Abstract
Recognition of axial spondyloarthritis (SpA) remains challenging, as no unique reference standard is available to ascertain diagnosis. Imaging procedures have been used for long in the field, in particular pelvic radiography, to capture structural changes evocative of sacroiliitis, the key feature in SpA. The introduction of MRI of the sacroiliac joints (SIJs) has led to a major shift in recognition of the disorder. MRI has been shown to detect the initial inflammatory processes, in particular osteitis depicted by bone marrow oedema, even in patients having not yet developed structural lesions. In addition, MRI has revealed a previously under-recognised very early clinical phase of the disease where patients have symptomatic axial involvement, but no structural changes. However, what constitutes a ‘positive MRI’ in SpA remains controversial, since both sensitivity and specificity show limitations, and interpretation of MRI lesions in daily practice is critically dependent on the clinical context. There is growing evidence that integration of the assessment of structural changes on dedicated T1 weighted-sequences on MRI may enhance diagnostic utility. The performance of MRI in detecting structural lesions in the SIJs may even be superior to traditional evaluation by pelvic radiography. These findings launched a debate on imaging in SpA, whether MRI, which is advancing early recognition of disease and shows superiority to detect structural changes, should replace traditional conventional radiography of the SIJs.
Keywords: spondyloarthritis, magnetic resonance imaging, ankylosing spondylitis
Key messages.
What is already known about this subject?
MRI of the sacroiliac joints (SIJs) is recognised as key imaging biomarker of axial spondyloarthritis (SpA), especially for recognition of emerging inflammation in the early phase of the disease.
What does this study add?
What constitutes a positive MRI for axial SpA remains controversial due to limited sensitivity and specificity of the ’suggestive bone marrow oedema' lesions. The performance of pelvic radiography for the detection of structural lesions in the SIJs is increasingly called into question by recent data on limited reliability and sensitivity, while dedicated T1-weighted MRI sequences have shown superior ability in assessing structural changes.
How might this impact on clinical practice?
Standardised and contextual evaluation of MRI of the SIJs, incorporating assessment of structural changes, may increase diagnostic utility. Whether MRI might eventually even replace conventional radiography is a matter of debate.
Introduction
The concept of non-radiographic axial spondyloarthritis
Diagnosis of spondyloarthritis (SpA) often remains challenging, especially in its axial form, when no objectively detectable sign of the disease can be observed by the consulting physician. Diagnosing an advanced axial or peripheral form of SpA in a patient referred for joint effusion, dactylitis or already ankylosed spine usually does not raise major concerns, but discrimination between non-specific chronic low back pain and inflammatory back pain due to early axial SpA may prove more challenging. Recent advances in management of SpA have made diagnostic ascertainment crucial, since innovative alternatives to nonsteroidal anti-inflammatory drugs (NSAIDs) have become available. Biological agents have proven major efficacy in axial SpA, which even in its early phase is associated with substantial impairment of quality of life and high burden of disease.1 This switch of interest to early axial SpA in the past two decades was further promoted by the advent of advanced imaging modalities such as MRI to detect early sacroiliitis before radiographic structural damage appears, and by the hypothesis of a ‘window of opportunity’ that treatment initiated in early disease might modify long-term outcome.2
Currently available criteria sets have, despite good global performances, undisputed limitations when applied in individual patients, and no unique disease-specific gold standard can be referred to in this context. These sets were developed to serve as classification rather than diagnostic criteria, although they are often used for disease recognition as well, as the same items are used in both settings.
The seminal importance of MRI in this context is its capability to reveal a hidden part of the disease, which could not be detected so far in patients with axial involvement without detectable structural radiographic features, but with signs or symptoms indicative of SpA. These patients can be classified as having ‘axial non-radiographic spondyloarthritis (nr-axSpA)’ according to criteria developed by the Assessment of Spondyloarthritis International Society (ASAS), which encompass chronic back pain (usually inflammatory back pain), associated with an evocative context, in particular presence of the HLA B27 antigen and/or sacroiliitis as detected by MRI.3 An advantage of MRI of sacroiliac joints (SIJs) is superiority to detect early phases of the disease versus conventional radiography, which in most cases has delayed sensitivity by several years after symptom onset, and which can even remain normal in some patients.4 A patient can also be classified as having nr-axSpA in the absence of imaging abnormalities (including MRI), by the so-called ‘clinical arm’ of ASAS classification criteria of axial SpA, provided the susceptibility HLA B27 gene is present and at least two features suggestive of SpA are observed during diagnostic evaluation. Criticism about the classification criteria included concerns about limited specificity given the high background prevalence of chronic non-specific back pain which may result in false positive assignments.5 6
MRI features are also considered a predictive factor of therapeutic response to anti-tumor necrosis factor (TNF) agents in axial SpA. The presence of unequivocal inflammatory lesions on SIJ MRI in patients with axial SpA was associated with a positive clinical response to anti-TNF drugs.7 8 These data provided the basis for the decision of the European Medicine Agency to restrict approval for TNF treatment indication to patients ‘having either detectable inflammatory lesions on MRI or elevated C-reactive protein’.9
The radiographic mNY criteria derived from patients with ankylosing spondylitis
Evaluation of SIJ on pelvic X-rays according to the modified New York (mNY) criteria served for decades as the gold standard to ascertain a diagnosis of ankylosing spondylitis (AS) at a given time point. In this classification system, patients are considered with AS if they report chronic inflammatory back pain and/or have limited mobility of lumbar spine or chest expansion, but the most discriminant element is the presence of either bilateral sclerosis or (limited) erosions, or at least unilateral severe erosions, widening of joint space or ankylosis of SIJ on pelvic X-Ray.
Actually, the mNY criteria were derived from a cohort of 183 HLA-B27 positive patients with AS, their HLA-B27-positive or HLA-B27 negative first-degree relatives, and population controls.10 Qualitative thresholds—with inherent subjectivity—to discriminate five grades of structural SIJ damage are applied, which promote inter-reader variability. Reliability of radiographic mNY criteria is further impaired by the lack of standardised and validated definitions for the five radiographic lesion types described originally in the Atlas of Standard Radiographs of Arthritis.11 There are limited data regarding radiographic SIJ lesion frequency and morphology in patients with mechanical back pain, in subjects with high physical activity, multiparous women, or regarding ‘background noise’ in healthy subjects. A primary back pain cohort from chiropractic practices in Canada showed degenerative SIJ changes in 35.2% of 142 women aged 18–60 years, which may contribute to reader disagreement in low grade sacroiliitis.12 Technical issues such as two-dimensional radiographic depiction of the complex three-dimensional joint anatomy or bowel structures superimposing the SIJ may also restrict reproducibility of SIJ evaluation on pelvic radiographs. In addition, features of radiographic sacroiliitis should not be applied in young subjects when pelvic growth plates are still open.13
How reliable are the radiographic mNY criteria in patients with early axial SpA?
Studies over the last three decades suggest that reliability of radiographic SIJ classification may depend on the frequency of subjects with already advanced radiographic joint damage. The higher the proportion of patients with AS in a given study sample, the better the concordance in radiographic SIJ classification. Substantial reliability with kappas (k)s of 0.66–0.69 between two trained rheumatologist readers was recorded in a study of 217 patients with AS who all met the mNY criteria.14 A cohort study about progression of radiographic sacroiliitis in axial SpA having 54.8% patients with AS showed moderate reliability between two trained rheumatology readers at baseline with a k value of 0.59.15 Agreement increased slightly to k of 0.67 at 2 years’ follow-up when additional 11.6% patients with non-radiographic axial SpA had progressed to AS. On the other hand, a cohort study in patients with inflammatory back pain suggestive of axial SpA, where only 21.1%–26.6% showed obvious radiographic sacroiliitis, showed k concordance for radiographic mNY criteria of 0.55 for central versus local radiologist and rheumatologist readings.16 Moreover, a report on an SpA inception cohort with just 15.7% patients with mNY criteria-positive back pain recorded only fair to, at best, moderate k reproducibility of 0.39 among seven radiology and rheumatology readers with varying experience in imaging in SpA.17 This study even suggested that erosion, which is widely regarded as a prototypical lesion indicating radiographic sacroiliitis, may be the primary driver of discordant classification. A study from Turkey applying the radiographic mNY criteria in a cohort of patients with Behçet’s disease reported low k values for pretraining/post-training agreement of 0.32/0.19, 0.32/0.36 and 0.44/0.41 for three reader pairs (one radiologist and two rheumatologists), respectively.18
A Dutch report concluded that training may not alter agreement in evaluation of radiographic sacroiliitis:19 Radiographic assessment of sacroiliitis by 100 rheumatologists and 23 radiologists showed only modest sensitivity and specificity and substantial intraobserver variation. Evaluation of the same image set after 3–6 months on individual training and workshops did not improve performance compared with the gold standard of scores by an expert panel of two rheumatologists, one epidemiologist and one radiologist.
How reliable is SIJ MRI versus radiography in adult and juvenile patients with early axial SpA?
A comparison of both imaging modalities in a cohort of patients with recent onset back pain clinically suspected to have early axial SpA highlighted only moderate agreement (k 0.54) of radiographic SIJ evaluation by central rheumatologist and radiologist readers.16 Conversely, assessment of SIJ MRI according to the ASAS definition based on active inflammatory lesions showed substantial agreement among central readers with a k value of 0.73.20 Moreover, even regarding structural lesions, a cross-sectional study in 68 patients with AS and 44 with non-radiographic axial SpA showed superior reliability in detection of the key lesion erosion on SIJ MRI (Κ 0.46 between two readers) than on pelvic radiographs (k 0.22).21 Superior performance of SIJ MRI over pelvic radiographs has also been reported in juvenile SpA.22 In a controlled retrospective cohort study of 26 patients with juvenile SpA, global assessment by SIJ MRI (k 0.80) was substantially more reliable compared with pelvic radiography (k 0.30).
Are the radiographic mNY criteria reliable to assess ‘progression’ in patients with early axial SpA?
The limited reliability of the mNY criteria may contribute to substantial measurement error when assessing progression of radiographic sacroiliitis in patients with early SpA.23 Blinded evaluation of baseline and follow-up pelvic radiographs in a cohort of 449 patients with clinically defined recent onset SpA showed progression over 2 years from mNY criteria negative to positive in 4.9% of subjects, but vice versa also regression from mNY criteria positive to negative of comparable magnitude of 5.7%. A similar analysis from the ASAS cohort24 recruited from many centres worldwide yielded even more puzzling results when comparing radiographic mNY grading by local readers (radiologists or rheumatologists, not necessarily the same reader at baseline and follow-up) after a mean follow-up of 4.4 years: of 975 patients at baseline, 357 had paired sacroiliac radiographs available at follow-up,24 and progression from radiographic mNY criteria negative to positive was observed in 18.3% (54/295 patients), and regression from positive to negative in 58.1% (36/62 patients). A potential explanation is that discrimination of true progression of radiographic sacroiliitis from measurement error over a mean interval of 4.4 years is challenging, which is a call to evaluate alternative imaging modalities to capture progression of SIJ damage.24 Regarding the limited sensitivity of pelvic radiography when applying mNY criteria, a higher, although still modest, rate of progression from ‘no AS’ to ‘AS’ has been reported when a cut-off of at least a change in one grade in at least one SIJ is applied, rather than a change from ‘not fulfilling mNY criteria’ to ‘fulfilment of radiographic criteria’.25 A cohort analysis on rates of radiographic sacroiliitis progression over 2 years in axial SpA reported 11.6% of patients transitioning from radiographic mNY criteria negative to positive, while 2.6% switched back from positive to negative.15 Sacroiliac radiographic progression over 5 years in an SpA inception cohort with evaluation according to the mNY criteria blinded to time order reported a small net progression rate in 5.1% of patients.25 Worsening by fulfilment of the radiographic criteria over time was observed in 5.8% of the patients, while a switch from radiographic to non-radiographic classification was recorded in 0.7%. Net radiographic progression was associated with inflammation on SIJ MRI assessed binarily as present or absent.
Limitations of MRI in axial SpA
Despite its superior reliability compared with radiography and its unanimously recognised relevance in the context of diagnostic assessment of a young patient referred for chronic back pain, MRI of SIJ has several limitations to take into account.
What constitutes a positive MRI in axial SpA?
First of all, although often regarded as an objective evaluation in SpA, MRI remains an imaging investigation that needs appropriate interpretation to provide the clinician with relevant information, whether ‘appropriate’ interpretation means ‘independent from’ or conversely ‘within a comprehensive clinical context’, needs further clarification. Indeed, the impressive specificity of MRI (97.3%) in a classification context as reported by the ASAS cohort aiming at developing the current classification criteria must be nuanced by the methodology that was applied. As no objective gold standard is available regarding the diagnosis of axial SpA, the final classification was made by experts in the field, after careful investigation of the patient including history, clinical signs, biological testing and MRI assessment. The latter information had a major impact on the expert’s final judgement, as it had led to a change in expert opinion in 21% of the patients.26 These criteria have been revisited after a mean follow-up of 4.4 years in a subsample of the ASAS cohort that was designed to develop the criteria set in patients with chronic low back pain less than 45 years at symptoms onset. The positive predictive value of the criteria was very high (92.2%), but the potential methodological limitation of partial ‘circular reasoning’ needs to be taken into account when applying the criteria set in clinical routine.27
Another important limitation encountered in clinical practice is the definition of a ‘positive MRI’ of SIJ. Interpretation by the radiologist and/or the rheumatologist is inherently subjective, and no objective reference standard could be defined in the development phase of the consensual criteria.26 Although it was stated in the original publication that only ‘inflammatory’ lesions, and especially bone marrow oedema/osteitis should be taken into account, no standardised definition of extent, location or intensity was provided. Paraphrasing the inflammatory lesion as being ‘highly suggestive of sacroiliitis associated with SpA’ remained the only anchor available to discriminate from mechanical back pain. Nevertheless, it was suggested that having one lesion on two consecutive slices or at least two lesions on a single slice could be regarded as sufficient for a positive SIJ MRI suggestive of sacroiliitis, although these cut-offs have not been formally validated, which might have resulted in false-positive assignments due to mechanical strain, vascular signals or artefacts on SIJ MRI.
Actually, both daily clinical practice and data from recent research in the field indicate that interpretation of MRI of SIJ is not an easy task. The expected binary response ‘positive’ or ‘negative’ SIJ MRI must often be replaced by varying levels of confidence. Indeed, the presence, number and extension of bone marrow oedema features are very heterogeneous across patients, their anatomical location is of crucial relevance (but not always taken into account by rheumatologists or radiologists), and the agreement for different lesion types can vary, even among trained and calibrated assessors.16 These discrepancies may translate into misclassification in both directions, with missed patients as well as excess diagnoses of SpA, in a small, but not negligible proportion of patients. On the background of the high prevalence of chronic back pain in the general population, discordant classification might result in substantial numbers of inappropriate therapeutic decisions.
Limited specificity
Presence of lesions on SIJ MRI indicative of SpA is rather common in patients with non-specific back pain, and is even possible in healthy subjects, which raises concerns about specificity of bone marrow oedema as detected by SIJ MRI. Multiple studies have reported that a sizeable proportion of non-SpA patients with back pain and healthy control subjects can be erroneously classified as having active sacroiliitis based on MRI scanning. Already in 2009, Marzo-Ortega et al reported a high prevalence of bone marrow oedema in up to 6/22 (27%) in a control sample of healthy volunteers and patients with mechanical back pain.28 A similar proportion of MRI lesions suggestive of SpA was recorded in MRI assessment of the spine. A study evaluating whole-body MRI in patients with SpA and healthy control subjects observed presence of bone marrow oedema in vertebral corners of 26% of healthy volunteers, as concordantly reported by a least two readers.29 In another study comparing the prevalence of various lesion types on MRI scans of patients with AS, recent onset inflammatory back pain, non-specific back pain and healthy subjects, presence of bone marrow oedema in SIJ meeting the ASAS criteria was confirmed concordantly by at least two of five independent readers in 64/75 (85.3%) of patients with AS, 18/27 (66.7%) of patients with inflammatory back pain, in 23.1% of patients with non-specific back pain and 6.8% of healthy subjects.30 As observed in this study, specificity will expectedly decrease when patients from the control group have a pretest condition evocative of the final disease, which is the prerequisite of a study aiming at developing diagnostic criteria, whereas the development of classification criteria may use healthy subjects as controls, and thus result in higher specificity.31 Other conditions like Diffuse Idiopathic Skeletal Hyperostosis (DISH) are associated with MRI features resembling SpA-associated sacroiliitis, although the MRI of SIJ remained more specific than the MRI of the spine in this systematic assessment of patients diagnosed with DISH.32 An evaluation of SIJ MRI in athletes showed bone marrow oedema compatible with ASAS standards as concordantly reported by at least two of three trained readers in 30%–35% of hobby runners and 41% of elite ice-hockey players, respectively.33 A topographical analysis showed an interesting clustering of these non-inflammatory bone marrow lesions in the posterior lower ilium, followed in frequency by the anterior upper sacrum. These results are consistent with the bone marrow oedema findings in SIJ of Belgian military recruits, who met the ASAS criteria in 23% and 36% before and 6 weeks after intensive physical training, respectively.34 A generic limitation to all proposals about positive SIJ MRI in SpA is the age range of 18 years to 45 years or 50 years of enrolled study subjects. This fact precludes extrapolation to subjects over age 50 years due to a lack of data.
Limited sensitivity
MRI lesions in patients with SpA may fluctuate, pointing out the limited sensitivity of MRI for diagnostic purposes in case it would be used as the sole evaluation tool. Two studies exploring diagnostic utility of SIJ MRI by two different gold standards consistently showed that sensitivity in the clinical setting of suspected early SpA reached, at best, 50%. In a first study testing the performance of SIJ MRI in 187 adult subjects aged less than 45 years (75 with AS, 27 with inflammatory back pain but no radiographic changes on pelvic X-rays, 26 with non-specific back pain and 59 healthy controls), global assessment of SIJ MRI yielded a mean sensitivity over five readers of 51% using physician expert opinion based on clinical, laboratory and radiographic examination as the independent gold standard.30 Another study applied histology on SIJ biopsies compatible with inflammation as reference standard in 109 patients clinically suspected to have early axial SpA, but who showed no or only minimal radiographic lesions of SIJ.35 In 77 patients, in whom also SIJ MRI was available, 23 subjects showed MRI evidence of sacroiliitis defined by bone marrow oedema alone, resulting in a sensitivity of 38% when compared with biopsy findings. This study did not explore a potential incremental contribution of structural SIJ lesions to diagnostic utility. Two other studies using physician expert opinion as gold standard reported sensitivities of SIJ MRI defined by presence of typical bone marrow oedema between ~35% and 42% in patients with back pain clinically considered to have early axial SpA.20 36 These data substantiate the possibility of recognition of axial SpA even in the absence of imaging abnormalities, rendering a positive SIJ MRI, an imaging biomarker of the disease, but not an independent diagnostic test by itself. In clinical practice, normal SIJ MRI findings cannot rule out a diagnosis of early axial SpA suspected on clinical grounds. Similarly, even in long-standing established disease in patients with radiographic evidence of axial involvement fulfilling the mNY criteria, and considered by their rheumatologist as having active disease requiring therapeutic escalation, inflammatory abnormalities were not found in all patients on MRI of the SIJ or spine: 15%–20% of patients with AS included in controlled trials of anti-TNF agents had no detectable active lesion on their baseline MRI scans.37 38
Integration of structural lesions in the assessment of SIJ MRI
Given these strengths and limitations of MRI in the diagnostic approach of patients with inflammatory back pain, it has been suggested to explore ’inflammatory' lesions on SIJ MRI, and to assess structural changes, which may confer additional clinically relevant information by contextual lesion interpretation simultaneously on T1-weighted and short tau inversion recovery (STIR) sequences.39 Structural lesions described for decades in AS by radiography of the pelvis and more reliably on CT scans, are mainly represented by erosion, partial or complete ankylosis of SIJ, and sclerosis. Another type of lesion, which cannot be seen on radiographic modalities, is fat metaplasia of the subchondral bone.
Several studies have been conducted, aiming at evaluating the ability of MRI of SIJ to detect active inflammatory lesions (bone marrow oedema), and various structural changes, including the key feature erosion. A preliminary international work based on SIJ MRI assessment by five readers (two radiologists and three rheumatologists) and a sample size of 187 subjects with either definite AS, inflammatory back pain without radiographic evidence of AS, non-specific back pain and healthy controls without symptoms suggestive of SpA showed that blinded and systematic assessment of MRI scans of SIJ had very good diagnostic utility for both radiographic and non-radiographic SpA with positive likelihood ratios of 44.6 and 26.0, respectively. The incorporation of erosion in the evaluation of SIJ MRI resulted in an increase in sensitivity from 67% to 81%, without affecting specificity with 88% in both settings, based on concordant findings by at least two out of five independent readers.30 It could also be concluded from this exercise that structural lesions can occur earlier than expected in the disease course with erosion being present in 59.3% of patients with non-radiographic SpA after a mean symptom duration of 29 months. Fat infiltration as well as bone marrow oedema were relatively non-specific, as they were also commonly observed in patients with non-specific back pain and even in healthy subjects. Actually, although fat infiltration showed good face validity, data-driven assessment of its diagnostic utility revealed disappointing performance, mainly due to redundant information conferred by simultaneous presence of bone marrow oedema and erosive lesions.40 The prevalence of the clinically relevant setting of erosive SIJ changes alone without concomitant bone marrow oedema in patients with non-radiographic SpA—as illustrated in figures 1–4—was comparable in a cross-sectional study and in an interventional trial with 13.0%41 and 10.9%42 patients with a mean symptom duration of 2.4 years and 2.5 years, respectively
Figure 1.
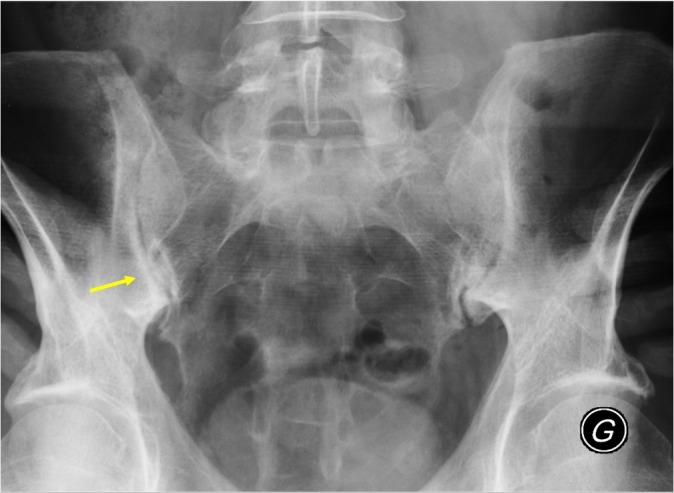
A 38-year-old man, with recurrent inflammatory back pain and alternating buttock pain for 2 years. No extra-articular manifestations nor family history suggestive of spondyloarthritis, moderately raised C-reactive protein (CRP) (37 mg/L, reference range <5 mg/L) and erythrocyte sedimentation rate (ESR) (33 mm/first hour). HLA B27 negative. Pelvic radiography shows minimal sclerosis and irregularities of the inferior part of the sacroiliac joints, especially on the right side (arrow), resulting in an 'equivocal finding', that is, insufficient confidence in a diagnosis of sacroiliitis.
Figure 2.
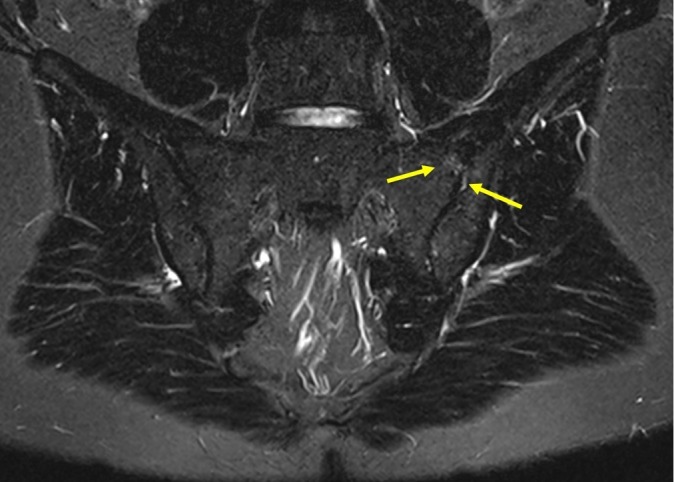
MRI, STIR sequence of sacroiliac joints (SIJs): minimal localised signal increase on both sides of the upper part of the left SIJ (arrows), which does not allow to discriminate between inflammation, local mechanical strain or vascular signal.
Figure 3.
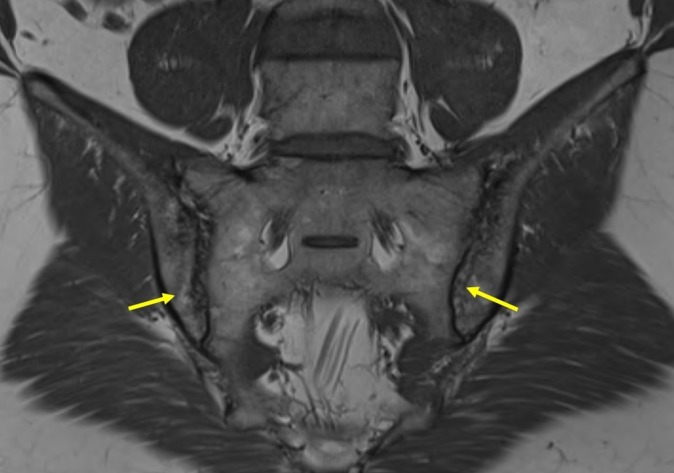
MRI, T1-weighted sequence of sacroiliac joints: presence of definite erosion in the ilium on both sides (arrows), perifocal fatty lesions of subchondral bone and localised sclerosis.
Figure 4.
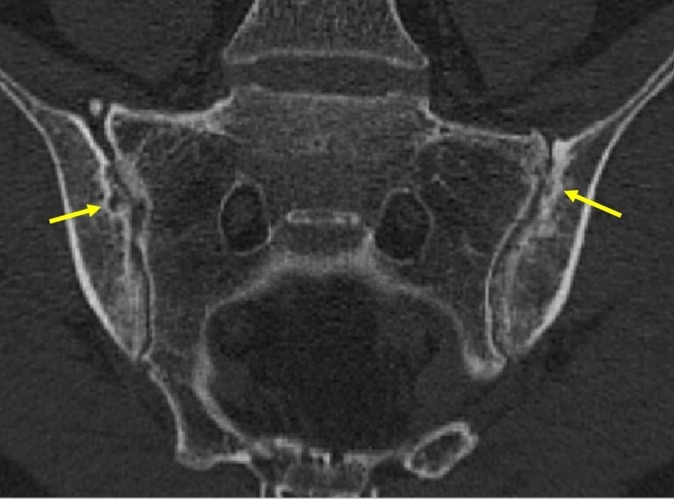
CT scan of sacroiliac joints (SIJs): confirmation of erosion of both SIJs and regional sclerosis of subchondral bone.
Another study, conducted in patients aged ≤50 years with either clinically suspected SpA or with acute anterior uveitis and chronic back pain, aimed at data-driven lesion-based definitions of a positive MRI of SIJ. The patients were evaluated by clinical examination, pelvic radiography and laboratory values (HLA B27 and C reactive protein), which were the basis for a ‘rheumatologist expert’ diagnosis serving as gold standard without the information obtained from MRI. MRI of SIJ of patients with SpA, as well as patients with non-specific back pain and healthy subjects, were independently scored by four blinded readers. The diagnostic utility of several lesions of potential interest, as well as their combinations, were assessed.43 The performance was optimal when both bone marrow oedema and erosion were taken into account, outperforming definitions based on bone marrow oedema lesions only. Erosion, which was a rare finding in controls (mean prevalence of ≥2 erosions in 2.5%–9.8% of controls), could be observed again in about 10% of patients (7.5%–11.3%) in the absence of active inflammatory lesions, making it a potentially relevant contribution to diagnostic utility due to high specificity.
A recent Danish study conducted in 1020 young patients with persistent low back pain provides interesting data for the apprehension of the diagnostic performance of different lesion types on SIJ MRI. Bone marrow oedema was observed in 21% of patients recruited from primary care with a high proportion of 42% showing lesions of limited extent, which were not associated with any of the clinical features of the ASAS criteria, but with older age.44 By contrast, the scarce finding of erosion in only 7.5% of patients with back pain was significantly associated with important clinical features of SpA such as presence of HLA B27 and response to NSAIDs. These findings highlight the limited specificity of ‘inflammatory’ bone marrow oedema lesions when applied in patients with long-standing back pain in primary care, especially in older subjects, conferring a potential risk of excess diagnosis of axial SpA when based solely on bone marrow oedema of limited extent. On the other hand, the results emphasise the crucial importance of contextual interpretation of SIJ MRI and the high specificity of erosion, which was not affected by older age.
Whether MRI can be used to reliably identify structural lesions (erosions, joint space narrowing, sclerosis) on SIJ was recently tested against low dose CT scans, the latter remaining the reference procedure to assess these features.45 Among 110 patients who were investigated in this study by Diekhoff et al, 58 were finally diagnosed as having axial SpA (35 with non-radiographic axial SpA, 23 with AS), and 52 received other diagnoses like osteoarthritis or osteitis condensans. MRI showed significantly higher sensitivity and concordance with CT scan than conventional pelvic radiography for all lesion types, except for sclerosis (the latter with non-significant difference).
The increasingly recognised relevance of structural lesions has been incorporated in a recent update of the ASAS definition of active sacroiliitis on SIJ MRI for classification of axial SpA.46 It is essential that MRI readers simultaneously review sequences designed to identify inflammation and structural damage. If an equivocal inflammatory bone marrow lesion is present, the decision may be influenced by the presence of concomitant structural damage, especially erosion. However, this recently accumulated evidence about how to assess MRI in SpA has not yet sufficiently permeated into daily routine due to a lack of dissemination among both radiologists and rheumatologists.47
How to apply in daily practice our expanding research skills on SIJ MRI assessment?
If SIJ MRI is taking the lead in daily practice for evaluation of patients with persisting low back pain, the uncritical application of classification proposals will likely result in overcalling a diagnosis of SpA, which entails overtreatment and expansion of healthcare cost. The Danish study mentioned above reflects this setting.42 In patients with chronic low back pain recruited from primary care without previous rheumatological assessment, 21% met the MRI classification criteria based on SIJ bone marrow oedema alone, but 42% of these lesions were small and of questionable clinical relevance as they showed no association with clinical SpA features. Revision of an earlier diagnosis of axial SpA, based mainly on minor changes on SIJ MRI, has become a common experience in the daily routine of rheumatologists. However, limited accessibility of the axial skeleton to clinical examination renders MRI of SIJ a very valuable tool for diagnosis, provided it is embedded as one element in a deductive process encompassing complementary clinical and paraclinical findings. The diagnostic approach to a multifaceted disorder such as SpA requires a process of pattern recognition, where a careful differential diagnosis on clinical grounds is as relevant as an MRI lesion signature. Diagnostic evaluation of SIJ MRI itself should not be based on bone marrow oedema alone, in particular if only minor lesions are present, but should adopt a contextual approach by taking into account structural lesions also, which appear early in the disease course and enhance specificity.
Should SIJ MRI supplant pelvic radiography in recognition of early axial SpA?
Pelvic radiographs are often performed as one element of rheumatological evaluation in the clinical setting of suspected early SpA, despite limited evidence whether they may enhance confidence in a diagnosis of early axial SpA. Cohort studies from secondary and tertiary care suggest that about 25%–50% of patients, who are clinically suspected of having axial SpA for less than 9 years, show definite radiographic SIJ changes compatible with AS.48–50 There is ongoing controversy as to whether SIJ MRI due to its superior reliability and ability to depict both active and structural lesions should be the preferred imaging modality in early disease over the traditional approach with pelvic radiographs. The EULAR recommendations for the use of imaging in the diagnosis and management of SpA in clinical practice state that conventional radiography of SIJ is recommended as the first imaging method to diagnose sacroiliitis.51 If the diagnosis of axial SpA cannot be established based on clinical features and conventional radiography, and axial SpA is still suspected, MRI of SIJ is recommended. This statement is supported by the European Society of Musculoskeletal Radiology.52
However, several studies applying the radiographic mNY criteria in patients with clinically suspected early axial SpA consistently showed limited agreement among trained readers with k values around 0.5.17 A post hoc analysis of pelvic radiographs by trained central readers in two interventional trials in non-radiographic axial SpA resulted in reclassification of 36% and 37% of the patients regarding fulfilment of the radiographic mNY criteria,53 54 which raised concerns in a public hearing of the Food and Drug Administration in 2013, whether radiographic classification is appropriate for clinical trials in early axial SpA.55 Furthermore, recent data shed doubt on reliability of the radiographic mNY criteria to evaluate progression of SIJ damage in patients with early axial SpA.23 By contrast, emerging evidence suggests superior reliability of MRI over radiography to assess sacroiliitis in adult and juvenile patients with clinically suspected early axial SpA, both by granular and global MRI evaluation.16 20 22
Radiographic assessment of SIJ following the mNY criteria derived from patients with advanced structural SIJ damage has substantial limitations regarding radiation exposure, reliability and assessment of progression, and may not be directly applicable to patients with back pain clinically suspected to have early axial SpA. The advantage of radiography is feasibility, as it is readily available and can be performed at low costs and minimal time loss. These feasibility issues do not apply to the potential alternative SIJ MRI, which has limited or no access in many parts of the world. However, a critical reappraisal of using pelvic radiographs in the clinical setting of suspected early SpA is warranted, and in healthcare settings, where SIJ MRI is readily available, it may be the preferred imaging modality in early axial SpA.
Conclusion
MRI of SIJ is a valuable adjunct tool in the workup of patients with back pain suspected to have early axial SpA, provided an appropriate clinical evaluation including differential diagnostic considerations has been made, and the context (age, sports activity, body mass index…) at the time of MRI is not associated with an expected deterioration of performances/metrological properties. MRI by itself cannot serve as the gold standard to make a diagnosis of early axial SpA due to limitations both in sensitivity and specificity, and because even an advanced imaging modality cannot capture the entire clinical spectrum of the disorder. Diagnosing early SpA remains a complex deductive process based on thorough clinical and paraclinical assessments. MRI may assist in confirming a clinical suspicion of SpA, but cannot replace a careful clinical examination and differential diagnostic assessment in the pattern recognition process towards a diagnosis of axial SpA. The role of pelvic radiography, the traditional imaging gold standard in AS, ought to be critically reappraised for growing concerns about reliability and sensitivity, particularly in the clinically challenging setting of suspected early SpA.
Contextual interpretation of structural and active MRI lesions simultaneously on T1-weighted and STIR sequences is key to enhance diagnostic utility of SIJ MRI. In the setting of low grade MRI changes, a diagnosis should not be made based on bone marrow lesions alone, as this constellation is common in back pain conditions not related to SpA and even in healthy subjects. Structural SIJ lesions are emerging to enhance confidence in a diagnosis of SpA, highlighted by the high specificity of erosion. Future research into the ‘background noise’ of different MRI features in patients with non-specific back pain and in healthy individuals with varying axial strain and further exploration of potential redundancy of various MRI lesions is needed towards a data-driven definition of a positive SIJ MRI in SpA.
Footnotes
Contributors: CL and UW wrote the original draft manuscript. CC and MD have reviewed, amended and corrected the different versions of the manuscript. All authors have validated the final submitted version of the text.
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Data sharing statement: The manuscript is a review of existing published data. Only the figures are original, aiming at illustrating the review from a personal anonymised clinical case of a patient.
References
- 1. Boonen A, Sieper J, van der Heijde D, et al. . The burden of non-radiographic axial spondyloarthritis. Semin Arthritis Rheum 2015;44:556–62. 10.1016/j.semarthrit.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 2. Maksymowych WP, Morency N, Conner-Spady B, et al. . Suppression of inflammation and effects on new bone formation in ankylosing spondylitis: evidence for a window of opportunity in disease modification. Ann Rheum Dis 2013;72:23–8. 10.1136/annrheumdis-2011-200859 [DOI] [PubMed] [Google Scholar]
- 3. Rudwaleit M, van der Heijde D, Landewé R, et al. . The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. 10.1136/ard.2009.108233 [DOI] [PubMed] [Google Scholar]
- 4. Rudwaleit M. New approaches to diagnosis and classification of axial and peripheral spondyloarthritis. Curr Opin Rheumatol 2010;22:375–80. 10.1097/BOR.0b013e32833ac5cc [DOI] [PubMed] [Google Scholar]
- 5. Robinson PC, Wordsworth BP, Reveille JD, et al. . Axial spondyloarthritis: a new disease entity, not necessarily early ankylosing spondylitis. Ann Rheum Dis 2013;72:162–4. 10.1136/annrheumdis-2012-202073 [DOI] [PubMed] [Google Scholar]
- 6. Deodhar A. Editorial: sacroiliac joint magnetic resonance imaging in the diagnosis of axial spondyloarthritis: “a tiny bit of white on two consecutive slices” may be objective, but not specific. Arthritis Rheumatol 2016;68:775–8. 10.1002/art.39549 [DOI] [PubMed] [Google Scholar]
- 7. Haibel H, Rudwaleit M, Listing J, et al. . Efficacy of adalimumab in the treatment of axial spondylarthritis without radiographically defined sacroiliitis: results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-two. Arthritis Rheum 2008;58:1981–91. 10.1002/art.23606 [DOI] [PubMed] [Google Scholar]
- 8. Rudwaleit M, Schwarzlose S, Hilgert ES, et al. . MRI in predicting a major clinical response to anti-tumour necrosis factor treatment in ankylosing spondylitis. Ann Rheum Dis 2008;67:1276–81. 10.1136/ard.2007.073098 [DOI] [PubMed] [Google Scholar]
- 9. EMA. Positive recommendations of Committee for Medicinal Products for Human Use (CHMP) on extensions of therapeutic indications for Humira. 2012. http://www.emea.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500129074 (accessed 1 Aug 2012).
- 10. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 11. Ball J, Jeffrey MR, Kellgren JH. The epidemiology of chronic rheumatism: volume 2: atlas of standard radiographs of arthritis. Blackwell Scientific Publications: Oxford, 1963. [Google Scholar]
- 12. O’Shea FD, Boyle E, Salonen DC, et al. . Inflammatory and degenerative sacroiliac joint disease in a primary back pain cohort. Arthritis Care Res 2010;62:447–54. 10.1002/acr.20168 [DOI] [PubMed] [Google Scholar]
- 13. Macrae IF, Haslock DI, Wright V. Grading of films for sacro-iliitis in population studies. Ann Rheum Dis 1971;30:58–66. 10.1136/ard.30.1.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spoorenberg A, de Vlam K, van der Linden S, et al. . Radiological scoring methods in ankylosing spondylitis. Reliability and change over 1 and 2 years. J Rheumatol 2004;31:125–32. [PubMed] [Google Scholar]
- 15. Poddubnyy D, Rudwaleit M, Haibel H, et al. . Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann Rheum Dis 2011;70:1369–74. 10.1136/ard.2010.145995 [DOI] [PubMed] [Google Scholar]
- 16. van den Berg R, Lenczner G, Feydy A, et al. . Agreement between clinical practice and trained central reading in reading of sacroiliac joints on plain pelvic radiographs. Results from the DESIR cohort. Arthritis Rheumatol 2014;66:2403–11. 10.1002/art.38738 [DOI] [PubMed] [Google Scholar]
- 17. Christiansen AA, Hendricks O, Kuettel D, et al. . Limited reliability of radiographic assessment of sacroiliac joints in patients with suspected early spondyloarthritis. J Rheumatol 2017;44:70–7. 10.3899/jrheum.160079 [DOI] [PubMed] [Google Scholar]
- 18. Yazici H, Turunç M, Ozdoğan H, et al. . Observer variation in grading sacroiliac radiographs might be a cause of ’sacroiliitis' reported in certain disease states. Ann Rheum Dis 1987;46:139–45. 10.1136/ard.46.2.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Tubergen A, Heuft-Dorenbosch L, Schulpen G, et al. . Radiographic assessment of sacroiliitis by radiologists and rheumatologists: does training improve quality? Ann Rheum Dis 2003;62:519–25. 10.1136/ard.62.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van den Berg R, Lenczner G, Thévenin F, et al. . Classification of axial SpA based on positive imaging (radiographs and/or MRI of the sacroiliac joints) by local rheumatologists or radiologists versus central trained readers in the DESIR cohort. Ann Rheum Dis 2015;74:2016–21. 10.1136/annrheumdis-2014-205432 [DOI] [PubMed] [Google Scholar]
- 21. Poddubnyy D, Gaydukova I, Hermann KG, et al. . Magnetic resonance imaging compared to conventional radiographs for detection of chronic structural changes in sacroiliac joints in axial spondyloarthritis. J Rheumatol 2013;40:1557–65. 10.3899/jrheum.130141 [DOI] [PubMed] [Google Scholar]
- 22. Jaremko JL, Liu L, Winn NJ, et al. . Diagnostic utility of magnetic resonance imaging and radiography in juvenile spondyloarthritis: evaluation of the sacroiliac joints in controls and affected subjects. J Rheumatol 2014;41:963–70. 10.3899/jrheum.131064 [DOI] [PubMed] [Google Scholar]
- 23. Dougados M, Demattei C, van den Berg R, et al. . Rate and predisposing factors for sacroiliac joint radiographic progression after a two-year follow-up period in recent-onset spondyloarthritis. Arthritis Rheumatol 2016;68:1904–13. 10.1002/art.39666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sepriano A, Rudwaleit M, Sieper J, et al. . Five-year follow-up of radiographic sacroiliitis: progression as well as improvement? Ann Rheum Dis 2016;75:1262–3. 10.1136/annrheumdis-2015-208964 [DOI] [PubMed] [Google Scholar]
- 25. Dougados M, Sepriano A, Molto A, et al. . Sacroiliac radiographic progression in recent onset axial spondyloarthritis: the 5-year data of the DESIR cohort. Ann Rheum Dis 2017;76:1823–8. 10.1136/annrheumdis-2017-211596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rudwaleit M, Jurik AG, Hermann KG, et al. . Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 2009;68:1520–7. 10.1136/ard.2009.110767 [DOI] [PubMed] [Google Scholar]
- 27. Sepriano A, Landewé R, van der Heijde D, et al. . Predictive validity of the ASAS classification criteria for axial and peripheral spondyloarthritis after follow-up in the ASAS cohort: a final analysis. Ann Rheum Dis 2016;75:1034–42. 10.1136/annrheumdis-2015-208730 [DOI] [PubMed] [Google Scholar]
- 28. Marzo-Ortega H, McGonagle D, O’Connor P, et al. . Baseline and 1-year magnetic resonance imaging of the sacroiliac joint and lumbar spine in very early inflammatory back pain. Relationship between symptoms, HLA-B27 and disease extent and persistence. Ann Rheum Dis 2009;68:1721–7. 10.1136/ard.2008.097931 [DOI] [PubMed] [Google Scholar]
- 29. Weber U, Hodler J, Kubik RA, et al. . Sensitivity and specificity of spinal inflammatory lesions assessed by whole-body magnetic resonance imaging in patients with ankylosing spondylitis or recent-onset inflammatory back pain. Arthritis Rheum 2009;61:900–8. 10.1002/art.24507 [DOI] [PubMed] [Google Scholar]
- 30. Weber U, Lambert RG, Østergaard M, et al. . The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum 2010;62:3048–58. 10.1002/art.27571 [DOI] [PubMed] [Google Scholar]
- 31. Dougados M, Gossec L. Classification criteria for rheumatic diseases: why and how? Arthritis Rheum 2007;57:1112–5. 10.1002/art.23015 [DOI] [PubMed] [Google Scholar]
- 32. Latourte A, Charlon S, Etcheto A, et al. . Imaging findings suggestive of axial spondyloarthritis in diffuse idiopathic skeletal hyperostosis. Arthritis Care Res 2018;70:145–52. 10.1002/acr.23244 [DOI] [PubMed] [Google Scholar]
- 33. Weber U, Jurik AG, Zejden A, et al. . Bone marrow oedema in sacroiliac joints of young athletes shows most frequently in the posterior inferior ilium. Ann Rheum Dis 2017;76(Suppl 2):101. [Google Scholar]
- 34. Varkas G, de Hooge M, Renson T, et al. . Presence of BME and structural lesions on MRI of the sacroiliac joints in young military recruits before and after 6 weeks of intensive physical training. [Abstract]. Arthritis Rheum;69(Suppl 10). [Google Scholar]
- 35. Gong Y, Zheng N, Chen SB, et al. . Ten years' experience with needle biopsy in the early diagnosis of sacroiliitis. Arthritis Rheum 2012;64:1399–406. 10.1002/art.33453 [DOI] [PubMed] [Google Scholar]
- 36. van den Berg R, de Hooge M, van Gaalen F, et al. . Percentage of patients with spondyloarthritis in patients referred because of chronic back pain and performance of classification criteria: experience from the Spondyloarthritis Caught Early (SPACE) cohort. Rheumatology 2013;52:1492–9. 10.1093/rheumatology/ket164 [DOI] [PubMed] [Google Scholar]
- 37. Braun J, Landewé R, Hermann KG, et al. . Major reduction in spinal inflammation in patients with ankylosing spondylitis after treatment with infliximab: results of a multicenter, randomized, double-blind, placebo-controlled magnetic resonance imaging study. Arthritis Rheum 2006;54:1646–52. 10.1002/art.21790 [DOI] [PubMed] [Google Scholar]
- 38. Rudwaleit M, Baraliakos X, Listing J, et al. . Magnetic resonance imaging of the spine and the sacroiliac joints in ankylosing spondylitis and undifferentiated spondyloarthritis during treatment with etanercept. Ann Rheum Dis 2005;64:1305–10. 10.1136/ard.2004.032441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weber U, Zubler V, Pedersen SJ, et al. . Development and validation of a magnetic resonance imaging reference criterion for defining a positive sacroiliac joint magnetic resonance imaging finding in spondyloarthritis. Arthritis Care Res 2013;65:977–85. 10.1002/acr.21893 [DOI] [PubMed] [Google Scholar]
- 40. Weber U, Pedersen SJ, Zubler V, et al. . Fat infiltration on magnetic resonance imaging of the sacroiliac joints has limited diagnostic utility in nonradiographic axial spondyloarthritis. J Rheumatol 2014;41:75–83. 10.3899/jrheum.130568 [DOI] [PubMed] [Google Scholar]
- 41. Weber U, Lambert RG, Pedersen SJ, et al. . Assessment of structural lesions in sacroiliac joints enhances diagnostic utility of magnetic resonance imaging in early spondylarthritis. Arthritis Care Res 2010;62:1763–71. 10.1002/acr.20312 [DOI] [PubMed] [Google Scholar]
- 42. Maksymowych WP, Wichuk S, Dougados M, et al. . MRI evidence of structural changes in the sacroiliac joints of patients with non-radiographic axial spondyloarthritis even in the absence of MRI inflammation. Arthritis Res Ther 2017;19:126 10.1186/s13075-017-1342-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weber U, Østergaard M, Lambert RG, et al. . Candidate lesion-based criteria for defining a positive sacroiliac joint MRI in two cohorts of patients with axial spondyloarthritis. Ann Rheum Dis 2015;74:1976–82. 10.1136/annrheumdis-2014-205408 [DOI] [PubMed] [Google Scholar]
- 44. Arnbak B, Grethe Jurik A, Hørslev-Petersen K, et al. . Associations between spondyloarthritis features and magnetic resonance imaging findings: a cross-sectional analysis of 1,020 patients with persistent low back pain. Arthritis Rheumatol 2016;68:892–900. 10.1002/art.39551 [DOI] [PubMed] [Google Scholar]
- 45. Diekhoff T, Hermann KG, Greese J, et al. . Comparison of MRI with radiography for detecting structural lesions of the sacroiliac joint using CT as standard of reference: results from the SIMACT study. Ann Rheum Dis 2017;76:1502–8. 10.1136/annrheumdis-2016-210640 [DOI] [PubMed] [Google Scholar]
- 46. Lambert RG, Bakker PA, van der Heijde D, et al. . Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis 2016;75:1958–63. 10.1136/annrheumdis-2015-208642 [DOI] [PubMed] [Google Scholar]
- 47. Bennett AN, Marzo-Ortega H, Kaur-Papadakis D, et al. . The use of magnetic resonance imaging in axial spondyloarthritis: time to bridge the gap between radiologists and rheumatologists. J Rheumatol 2017;44:780–5. 10.3899/jrheum.161337 [DOI] [PubMed] [Google Scholar]
- 48. Rudwaleit M, Landewé R, van der Heijde D, et al. . The development of assessment of spondyloarthritis international society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–6. 10.1136/ard.2009.108217 [DOI] [PubMed] [Google Scholar]
- 49. Dougados M, d’Agostino MA, Benessiano J, et al. . The DESIR cohort: a 10-year follow-up of early inflammatory back pain in France: study design and baseline characteristics of the 708 recruited patients. Joint Bone Spine 2011;78:598–603. 10.1016/j.jbspin.2011.01.013 [DOI] [PubMed] [Google Scholar]
- 50. Poddubnyy D, Brandt H, Vahldiek J, et al. . The frequency of non-radiographic axial spondyloarthritis in relation to symptom duration in patients referred because of chronic back pain: results from the Berlin early spondyloarthritis clinic. Ann Rheum Dis 2012;71:1998–2001. 10.1136/annrheumdis-2012-201945 [DOI] [PubMed] [Google Scholar]
- 51. Mandl P, Navarro-Compán V, Terslev L, et al. . EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis 2015;74:1327–39. 10.1136/annrheumdis-2014-206971 [DOI] [PubMed] [Google Scholar]
- 52. Sudoł-Szopińska I, Jurik AG, Eshed I, et al. . Recommendations of the ESSR Arthritis Subcommittee for the use of magnetic resonance imaging in musculoskeletal rheumatic diseases. Semin Musculoskelet Radiol 2015;19:396–411. 10.1055/s-0035-1564696 [DOI] [PubMed] [Google Scholar]
- 53. U.S. Food and Drug Administration, Department of Health & Human Services. Arthritis Advisory Committee Meeting: Adalimumab (Humira®) for active non-radiographic axial spondyloarthritis (nr-axSpA) in adults with objective signs of inflammation by elevated CRP or MRI who have had inadequate response or are intolerant to NSAIDs. 2013. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM361563.pdf (accessed 22 Jan 2016).
- 54. U.S. Food and Drug Administration, Department of Health & Human Services. Arthritis Advisory Committee Meeting: Certolizumab (Cimzia®) for active axial spondyloarthritis including ankylosing spondylitis. 2013. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM361565.pdf (accessed 22 Jan 2016).
- 55. Deodhar A, Reveille JD, van den Bosch F, et al. . The concept of axial spondyloarthritis: joint statement of the spondyloarthritis research and treatment network and the Assessment of SpondyloArthritis International Society in response to the US Food and Drug Administration’s comments and concerns. Arthritis Rheumatol 2014;66:2649–56. 10.1002/art.38776 [DOI] [PubMed] [Google Scholar]


