Abstract
Background
Significant associations between visceral fat and alterations in plasma fatty acids have been identified in overweight individuals. However, there are scant data regarding the relationships of the visceral fat area (VFA) with the plasma fatty acid profiles and desaturase activities following weight loss. We investigated the effect of weight loss with mild calorie restriction on the circulating fatty acid profiles and desaturase activities in nondiabetic overweight subjects with high VFA.
Methods
Eighty overweight subjects with high VFA (L4 VFA ≥100 cm2) were randomized into the 12-week mild-calorie-restriction (300 kcal/day) or control groups.
Results
Comparison of the percent of body weight changes between groups revealed that the weight-loss group had greater reductions in body weight. The VFA decreased by 17.7 cm2 from baseline in the weight-loss group (P < 0.001). At follow-up, the weight-loss group showed greater reductions in serum triglycerides, insulin, and HOMA-IR than the control group. Significantly greater reductions in total saturated fatty acids, palmitic acid, stearic acid, total monounsaturated fatty acids, palmitoleic acid, oleic acid, eicosadienoic acid, and dihomo-γ-linolenic acid levels were detected in the weight-loss group compared with the control group after adjusting for baseline values. Following weight loss, C16 Δ9-desaturase activity was significantly decreased and Δ5-desaturase activity was significantly increased, and the changes were greater in the weight-loss group than in the control group.
Conclusions
The results suggest that mild weight loss improves abdominal obesity, overall fatty acid profiles, and desaturase activities; therefore, mild calorie restriction has potential health benefits related to obesity-related diseases in overweight subjects with high VFA.
Trial registration
NCT02992639. Retrospectively registered 11 December 2016.
Keywords: Fatty acid, Fatty acid desaturase, Visceral fat, Weight loss, Obesity-related disease
Background
A visceral fat area (VFA) cutoff of 100 cm2 was established to predict the risk of obesity-related health risks, including insulin resistance (IR), metabolic syndrome, and diabetes, in Asian populations [1]. Compared with abdominal subcutaneous fat, visceral fat is more metabolically active in the fasting state [2, 3]. Compared with other weight-loss methods, calorie restriction tends to reduce more visceral than subcutaneous adipose tissue [4]. It is known that plasma fatty acid composition mirrors not only dietary fatty acid composition but also endogenous fatty acid synthesis, in which desaturases play important roles [5]. In endogenous fatty acid synthesis, C16 Δ9-desaturase (C16:1/C16:0) activity is known to be high in conditions of diabetes and abdominal obesity. Additionally, an inverse relation between Δ5-desaturase activity (C20:4, n-6/C20:3, n-6) and diabetes risk and a direct relation between Δ6-desaturase activity (C18:3, n-6/C18:2, n-6) and IR have been reported. Pan et al. [6] reported that obesity is related to increased Δ9-desaturase activity and reduced Δ5-desaturase activity. Several previous studies observed that low Δ5-desaturase activity is linked to increased risk of type 2 diabetes and insulin resistance among Japanese adults [7, 8]. Zhao et al. [9] also noted that Δ5-desaturase activity is inversely associated with metabolic abnormalities among obese Chinese subjects. In a Swedish study, Δ5-desaturase activity was found to be inversely associated with obesity and insulin resistance, whereas Δ6-desaturase activity demonstrated positive associations [10].
Significant associations between visceral fat amount and alterations in serum fatty acids have been identified in overweight individuals [11]. However, there are scant data regarding the relations between changes in VFA and changes in circulating fatty acid profiles and desaturase activities following weight loss. Research is required to determine the amount of visceral fat reduction with calorie restriction that is necessary to induce favorable metabolic changes in the overall fatty acid profile. Therefore, in this study, we investigated the effects of weight loss with mild calorie restriction (a 300 kcal/day intake reduction) on circulating fatty acid profiles and desaturase activities in nondiabetic overweight subjects with high VFA [VFA at the 4th lumbar vertebrae (L4 VFA) ≥ 100 cm2].
Methods
Study subjects
Study subjects were recruited through advertisements posted in Seoul. Based on the data screened by the Clinical Nutrition Lab, Yonsei University, overweight subjects [25.0 kg/m2 ≤ body mass index (BMI) < 30 kg/m2] were referred to the Department of Internal Medicine, Yonsei University Severance Hospital. After their health and basic blood tests, including serum glucose, were rechecked, individuals who met the study criteria were recommended for study participation. The inclusion criteria were as follows: age between 20 and 60 years; absence of pregnancy or breastfeeding; stable body weight (body weight change < 1 kg in the 3 months before screening); BMI between 25 and 30 kg/m2; high L4 VFA (L4 VFA ≥ 100 cm2); absence of hypertension, type 2 diabetes, cardiovascular disease, and thyroid disease; and no use of medication affecting body weight, energy expenditure, or glucose control for 6 months prior to screening. Subjects were excluded if they had a history of Cushing syndrome, malignancy, or liver disease, including chronic viral hepatitis, autoimmune hepatitis, primary biliary cirrhosis, and drug-induced liver disease. Male and female subjects whose alcohol consumption was > 40 and > 20 g/day, respectively, and subjects with a history of intentional weight reduction in the 6 months before the current study were excluded. Finally, those who consented to participate in the program were included in this study. The purpose of the study was carefully explained to all participants, and written consent was obtained prior to their participation. The protocol was approved by the Institutional Review Board of Yonsei University and Yonsei University Severance Hospital in accordance with the Helsinki Declaration.
Study design and intervention
The basic framework of the present study was based on a previous study [12]. A 12-week, placebo-controlled, randomized study was conducted with 80 high VFA, nondiabetic overweight subjects (L4 VFA ≥ 100 cm2). Subjects were divided into two groups: a weight-loss group with 12 weeks of mild calorie restriction (a 300 kcal/day intake reduction) or a control group with no treatment (NCT02992639; http://www.clinicaltrials.gov/). Randomization was achieved by computer-generated block randomization (placebo:test = 1:1).
Protocol for weight loss
The subjects in the weight-loss group followed a 12-week weight-loss program comprising a 300 kcal/day reduction of their usual caloric intake. Basically, the subjects were instructed to remove 1/3 of a bowl of rice (approximately 100 kcal; a bowl of rice is approximately 300 kcal per meal, according to the food composition table from the Rural Development Administration of Korea [13]) from one meal a day for easier application of the 300 kcal/day deficit. Moreover, based on each subject’s reported food intake, an individualized and nutritionally balanced diet plan was produced by a trained nutritionist to achieve the goal of losing a minimum of 3% of initial body weight. The instructions included food choices, cooking methods, reductions in snack consumption frequency, low-calorie substitutions for high-calorie foods, low-fat foods, and limitations on simple sugar consumption. The subjects in the control group were instructed to maintain their usual dietary and physical activity habits during the study period.
Daily energy intake and physical activity measurements
At baseline, the subjects’ usual dietary intakes were assessed via 24-h recall and semiquantitative food frequency questionnaires completed with the nutritionist’s assistance. In addition, a standardized 3-day dietary record was obtained from each participant at week 6 and 12. These records were completed at home following detailed instructions from the nutritionist. A computerized version of the Korean Nutrition File (Can-Pro 3.0; The Korean Nutrition Society, Seoul, Korea) was used to determine the macronutrient contents of foods and total daily energy intakes. On the same days as the dietary records, 3-day physical activity records were completed at home. The total energy expenditure (TEE) (kcal/day) was calculated from activity patterns, including the basal metabolic rate (BMR), physical activity for 24 h, and the specific dynamic action of food. Each subject’s BMR was calculated using the Harris-Benedict equation.
Anthropometric parameters and body composition measurements
Detailed information was previously published [14]. Briefly, all data were acquired at baseline and week 12. Body weight (Inbody370; Biospace, Cheonan, Korea), height, waist circumference, and blood pressure (BP) were measured. BMI was calculated in units of kilogram per square meter (kg/m2). The abdominal fat distribution was measured at L4 by computed tomography (CT). The scanning parameters were a slice thickness of 1 mm at 200 mA and 120 kVp, with a 48-cm field of view. Abdominal adipose tissue was determined using the attenuation range from − 150 to − 50 Hounsfield units in the CT images. The body composition of the study participants was measured via dual-energy X-ray absorptiometry (DEXA) to determine the fat percentage, fat mass, and lean body mass.
Blood collection and biochemical assessments
Details were provided previously [14]. Blood samples were collected after an overnight fast of at least 12 h. Venous blood specimens were collected in EDTA-treated tubes and serum tubes. The blood samples were centrifuged to obtain plasma and serum samples, which were then stored at − 70 °C. The levels of serum-fasting glucose, insulin, triglycerides (TGs), total cholesterol, and high-density lipoprotein (HDL) cholesterol were measured [15]. The IR and low-density lipoprotein (LDL) cholesterol levels were calculated using the equations of homeostatic model assessment (HOMA) and the Friedewald formula, respectively: HOMA-IR = [fasting insulin (μIU/mL) × fasting glucose (mg/dL)]/405; Friedewald formula: LDL cholesterol = total cholesterol – [HDL cholesterol + (TG/5)].
Metabolite extraction and gas chromatography–mass spectrometry (GC-MS) analysis
The details of the GC-MS procedure have been previously published [16]. Briefly, plasma samples (100 μL) were placed into PTEE screw-capped Pyrex tubes. An internal standard compound containing heptadecanoic acid (500 μL, 25 ppm in n-hexane) and methanol (2 mL) were added to the samples. After vortexing, acetyl chloride (100 μL) was added slowly, and the tubes were then heated at 95 °C for 1 h. After cooling on ice, 6% potassium carbonate (5 mL) was added to the tubes. The tubes were centrifuged (4 °C, 3000 rpm, 5 min), and the clear n-hexane top layer (100 μL), including the fatty acid methyl esters (FAME), was then transferred into a vial prior to GC-MS analysis.
GC-MS analyses were performed on an Agilent Technologies 7890 N gas chromatograph coupled with an Agilent Technologies 5977A quadrupole mass selective spectrometer with a triple-axis detector (Agilent, Palo Alto, CA, USA) in the electron ionization (70 eV) and full scan monitoring (m/z 50–800) modes. The injection volume of the samples was 1 μL in the splitless mode. The fatty acid methyl esters of all samples were separated on a VF-WAX column (Agilent Technologies, Middelburg, Netherlands) with helium as a carrier gas. The temperature program was as follows: (1) initial temperature of 50 °C for 2.3 min, (2) increase to 175 °C at 50 °C/min, and (3) increase to 230 °C at 2 °C/min. The metabolites in the samples were identified by comparing their relative retention times and mass spectra with those of authentic reference standards. The relative levels of metabolites were calculated by comparing their peak areas to those of the internal standard compound.
Statistical analysis
The statistical analyses were conducted with SPSS version 23.0 software (IBM/SPSS, Chicago, IL, USA). Skewed variables were logarithmically transformed. An independent t-test compared continuous variables between the control and weight-loss groups. A paired t-test was used to compare continuous variables at baseline and follow-up within each group. For the comparison of categorical variables, a chi-squared test was conducted. A general linear model test was performed to adjust the baseline values of the changed values. A Pearson’s correlation coefficient was used to examine the relations among variables. A heat map was created using Multiple Experiment Viewer (MeV) version 4.9.0 (http://www.tm4.org/) to visualize and evaluate the relations among variables in the study population. The results are expressed as the mean ± standard error (SE). A two-tailed P < 0.05 was considered statistically significant.
Results
The dropout rate was less than 10% in the control (n = 2) and weight-loss (n = 3) groups. Participants were excluded for personal reasons (n = 4) and lack of a blood sample for GC-MS analysis (n = 1).
Effects of mild calorie restriction on clinical and biochemical characteristics at baseline and follow-up
The age, sex distribution, smoking, and drinking did not significantly differ between the two groups (Table 1). At baseline, no significant differences were observed in all clinical and biochemical parameters between the control and weight-loss groups; after the 12-week intervention, the BMI, serum insulin levels, HOMA-IR score, and serum TG levels were significantly decreased in the weight-loss group compared with the control group (Table 1). In the control group, the subjects showed slight but significant weight gain (weight and BMI; all P < 0.001) during the intervention, whereas the subjects in the weight-loss group showed significant reductions in not only the weight and BMI (both P < 0.001) but also the waist circumference (P < 0.001), systolic BP (P = 0.049), insulin levels (P = 0.001), and HOMA-IR score (P = 0.003) following the intervention (Table 1). In addition, these reductions in weight, BMI, and waist circumference in the weight-loss group were greater than those in the control group after adjusting for the baseline values (all P < 0.001) (Table 1). The serum insulin levels, HOMA-IR score, and serum TG levels also showed significantly greater decreases in the weight-loss group than in the control group after adjusting for the baseline values (P = 0.020, P = 0.034, and P = 0.022, respectively) (Table 1). The estimated TEE (2144.6 ± 45.3 kcal/d vs. 2201.3 ± 59.3 kcal/d; P = 0.519), total calorie intake (TCI) (2105.8 ± 40.0 kcal/d vs. 2200.5 ± 50.3 kcal/d; P = 0.159), and percentage of macronutrient intakes (%CHO:%PRO:%FAT = 61:15:22 in both groups) did not significantly differ between the two groups at baseline (data not shown). The subjects’ overall compliance with the weight-loss plan was good. The estimated TCI of the weight-loss group at 12-week follow-up was, on average, 1949.4 kcal (%CHO:%PRO:%FAT = 59:19:22); and the types of dietary fats [saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs)] ingested by the study participants did not demonstrate any changes during the intervention (data not shown).
Table 1.
Clinical and biochemical characteristics of the weight-maintenance (control) and weight-loss groups at baseline and 12-week follow-up
| Weight-maintenance group (n = 38) |
Weight-loss group (n = 37) |
P a | P b | P c | P d | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |||||||
| Age (year) | 46.0 ± 1.35 | 44.1 ± 1.92 | 0.421 | |||||||
| Male/female n, (%) | 11 (28.9)/27 (71.1) | 15 (40.5)/22 (59.5) | 0.292 | |||||||
| Cigarette smoker n, (%) | 4 (10.5) | 5 (13.5) | 0.691 | |||||||
| Drinker of alcohol n, (%) | 21 (55.3) | 22 (59.5) | 0.713 | |||||||
| Weight (kg) | 71.6 ± 1.51 | 72.1 ± 1.49*** | 73.8 ± 1.54 | 71.4 ± 1.52*** | 0.321 | 0.743 | ||||
| Change | 0.44 ± 0.10 | − 2.42 ± 0.24 | < 0.001 | < 0.001 | ||||||
| BMI (kg/m2) | 27.3 ± 0.25 | 27.4 ± 0.24*** | 27.3 ± 0.23 | 26.4 ± 0.24*** | 0.994 | 0.002 | ||||
| Change | 0.17 ± 0.04 | − 0.90 ± 0.08 | < 0.001 | < 0.001 | ||||||
| Waist (cm) | 93.6 ± 0.82 | 93.4 ± 0.83 | 93.0 ± 0.86 | 91.5 ± 0.80*** | 0.648 | 0.103 | ||||
| Change | − 0.13 ± 0.21 | − 1.48 ± 0.31 | 0.001 | < 0.001 | ||||||
| Systolic BP (mmHg) | 122.4 ± 1.99 | 123.0 ± 2.49 | 126.1 ± 2.20 | 122.9 ± 2.09* | 0.214 | 0.431 | ||||
| Change | − 2.09 ± 1.87 | − 3.23 ± 1.59 | 0.645 | 0.919 | ||||||
| Diastolic BP (mmHg) | 77.6 ± 1.67 | 76.9 ± 1.60 | 78.0 ± 1.52 | 76.7 ± 1.47 | 0.871 | 0.930 | ||||
| Change | − 0.70 ± 1.26 | − 1.26 ± 1.30 | 0.758 | 0.794 | ||||||
| Glucose (mg/dL)∮ | 89.5 ± 1.46 | 90.8 ± 1.45 | 89.5 ± 1.65 | 89.9 ± 1.45 | 0.940 | 0.638 | ||||
| Change | 1.32 ± 1.40 | 0.41 ± 1.44 | 0.651 | 0.303 | ||||||
| Insulin (μIU/dL)∮ | 15.5 ± 1.19 | 17.5 ± 2.25 | 13.5 ± 0.92 | 11.0 ± 0.75** | 0.240 | < 0.001 | ||||
| Change | 1.99 ± 1.91 | − 2.61 ± 0.81 | 0.031 | 0.020 | ||||||
| HOMA-IR∮ | 3.43 ± 0.26 | 4.00 ± 0.59 | 2.99 ± 0.20 | 2.42 ± 0.17** | 0.256 | < 0.001 | ||||
| Change | 0.57 ± 0.52 | − 0.57 ± 0.19 | 0.046 | 0.034 | ||||||
| Triglycerides (mg/dL)∮ | 166.4 ± 18.4 | 159.6 ± 12.9 | 135.9 ± 9.38 | 115.3 ± 7.56 | 0.271 | 0.010 | ||||
| Change | − 6.74 ± 14.1 | − 16.3 ± 8.36 | 0.565 | 0.022 | ||||||
| Total-cholesterol (mg/dL)∮ | 220.30 ± 7.10 | 220.0 ± 6.93 | 213.8 ± 5.46 | 216.0 ± 6.18 | 0.577 | 0.738 | ||||
| Change | − 0.37 ± 4.22 | 2.22 ± 4.90 | 0.690 | 0.857 | ||||||
| HDL-cholesterol (mg/dL)∮ | 51.0 ± 1.97 | 51.6 ± 1.86 | 52.0 ± 1.60 | 54.0 ± 1.92 | 0.568 | 0.409 | ||||
| Change | 0.53 ± 1.34 | 2.00 ± 1.27 | 0.427 | 0.352 | ||||||
| LDL-cholesterol (mg/dL)∮ | 136.0 ± 6.00 | 136.5 ± 6.31 | 134.6 ± 4.78 | 135.9 ± 4.90 | 0.911 | 0.855 | ||||
| Change | 0.45 ± 3.51 | 1.10 ± 3.86 | 0.901 | 0.933 | ||||||
Mean ± SE. ∮tested following logarithmic transformation. Pa-values derived from an independent t-test between the groups at baseline. Pb-values derived from an independent t-test between the groups at follow-up. Pc-values derived from an independent t-test between the groups at changed values. Pd-values are Pc-values adjusted with the baseline values. *P < 0.05, **P < 0.01, and ***P < 0.001 derived from a paired t-test
Effects of a 12-week mild calorie restriction on percent of body weight change, abdominal fat areas (CT), and body composition (DEXA)
Table 2 presents the effects of a 12-week mild calorie-restricted diet on the percent of body weight change, abdominal fat area measured by CT, and body composition measured by DEXA. Comparison of the percent of body weight changes (differences from baseline) revealed that the weight-loss group had greater reductions in the percent of body weight change than the control group (0.63 ± 0.15 vs. -3.44 ± 0.34; P < 0.001). Regarding the results of CT, no significant differences were observed in the L4 abdominal fat area (whole fat area and VFA), L4 subcutaneous fat area, or visceral-to-subcutaneous area ratio (VSR) at baseline between the control and weight-loss groups. At follow-up, the L4 VFA in the weight-loss group was lower than in the control group (P < 0.001). The VFA decreased by 17.7 cm2 (15%) from baseline in the weight-loss group (P < 0.001), and the VSR decreased by 0.09 from baseline in the weight-loss group (P < 0.001). Significantly greater reductions in the L4 VFA and VSR were observed in the weight-loss group compared with the control group (all P < 0.001). No statistically significant shifts in the VFA, subcutaneous fat area, or VSR were identified in the control group (Table 2). With regard to the DEXA results, there were no significant differences in the fat percentage, fat mass, or lean body mass between the two groups at both baseline and follow-up (Table 2). After the intervention, significant decreases in the fat percentage and fat mass were observed in both the control and weight-loss groups; however, greater reductions in the fat percentage and fat mass were observed in the weight-loss group than in the control group after adjustment for the baseline values (P = 0.006 and P < 0.001, respectively). The control group showed a significant increase in the lean body mass, whereas the weight-loss group showed a significant decrease in the lean body mass at the 12-week follow-up; these changes were significantly different between the two groups (P < 0.001) (Table 2).
Table 2.
CT and DEXA evaluation of the weight-maintenance (control) and weight-loss groups at baseline and 12-week follow-up
| Weight-maintenance group (n = 38) |
Weight-loss group (n = 37) |
P a | P b | P c | P d | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |||||||
| Percent weight change | 0.63 ± 0.15 | − 3.44 ± 0.34 | < 0.001 | |||||||
| CT evaluation (L4) | ||||||||||
| Whole fat area (cm2) | 327.0 ± 6.32 | 333.3 ± 6.23 | 319.8 ± 6.65 | 298.5 ± 7.23*** | 0.435 | < 0.001 | ||||
| Change | 6.34 ± 4.72 | − 21.3 ± 4.70 | < 0.001 | < 0.001 | ||||||
| Visceral fat area (cm2) | 118.2 ± 2.34 | 121.9 ± 3.26 | 118.3 ± 3.14 | 100.6 ± 3.67*** | 0.989 | < 0.001 | ||||
| Change | 3.65 ± 2.31 | − 17.7 ± 3.30 | < 0.001 | < 0.001 | ||||||
| Subcutaneous fat area (cm2) | 208.7 ± 6.98 | 211.4 ± 6.24 | 201.5 ± 7.06 | 197.8 ± 6.90 | 0.468 | 0.148 | ||||
| Change | 2.69 ± 4.17 | − 3.66 ± 2.54 | 0.200 | 0.099 | ||||||
| Visceral/subcutaneous fat ratio (%) | 0.60 ± 0.03 | 0.60 ± 0.03 | 0.62 ± 0.03 | 0.53 ± 0.03*** | 0.556 | 0.101 | ||||
| Change | 0.00 ± 0.02 | − 0.09 ± 0.02 | < 0.001 | < 0.001 | ||||||
| DEXA evaluation | ||||||||||
| Fat percentage (%) | 31.5 ± 0.94 | 31.0 ± 0.94** | 29.8 ± 1.03 | 28.5 ± 0.98*** | 0.209 | 0.067 | ||||
| Change | − 0.50 ± 0.18 | − 1.26 ± 0.24 | 0.014 | 0.006 | ||||||
| Fat mass (g) | 22,826.7 ± 616.6 | 22,536.71 ± 606.1* | 22,038.8 ± 685.1 | 20,768.91 ± 651.1*** | 0.395 | 0.050 | ||||
| Change | − 290.0 ± 128.4 | − 1269.9 ± 221.4 | < 0.001 | < 0.001 | ||||||
| Lean body mass (g) | 48,289.8 ± 1471.4 | 48,934.6 ± 1497.4*** | 50,875.5 ± 1598.1 | 49,726.2 ± 1567.7*** | 0.237 | 0.716 | ||||
| Change | 644.8 ± 168.6 | − 1149.3 ± 164.3 | < 0.001 | < 0.001 | ||||||
Mean ± SE. ∮tested following logarithmic transformation. Pa-values derived from an independent t-test between the groups at baseline. Pb-values derived from an independent t-test between the groups at follow-up. Pc-values derived from an independent t-test between the groups at changed values. Pd-values are Pc-values adjusted with the baseline values. *P < 0.05, **P < 0.01, and ***P < 0.001 derived from a paired t-test
Effects of mild calorie restriction on circulating fatty acid profiles and desaturase activities at baseline and follow-up
Table 3 presents the effects of a 12-week mild calorie-restricted diet on plasma fatty acid profiles. At baseline, no significant difference was observed in the SFAs, MUFAs, n-6 PUFAs, and n-3 PUFAs between the control and weight-loss groups. The total SFAs, including palmitic acid (C16:0) and stearic acid (C18:0), decreased from baseline in the weight-loss group; in addition, these reductions were significantly greater than those in the control group after adjusting for the baseline values (P = 0.027, P = 0.033, and P = 0.021, respectively). At follow-up, the weight-loss group showed significantly lower levels of total SFAs (P = 0.029), lauric acid (C12:0) (P = 0.032), and palmitic acid (C16:0) (P = 0.028) than the control group. Similarly, the total MUFAs, including palmitoleic acid (C16:1, n-7) and oleic acid (C18:1, n-9), decreased from baseline in the weight-loss group, and these decreases were significantly greater than those in the control group after adjustment for the baseline values (P = 0.017, P = 0.006, and P = 0.018, respectively). At follow-up, the weight-loss group showed significantly lower levels of total MUFAs (P = 0.021), palmitoleic acid (C16:1, n-7) (P = 0.018), and oleic acid (C18:1, n-9) (P = 0.024) than did control group. Although the total n-6 and n-3 PUFA levels did not significantly change, eicosadienoic acid (C20:2, n-6) and dihomo-γ-linolenic acid (C20:3, n-6) decreased from baseline in the weight-loss group. Both showed greater reductions in the weight-loss group than in the control group after adjusting for the baseline values (P < 0.001 and P = 0.003, respectively). At follow-up, the weight-loss group showed significantly lower levels of eicosadienoic acid (C20:2, n-6) (P < 0.001) and dihomo-γ-linolenic acid (C20:3, n-6) (P = 0.001) than the control group (Table 3).
Table 3.
GC-MS analysis of fatty acids in the weight-maintenance (control) and weight-loss groups at baseline and 12-week follow-up
| Fatty acids (Relative peak area) | Weight-maintenance group (n = 38) |
Weight-loss group (n = 37) |
P a | P b | P c | P d | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |||||||
| Saturated fatty acids | 7.292 ± 0.211 | 7.287 ± 0.215 | 7.166 ± 0.177 | 6.565 ± 0.243* | 0.650 | 0.029 | ||||
| Change | − 0.005 ± 0.216 | − 0.601 ± 0.228 | 0.062 | 0.027 | ||||||
| Lauric acid (C12:0) | 0.033 ± 0.003 | 0.040 ± 0.005 | 0.027 ± 0.002 | 0.027 ± 0.003 | 0.063 | 0.032 | ||||
| Change | 0.007 ± 0.005 | 0.000 ± 0.003 | 0.313 | 0.067 | ||||||
| Myristic acid (C14:0) | 0.307 ± 0.022 | 0.319 ± 0.029 | 0.260 ± 0.017 | 0.241 ± 0.031 | 0.105 | 0.068 | ||||
| Change | 0.013 ± 0.028 | − 0.019 ± 0.029 | 0.435 | 0.210 | ||||||
| Pentadecylic acid (C15:0) | 0.045 ± 0.002 | 0.046 ± 0.003 | 0.041 ± 0.002 | 0.046 ± 0.006 | 0.182 | 0.978 | ||||
| Change | 0.001 ± 0.002 | 0.005 ± 0.006 | 0.505 | 0.466 | ||||||
| Palmitic acid (C16:0) | 4.762 ± 0.148 | 4.729 ± 0.135 | 4.646 ± 0.118 | 4.333 ± 0.113* | 0.545 | 0.028 | ||||
| Change | − 0.033 ± 0.152 | − 0.313 ± 0.120 | 0.155 | 0.033 | ||||||
| Stearic acid (C18:0) | 2.011 ± 0.055 | 2.014 ± 0.059 | 2.054 ± 0.048 | 1.778 ± 0.108** | 0.557 | 0.058 | ||||
| Change | 0.004 ± 0.056 | − 0.276 ± 0.100 | 0.016 | 0.021 | ||||||
| Arachidic acid (C20:0) | 0.040 ± 0.002 | 0.041 ± 0.002 | 0.041 ± 0.001 | 0.041 ± 0.002 | 0.623 | 0.859 | ||||
| Change | 0.001 ± 0.002 | 0.000 ± 0.003 | 0.808 | 0.978 | ||||||
| Behenic acid (C22:0) | 0.096 ± 0.005 | 0.098 ± 0.004 | 0.098 ± 0.004 | 0.099 ± 0.004 | 0.663 | 0.880 | ||||
| Change | 0.003 ± 0.004 | 0.001 ± 0.005 | 0.759 | 0.938 | ||||||
| Monounsaturated fatty acids | 1.472 ± 0.052 | 1.457 ± 0.054 | 1.442 ± 0.046 | 1.235 ± 0.078** | 0.663 | 0.021 | ||||
| Change | − 0.015 ± 0.050 | − 0.207 ± 0.068 | 0.026 | 0.017 | ||||||
| Palmitoleic acid (C16:1, n-7) | 0.196 ± 0.011 | 0.197 ± 0.012 | 0.192 ± 0.011 | 0.156 ± 0.012** | 0.777 | 0.018 | ||||
| Change | 0.001 ± 0.011 | − 0.035 ± 0.009 | 0.013 | 0.006 | ||||||
| cis-10-Heptadecenoic acid (C17:1, n-7) | 0.015 ± 0.001 | 0.014 ± 0.001 | 0.014 ± 0.001 | 0.014 ± 0.003 | 0.264 | 0.998 | ||||
| Change | 0.000 ± 0.001 | 0.001 ± 0.003 | 0.675 | 0.709 | ||||||
| Oleic acid (C18:1, n-9) | 1.193 ± 0.041 | 1.176 ± 0.042 | 1.171 ± 0.036 | 0.997 ± 0.066** | 0.693 | 0.024 | ||||
| Change | − 0.016 ± 0.039 | − 0.174 ± 0.057 | 0.025 | 0.018 | ||||||
| Eicosenoic acid (C20:1, n-9) | 0.011 ± 0.001 | 0.012 ± 0.001 | 0.010 ± 0.001 | 0.011 ± 0.002 | 0.158 | 0.638 | ||||
| Change | 0.001 ± 0.001 | 0.001 ± 0.001 | 0.889 | 0.947 | ||||||
| Erucic acid (C22:1, n-9) | 0.008 ± 0.001 | 0.007 ± 0.001 | 0.007 ± 0.001 | 0.008 ± 0.001 | 0.576 | 0.476 | ||||
| Change | − 0.001 ± 0.001 | 0.001 ± 0.001 | 0.278 | 0.321 | ||||||
| Nervonic acid (C24:1, n-9) | 0.049 ± 0.002 | 0.050 ± 0.003 | 0.048 ± 0.002 | 0.049 ± 0.002 | 0.669 | 0.731 | ||||
| Change | 0.001 ± 0.002 | 0.000 ± 0.003 | 0.979 | 0.824 | ||||||
| Polyunsaturated fatty acids (n-6) | 2.965 ± 0.065 | 2.971 ± 0.069 | 2.844 ± 0.070 | 2.800 ± 0.067 | 0.208 | 0.080 | ||||
| Change | 0.006 ± 0.055 | − 0.044 ± 0.071 | 0.577 | 0.212 | ||||||
| Linoleic acid (C18:2, n-6) | 2.203 ± 0.052 | 2.194 ± 0.054 | 2.097 ± 0.051 | 2.089 ± 0.050 | 0.149 | 0.156 | ||||
| Change | − 0.009 ± 0.049 | − 0.008 ± 0.052 | 0.992 | 0.449 | ||||||
| γ-linolenic acid (C18:3, n-6) | 0.038 ± 0.003 | 0.041 ± 0.005 | 0.030 ± 0.002 | 0.028 ± 0.002 | 0.065 | 0.033 | ||||
| Change | 0.003 ± 0.004 | − 0.002 ± 0.002 | 0.222 | 0.220 | ||||||
| Eicosadienoic acid (C20:2, n-6) | 0.022 ± 0.001 | 0.023 ± 0.001 | 0.021 ± 0.001 | 0.016 ± 0.001*** | 0.519 | < 0.001 | ||||
| Change | 0.001 ± 0.001 | − 0.005 ± 0.001 | < 0.001 | < 0.001 | ||||||
| Dihomo-γ-linolenic acid (C20:3, n-6) | 0.122 ± 0.005 | 0.125 ± 0.006 | 0.111 ± 0.005 | 0.096 ± 0.006* | 0.168 | 0.001 | ||||
| Change | 0.004 ± 0.005 | − 0.015 ± 0.007 | 0.028 | 0.003 | ||||||
| Arachidonic acid (C20:4, n-6) | 0.547 ± 0.018 | 0.553 ± 0.020 | 0.550 ± 0.020 | 0.537 ± 0.021 | 0.927 | 0.584 | ||||
| Change | 0.006 ± 0.013 | − 0.013 ± 0.019 | 0.421 | 0.419 | ||||||
| Docosatetraenoic acid (C22:4, n-6) | 0.033 ± 0.002 | 0.034 ± 0.002 | 0.034 ± 0.002 | 0.033 ± 0.002 | 0.826 | 0.670 | ||||
| Change | 0.001 ± 0.002 | − 0.001 ± 0.002 | 0.490 | 0.505 | ||||||
| Polyunsaturated fatty acids (n-3) | 0.477 ± 0.025 | 0.496 ± 0.040 | 0.477 ± 0.028 | 0.469 ± 0.034 | 0.999 | 0.619 | ||||
| Change | 0.018 ± 0.035 | − 0.008 ± 0.025 | 0.542 | 0.541 | ||||||
| α-linolenic acid (C18:3, n-3) | 0.115 ± 0.010 | 0.121 ± 0.009 | 0.099 ± 0.007 | 0.093 ± 0.010 | 0.182 | 0.037 | ||||
| Change | 0.005 ± 0.008 | - 0.007 ± 0.008 | 0.296 | 0.114 | ||||||
| Eicosapentaenoic acid (C20:5, n-3) | 0.112 ± 0.008 | 0.115 ± 0.016 | 0.110 ± 0.010 | 0.116 ± 0.013 | 0.867 | 0.978 | ||||
| Change | 0.003 ± 0.015 | 0.006 + ±0.012 | 0.894 | 0.926 | ||||||
| Docosapentaenoic acid (C22:5, n-3) | 0.048 ± 0.003 | 0.050 ± 0.004 | 0.046 ± 0.002 | 0.044 ± 0.003 | 0.696 | 0.193 | ||||
| Change | 0.002 ± 0.004 | − 0.003 ± 0.002 | 0.257 | 0.195 | ||||||
| Docosahexaenoic acid (C22:6, n-3) | 0.203 ± 0.016 | 0.210 ± 0.024 | 0.223 ± 0.019 | 0.217 ± 0.018 | 0.425 | 0.808 | ||||
| Change | 0.007 ± 0.015 | − 0.005 ± 0.012 | 0.513 | 0.527 | ||||||
| Ratio n-6/n-3 | 6.786 ± 0.352 | 7.243 ± 0.493 | 6.600 ± 0.346 | 7.519 ± 0.673 | 0.707 | 0.742 | ||||
| Change | 0.457 ± 0.421 | 0.919 ± 0.544 | 0.502 | 0.513 | ||||||
| C16 Δ9-desaturasea | 0.041 ± 0.001 | 0.041 ± 0.002 | 0.041 ± 0.002 | 0.036 ± 0.002** | 0.992 | 0.058 | ||||
| Change | 0.000 ± 0.001 | − 0.005 ± 0.002 | 0.016 | 0.014 | ||||||
| C18 Δ9-desaturaseb | 0.593 ± 0.012 | 0.583 ± 0.011 | 0.569 ± 0.009 | 0.561 ± 0.010 | 0.111 | 0.144 | ||||
| Change | − 0.010 ± 0.013 | − 0.008 ± 0.012 | 0.903 | 0.331 | ||||||
| Δ6-desaturasec | 0.017 ± 0.002 | 0.019 ± 0.002 | 0.014 ± 0.001 | 0.014 ± 0.001 | 0.122 | 0.049 | ||||
| Change | 0.001 ± 0.002 | − 0.001 ± 0.001 | 0.264 | 0.211 | ||||||
| Δ5-desaturased | 4.725 ± 0.201 | 4.689 ± 0.214 | 5.141 ± 0.190 | 6.157 ± 0.336** | 0.137 | < 0.001 | ||||
| Change | − 0.035 ± 0.154 | 1.017 ± 0.351 | 0.008 | 0.001 | ||||||
| Elongasee | 0.426 ± 0.008 | 0.428 ± 0.007 | 0.444 ± 0.006 | 0.406 ± 0.014* | 0.092 | 0.161 | ||||
| Change | 0.001 ± 0.010 | − 0.038 ± 0.014 | 0.024 | 0.101 | ||||||
Mean ± SE. ∮tested following logarithmic transformation. Pa-values derived from an independent t-test between the groups at baseline. Pb-values derived from an independent t-test between the groups at follow up. Pc-values derived from an independent t-test between the groups at changed values. Pd-values are Pc-values adjusted with the baseline values. *P < 0.05, **P < 0.01, and ***P < 0.001 derived from a paired t-test. aC16 Δ9-desaturase = palmitoleic acid/palmitic acid. bC18 Δ9-desaturase = oleic acid/stearic acid. cΔ6-desatuarse = γ-linolenic acid/linoleic acid. dΔ5-desaturase = arachidonic acid/dihomo-γ-linolenic acid. eElongase activity = stearic acid/palmitic acid
C16 Δ9-desaturase activity significantly decreased from baseline in the weight-loss group, and this reduction was greater in the weight-loss group than in the control group after adjusting for the baseline values (P = 0.014). The activity of Δ5-desaturase significantly increased from baseline in the weight-loss group, and this increase was greater in the weight-loss group than in the control group after adjusting for the baseline values (P = 0.001). In addition, at follow-up, Δ5-desaturase activity was significantly higher in the weight-loss group than in the control group (P < 0.001). Elongase activity significantly decreased after the intervention (Table 3).
Relations between changes in L4 VFA and changes in clinical/biochemical characteristics and fatty acid levels
Overall, 22 variables showed significant changes in the weight-loss group compared with the control group after adjusting for the baseline values [Table 1: weight, BMI, waist circumference, insulin, HOMA-IR, and TGs; Table 2: whole fat area, VFA, VSR, fat percentage, fat mass, and lean body mass; Table 3: SFAs, palmitic acid (C16:0), stearic acid (C18:0), MUFAs, palmitoleic acid (C16:1, n-7), oleic acid (C18:1, n-9), eicosadienoic acid (C20:2, n-6), dihomo-γ-linolenic acid (C20:3, n-6), C16 Δ9-desaturase, and Δ5-desaturase]. A correlation analysis between changes in L4 VFA and changes in these variables was performed. Of the total study subjects (n = 75), the changes in L4 VFA demonstrated significant positive correlations with changes in weight (r = 0.596, P < 0.001), BMI (r = 0.596, P < 0.001), waist circumference (r = 0.317, P = 0.006), insulin (r = 0.259, P = 0.025), fat percentage (r = 0.241, P = 0.037), fat mass (r = 0.381, P = 0.001), lean body mass (r = 0.464, P < 0.001), and dihomo-γ-linolenic acid (C20:3, n-6) (r = 0.235, P = 0.042) and a significant negative correlation with changes in Δ5-desaturase activities (r = −0.277, P = 0.016) (Fig. 1).
Fig. 1.
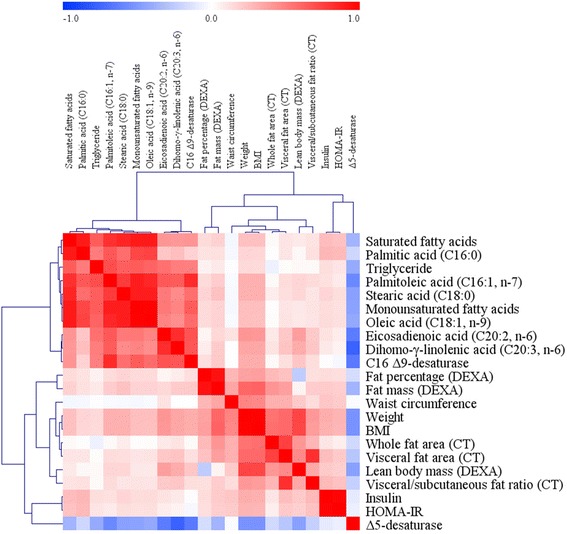
Correlation matrix among major clinical parameters, fatty acids, and fatty acid desaturases (changed values). Correlations were obtained by identifying a Pearson’s correlation coefficient. Red indicates a positive correlation, and blue indicates a negative correlation
Discussion
VFA is associated with plasma fatty acid profiles and lifestyle patterns [17]. We investigated the effects of weight loss by mild calorie restriction (a 300 kcal/day intake reduction) on circulating fatty acid profiles and desaturase activities in nondiabetic and overweight subjects with high VFA (L4 VFA ≥100 cm2). The 12-week intervention led to a 3.4% body weight loss and a 15% VFA loss in the participants in the weight-loss group. In addition, the study showed enhancement of plasma fatty acid profiles (SFAs and MUFAs), greater decreases in C16 Δ9-desaturase activity, and greater increases in Δ5-desaturase activity in the weight-loss group compared with the control group.
An inverse relation between Δ5-desaturase activity and diabetes risk [18] and direct relations among C16 Δ9-desaturase activity, insulin resistance, and abdominal obesity [5] have been reported. In the present study, a VFA loss was significantly related to Δ5-desaturase activity but not to C16 Δ9-desaturase activity. Although some evidence supports the inverse relation between Δ5-desaturase activity and diabetes risk [18–22], the Δ5-desaturase activity of this study did not correlate to insulin and HOMA-IR, which are risk factors of type 2 diabetes. However, the weight-loss group showed greater reductions in the serum insulin levels and HOMA-IR scores than the control group; furthermore, changes in insulin and HOMA-IR were positively correlated with changes in weight and BMI, which showed a significant negative correlation with changes in Δ5-desaturase activity. Because weight and BMI are also closely related to the risk of type 2 diabetes [23, 24], our results might indicate an indirect inverse association between Δ5-desaturase activity and the risk of type 2 diabetes (Fig. 2). Consequently, mild calorie-restriction-induced weight loss in individuals with high VFA might lead to the potential benefit of a reduced risk of type 2 diabetes by improving fatty acid desaturase activity.
Fig. 2.
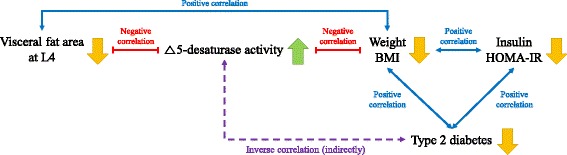
Summary of an indirect association between Δ5-desaturase activity and the risk of type 2 diabetes in the present study. Although no significant and direct correlation between Δ5-desaturase activity and the risk of type 2 diabetes was observed in this study, connections among Δ5-desaturase activity, weight, BMI, serum insulin level, and HOMA-IR score indicate an indirect inverse correlation between Δ5-desaturase activity and the risk of type 2 diabetes. Because the visceral fat area (VFA) has a significant negative correlation with Δ5-desaturase activity and Δ5-desaturase activity is inversely related to the risk of type 2 diabetes, VFA and the risk of type 2 diabetes might be positively correlated. Thus, mild calorie-restriction-induced weight loss that affects VFA at L4 decrease might lead to health benefits
Estimated Δ5-desaturase activity was expressed as the ratio of arachidonic acid (20:4, n-6) to dihomo-γ-linolenic acid (20:3, n-6). The increase in Δ5-desaturase activity observed in the weight-loss group might result from the greater decrease of dihomo-γ-linolenic acid (20:3, n-6) than that observed in the control group because there were no significant changes in arachidonic acid (20:4, n-6) in the control and weight-loss groups. Indeed, changes in VFA were positively correlated with changes in dihomo-γ-linolenic acid (20:3, n-6) (Fig. 1). Therefore, the study indicated that the inverse relation between VFA and Δ5-desaturase activity might be mediated by an alteration of dihomo-γ-linolenic acid (20:3, n-6), which is also significantly associated with VFA.
Although the total n-6 PUFAs did not significantly change during the intervention, the subjects in the weight-loss group showed decreases in not only dihomo-γ-linolenic acid (C20:3, n-6) but also eicosadienoic acid (C20:2, n-6); and these reductions were greater than the reductions detected in the control group (Table 3). Consistent with our results, other studies have found that obese individuals showed higher levels of dihomo-γ-linolenic acid (C20:3, n-6) in plasma phospholipids, TGs, and sterol esters than the controls [25, 26]. A recent study emphasized the proadipogenic properties of n-6 PUFAs [27]; indeed, several studies have found that n-6 PUFAs promote adipogenesis and increase the expression of lipogenic genes [27, 28]. Muhlhausler BS et al. [27] concluded that n-6 PUFAs promote the expansion of fat depots by up-regulating both hyperplasia and hypertrophy; in other words, an accumulation of n-6 PUFAs might exacerbate an obese status by enhanced adipogenesis, which could lead to several obesity-related health risks, including IR, metabolic syndrome, and diabetes [1]. Therefore, lowering the n-6 PUFA levels by calorie-restriction-induced weight loss is necessary, and the reduction of VFA to lower n-6 PUFAs is important.
In this study, the weight-loss group showed significant decreases in total SFAs, including palmitic acid (C16:0) and stearic acid (C18:0), which have previously been observed to be related to the incidence of type 2 diabetes [29, 30]. Total MUFAs, including palmitoleic acid (C16:1, n-7) and oleic acid (C18:1, n-9), were also significantly decreased in the weight-loss group of this study. The palmitoleic acid (C16:1, n-7) plasma concentrations mostly showed de novo hepatic fatty acid synthesis from palmitic acid (C16:0) by C16 Δ9-desaturase [31, 32]; in the present study, both palmitic acid (C16:0) and C16 Δ9-desaturase activity were significantly decreased during the 12-week intervention in the weight-loss group, which indicates that these alterations might affect plasma concentrations of palmitoleic acid (C16:1, n-7). Mice supplemented with palmitoleic acid exhibit higher fat deposition, hepatic steatosis, and increased hepatic expression of sterol regulatory element-binding protein 1c and fatty acid synthase, demonstrating the pro-lipogenic effect of this MUFA [33]. Moreover, studies conducted on humans have observed a detrimental influence of this MUFA on health [34, 35]. A previous study found that high levels of palmitoleic acid (C16:1, n-7) are associated with an increased risk of cardiovascular disease [34] and are positively associated with metabolic syndrome [35], including hypertriglyceridemia [34] and abdominal obesity [36]. In the present study, the weight-loss group, which presented significantly decreased levels of palmitoleic acid (C16:1, n-7), showed greater reductions in serum TGs and waist circumference than the control group. Therefore, reduced levels of SFAs and MUFAs via calorie-restriction-induced weight and VFA loss might yield future health benefits.
One of the major points of the present study is the correlation of weight loss with a significant reduction of VFA. As discussed thus far, many changes observed in the weight-loss group of this study might be explained by the VFA change. However, there is controversy regarding whether visceral fat is more harmful to circulating fatty acids than subcutaneous fat. Jensen MD [37] reported that visceral fat is not a source of excess systemic free fatty acid availability; conversely, Björntorp P [38] concluded that visceral fat tissue could be a significant source of free fatty acids that might exert complex metabolic effects. Indeed, several studies have suggested that large visceral fat depots flooded the liver and the systemic circulation in the form of free fatty acids [39, 40]. In addition, Klein S [41] observed that excess visceral fat is more harmful than excess subcutaneous fat because the lipolysis of TGs in visceral adipose tissue releases free fatty acids into the portal vein, which directly delivers them to the liver. Nielsen et al. [42] demonstrated that the contribution of free fatty acids derived from visceral fat to the portal and systemic circulation increases with increases in the visceral fat mass. Because evidence that supports our results exists, we believe that visceral fat is an important factor affecting the circulating fatty acid profiles.
A limitation of this study is its small sample size. Additionally, our results might not be generalizable because the study subjects were limited to nondiabetic overweight Korean subjects. Finally, we did not exclude subjects who took a medication that affects lipid metabolism. Only two subjects in the control group took a lipid-lowering medication; the clinical/biochemical parameters and fatty acid profiles did not show any significant differences between the control groups according to medication [n = 38 (original control group) vs. n = 36 (control group w/o medication)] (data not shown). In addition, the baseline value-adjusted statistical significance of the changed values between the control group without medication and the weight-loss group was identical to the present results (data not shown). Therefore, in this study, medication was not considered a confounding factor in interpreting the research results. Despite these limitations, this study demonstrates that a weight-loss intervention based on a hypocaloric diet in overweight subjects with high visceral fat levels improved not only the anthropometric and biochemical parameters but also the fatty acids plasma levels.
Conclusions
An analysis of plasma fatty acid profiles identified significant decreases in palmitic acid (C16:0), stearic acid (C18:0), palmitoleic acid (C16:1), oleic acid (C18:1), eicosadienoic acid (C20:2, n-6), and dihomo-γ-linolenic acid (C20:3, n-6) as well as a decrease in C16 Δ9-desaturase activity and an increase in Δ5-desaturase activity. These results suggest that maintenance of high VFA levels without treatment does not produce changes in the plasma fatty acid profiles, whereas even mild weight loss with reduced VFA improves the overall plasma fatty acid profile and desaturase activities. Through enhancement of the plasma fatty acid profiles, individuals may receive potential health benefits of reduced future risks of type 2 diabetes and cardiovascular disease. Moreover, eating behavior modification (mild calorie restriction) might be an effective therapy for the treatment of diseases such as type 2 diabetes and cardiovascular disease.
Acknowledgements
Not applicable
Funding
This study was funded by the Bio-Synergy Research Project (NRF-2012 M3A9C4048762) and the Mid-Career Researcher Program (NRF-2016R1A2B4011662) of the Ministry of Science, ICT and Future Planning through the National Research Foundation, Republic of Korea and by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C2686010116 and HI14C2686).
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author [JHL].
Abbreviations
- BMI
Body mass index
- BMR
Basal metabolic rate
- BP
Blood pressure
- CT
Computed tomography
- DEXA
Dual-energy X-ray absorptiometry
- HDL
High-density lipoprotein
- HOMA-IR
Homeostasis model assessment insulin resistance
- IR
Insulin resistance
- L4
4th lumbar
- LDL
Low-density lipoprotein
- MUFA
Monounsaturated fatty acid
- PUFA
Polyunsaturated fatty acid
- SE
Standard error
- SFA
Saturated fatty acid
- TCI
Total calorie intake
- TEE
Total energy expenditure
- TG
Triglyceride
- VFA
Visceral fat adiposity
- VSR
Visceral-to-subcutaneous area ratio
Authors’ contributions
YJL contributed to the acquisition, analysis, and interpretation of the data and helped draft the manuscript. HJY, MK, and MK contributed to the analysis and interpretation of the data and helped draft the manuscript. AL contributed to the acquisition and analysis of the data. SHJ contributed to the conception and design of the study. DYS contributed to the acquisition of the data. JHL contributed to the conception and design of the study and to the analysis and interpretation of the data and helped draft the manuscript. All authors critically revised, read, and approved the final manuscript and agreed to be fully accountable for ensuring the integrity and accuracy of the work.
Ethics approval and consent to participate
The purpose of the study was carefully explained to all participants, and written consent was obtained prior to their participation. The protocol was approved by the Institutional Review Board of Yonsei University and Yonsei University Severance Hospital in accordance with the Helsinki Declaration.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Young Ju Lee, Email: juny9558@naver.com.
Ayoung Lee, Email: ao0511@naver.com.
Hye Jin Yoo, Email: hyejin10432@hanmail.net.
Minjoo Kim, Email: minjookim@yonsei.ac.kr.
Minkyung Kim, Email: mkkim0106@yonsei.ac.kr.
Sun Ha Jee, Email: JSUNHA@yuhs.ac.
Dong Yeob Shin, Email: SHINDONGYI@yuhs.ac.
Jong Ho Lee, Phone: +82-2-2123-3122, Email: jhleeb@yonsei.ac.kr.
References
- 1.Katsuki A, Sumida Y, Urakawa H, et al. Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in Japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care. 2003;26:2341–2344. doi: 10.2337/diacare.26.8.2341. [DOI] [PubMed] [Google Scholar]
- 2.Hannukainen JC, Kalliokoski KK, Borra RJ, et al. Higher free fatty acid uptake in visceral than in abdominal subcutaneous fat tissue in men. Obesity. 2010;18:261–265. doi: 10.1038/oby.2009.267. [DOI] [PubMed] [Google Scholar]
- 3.Bucci M, Borra R, Någren K, et al. Human obesity is characterized by defective fat storage and enhanced muscle fatty acid oxidation, and trimetazidine gradually counteracts these abnormalities. Am J Physiol Endocrinol Metab. 2011;301:E105–E112. doi: 10.1152/ajpendo.00680.2010. [DOI] [PubMed] [Google Scholar]
- 4.Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes. 2008;32:619–628. doi: 10.1038/sj.ijo.0803761. [DOI] [PubMed] [Google Scholar]
- 5.Warensjö E, Ohrvall M, Vessby B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr Metab Cardiovasc Dis. 2006;16:128–136. doi: 10.1016/j.numecd.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Pan DA, Hulbert AJ, Storlien LH. Dietary fats, membrane phospholipids and obesity. J Nutr. 1994;124:1555–1565. doi: 10.1093/jn/124.9.1555. [DOI] [PubMed] [Google Scholar]
- 7.Akter S, Kurotani K, Sato M, et al. High serum phospholipid dihomo-γ-linoleic acid concentration and low Δ5-desaturase activity are associated with increased risk of type 2 diabetes among Japanese adults in the Hitachi health study. J Nutr. 2017;147:1558–1566. doi: 10.3945/jn.117.248997. [DOI] [PubMed] [Google Scholar]
- 8.Poudel-Tandukar K, Sato M, Ejima Y, et al. Relationship of serum fatty acid composition and desaturase activity to C-reactive protein in Japanese men and women. Atherosclerosis. 2012;220:520–524. doi: 10.1016/j.atherosclerosis.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Zhao L, Ni Y, Ma X, et al. A panel of free fatty acid ratios to predict the development of metabolic abnormalities in healthy obese individuals. Sci Rep. 2016;6:28418. doi: 10.1038/srep28418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warensjö E, Rosell M, Hellenius ML, et al. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: links to obesity and insulin resistance. Lipids Health Dis. 2009;8:37. doi: 10.1186/1476-511X-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishino T, Watanabe K, Urata T, et al. Visceral fat thickness in overweight men correlates with alterations in serum fatty acid composition. Clin Chim Acta. 2008;398:57–62. doi: 10.1016/j.cca.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Shin MJ, Hyun YJ, Kim OY, et al. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes. 2006;30:1529–1534. doi: 10.1038/sj.ijo.0803304. [DOI] [PubMed] [Google Scholar]
- 13.Department of Agrofood Resources . 8th revision standard food composition table. Suwon: Rural Development Administration; 2011. [Google Scholar]
- 14.Jung S, Lee YJ, Kim M, et al. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduced body adiposity and Lp-PLA2 activity in overweight subjects. J Funct Foods. 2015;19:744–52.
- 15.Kim M, Kim M, Lee YJ, et al. Supplementation with nutrients modulating insulin-like growth factor-1 negatively correlated with changes in the levels of pro-inflammatory cytokines in community-dwelling elderly people at risk of undernutrition. J Hum Nutr Diet. 2017;30:27–35. doi: 10.1111/jhn.12447. [DOI] [PubMed] [Google Scholar]
- 16.Kim M, Kim M, Lee YJ, et al. Effects of α-linolenic acid supplementation in perilla oil on collagen-epinephrine closure time, activated partial thromboplastin time and Lp-PLA2 activity in non-diabetic and hypercholesterolaemic subjects. J Funct Foods. 2016;23:95–104.
- 17.Oshima Y, Rin S, Kita H, et al. The frequency of fish-eating could negatively associate with visceral adiposity in those who eat moderately. J Nutr Sci Vitaminol (Tokyo) 2015;61:426–431. doi: 10.3177/jnsv.61.426. [DOI] [PubMed] [Google Scholar]
- 18.Kröger J, Schulze MB. Recent insights into the relation of Δ5 desaturase and Δ6 desaturase activity to the development of type 2 diabetes. Curr Opin Lipidol. 2012;23:4–10. doi: 10.1097/MOL.0b013e32834d2dc5. [DOI] [PubMed] [Google Scholar]
- 19.Kröger J, Zietemann V, Enzenbach C, et al. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European prospective investigation into cancer and nutrition (EPIC)-Potsdam study. Am J Clin Nutr. 2011;93:127–142. doi: 10.3945/ajcn.110.005447. [DOI] [PubMed] [Google Scholar]
- 20.Krachler B, Norberg M, Eriksson JW, et al. Fatty acid profile of the erythrocyte membrane preceding development of type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2008;18:503–510. doi: 10.1016/j.numecd.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Patel PS, Sharp SJ, Jansen E, et al. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European prospective investigation into cancer and nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr. 2010;92:1214–1222. doi: 10.3945/ajcn.2010.29182. [DOI] [PubMed] [Google Scholar]
- 22.Chow LS, Li S, Eberly LE, et al. Estimated plasma stearoyl co-a desaturase-1 activity and risk of incident diabetes: the atherosclerosis risk in communities (ARIC) study. Metabolism. 2013;62:100–108. doi: 10.1016/j.metabol.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S, Kuk JL, Davidson LE, et al. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without type 2 diabetes. J Appl Physiol (1985) 2005;99:1220–1225. doi: 10.1152/japplphysiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 24.Kopp HP, Krzyzanowska K, Möhlig M, et al. Effects of marked weight loss on plasma levels of adiponectin, markers of chronic subclinical inflammation and insulin resistance in morbidly obese women. Int J Obes. 2005;29:766–771. doi: 10.1038/sj.ijo.0802983. [DOI] [PubMed] [Google Scholar]
- 25.Decsi T, Molnár D, Koletzko B. Long-chain polyunsaturated fatty acids in plasma lipids of obese children. Lipids. 1996;31:305–311. doi: 10.1007/BF02529877. [DOI] [PubMed] [Google Scholar]
- 26.Decsi T, Csábi G, Török K, et al. Polyunsaturated fatty acids in plasma lipids of obese children with and without metabolic cardiovascular syndrome. Lipids. 2000;35:1179–1184. doi: 10.1007/s11745-000-0634-7. [DOI] [PubMed] [Google Scholar]
- 27.Muhlhausler BS, Ailhaud GP. Omega-6 polyunsaturated fatty acids and the early origins of obesity. Curr Opin Endocrinol Diabetes Obes. 2013;20:56–61. doi: 10.1097/MED.0b013e32835c1ba7. [DOI] [PubMed] [Google Scholar]
- 28.Lorente-Cebrián S, Costa AG, Navas-Carretero S, et al. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: a review of the evidence. J Physiol Biochem. 2013;69:633–651. doi: 10.1007/s13105-013-0265-4. [DOI] [PubMed] [Google Scholar]
- 29.Iggman D, Arnlöv J, Vessby B, et al. Adipose tissue fatty acids and insulin sensitivity in elderly men. Diabetologia. 2010;53:850–857. doi: 10.1007/s00125-010-1669-0. [DOI] [PubMed] [Google Scholar]
- 30.Kurotani K, Sato M, Ejima Y, et al. High levels of stearic acid, palmitoleic acid, and dihomo-γ-linolenic acid and low levels of linoleic acid in serum cholesterol ester are associated with high insulin resistance. Nutr Res. 2012;32:669–675.e3. doi: 10.1016/j.nutres.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res. 2004;43:91–104. doi: 10.1016/S0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 32.Zong G, Ye X, Sun L, et al. Associations of erythrocyte palmitoleic acid with adipokines, inflammatory markers, and the metabolic syndrome in middle-aged and older Chinese. Am J Clin Nutr. 2012;96:970–976. doi: 10.3945/ajcn.112.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo X, Li H, Xu H, et al. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS One. 2012;7:e39286. doi: 10.1371/journal.pone.0039286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paillard F, Catheline D, Duff FL, et al. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr Metab Cardiovasc Dis. 2008;18:436–440. doi: 10.1016/j.numecd.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Warensjö E, Risérus U, Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. 2005;48:1999–2005. doi: 10.1007/s00125-005-1897-x. [DOI] [PubMed] [Google Scholar]
- 36.Gong J, Campos H, McGarvey S, et al. Adipose tissue palmitoleic acid and obesity in humans: does it behave as a lipokine? Am J Clin Nutr. 2011;93:186–191. doi: 10.3945/ajcn.110.006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen MD. Is visceral fat involved in the pathogenesis of the metabolic syndrome? Human Model Obesity. 2006;14:20S–4S. [DOI] [PubMed]
- 38.Björntorp P. Metabolic difference between visceral fat and subcutaneous abdominal fat. Diabetes Metab. 2000;26:10–12. [PubMed] [Google Scholar]
- 39.Björntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arterioscler Thromb Vasc Biol. 1990;10:493–496. doi: 10.1161/01.ATV.10.4.493. [DOI] [PubMed] [Google Scholar]
- 40.Kissebah AH, Peiris AN. Biology of regional body fat distribution: relationship to non-insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1989;5:83–109. doi: 10.1002/dmr.5610050202. [DOI] [PubMed] [Google Scholar]
- 41.Klein S. The case of visceral fat: argument for the defense. J Clin Invest. 2004;113:1530–1532. doi: 10.1172/JCI200422028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen S, Guo Z, Johnson CM, et al. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author [JHL].


