Abstract
Objective
Occupational exposure to indium compounds including indium-tin oxide (ITO) can result in potentially fatal indium lung disease. We compared plasma, serum and whole blood indium concentrations (InP, InS and InB) from workers at a single ITO production facility to assess the comparability of these matrices used for biological monitoring of indium exposure.
Method
InP, InS and InB were measured using inductively coupled mass spectrometry from consenting workers at an ITO production facility with specimen collection occurring during June–July 2014. Matched pairs from workers were assessed to determine the matrix relationships using the Pearson correlation, paired t-tests, per cent difference, linear regression and κ statistics.
Results
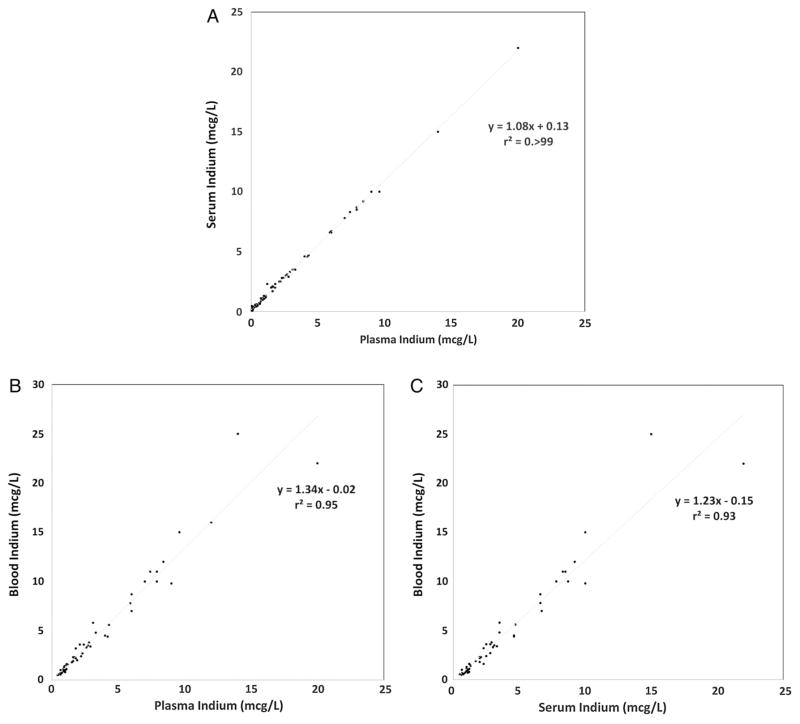
Indium matrices were collected from 80 workers. Mean (SD) InP, InS and InB were 3.48 (3.84), 3.90 (4.15) and 4.66 (5.32) mcg/L, respectively. The InS–InP difference was 14%; InS was higher in all but two workers. InP and InS were highly correlated (r=>0.99). The InB–InS difference was 19%; InB was higher in 85% of workers. The InB–InP difference was 34%; InB was higher in 66% of workers. InB was highly correlated with both InP and InS (r=0.97 and 0.96, respectively). κ Statistics were 0.84, 0.83 and 0.82 for InP, InS and InB, respectively, for individuals with each matrix ≥1 mcg/L (p<0.01).
Conclusions
While all matrices were highly correlated, we encourage the use of InP and InS to reliably compare studies across different populations using different matrices. The higher per cent difference and increased variability of InB may limit its utility in comparisons with InP and InS in different populations.
Occupational exposure to indium compounds including indium-tin oxide (ITO) can result in potentially fatal indium lung disease. The spectrum of disease includes pulmonary alveolar proteinosis that may progress to fibrosis with or without emphysema.1 Biological monitoring of indium using plasma, serum or blood has served as the foundation to assess exposure and prevent adverse health effects in workers exposed to ITO and other indium compounds. However, as there are no reference values for indium in these matrices, the extent to which the selected matrix affects the measurements is unknown.
Exposure to indium is rare and likely occupational in nature when it occurs. However, knowledge of the absorption, distribution and elimination of indium in the human body is limited. The fate of inhaled indium in the body is likely influenced by the chemical form and duration of exposure.2 Ionic indium that is absorbed into the blood may bind to blood proteins. In one study, the estimated biological half-life of indium in serum was 8.1 years for workers who had quit handling indium for >3 years, but the half-life varied and was longer for those with a higher initial indium burden.3 Another study has shown that former workers had similar levels of indium in the serum as current workers an average of nearly 5 years since exposure to indium, suggesting indium in the blood represents cumulative exposure with an extended half-life.4
To date, epidemiological studies have measured indium concentrations in plasma (InP) or serum (InS) to evaluate worker exposure to indium. Results of these studies have been used to establish recommendations and for regulation. In the USA, adverse health outcomes were associated with ≥1 mcg/L InP.5 In Japan, a biological exposure limit of 3 mcg/L InS has been established.6 Other countries, including Germany and Korea, are also considering developing standards. Whole blood indium (InB) has been used in the clinical setting to monitor indium exposure. Yet the equivalence of these different matrices has not been evaluated. To assess comparability of these matrices, we analysed InP, InS and InB from workers at a single ITO production facility measured during a narrow timeframe.
METHODS
As part of a longitudinal research study approved by the Institutional Review Board of the National Institute for Occupational Safety and Health (NIOSH), InP and InS were collected from consenting workers at an ITO production facility during June–July 2014. Separately, InB was measured during June–July 2014 as part of the company’s medical surveillance programme, and results were provided to NIOSH with study participants’ permission.
All measurements were performed by one commercial laboratory using inductively coupled plasma mass spectrometry. For InP and InS, the indium analytical method had a limit of detection (LOD) of 0.03 mcg/L and a limit of quantification (LOQ) of 0.1 mcg/L. InP and InS values between the LOD and LOQ were reported by the laboratory. For InB, the LOD was not available and the LOQ was 0.5 mcg/L. Values below the LOQ were not reported by the laboratory.
We compared matched pairs from workers for each matrix. For comparison to InB, we selected only those who had InB levels greater than its LOQ. We analysed the subset of workers with each matrix collected in order to assess all three substrates together. We measured matrix agreement for identifying individuals with InP, InS and InB ≥1 mcg/L. We stratified the mean differences of InB–InP and InB–InS by ≥6 days and <6 days between specimen collection times. Matrix relationships were evaluated using the Pearson correlation, paired t-tests, per cent difference, linear regression and κ statistics using JMP software V.10.0.1 (SAS Institute, Cary, North Carolina, USA). p Values reported are two sided. We considered p≤0.05 to be significant.
RESULTS
Indium matrices were collected from 80 workers at the ITO production facility during June–July 2014. Of these workers, 86% were men. Sixty-six per cent were white, 8% were black and 10% were Asian; 16% were Hispanic. Median age was 45 years (range: 19–66 years). Median tenure at the ITO facility was 3.4 years (range: 0.1–38 years). Our analyses included 76 workers (95%) with InP and InS measured, 53 (66%) with InP and InB measured and 50 (63%) with InS and InB measured. After removing those without each matrix measured (n=12) and those with InB below an LOQ of 0.5 mcg/L (n=18), 50 workers were included in the analyses comparing InP, InS and InB. For those individuals with each matrix measured, whole blood was collected at a median of 5.6 days before simultaneous plasma and serum collection, with whole blood collection ranging from 20 days before to 34 days after plasma and serum collection.
Mean (SD) InP, InS and InB were 3.48 (3.84), 3.90 (4.15) and 4.66 (5.32) mcg/L, respectively, for the 50 workers with each exposure matrix collected. The InS–InP difference was 14% on average, with individual matched pairs ranging from −0.02 to 2 mcg/L difference; InS was higher than InP in all but two workers. InP and InS were highly correlated (r=>0.99) (figure 1A). InB was more variable when compared with both InP and InS. The InB–InS difference was 19% on average (range: −0.70 to 10 mcg/L difference); InB was higher in 85% of workers. The InB–InP difference was 34% on average (range: −0.21 to 11 mcg/L difference); InB was higher in 66% of workers. InB was highly correlated with both InP and InS (r=0.97 and 0.96, respectively) (figure 1B, C). κ Statistics were 0.84, 0.83 and 0.82 for InP and InS, InP and InB and InS and InB, respectively, for individuals with each matrix ≥1 mcg/L (p<0.01). The InB–InP and InB–InS mean differences were not significantly different in workers with specimen collection occurring ≥6 days and <6 days apart (0.84 and 1.47 mcg/L (p=0.22); 0.33 and 1.10 mcg/L (p=0.11), respectively).
Figure 1.
Blood matrices scatterplots with linear regression lines: (A) InP and InS (N=76), (B) InP and InB (N=53) and (C) InS and InB (N=50). InB, blood indium; InP, plasma indium; InS, serum indium.
DISCUSSION
Our study comparing indium in plasma, serum and blood of workers from a single facility during a narrow timeframe is the first to the best of our knowledge characterising relationships among these three matrices. While InP, InS and InB were highly correlated, we found meaningful differences that should be considered before comparing indium exposures estimated using different matrices.
On average, InP and InS had the smallest relative difference of 14%. Seventy-four per cent (97%) of the 76 workers had higher InS than InP, suggesting InP and InS could be reliably used when comparing indium exposures. Owing to the higher per cent difference and variability, InB may not be as useful for comparisons to either InP or InS in different workforces using the different matrices. InB was 34% higher than InP on average; however, in more than one-third (34%) of workers InB was lower than InP, InB shared a similar relationship with InS, with InB being 19% higher on average, but lower in 15% of workers. This is consistent with a 2003 study by Miyaki et al7 comparing biological monitoring of indium using blood, serum and urine in which mean InB was higher than InS. Agreement statistics between each matrix were high. Individuals with InP ≥1 mcg/L, a value associated with adverse health outcomes,5 generally had InS and InB ≥1 mcg/L, suggesting each matrix could be used to detect overexposure to indium.
There are several plausible explanations for indium measuring slightly higher in serum than plasma. InP was measured using trace metal-free specimen collection tubes. It is possible that the higher mean InS we observed was due to positive interference during analysis by elements contained in serum collection tubes. Specimen collection and storage can interfere with trace metal measurement.8 An effect of sample concentration may also account for the higher indium measured in serum than plasma. Fibrinogen is the principal component present in plasma but removed from serum during coagulation.9 As it lacks fibrinogen, serum is more concentrated than plasma, making it possible that InS has a higher mean due to the smaller volume fraction in serum than in plasma.
The higher mean and increased variability of InB compared with both InP and InS are likely multifactorial. In addition to the potential issue of elements contained in the collection tubes described above, these differences could involve interaction between indium compounds and the cellular compartment of blood consisting predominantly of red blood cells. However, the higher mean InB is consistent with a recent study in which 9 (82%) of the 11 metals and trace elements were more concentrated in the whole blood than in the serum.10 Other factors that could contribute to variability include varying sampling times, and that different instruments with different sensitivities were used. We believe these factors would not substantially affect our results. Most samples were collected during the same week and indium levels in these matrices are indicative of long-term exposure and unlikely to fluctuate during our study window.11 When we compared the InB–InP and InB–InS mean differences in workers with sample collection occurring ≥6 days and <6 days apart, we found no significant differences, providing further evidence that sample collection time had limited influence on the results. Moreover, by excluding InB values below the LOQ in our analyses, the different sensitivities of the instruments unlikely contributed much to variability. Nonetheless, the possibility remains that some of the observed differences reflect when and how blood samples were collected and analysed. Ultimately, further investigation into the absorption, distribution and elimination of inhaled indium compounds may provide a clearer explanation for these differences.
The small sample size precluded the development of correction factors that could be used to adjust concentrations and allow accurate comparisons across populations. However, a factor to account for the consistently higher fraction of indium identified in the serum compared with plasma may be possible with additional data from different workplaces. Likewise, a larger sample size from different workplaces is needed to develop a reliable correction factor for whole blood compared with both plasma and serum, particularly given the variability of indium measured in whole blood compared with the other matrices in our study. While environmental health studies increasingly are quantifying certain metals using various matrices in different populations,10,12 it is unlikely that reference values will be established for indium as it is not an essential trace element.
InP, InS and InB have been used clinically and/or for epidemiological studies to assess exposure to indium. We measured these matrices that are currently used for biological monitoring in a single workplace during a narrow timeframe. While all three matrices were highly correlated, our study suggests InP and InS can be used to reliably compare studies across different populations. While InB may have value in clinical settings to serially monitor indium exposure of an individual, the higher per cent difference and increased variability measured in this ITO production facility workforce imply limited utility in comparisons with InP and InS in different populations using these different matrices.
What this paper adds.
Indium in plasma, serum and whole blood has been used for biological monitoring of indium exposure in epidemiological studies and/or clinical settings. As there are no reference values for indium in these matrices, the extent to which the selected matrix affects the measurements is unknown.
While InP, InS and InB were highly correlated, we conclude InP and InS are suitable to reliably compare studies across different populations. Owing to the higher per cent difference and increased variability of InB relative to InP and InS in this population, we would urge caution in comparing InB to InP or InS.
As countries have either already established or are considering standards to limit exposure to indium in occupational settings, our paper helps determine the relationship of different blood matrices used for biological monitoring of indium exposure.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of NIOSH. The authors acknowledge the members of the NIOSH field teams for their contributions to data acquisition and Drs David Blackley and Bill Chisholm of NIOSH for their valuable comments on the manuscript.
Funding This work was supported by the intramural National Occupational Research Agenda (NORA) funding from the National Institute for Occupational Safety and Health (NIOSH).
Footnotes
Competing interests None declared.
Patient consent Obtained.
Ethics approval NIOSH HSRB.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Cummings KJ, Nakano M, Omae K, et al. Indium lung disease. Chest. 2012;141:1512–21. doi: 10.1378/chest.11-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim CH, Han JH, Cho HW, et al. Studies on the toxicity and distribution of indium compounds according to particle size in Sprague-Dawley rats. Toxicol Res. 2014;30:55–63. doi: 10.5487/TR.2014.30.1.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amata A, Chonan T, Omae K, et al. High levels of indium exposure relate to progressive emphysematous changes: a 9-year longitudinal surveillance of indium workers. Thorax. 2015;70:1040–6. doi: 10.1136/thoraxjnl-2014-206380. [DOI] [PubMed] [Google Scholar]
- 4.Nakano M, Omae K, Tanaka A, et al. Causal relationship between indium compound inhalation and effects on the lungs. J Occup Health. 2009;51:513–21. doi: 10.1539/joh.l9077. [DOI] [PubMed] [Google Scholar]
- 5.Cummings KJ, Virji MA, Trapnell BC, et al. Early changes in clinical, functional, and laboratory biomarkers in workers at risk of indium lung disease. Ann Am Thorac Soc. 2014;11:1395–403. doi: 10.1513/AnnalsATS.201407-346OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Japan Society for Occupational H. Recommendation of occupational exposure limits (2007–2008) J Occup Health. 2007;49:328–44. [PubMed] [Google Scholar]
- 7.Miyaki K, Hosoda K, Hirata M, et al. Biological monitoring of indium by means of graphite furnace atomic absorption spectrophotometry in workers exposed to particles of indium compounds. J Occup Health. 2003;45:228–30. doi: 10.1539/joh.45.228. [DOI] [PubMed] [Google Scholar]
- 8.Moyer TP, Mussmann GV, Nixon DE. Blood-collection device for trace and ultra-trace metal specimens evaluated. Clin Chem. 1991;37:709–14. [PubMed] [Google Scholar]
- 9.Yu Z, Kastenmüller G, He Y, et al. Differences between human plasma and serum metabolite profiles. PLoS ONE. 2011;6:e21230. doi: 10.1371/journal.pone.0021230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultze B, Lind PM, Larsson A, et al. Whole blood and serum concentrations of metals in a Swedish population-based sample. Scand J Clin Laboratory Invest. 2014;74:143–8. doi: 10.3109/00365513.2013.864785. [DOI] [PubMed] [Google Scholar]
- 11.Hoet P, De Graef E, Swennen B, et al. Occupational exposure to indium: what does biomonitoring tell us? Toxicol Lett. 2012;213:122–8. doi: 10.1016/j.toxlet.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Kucera J, Bencko V, Sabbioni E, et al. Review of trace elements in blood, serum and urine for the Czech and Slovak populations and critical evaluation of their possible use as reference values. Sci Total Environ. 1995;166:211–34. doi: 10.1016/0048-9697(95)04425-z. [DOI] [PubMed] [Google Scholar]