Abstract
It is believed that therapy for rheumatoid arthritis (RA) is the most effective and beneficial within a short time frame around RA diagnosis. This insight has caused a shift from research in patients with established RA to patients at risk of developing RA and recently diagnosed patients. It is important for improvement of RA therapy to understand when and what changes occur in patients developing RA. This is true for both seropositive and seronegative patients. Activation of the immune system as presented by autoantibodies, increased cytokine and chemokine production, and alterations within several immune cells occur during RA development. In this review we describe RA pathogenesis with a focus on knowledge obtained from patients with arthralgia, pre-RA and recently diagnosed RA. Connections are proposed between altered immune cells, cytokines and chemokines, and events like synovial hyperplasia, pain and bone damage.
Keywords: early rheumatoid arthritis, cytokines, inflammation
Key messages.
Seropositive (arthralgia) patients display an array of altered cytokines and chemokines especially compared with seronegative (arthralgia) patients.
There is a knowledge gap concerning mechanisms of seronegative rheumatoid arthritis (RA) development.
RA is a dynamic disease in its development, which is best studied in large longitudinal cohorts.
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory autoimmune disease characterised by inflamed joints and bone damage. As with any disease RA has a series of events leading up to its diagnosis. There are six phases that a patient can experience throughout the development of RA. These phases are ‘genetic risk factors’, ‘environmental risk factors’, ‘systemic autoimmunity’, ‘symptoms without arthritis’, ‘undifferentiated arthritis’ and ‘RA’.1 Pre-RA patients are patients who are at risk for developing RA and who in the future will be diagnosed with RA when multiple criteria are met.2 Pre-RA patients can have autoantibodies such as anticitrullinated protein antibodies (ACPA) or rheumatoid factor (RF) but can also remain seronegative for these autoantibodies. Within the time frame of being a patient with arthralgia and receiving RA as diagnosis, multiple processes can take place causing autoimmunity, pain and bone erosions. Full comprehension of how RA develops over time is of importance. By understanding this, therapies can be developed to prevent RA instead of treating symptoms. In this review we describe RA pathogenesis with a focus on knowledge obtained from patients with arthralgia, pre-RA and recently diagnosed RA. We describe the pathogenesis for seropositive as well as seronegative patients. The complex interactions that compose inflammation are subdivided in the next sections and describe serum markers such as cytokines and chemokines, inflammatory cells and local changes in the synovium. Connections are proposed between altered immune cells, cytokines and chemokines, and events like synovial hyperplasia, pain and bone damage.
Cytokines and chemokines from arthralgia to RA
Activation of the immune system during RA development can be monitored via measuring proteins within the blood, such as cytokines and chemokines. As blood is easily accessible, it represents a medium that might be ideal for diagnosis and monitoring disease progress.
Already prior to the development of autoantibody positivity changes in cytokine and chemokine levels have been observed within blood. Cytokines preceding CCP positivity in pre-RA patients are interleukin (IL)-1alpha, IL-6 and IP-10.3 The proportion of pre-RA patients positive for these cytokines was higher compared with controls. The first significantly elevated count of cytokines and chemokines was found about 7.2 years prior to receiving RA as diagnosis. A relationship was found between the number of elevated cytokines and chemokines and time of diagnosis, with higher counts being associated with a shorter time remaining to diagnosis.3 This increase in number of elevated cytokines has also been reported by others where a rise of cytokines was observed from 2 to 3 years prior to RA diagnosis independent of the presence or absence of CCP.4
Insight into which cytokines and chemokines play a role in the development of RA is of interest to understand RA pathogenesis. Altered cytokine and chemokine levels were found in patients with arthralgia. IL-17A for example was increased in patients with seropositive arthralgia compared with healthy controls.5 Additionally IL-5 is an elevated cytokine in pre-RA patients positive for RF compared with controls. IL-5 is also elevated in pre-RA patients compared with patients with seropositive arthralgia not receiving RA as diagnosis.5 6 This indicates that IL-5 and IL-17A play a role in RA development. However, IL-17A and IL-5 were not found to be significantly altered in seronegative patients. More importantly the significance of cytokine and chemokine levels was found to be influenced by the presence of autoantibodies. Indeed stratification for autoantibodies reveals major differences between seropositive and seronegative (pre-)RA patients. After stratification for RF the only significantly dysregulated cytokines were IL-8 and IL-13 in RF-negative pre-RA patients.5 In seropositive (pre-)RA patients multiple cytokines and chemokines are differentially expressed compared with healthy controls. For patients with RF-positive arthralgia compared with controls, IL-2RA, IL-5, IL-9, IL-13, IL-17 and MIG became significantly different after stratification for RF positivity.5 This could indicate different processes underlying the development of RA in seronegative patients compared with seropositive patients.
In seronegative patients with RA, IL-10 was increased while eotaxin and Rantes were decreased compared with healthy controls.6 Overlap in differential expression compared with controls between patients with seropositive arthralgia and patients with seropositive RA consists of IL-1beta, IL-2, IL-1RA, IL-17, IL-4, IL-15 and IL-2R.6 Other cytokines and chemokines that become significantly different after RA diagnosis are tumour necrosis factor (TNF)α, interferon (IFN)α, MCP-1 and MIP-1α.6
Overall, the cytokine and chemokine data do confirm activation of the immune system prior to RA diagnosis. Nonetheless, it is difficult to determine the exact source of the detected cytokines and chemokines within blood as multiple cells can secrete the same cytokine or chemokine. In addition, the consequences of some elevated cytokines are difficult to interpret due to simultaneous increase of their antagonists such as IL-1R and IL-2R for, respectively, IL-1 and IL-2. An interesting effect of elevated cytokines and inflammation-related factors such as prostaglandin E2 is their ability to increase nociceptor neuron sensitivity, resulting in pain signalling.7 Both seropositive and seronegative patients with arthralgia experience pain. The limited data so far suggest that less cytokines are differentially expressed within seronegative pre-RA patients compared with the differential expression in seropositive pre-RA patients. This raises the question whether the elevation of IL-8 is enough to explain the pain complaints in patients with seronegative arthralgia or whether a more elaborate inflammation such as seen in seropositive patients is needed to result in inflammation-induced pain.
Individual levels of cytokines and chemokines might rise over time within a single patient. These increases in cytokine and chemokine levels over time during RA development need to be further examined in follow-up studies as most of the current data are derived from cross-sectional studies. Additionally, future studies should broaden or extend the array of cytokines and chemokines measured and correct them for autoantibody presence. The studies should preferably take patients with both seropositive and seronegative arthralgia and follow these patients to determine time to RA diagnosis. These studies can elucidate which cytokines and chemokines are altered at which time point during RA development and just after RA diagnosis. TNFα for example is a cytokine that is altered after RA diagnosis. The knowledge that TNFα so far has not been reported differently prior to RA diagnosis is of interest when it concerns treatment. Inhibition of TNFα already occurs in daily practice in patients with diagnosed RA. It would be interesting to know whether inhibition of TNFα in patients with arthralgia would influence their chance on developing RA by inhibiting a rise of TNFα levels. Future studies should therefore also focus on the effectiveness of treatment prior to RA diagnosis.
Immune cells in the development of RA
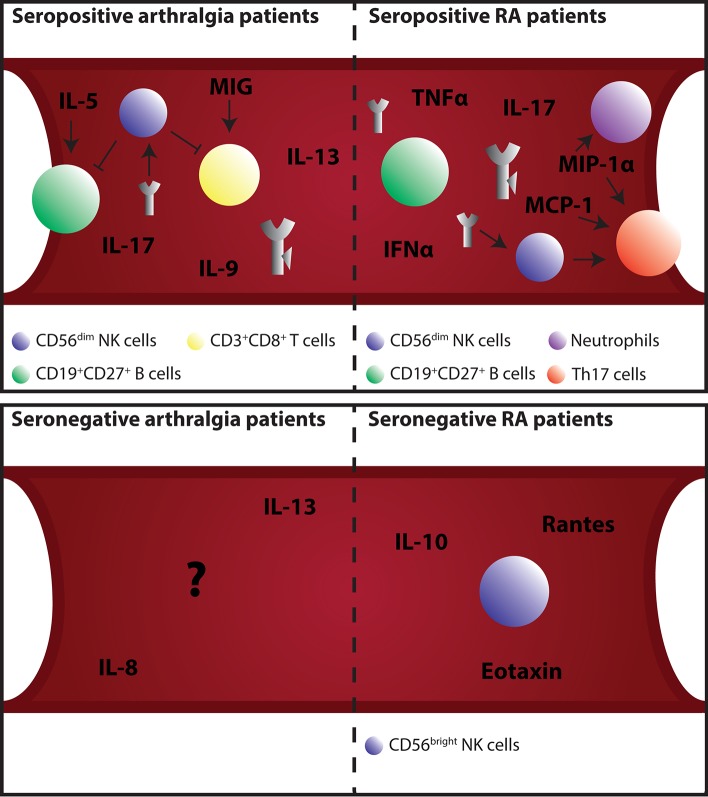
Immune cells play an important role in the development of RA. Multiple cell types can become involved at different time points during RA development and progression. In the next section different types of immune cells that have been examined in the blood of patients with arthralgia or RA will be discussed. Possible links between cell types or above-reported cytokines and chemokines and their effects will be described (see figure 1).
Figure 1.
Schematic overview of different immune processes in blood during the development of RA. Upper left: CD56dim NK cells are capable of killing autoimmune cells like B cells and CD8 T cells. RF immune complexes bind to the FcγRIIIa receptor on NK cells causing apoptosis and thereby reduce CD56dim NK cell number. Cytokines like IL-5 exert their influence on B cells resulting in more immunoglobulin secretion. MIG attracts CD8 T cells via the CXCR3 receptor to lymph nodes where these cells become short-lived effector cells. B cells migrate out of the bloodstream. IL-13 prevents synovial fibroblasts from apoptosis induced by NO. Synovial hyperplasia will develop. Upper right: Levels of autoantibodies have risen, epitope spreading has occurred, and antibody modifications like glycosylation, sialylation and galactosylation are altered. CD56dim NK cell number is still reduced and start to dysfunction by lowered IFNγ production. Reduction of IFNγ results in skewing towards Th17 cell differentiation. Th17 cells and Th17 subpopulations migrate from blood under the influence of MIG, MIP-1α and MCP-1. Neutrophils are activated and recruited from blood via MIP-1α. Lower left: The cytokines IL-8 and IL-13 are altered in the blood of patients with seronegative arthralgia. IL-8 could induce pain by binding to receptors on nociceptor sensory neurons. IL-13 prevents synovial fibroblasts from apoptosis induced by NO. Synovial hyperplasia will develop. Lower right: Increased IL-10 and decreased Rantes and eotaxin are detected. CD56bright NK cell number is increased. These cells are capable of secreting proinflammatory cytokines like IL-13. Any type of arrow displayed within the figure are suggested links between antibodies, immune cells and cytokines or chemokines. The indicated cytokines and chemokines are not necessarily secreted by the depicted immune cells. IFNγ, interferon gamma; IL, interleukin; RA, rheumatoid arthritis; RF, rheumatoid factor.
Patients with arthralgia
Studies reporting on changes within immune cells at the arthralgia phase are limited. The information available on immune cells is somewhat contradictive and only focused on patients with seropositive arthralgia (see table 1). Two studies from the same group showed the lack of differences on absolute numbers of naïve T cells, T central memory cells, effector memory and terminal differentiated effector memory T cells, as well as CD19+ B cells, compared with healthy controls.8 9 These studies were based on cross-sectional comparisons of patients with seropositive arthralgia with healthy controls. A limitation of these studies is that it is not reported whether these patients with seropositive arthralgia developed arthritis or RA, leaving open the question whether the lack of difference is due to non-convertment of these patients with seropositive arthralgia into patients with RA. Some studies did report on patients with arthralgia who converted to patients with the diagnosis of RA.
Table 1.
Changes within immune cells from patients with arthralgia
| First author (ref) | Type of cell | Participants | Findings |
| Chalan et al9 | Absolute number of naïve T cells, T central memory, effector memory and terminally differentiated effector memory T cells | Patients with seropositive arthralgia | Compared with healthy individuals no differences in these cells |
| Effector memory T cells and terminally differentiated effector memory T cells | Patients with seropositive arthralgia and patients with newly diagnosed RA | Elevated in patients with seropositive arthralgia compared with RA | |
| CD4+CD161+ T cells | Patients with seropositive arthralgia | Increased in patients with seropositive arthralgia compared with healthy controls and patients with RA | |
| Th17/Th1 double positive cells | Patients with seropositive arthralgia | Increased in patients with seropositive arthralgia compared with healthy controls and patients with RA | |
| Chalan et al8 | Absolute and frequency of CD3+CD4+ T cells CD3+CD8+ T cells CD19+ B cells |
Seropositive patients with arthralgia, healthy controls, patients with early seropositive RA and patients with seronegative RA | No differences |
| NK cells | Patients with seropositive arthralgia, seropositive RA and healthy controls | Less NK cells in patients with seropositive arthralgia and seropositive RA compared with healthy controls | |
| CD56dim NK cells | Patients with seropositive arthralgia, patients with seropositive RA and healthy controls | Number not frequency decreased in seropositive arthralgia and seropositive RA compared with healthy controls | |
| CD56bright NK cells | Patients with seropositive arthralgia, patients with seropositive RA and healthy controls | Number not different | |
| Janssen et al10 | Fr I (CD45RA+FoxP3low) Fr II (CD45RA+FoxP3high) |
Patients with seropositive arthralgia (n=34 of whom 14 developed RA) | No differences |
| Fr III (CD45RA−FoxP3low) | Patients with seropositive arthralgia (n=34 of whom 14 developed RA) | Increased compared with healthy control | |
| Lübbers et al11 | Conventional memory CD27+ B cells activated CD80+ B cells | Patients with seropositive arthralgia (22 developed arthritis within ≤1 year, 18 developed arthritis after >1 year and 73 did not develop arthritis) | Decrease of these cells early RA to healthy control |
| Ramwadhdoebe et al31 | CD4+CD69+ (activated or tissue resident CD4 T cells) | At risk (patients with arthralgia but within follow-up no arthritis developed) | CD4+CD69+ more in at risk compared with healthy controls in blood but not in lymph nodes (LN) |
| CD4+IL-17+IL-10+and CD4+IFNg+IL-10+ | At risk and healthy controls | In LN tissue decreased in at risk compared with healthy controls (frequencies very low) |
IFN, interferon; IL, interleukin; RA, rheumatoid arthritis.
Regulatory T cells
CD4+CD25+FoxP3+ T cells (Tregs) can suppress other immune cells by regulating their proliferation and cytokine production. In a study on patients with seropositive arthralgia, no differences were detected in the percentage and number of naïve CD4+CD25+FoxP3+T cells compared with healthy controls.10 The same was found for activated CD4+CD25+FoxP3+ T cells. Comparing the CD4+CD25+FoxP3+ T cells from patients with seropositive arthralgia who converted to patients with RA with those who did not resulted again in no detectable differences. These results indicate that Tregs are not altered on the level of numbers and frequencies within the blood of patients with arthralgia. Whether Tregs are functionally different in patients with arthralgia is still unknown.
CD8+ T cells
A study comparing patients with seropositive arthralgia who converted to patients with RA with non-converters showed decreased CD8+ cytotoxic T cells approximately 24 months prior to arthritis development.11 The CD8+ cytotoxic T cells described in the article are determined by CD3 and CD8 positivity and represent a rather diverse group. Not all CD8+ T cells have the same ability to be cytotoxic. Additionally there is a description of CD8+ T cells to be suppressive or regulatory. These cells are characterised by the production of IL-10 and TGFβ. The effects of reduced CD3+CD8+ T cells in patients with arthralgia need to be further investigated with focus on specific subsets and their function. Of interest would also be whether these cells migrate to other sites of the body. The migration and longevity of CD8+ T cells could be influenced by chemokines like MIG, which binds to the receptor CXCR3. As described in the previous section about cytokines and chemokines, patients with RF seropositive arthralgia have elevated levels of MIG. Signalling via CXCR3 receptor leads to the accumulation of CD8+ T cells in the marginal zone where these cells differentiate into short-lived effector cells.12 An alternative reason for decreased CD8+ T cells could be the elimination of autoreactive CD8+ T cells by other immune cells.
CD56dim NK cells
The suggested explanation of CD3+CD8+ T cells elimination by other immune cells could be due to NK cells. NK cells have been shown to exhibit cytotoxic abilities, especially the CD56dim NK cells. NK cells could influence autoimmune cells via direct lysis or indirectly via targeting DCs. A single study reported less NK cells in patients with seropositive arthralgia compared with controls.8 More importantly CD56dim NK cells were decreased, not the CD56bright NK cells. The decrease in CD56dim NK cells is likely the consequence of apoptosis induced by binding of RF to the FcγRIIIa. Apoptosis was in vitro further induced when IL-2 was added. As described above IL-2 is increased in the blood of patients with seropositive arthralgia. The reduction of CD56dim NK cells was measured in patients with seropositive arthralgia of which it is not described whether they developed arthritis. Therefore it is only guessing when these changes in NK cells occur during RA development. The reduction in NK cells is likely to occur at a later phase if these CD56dim NK cells are responsible for the decrease in CD3+CD8+T cells within patients with arthralgia. The reduction of CD56dim NK cells could also impact the presence of other (autoimmune) cells such as Th17 cells and B cells.
Th17 cells and Th17 cell subpopulations
A single study has investigated Th17 cells in patients with arthralgia. CD4+CD161+ cells are known for their ability to differentiate into IL-17-producing cells. Additionally CD4+CD161+ cells include IL-17+IFNγ+ double positive cells and the non-classical Th1 cells. In patients with arthralgia higher percentage and absolute number of CD4+CD161+ cells were reported compared with healthy controls. Additionally the IL-17+IFNγ+ double positive cells were reported to be increased in patients with seropositive arthralgia versus healthy controls.9 Unfortunately it was not reported whether the patients with seropositive arthralgia within the study developed RA. It therefore remains a question whether the differences found is due to RA development or whether it is a common finding in patients with arthralgia. Direct data on Th17 cells within the arthralgia phase are still missing, although these indirect results on CD4+CD161+ cells give a first indication of a possible increase in Th17 cells in patients with seropositive arthralgia.
B cells
B cells can differentiate into plasma cells, producing antibodies that will bind to antigens and activate the immune system. Approximately 10 years prior to RA diagnosis, autoantibodies can be detected in individuals.13 Examples of autoantibodies are RF, ACPA and antibodies against carbamylated antigens (anti-CarP). Even though these antibodies are described as autoantibodies, no clinical disease is detected shortly after the first appearance of these antibodies. This observation questions whether these autoantibodies are as pathogenic as they have been described. The pathogenicity might depend on the glycosylation states of autoantibodies.14 15 The number of CD27+ memory B cells is decreased in patients with seropositive arthralgia who converted to patients with RA compared with non-converters.11 This decrease was reported in patients with arthralgia who developed RA within 12 months but not in those who converted at a later time point.
The start of epitope spreading of autoantibody responses is reported approximately 2–3 years prior to RA diagnosis.4 Along this time frame changes within bones also occur. During the development of RA cortical bone thickness but not trabecular bone decreases in patients with ACPAs.16 Cortical fenestrations are additionally more abundant in ACPA-positive participants. Although reduction of bone mineral density occurs at an early stage, only at time of diagnosis the changes are such that they differ from other non-inflammatory joint diseases like osteoarthritis.17 It is a question whether the early changes in bone mineral density as seen in patients with arthralgia already contribute to the induction of pain. Recently certain types of ACPA have been described to induce osteoclastogenesis. A subset of ACPAs that are reactive to enolase and vimentin can induce osteoclastogenesis and osteoclast activation.18 19 IL-8 can be secreted by osteoclasts, which can subsequently result in pain via binding to the IL-8 receptor on nociceptor sensory neurons.20
The ACPA against vimentin was shown to be sialylated. This is interesting in the context that the percentage of glycosylation as well as sialylation of total IgG1 and total ACPAs was reduced in asymptomatic ACPA-positive patients who were diagnosed with RA within 12 months compared with those who were not diagnosed with RA within 12 months.14 Additionally, galactosylation of total ACPAs was decreased 3 months prior to RA diagnosis.15 Data on the sequential order of events about appearance, modifications such as glycosylation, sialylation and galactosylation, and concentration of these possible pathogenic subsets of ACPAs and bone erosions within the same patients at the same time are lacking. The importance of these ACPAs and whether they indeed induce bone erosion in humans need further investigation.
Patients with RA
In comparison with patients with arthralgia, more is known about immune cells in patients with RA. The immune cells described below are derived from the blood of patients with RA (see figure 1 and table 2).
Table 2.
Changes within immune cells from patients with RA
| First author (ref) | Type of cell | Participants | Findings |
| Colin et al28 | IL-17+CD4+ T cells, IL-17+CD4+CD45RO+ T cells, IL22+CD4+ T cells, IL22+CD4+CD45RO+ T cells and TNFα+CD4+CD45RO+ T cells | Patients with recently diagnosed RA and healthy controls | IL-17+CD4+ T cells, IL-17+CD4+CD45RO+ T cells and IL-22+CD4+ T cells were increased in patients with recently diagnosed RA compared with healthy control. No differences in IL-22+CD4+CD45RO+ T and TNFα+CD4+CD45RO+ T cells |
| Chalan et al9 | CD4+CD161+ T cells | Patients with newly diagnosed RA | Decreased in newly diagnosed RA compared with healthy controls and patients with seropositive arthralgia |
| Th17 cells | Newly diagnosed RA | Increased compared with healthy controls | |
| Non-classical Th1 cells | Patients with newly diagnosed RA and seropositive arthralgia | Decreased in patients with newly diagnosed RA compared with patients with seropositive arthralgia | |
| CD4+CD161+ cells | Synovial tissue sections in patients with RA | CD161 expression in area s infiltrated by CD3, CD4 expressing cells | |
| CD4+CD161+ cells | RA late stage | Increased in synovial fluid compared with peripheral blood. Increased in synovia l tissue compared with blood | |
| CD4+CD161+ cells | RA late stage | Percentage of IL-17 producing cells higher in blood derived CD4+CD161+ subset than synovial fluid derived subset. IFNγ and IL-17 double positive producers were similar. Significant increase in frequency of IFNγ cells (non-classical Th1 cells) in synovial fluid vs blood |
|
| Chalan et al8 | NK cells | Patients with seropositive arthralgia, seropositive RA and healthy controls | Less NK cells in patients with seropositive arthralgia and seropositive RA compared with healthy controls |
| NK cells | Seronegative RA | Number and proportion not altered | |
| CD56 dim NK cells | Patients with seropositive arthralgia, patients with seropositive RA and healthy controls | Number not frequency decreased in patients with seropositive arthralgia and patients with seropositive RA compared with controls | |
| CD56 bright NK cells | Patients with seropositive arthralgia, patients with seropositive RA and healthy controls | Number not different | |
| CD56 bright NK cells | Seronegative RA and healthy controls | Number and frequency higher in seronegative RA compared with healthy controls | |
| NK cells | Patients with seropositive RA, seropositive arthralgia | In seropositive RA NK cells produce less IFNγ compared with healthy controls. This is not observed in patients with seropositive arthralgia | |
| Janssen et al10 | Fr III (CD45RA−FoxP3low) | RA (n=12 newly diagnosed) | Not different from healthy control |
| Kotake et al46 | Th17 cell-derived Th1 cells (non-classical Th1) | Patients with RA | Th17 cell-derived Th1 cells to Th17 cells were elevated in patients with recent onset RA compared with osteoarthritis. |
| Leipe et al27 | IL-17+CD4+ T cells, Th1 cells, IL-17+IFNγ+ T cells and IFNγ+ T cells | Patients with RA, healthy controls, osteoarthritis and psoriatic arthritis | Increased IL-17+CD4+ T cells in patients with RA and psoriatic arthritis in blood compared with controls and osteoarthritis No differences in Th1 cells, IL-17+IFNγ+ T cells and IFNγ+ T cells within blood |
| IL-17+CD4+ T cells, IL-17+IFNγ+ T cells, CD4+CCR4+CCR6+ T cells and Th1 cells | Patients with early RA and psoriatic arthritis | Increased Th17 cells, IL-17+IFNγ+ double producers and CD4+CCR4+CCR6+ T cells in synovial fluid compared with paired blood samples No differences in Th1 cells |
|
| Lübbers et al11 | CD3+ T cell, CD3+CD56+CD16+ activated T cells, conventional memory CD27+ B cells, activated CD80+ B cells | RA (n=89, less than 6 months no DMARD of biological agent) | Decrease of these cells early RA to healthy control |
| Paulissen et al32 | Th22, Th17.1 and CCR4+CXCR3+ DP cells | Patients with RA | Proportions of these cells were increased in patients with ACPA+ RA compared with ACPA− patients. CCR6+ Th cell proportions inversely correlate with disease duration in ACPA− not ACPA+ patients. |
| Ramwadhdoebe et al31 | CXCR3+CCR6−CCR4−Th1 | Patients with RA | Higher in early RA compared with healthy controls but not in at risk |
| CD4+CCR7+ | Patients with RA | In lymph nodes (LN) lower in early RA compared with at risk | |
| CD4+IL-17+ | Early RA, at risk and healthy controls | In blood but not LN higher in early RA compared with at risk and healthy controls | |
| CD4+IL-17+IL-10+ and CD4+IFNg+IL-10+ | At risk and healthy controls | In LN tissue decreased in at risk compared with healthy controls (frequencies very low) | |
| Tudhope et al21 | Invariant NK T cell | Patients with RA | Lower frequency in RA compared with healthy controls Untreated patients with RA less iNKT frequency and number Proliferation towards alpha-galactosylceramide impaired |
| van Hamburg et al29 | CD4+ T cells, CCR6+ memory T cells, IL-17A+CCR6+CD45RO+ T cells | Patients with recently diagnosed RA, healthy controls | Increased CD4+ T cells, CCR6+ memory T cells, IL-17A+CCR6+CD45RO+ T cells to healthy controls |
ACPA, anticitrullinated protein antibodies; IFNγ, interferon gamma; IL, interleukin; RA, rheumatoid arthritis; TNF, tumour necrosis factor.
Invariant NK T cells
A single study investigated invariant NK T cells within patients with recently diagnosed RA and found a reduced frequency of invariant NK T cells.21 Interestingly these cells from patients with RA proliferated less when exposed to alpha-galactosylceramide. This reveals that invariant NK T cells from patients with RA are not only decreased in frequency but also harbour functional changes. Whether invariant NK T cells play a pathogenic role in RA is still unclear. Decreased invariant NK T cells are also found in osteoarthritis, indicating that a decrease in these cells is not specific for RA.21
NK cells
In agreement with findings in patients with arthralgia, NK cells were decreased in patients with RA compared with healthy controls.8 Different subtypes of NK cells were affected in patients with seropositive RA compared with patients with seronegative RA. In patients with seropositive RA, the CD56dim NK cells were decreased compared with healthy controls. Additionally the NK cells from patients with seropositive RA produce less IFNγ compared with healthy controls. This difference in IFNγ production was not observed in patients with seropositive arthralgia. The reduced IFNγ production by NK cells could lead to an increase in cells differentiating to Th17 cells.22 For patients with seronegative RA, the CD56bright NK cells were found to be increased compared with healthy controls. CD56bright NK cells are producers of multiple cytokines and dependent on the stimulation can secrete IL-10 and IL-13.23 These cytokines, as discussed before, are dysregulated in seronegative (arthralgia) patients.
Neutrophils
Neutrophils are recruited from the blood by chemokines like IL-8 and MIP-1α and are numerous in RA synovial fluid. Neutrophils from patients with RA are functionally different from healthy controls. They display a more activated status and are primed to produce reactive oxygen species.24 Additionally neutrophils from patients with RA form more often neutrophil extracellular traps (NETosis) compared with neutrophils from healthy controls.25 Furthermore, neutrophils of patients with RA have an expanded life span. Most of the described features can be explained by the inflammatory environment in which RA neutrophils reside. IL-8 for example is, besides a neutrophil attractant, also a factor that delays neutrophil apoptosis. TNFα is another antiapoptotic cytokine for neutrophils. TNFα as well as IL-17 are capable in promoting NETosis.25 Interestingly normal neutrophils needed TNFα priming before IL-17A can induce NETosis. TNFα levels are however reported to be altered in patients with RA but not in patients with arthralgia. This raises the question whether the rise of TNFα levels is accompanied by increased NETosis, and if not whether the rise in RF and ACPA levels or specific modifications of these antibodies is enough to induce NETosis. The NETosis could then result in epitope spreading of antibody responses due to exposed citrullinated proteins on NETs. Binding of autoantibodies to exposed citrullinated proteins could then lead to an escalation of inflammation and subsequently secretion of additional cytokines like TNFα. Neutrophils are, besides a source of autoantigens, capable of secreting matrix metalloproteinases (MMPs) such as MMP-8 and MMP-9. These MMPs cause degradation of collagens within the extracellular matrix and activation of IL-1 beta. The NETs from neutrophils can additionally activate synovial fibroblasts.25 Synovial fibroblasts are a source for, for example, MMP-1 and MMP-3. The cocktail of MMPs causes tissue damage. Neutrophils are thus multifactorial contributors to RA pathology.
Th17 cells and Th17 cell subpopulations
In patients with recently diagnosed RA, the role of Th17 cells has been more extensively examined than in patients with arthralgia. Th17 cells are a heterogeneous group composed, among others, of non-classical Th1 or Th17.1 cells.26 Th17 cells are increased in the peripheral blood of patients with RA compared with healthy controls.27–30 This increase was associated with increased disease activity as measured by DAS28 and CRP.27 CD4+CD161+ T cells were decreased in numbers in patients with RA compared with healthy controls and patients with seropositive arthralgia.9 Within these CD4+CD161+ cells, Th17 cells were increased in patients with RA compared with controls, while non-classical Th1 cells were decreased in patients with newly diagnosed RA compared with patients with seropositive arthralgia. The increase of CD4+IL-17+ cells was confirmed by others within blood but not lymph nodes of patients with early RA compared with at risk individuals and healthy controls.31 Remarkably there is a possible time dependency of Th17 cells involvement for patients with ACPA-negative RA. This is based on the observation that more Th17 cells are correlated with a shorter disease duration,32 while for ACPA-positive patients this correlation does not hold and might account for the possibility to distinguish ACPA-positive patients from ACPA-negativepatients based on their increased levels of Th17 cells.
The importance of Th17 cells after the development of RA is still a matter of debate. Within patients with early RA, Th17 cells, IL-17+IFNγ+ double producers and CD4+CCR4+CCR6+ T cells were increased in synovial fluid compared with paired blood samples.27 However at late-stage RA the percentage of IL-17 producing cells within CD4+CD161+ cells was found to be higher in blood than in synovial fluid.9 IFNγ and IL-17 double producers were similar, while non-classical Th1 cells were found to be increased in synovial fluid compared with blood. These observations lead to the question whether Th17 cells within patients with RA remain Th17 cells that migrate to the joints. Th17 cells could also change characteristics at sites of local inflammation such as the joints. Another possibility is the specific attraction of Th17 cell subpopulations to the joints. In addition to MIG, MIP-1α and MCP-1 become altered in the blood of patients with RA. MIP-1α and MCP-1 are both able to induce migration of cells that express the CCR4 receptor. The combination of MIG, which signals via CXCR3, with MIP-1α and MCP-1 could be the mix of attractants to induce migration of Th17, CCR4+CXCR3+DP cells and non-classical Th1 cells from blood towards the joints.
The IL-17 produced by Th17 cells and Th17 cell subpopulations contributes to RA pathogenesis by activating other immune cells like macrophages but also synovial fibroblasts. The activation of these cells leads to the expression of receptor activator of nuclear factor kappa-B ligand (RANKL) that can induce osteoclastogenesis via binding to receptor activator of nuclear factor kappa-B (RANK) on osteoclast precursors. These osteoclast precursors then differentiate into osteoclasts. Additionally cytokines like IL-1 can bind to the IL-1 receptor on osteoclasts and activate them.33 The combined actions of Th17 cells, Th17 cell subpopulations, macrophages, neutrophils and synovial fibroblasts and their secreted proinflammatory cytokines are likely the cause of changes in bone mineral density detected in, for example, hands of patients with recently diagnosed RA.34–37
B cells
Similar to what is found in patients with arthralgia, conventional memory CD27+ B cells were lower in patients with RA compared with healthy controls. Additionally activated CD80+ B cells were found to be decreased in patients with RA.11 Autoantibody production occurs throughout the development as well as the progression phase of RA. The functionality of B cells from patients with RA is likely not different compared with B cells from patients with arthralgia. The function of B cells besides the production of antibodies should be further examined. Possible research directions of future studies are the function of B cells as antigen-presenting cells and the function of B cell-derived cytokines.38
Synovial inflammation in the development of RA
Synovial biopsies from patients with systemic autoimmunity (ACPA-positive or RF-positive) at risk of RA showed minimal infiltration by T cells but absence of clear synovial inflammation. No markers for infiltrating cells were found to explain arthritis development. CD3+CD8+ T cells however showed a trend for an association with arthritis development, although this trend was found in uninvolved joints in individuals developing arthritis.39 Absence of cellular infiltration has also been reported in newly diagnosed treatment-naïve patients with RA, where only 4 out of 15 patients showed clear immune cell infiltration in their biopsies.9 In the case of clear immune cell infiltration mostly B cells, T cells and macrophages are found.40 The influx of immune cells in the synovium of patients with early RA with a disease duration of less than 1 year did not differ in the type of cellular influx compared with the synovium of patients with RA with a duration of more than 5 years.41 Because of the heterogeneity between the reports showing either presence or absence of immune cell infiltration in the synovium, it remains to be determined when and which cell types infiltrate the synovium during the development of RA.
Interestingly, besides changes in blood vessels, the most reported feature of the synovium from uninvolved knee joints from patients with RA is increased numbers of synoviocytes or synovial lining cell hyperplasia.42 This could indicate early changes in the synovial fibroblasts prior to clear synovial inflammation by the influx of immune cells. Synovial fibroblasts were found to be activated as seen by the expression of HLA-DR in biopsies without cellular infiltration. Additionally, previously activated synovial fibroblasts produced more cytokines such as IL-6 on a second stimulation.43 This enhanced reaction on a first activation or proinflammatory memory lasted for multiple days and was accompanied by prolonged NF-KB signalling. Of interest to note is the altered expression of IL-13 in the blood of patients with both seropositive and seronegative arthralgia, as well as IL-4 expression in patients with seropositive arthralgia. Both IL-13 and IL-4 are increased in synovial fluid from patients with a symptom duration of less than 3 months who developed RA.44 This increase in IL-4 and IL-13 in synovial fluid was transient as it was not detected in synovial fluid of patients with established RA. The effect of IL-4 and IL-13 however is important as it protects synovial apoptosis.45 This protection allows synovial fibroblasts to survive NO generated by, for example, neutrophils. Protection against apoptosis can subsequently result in synovial hyperplasia and increased production of proinflammatory cytokines, RANKL expression and tissue damage by MMPs secretion. These findings indicate that synovial fibroblasts might play a role in the earliest phases of RA development and warrant additional research on these cells.
Conclusions and future directions
In this review we build a model for RA pathogenesis for both seropositive and seronegative patients (see figure 1). We focused on data obtained from studies reporting on patients with arthralgia, pre-RA and recently diagnosed RA. Most of the reviewed studies on patients with arthralgia were cross-sectional in design, which leads to information about that specific time point within RA development. Due to the study design it is often unknown who of the patients with arthralgia developed RA and how much time there is between the presented data and the actual RA diagnosis. Therefore most of the obtained results are hard to place on a timeline of RA development. An example is the contradicting data on cellular influx of the synovium. Additionally, due to the limited number of studies on patients with arthralgia, most changes have been reported only once. Confirming these immunological changes is important to determine if these alterations truly occur during RA development and whether they are pathological and a cause rather than consequence.
Future studies should include a large follow-up study with repetitive blood drawings for inflammatory measures, biopsies, as well as pain and bone measurements to fully map changes over time in inflammation and bones. The focus should be on altered cell number or frequency and include changes in cell functionality. This will lead to a better understanding of RA development instead of extending data from mechanistic in vitro studies towards earlier prediagnosis phases. Additionally there is a lack of information regarding patients with ACPA-negative arthralgia and ACPA-negative RA. The limited data so far do indicate differences in cytokines and chemokines, as well as immune cells, besides the presence or absence of autoantibodies between patients with seronegative and seropositive RA. Patients with seronegative arthralgia should therefore be included in follow-up studies as described above and followed until the diagnosis of RA is set.
Furthermore, studies directed to limit or prevent RA development via medication should take notion of differences in cytokines, chemokines and immune cell alterations between patients with arthralgia and patients with recently diagnosed RA. These differences are a starting point to prevent, for example, the rise of cytokines or chemokines, or more ideally revert immune cell function and number to normal. Overall, the current knowledge of how RA develops in patients with seropositive as well as seronegative arthralgia is still slim. Large follow-up studies are needed to provide new insights into RA development.
Footnotes
Contributors: MM performed the literature research, designed the layout and wrote the review. JMWH designed the layout, contributed to the clinical sections and revised the manuscript. EL designed the layout, contributed to immunological sections and revised the manuscript.
Funding: This study was funded by the Dutch Arthritis Foundation (DAF 12-2-409) to EL.
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Gerlag DM, Raza K, van Baarsen LG, et al. . EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the study group for risk factors for rheumatoid arthritis. Ann Rheum Dis 2012;71:638–41. 10.1136/annrheumdis-2011-200990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aletaha D, Neogi T, Silman AJ, et al. . 2010 rheumatoid arthritis classification criteria: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. 10.1136/ard.2010.138461 [DOI] [PubMed] [Google Scholar]
- 3.Deane KD, O’Donnell CI, Hueber W, et al. . The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum 2010;62:3161–72. 10.1002/art.27638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokolove J, Bromberg R, Deane KD, et al. . Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One 2012;7:e35296 10.1371/journal.pone.0035296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokkonen H, Söderström I, Rocklöv J, et al. . Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum 2010;62:83–91. 10.1002/art.27186 [DOI] [PubMed] [Google Scholar]
- 6.Chalan P, Bijzet J, van den Berg A, et al. . Analysis of serum immune markers in seropositive and seronegative rheumatoid arthritis and in high-risk seropositive arthralgia patients. Sci Rep 2016;6:26021 10.1038/srep26021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinho-Ribeiro FA, Verri WA, Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol 2017;38:5–19. 10.1016/j.it.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalan P, Bijzet J, Kroesen BJ, et al. . altered natural killer cell subsets in seropositive arthralgia and early rheumatoid arthritis are associated with autoantibody status. J Rheumatol 2016;43:1008–16. 10.3899/jrheum.150644 [DOI] [PubMed] [Google Scholar]
- 9.Chalan P, Kroesen BJ, van der Geest KS, et al. . Circulating CD4+CD161+ T lymphocytes are increased in seropositive arthralgia patients but decreased in patients with newly diagnosed rheumatoid arthritis. PLoS One 2013;8:e79370 10.1371/journal.pone.0079370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssen KM, Westra J, Chalan P, et al. . Regulatory CD4+ T-Cell Subsets and anti-citrullinated protein antibody repertoire: potential biomarkers for arthritis development in seropositive arthralgia patients? PLoS One 2016;11:e0162101 10.1371/journal.pone.0162101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lübbers J, van Beers-Tas MH, Vosslamber S, et al. . Changes in peripheral blood lymphocyte subsets during arthritis development in arthralgia patients. Arthritis Res Ther 2016;18:205 10.1186/s13075-016-1102-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurachi M, Kurachi J, Suenaga F, et al. . Chemokine receptor CXCR3 facilitates CD8(+) T cell differentiation into short-lived effector cells leading to memory degeneration. J Exp Med 2011;208:1605–20. 10.1084/jem.20102101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielen MM, van Schaardenburg D, Reesink HW, et al. . Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004;50:380–6. 10.1002/art.20018 [DOI] [PubMed] [Google Scholar]
- 14.Pfeifle R, Rothe T, Ipseiz N, et al. . Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat Immunol 2017;18:104–13. 10.1038/ni.3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rombouts Y, Ewing E, van de Stadt LA, et al. . Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis 2015;74:234–41. 10.1136/annrheumdis-2013-203565 [DOI] [PubMed] [Google Scholar]
- 16.Kleyer A, Finzel S, Rech J, et al. . Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis 2014;73:854–60. 10.1136/annrheumdis-2012-202958 [DOI] [PubMed] [Google Scholar]
- 17.Haugeberg G, Green MJ, Quinn MA, et al. . Hand bone loss in early undifferentiated arthritis: evaluating bone mineral density loss before the development of rheumatoid arthritis. Ann Rheum Dis 2006;65:736–40. 10.1136/ard.2005.043869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harre U, Georgess D, Bang H, et al. . Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest 2012;122:1791–802. 10.1172/JCI60975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnamurthy A, Joshua V, Haj Hensvold A, et al. . Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann Rheum Dis 2016;75:721–9. 10.1136/annrheumdis-2015-208093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wigerblad G, Bas DB, Fernades-Cerqueira C, et al. . Autoantibodies to citrullinated proteins induce joint pain independent of inflammation via a chemokine-dependent mechanism. Ann Rheum Dis 2016;75:730–8. 10.1136/annrheumdis-2015-208094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tudhope SJ, von Delwig A, Falconer J, et al. . Profound invariant natural killer T-cell deficiency in inflammatory arthritis. Ann Rheum Dis 2010;69:1873–9. 10.1136/ard.2009.125849 [DOI] [PubMed] [Google Scholar]
- 22.Lo CK, Lam QL, Sun L, et al. . Natural killer cell degeneration exacerbates experimental arthritis in mice via enhanced interleukin-17 production. Arthritis Rheum 2008;58:2700–11. 10.1002/art.23760 [DOI] [PubMed] [Google Scholar]
- 23.Cooper MA, Fehniger TA, Turner SC, et al. . Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 2001;97:3146–51. 10.1182/blood.V97.10.3146 [DOI] [PubMed] [Google Scholar]
- 24.Eggleton P, Wang L, Penhallow J, et al. . Differences in oxidative response of subpopulations of neutrophils from healthy subjects and patients with rheumatoid arthritis. Ann Rheum Dis 1995;54:916–23. 10.1136/ard.54.11.916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, et al. . NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 2013;5:178ra40 10.1126/scitranslmed.3005580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lubberts E. The IL-23-IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol 2015;11:415–29. 10.1038/nrrheum.2015.53 [DOI] [PubMed] [Google Scholar]
- 27.Leipe J, Grunke M, Dechant C, et al. . Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum 2010;62:2876–85. 10.1002/art.27622 [DOI] [PubMed] [Google Scholar]
- 28.Colin EM, Asmawidjaja PS, van Hamburg JP, et al. . 1,25-dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum 2010;62:132–42. 10.1002/art.25043 [DOI] [PubMed] [Google Scholar]
- 29.van Hamburg JP, Asmawidjaja PS, Davelaar N, et al. . Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum 2011;63:73–83. 10.1002/art.30093 [DOI] [PubMed] [Google Scholar]
- 30.van Hamburg JP, Corneth OB, Paulissen SM, et al. . IL-17/Th17 mediated synovial inflammation is IL-22 independent. Ann Rheum Dis 2013;72:1700–7. 10.1136/annrheumdis-2012-202373 [DOI] [PubMed] [Google Scholar]
- 31.Ramwadhdoebe TH, Hähnlein J, Maijer KI, et al. . Lymph node biopsy analysis reveals an altered immunoregulatory balance already during the at-risk phase of autoantibody positive rheumatoid arthritis. Eur J Immunol 2016;46:2812–21. 10.1002/eji.201646393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulissen SM, van Hamburg JP, Davelaar N, et al. . CCR6(+) Th cell populations distinguish ACPA positive from ACPA negative rheumatoid arthritis. Arthritis Res Ther 2015;17:344 10.1186/s13075-015-0800-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura I, Kadono Y, Takayanagi H, et al. . IL-1 regulates cytoskeletal organization in osteoclasts via TNF receptor-associated factor 6/c-Src complex. J Immunol 2002;168:5103–9. 10.4049/jimmunol.168.10.5103 [DOI] [PubMed] [Google Scholar]
- 34.Hoff M, Haugeberg G, Odegård S, et al. . Cortical hand bone loss after 1 year in early rheumatoid arthritis predicts radiographic hand joint damage at 5-year and 10-year follow-up. Ann Rheum Dis 2009;68:324–9. 10.1136/ard.2007.085985 [DOI] [PubMed] [Google Scholar]
- 35.Forslind K, Kälvesten J, Hafström I, et al. . Does digital X-ray radiogrammetry have a role in identifying patients at increased risk for joint destruction in early rheumatoid arthritis? Arthritis Res Ther 2012;14:R219 10.1186/ar4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Black RJ, Spargo L, Schultz C, et al. . Decline in hand bone mineral density indicates increased risk of erosive change in early rheumatoid arthritis. Arthritis Care Res 2014;66:515–22. 10.1002/acr.22199 [DOI] [PubMed] [Google Scholar]
- 37.Güler-Yüksel M, Klarenbeek NB, Goekoop-Ruiterman YP, et al. . Accelerated hand bone mineral density loss is associated with progressive joint damage in hands and feet in recent-onset rheumatoid arthritis. Arthritis Res Ther 2010;12:R96 10.1186/ar3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodríguez-Pinto D. B cells as antigen presenting cells. Cell Immunol 2005;238:67–75. 10.1016/j.cellimm.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 39.de Hair MJ, van de Sande MG, Ramwadhdoebe TH, et al. . Features of the synovium of individuals at risk of developing rheumatoid arthritis: implications for understanding preclinical rheumatoid arthritis. Arthritis Rheumatol 2014;66:513–22. 10.1002/art.38273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraan MC, Haringman JJ, Post WJ, et al. . Immunohistological analysis of synovial tissue for differential diagnosis in early arthritis. Rheumatology 1999;38:1074–80. 10.1093/rheumatology/38.11.1074 [DOI] [PubMed] [Google Scholar]
- 41.Hitchon CA, el-Gabalawy HS. The histopathology of early synovitis. Clin Exp Rheumatol 2003;21(5 Suppl 31):S28–36. [PubMed] [Google Scholar]
- 42.Zvaifler NJ, Boyle D, Firestein GS. Early synovitis – synoviocytes and mononuclear cells. Semin Arthritis Rheum 1994;23(6 Suppl 2):11–16. 10.1016/0049-0172(94)90080-9 [DOI] [PubMed] [Google Scholar]
- 43.Crowley T, O’Neil JD, Adams H, et al. . Priming in response to pro-inflammatory cytokines is a feature of adult synovial but not dermal fibroblasts. Arthritis Res Ther 2017;19:35 10.1186/s13075-017-1248-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raza K, Saber TP, Kvien TK, et al. . Timing the therapeutic window of opportunity in early rheumatoid arthritis: proposal for definitions of disease duration in clinical trials. Ann Rheum Dis 2012;71:1921–3. 10.1136/annrheumdis-2012-201893 [DOI] [PubMed] [Google Scholar]
- 45.Relic B, Guicheux J, Mezin F, et al. . Il-4 and IL-13, but not IL-10, protect human synoviocytes from apoptosis. J Immunol 2001;166:2775–82. 10.4049/jimmunol.166.4.2775 [DOI] [PubMed] [Google Scholar]
- 46.Kotake S, Nanke Y, Yago T, et al. . Elevated ratio of Th17 Cell-Derived Th1 Cells (CD161(+)Th1 Cells) to CD161(+)Th17 cells in peripheral blood of early-onset rheumatoid arthritis patients. Biomed Res Int 2016;2016:1–5. 10.1155/2016/4186027 [DOI] [PMC free article] [PubMed] [Google Scholar]