by cyclosarin. J Braz Chem Soc. 2011;22:1999–2004. [Google Scholar]
- 41.Ramalho TC, Caetano MS, da Cunha EF, Souza TC, Rocha MV. Construction and assessment of reaction models of class I EPSP synthase: molecular docking and density functional theoretical calculations. J Biomol Struct Dyn. 2009;27(2):195–207. doi: 10.1080/07391102.2009.10507309. [DOI] [PubMed] [Google Scholar]
- 42.da Cunha EF, Ramalho TC, Reynolds RC. Binding mode analysis of 2,4-diamino-5-methyl-5-deaza-6-substituted pteridines with Mycobacterium tuberculosis and human dihydrofolate reductases. J Biomol Struct Dyn. 2008;25(4):377–385. doi: 10.1080/07391102.2008.10507186. [DOI] [PubMed] [Google Scholar]
- 43.da Cunha EE, Barbosa EF, Oliveira AA, Ramalho TC. Molecular modeling of Mycobacterium tuberculosis DNA gyrase and its molecular docking study with gatifloxacin inhibitors. J Biomol Struct Dyn. 2010;27(5):619–625. doi: 10.1080/07391102.2010.10508576. [DOI] [PubMed] [Google Scholar]
- 44.Borman SA. Much to do about enzyme mechanism. Chem Eng News. 2004;8:35–39. doi: 10.1021/cen-v082n008.p035. [DOI] [Google Scholar]
- 45.Heyden A, Lin H, Truhlar DG. Adaptive partitioning in combined quantum mechanical and molecular mechanical calculations of potential energy functions for multiscale simulations. J Phys Chem B. 2007;111:2231–2241. doi: 10.1021/jp0673617. [DOI] [PubMed] [Google Scholar]
- 46.Ramalho TC, Da Cunha EFF, De Alencastro RB. Solvent effects on 13 C and 15 N shielding tensors of nitroimidazoles in the condensed phase: a sequential molecular dynamics/quantum mechanics study. J Phys Condens Matter. 2004;16:6159–6170. doi: 10.1088/0953-8984/16/34/015. [DOI] [Google Scholar]
- 47.Matos KS, da Cunha EFF, Abagyan R, Ramalho TC. Computational evidence for the reactivation process of human acetylcholinesterase inhibited by carbamates. Comb.Chem. High Throughput Screen. 2014;17(6):554–564. doi: 10.2174/1386207316666131217100416. [DOI] [PubMed] [Google Scholar]
- 48.Singh UC. Kollman PA An approach to computing electro-. static charges for molecules. J Comput Chem. 1984;5:129–134. doi: 10.1002/jcc.540050204. [DOI] [Google Scholar]
- 49.Besler BH, Merz KM, Kollman PA. Atomic charges derived from semiempirical methods. J Comput Chem. 1990;11:431–439. doi: 10.1002/jcc.540110404. [DOI] [Google Scholar]
- 50.Gustin DJ, Mattei P, Kast P, Wiest O, Lee L, Cleland WW, Hilvert D. Heavy atom isotope effects reveal a highly polarized. transition state for chorismate mutase. J Am Chem Soc. 1999;121:1756–1765. doi: 10.1021/ja9841759. [DOI] [Google Scholar]
- 51.Rutkowska-Zbik D, Witko M. Following nature-theoretical studies on factors modulating catalytic activity of porphyrins. J Mol Catal Chem. 2006;258(1-2):376–380. doi: 10.1016/j.molcata.2006.07.017. [DOI] [Google Scholar]
- 52.Giacoppo JOS, França TCC, Kuca K, da Cunha EFF, Abagyan R, Mancini DT, Ramalho TC. Molecular modeling and in vitro reactivation study between the oxime BI-6 and acetyl- cholinesterase inhibited by different nerve agents. J Biomol Struct Dyn. 2015;33:2048–2058. doi: 10.1080/07391102.2014.989408. [DOI] [PubMed] [Google Scholar]
- 53.Li R, Liu Y, Zhang J, Chen K, Li S, Jiang J. An isofenphos-. methyl hydrolase (Imh) capable of hydrolyzing the P–O–Z moiety. of organophosphorus pesticides containing an aryl or heterocyclic group. Appl Microbiol Biotechnol. 2012;94(6):1553–64. [DOI] [PubMed]
- 54.Gorecki L, Korabecny J, Musilek K, Malinak D, Nepovimova E, Dolezal R, Jun D, Soukup O. Kuca K.¨ SAR study to find optimal cholinesterase reactivator against organophosphorous nerve agents and pesticides. Arch Toxicol. 2016;90(12):2831–2859. doi: 10.1007/s00204-016-1827-3. [DOI] [PubMed] [Google Scholar]
- 55.Nepovimova E, Korabecny J, Dolezal R, Nguyen TD, Jun D, Soukup O, Pasdiorova M, Jost P, Muckova L, Malinak D, Gorecki L, Musilek K, Kuca K. A 7-methoxytacrine–4-pyridinealdoxime hybrid as a novel prophylactic agent with reactivation properties in organophosphate. Toxicol res. 2016;4(5):1012–1016. doi: 10.1039/C6TX00130K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuca K, Cabal J. Evaluation of newly synthesized reactivators of the brain cholinesterase inhibited by sarin nerve agent. Toxicol Mech Methods. 2005;15(4):247–252. doi: 10.1080/15376520590968770. [DOI] [PubMed] [Google Scholar]
- 57.Kuca K, Jun D, Musilek K. Structural requirements of acetylcholinesterase reactivators. Mini Rev Med Chem. 2006;6(3):269–277. doi: 10.2174/138955706776073510. [DOI] [PubMed] [Google Scholar]
- 58.Kalász H, Hasan MY, Sheen R, Kuca K, Petroianu GA, Ludány K, Gergely A, Tekes K. HPLC Analysis of K-48 concentration in plasma. Anal Bioanal Chem. 2006;385(6):1062–1067. doi: 10.1007/s00216-006-0490-6. [DOI] [PubMed] [Google Scholar]
- 59.Tekes K, Hasan MY, Sheen R, Kuca K, Petroianu G, Ludányi K, Kalász H. HPLC determination of the serum concentration of K-27, a novel oxime-type cholinesterase reactivator. J Chromatogr A. 2006;1122(1-2):84–87. doi: 10.1016/j.chroma.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Petroianu GA, Arafat K, Nurulain SM, Kuca K, Kassa J. In vitro oxime reactivation of red blood cell acetylcholinesterase inhibited by methyl-paraoxon. J Appl Toxicol. 2007;27(2):168–175. doi: 10.1002/jat.1189. [DOI] [PubMed] [Google Scholar]
- 61.Petroianu GA, Nurulain SM, Nagelkerke N, Shafiullah M, Kassa J, Kuca K. Five oximes (K-27, K-48, obidoxime, HI-6 and trimedoxime) in comparison with pralidoxime: survival in rats exposed to methyl-paraoxon. J Appl Toxicol. 2007;27(5):453–457. doi: 10.1002/jat.1224. [DOI] [PubMed] [Google Scholar]
- 62.Kovarik Z, Lucić VA, Berend S, Katalinić M, Kuca K, Musilek K, Radić B. Evaluation of oxime K203 as antidote in tabun poisoning. Arhiv za Higijenu Rada i Toksikologiju. 2009;60(1):19–26. doi: 10.2478/10004-1254-60-2009-1890. [DOI] [PubMed] [Google Scholar]
- 63.Lorke DE, Hasan MY, Nurulain SM, Sheen R, Kuca K, Petroianu GA. Entry of two new asymmetric bispyridinium oximes (K-27 and K-48) into the rat brain: comparison with obidoxime. J Appl Toxicol. 2007;27(5):482–490. doi: 10.1002/jat.1229. [DOI] [PubMed] [Google Scholar]
- 64.Petroianu GA, Arafat K, Kuca K, Kassa J. Five oximes (K-27, K-33, K-48, BI-6 and methoxime) in comparison with pralidoxime: in vitro reactivation of red blood cell acetylcholinesterase inhibited by paraoxon. J Appl Toxicol. 2006;26(1):64–71. doi: 10.1002/jat.1108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request. Oxime K203 is available by authors for non-commercial use.


