Abstract
Human apolipoprotein A-I (apoA-I) mimetic L-4F inhibits acute inflammation in endotoxemic animals. Since neutrophils play a crucial role in septic inflammation, we examined the effects of L-4F, compared to apoA-I, on lipopolysaccharide (LPS)-mediated activation of human neutrophils. We performed bioassays in human blood, isolated human neutrophils (incubated in 50 % donor plasma), and isolated human leukocytes (incubated in 5 and 50 % plasma) in vitro. In whole blood, both L-4F and apoA-I inhibited LPS-mediated elevation of TNF-α and IL-6. In LPS-stimulated neutrophils, L-4F and apoA-I (40 μg/ml) also decreased myeloperoxidase and TNF-α levels; however, L-4F tended to be superior in inhibiting LPS-mediated increase in IL-6 levels, membrane lipid rafts abundance and CD11b expression. In parallel experiments, when TNF-α and IL-8, instead of LPS, was used for cell stimulation, L-4F and/or apoA-I revealed only limited efficacy. In LPS-stimulated leukocytes, L-4F was as effective as apoA-I in reducing superoxide formation in 50 % donor plasma, and more effective in 5 % donor plasma (P<0.05). Limulus ambocyte lysate (LAL) and surface plasmon resonance assays showed that L-4F neutralizes LAL endotoxin activity more effectively than apoA-I (P<0.05) likely due to avid binding to LPS. We conclude that (1) direct binding/neutralization of LPS is a major mechanism of L-4F in vitro; (2) while L-4F has similar efficacy to apoA-I in anti-endotoxin effects in whole blood, it demonstrates superior efficacy to apoA-I in aqueous solutions and fluids with limited plasma components. This study rationalizes the utility of L-4F in the treatment of inflammation that is mediated by endotoxin-activated neutrophils.
Keywords: L-4F peptide, human neutrophil, apolipoprotein A-I, high-density lipoprotein, lipopolysaccharide, inflammation, apoA-I mimetic
INTRODUCTION
Neutrophils represent the most abundant pool of leukocytes in human blood and play a crucial role in inflammation [1, 2]. Neutrophils are able to degrade internalized pathogenic bacteria due to their large stores of proteolytic enzymes and rapid production of reactive oxygen species [1, 2]. Neutrophils-released superoxides and myeloperoxidase (MPO) lead to formation of secondary oxidants with potent cytotoxic activity [3]. In case of severe neutrophil activation, tissue-infiltrating neutrophils release these lytic factors or pro-inflammatory cytokines causing organ tissue damage and failure [2, 3]. Gram-negative bacteria are major pathogens responsible for neutrophil activation in human inflammatory conditions including sepsis [4]. During entire life span, humans are persistently exposed to endotoxin or lipopolysaccharide (LPS), which is a toxic component of the outer membrane of Gram-negative bacteria. Toll-like receptor (TLR4), major cellular target for LPS in humans, has been shown to play a role in the pathogenesis of inflammation [4, 5]. In neutrophils, the stimulation of TLR4 by LPS activates CD11b/CD18 expression [6]. This is associated with neutrophil activation and adhesion to target cells [2].
Apolipoprotein A-I (apoA-I), the main protein constituent of high-density lipoproteins (HDL), has strong anti-inflammatory and anti-oxidant activity [7, 8]. Earlier (in 1991), our laboratory showed that human apoA-I and certain amphipathic helical peptides inhibit neutrophil adhesion, activation, degranulation, and superoxide production, which was further supported by others [9–11]. The effect of apoA-I on neutrophil activation appears to be mediated via the ATP-binding cassette transporter ABCA1 (membrane protein involved in apoA-I mediated cholesterol efflux) and a reduction in the abundance of lipid rafts and CD11b expression [11]. The infusion of human apoA-I or recombinant HDL resulted in beneficial effects in animal models of inflammation [12–14] or in clinical studies [15, 16]. In experiments that utilized LPS to induce inflammation, HDL/apoA-I have been shown to neutralize effects of LPS in vivo and in vitro by mechanisms that are at least in part mediated by direct binding of HDL/apoA-I to LPS [12, 15, 17]. Since apoA-I is a physiologically relevant molecule with anti-inflammatory properties, apoA-I infusion represents a natural choice in the development of HDL replacement therapy [18]. However, obtaining reconstituted HDL/apoA-I in therapeutic quantities is expensive and therefore impractical at present.
ApoA-I mimetic peptides with chain length as small as 18 amino acids have been developed as a feasible approach for plasma HDL replacement therapy [19, 20]. 4F, an apo A-I mimetic peptide with the sequence Ac-DWFKAFYDKVAEKFKEAF-NH2, mimics the helical repeating domains of apoA-I and exerts strong anti-inflammatory properties [19–21]. In animal models of acute inflammation, L-4F reduces inflammatory response and improves survival [22–25]. L-4F also inhibits activation of human neutrophils stimulated by septic patient serum [24]. Importantly, our previous experiments strongly suggest that L-4F binds and neutralizes LPS [21, 24]. Therefore, we hypothesized that L-4F may have effects on LPS-mediated neutrophil activation, analogous to the effects of human apoA-I. To test this hypothesis, we performed in vitro experiments in isolated human neutrophils, in isolated human leukocytes, and in human blood. In addition, we sought to measure L-4F and human apoA-I binding characteristics to LPS and compare their potential to neutralize endotoxin activity. We found that L-4F neutralizes LAL endotoxin activity more effectively than apoA-I and binds to LPS more avidly. We also found that L-4F and apoA-I likewise inhibit LPS-mediated inflammatory response in human blood and isolated neutrophils, incubated with high levels of plasma components. However, anti-endotoxin effects of L-4F appear to be superior to apoA-I in aqueous solutions and fluids with limited plasma components. Based on results of parallel experiments when human TNF-α and IL-8 were used for cell stimulation, we suggest that direct binding/neutralization of LPS appears to be an important mechanism of L-4F effects in our LPS experiments. This study rationalizes the utility of L-4F in the treatment of inflammation that is mediated by endotoxin-activated neutrophils.
METHODS
LPS, apoA-I Mimetic Peptide Synthesis and Isolation of Human apoA-I
L-4F, with the sequence Ac-DWFKAFYDKVAEKFKEAF-NH2, was synthesized by the solid phase peptide synthesis method as previously described [26]. Human apoA-I was isolated and purified using the methods described previously [27]. LPS (Escherichia coli 026:B6) was obtained from Sigma-Aldrich (Saint Louis, MO).
Justification of Reagent Concentrations Used in the Experiments
In initial experiments using whole human blood (Fig. S1), we tested a dose-dependent effect of LPS from E. coli 026:B6 (Sigma) to induce a robust inflammatory response as assessed by plasma elevation of IL-6. We found that LPS at 1 μg/ml produced such effect (P<0.05 vs. control). This LPS dose was previously used by us in experiments studying effects of L-4F in human cells in vitro [21, 28] or effects of human apoA-I in isolated human neutrophils [11]. In similar experiments (Fig. S2), we tested a dose-dependent effect of L-4F to inhibit LPS-induced inflammatory response. While L-4F at 50 μg/ml (concentration routinely used in cell culture experiments [21, 28, 29]) produced strong inhibition (by 30 %, P< 0.05 vs. LPS), less dose at 10 μg/ml had only marginal inhibition (by 10 %, nonsignificant vs. LPS). However, because both, human apoA-I [11] and L-4F [24], are effective at 40 μg/ml, we chose this concentration to competitively study the effects of these agents in the present work.
Experiments Using Human Blood Samples
All human studies were approved by the University of Alabama at Birmingham Institutional Review Board. All subjects provided written informed consent. Blood samples from healthy volunteers were collected by venipuncture into heparinized Vacutainer™ tubes (Becton Dickinson).
In Vitro Experiments with Human Blood
To 0.5 ml of blood from a healthy volunteer either: (1) saline; (2) L-4F (40 μg/ml); (3) apoA-I (40 μg/ml); (4) LPS (1 μg/ml); (5) LPS (1 μg/ml) and L-4F (40 μg/ml); or (6) LPS (1 μg/ml) and apoA-I (40 μg/ml) were added and incubated for 3 h at 37 °C in 5 %CO2. The blood was then centrifuged at 4 °C for 20 min at 300×g. Collected plasma aliquots were analyzed for TNF-α and IL-6 levels using commercially obtained ELISA kit (BD Biosciences) specific for humans. In a separate similar series of experiments, instead of LPS, we used 10 ng/ml human TNF-α (eBioscience) to stimulate whole human blood for 6 h at 37 °C in 5 %CO2. We then measured plasma levels of IL-6 induced by TNF-α in the absence and presence of L-4F or apoA-I.
In Vitro Experiments with Isolated Neutrophils
Neutrophils were isolated from peripheral blood of healthy volunteers as previously described [30] by separation on a Ficoll-Histopaque gradient (Sigma-Aldrich). The final suspension of neutrophils contained more than 96 % of viable cells as evaluated by trypan blue exclusion. The prewarmed neutrophil suspensions (at 37 °C, 3.5×106 cells/ml) were incubated in healthy donor plasma [50 % plasma in phosphate buffered saline (PBS)] with 1 μg/ml LPS in the absence or presence of L-4F (40 μg/ml) or human apoA-I (40 μg/ml) for 30 min (n=5), 1 h (n=4), and 3 h (n=4) at 37 °C in 5 % CO2. Following incubation, neutrophils were isolated by gentle centrifugation and stained for measuring CD11b and lipid raft expression as described below, and cell-free supernatant was analyzed for TNF-α, IL-6, and MPO levels. In a similar separate experiment, we used human IL-8 (R&D Systems) to stimulate neutrophils for 1 h, instead of LPS.
Measurement of TNF-α, IL-6, and MPO Levels in Cell-Free Supernatant
The concentration of TNF-α and IL-6 were quantified by using commercially obtained enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences) specific for humans. A concentration of specific active human MPO was quantified by using the Calbiochem® InnoZyme™ myeloperoxidase activity kit purchased from EMD Millipore (Billerica, MA).
Flow Cytometry for CD11b and Lipid Rafts Detection
Following incubation with LPS, neutrophils were washed with PBS and incubated with FACS buffer [PBS containing 0.25 % bovine serum albumin (BSA), and 2.1 mM EDTA] for 15 min at room temperature. Neutrophils were again washed and incubated in FACS buffer with 1 μg/ml FITC-cholera toxin B (Sigma) for 30 min at room temperature. Mouse anti-human CD11b (activation epitope) allophycocyanin (APC; eBioscience) was added to samples and neutrophils were further incubated for 30 min at 4 °C. After washing with PBS, the cells were fixed in 2 % paraformaldehyde. In control experiments, cells were treated in the presence and absence of mouse IgG1 K Isotype Control (APC, eBioscience). CD11b and lipid raft expressions were measured by flow cytometry using BD LRSII (Becton Dickinson). Analysis was performed using FlowJo software (version 7.6.5, Tree Star, Ashland, OR). Results were normalized to un-stimulated saline-treated control and expressed as a percentage change compared to control.
In Vitro Measurement of Oxidative Stress Using Lucigenin-Amplified Chemiluminescence
Fresh human leukocytes were collected from the blood of healthy volunteers by aspiration of a buffy coat. Any remaining red blood cells in buffy coat were lysed and removed from the leukocytes by centrifugation and washing using PBS. Following isolation, leukocytes (1–2× 106 cell/ml) were incubated in healthy donor plasma (5 and 50 % plasma in PBS, in parallel) with 1 μg/ml LPS in the absence and presence of 40 μg/ml L-4F or 40 μg/ml apoA-I (n=3). Lucigenin (10 μM) was added 2 min before adding L-4F or apoA-I and onset of measurements. Lucigenin-amplified chemiluminescence was measured at 37 °C for 3 h as a marker of superoxide formation [24, 31]. Area under curves (AUC) of relative photon emission (RPE) over time were calculated and compared.
Measurements of L-4F and apoA-I Interaction with LPS in Aqueous Solution
Effects on Endotoxin Activity
Endotoxin activity of LPS in aqueous solutions (saline) in the absence and presence of indicated concentrations of L-4F or apoA-I was measured by limulus amoebocyte lysate (LAL) assay using kinetic–chromogenic test (Endochrome-K, Charles River) in accordance with manufacturer protocol (n=3).
Measurements of Binding Kinetics Using SPR
Binding of LPS to L-4F and human apoA-I were assessed using a Biacore T200 system (BiaCore, Piscataway, NJ) as previously described [32]. Briefly, L-4F and apoA-I were immobilized on two different surfaces of a CM5 sensor chip activated per the manufacturer’s protocol, whereas another chip surface was left blank to serve as a reference surface. 30 μl of LPS, diluted in HEPES buffered saline, flowed over the CM5 chip at a rate of 20 μl/min at a concentration of 0, 0.05, 0.1, 0.2, and 0.4 mg/ml (in duplicates). The interaction was monitored as the changes of surface plasmon resonance (SPR) response for 2.5 min at 25 °C. The same buffer was then introduced onto the sensor chip without LPS to start the dissociation. Surface regeneration was done by washing the chip with 10 mM glycine, pH 2.5. Bovine serum albumin (BSA, 0.1 mg/ml) was used as a negative control. The dissociation constant (Kd) was determined as kd/ka by Biacore T200 Evaluation Software version 1.0 based on 1:1 binding kinetics and LPS molecular weight of 1,000 kDa.
Statistical Analysis
All results, unless otherwise specified, are reported as the mean±SEM. Statistical analysis was performed using GraphPad Prism V.4.0.1 (GraphPad Software). Differences between groups were assessed by one-way or two-way ANOVA followed by Bonferroni’s post hoc testing. A value of P<0.05 was considered to be statistically significant.
RESULTS
Effects of L-4F and apoA-I on LPS-Induced TNF-α and IL-6 Production in Human Blood
We first compared the effects of L-4F and apoA-I on LPS-stimulated inflammatory response in freshly isolated peripheral whole human blood. We incubated 1 μg/ml LPS in human blood in vitro for 3 h with or without 40 μg/ml L-4F or apoA-I (n=4). LPS alone causes elevation of TNF-α and IL-6 levels in plasma (P<0.001 vs. controls, Fig. 1). Both L-4F and apoA-I inhibits inflammatory response to LPS in blood to an equal extent (Fig. 1).
Fig. 1.
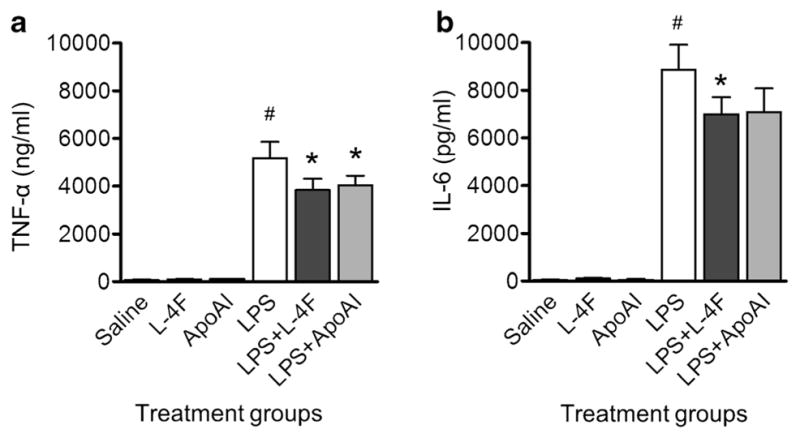
Effects of L-4F and apoA-I on LPS-mediated production of inflammatory cytokines in whole human blood. L-4F or apoA-I (40 μg/ml) reduces plasma levels of TNF-α (A) and IL-6 (B) in the healthy whole human blood incubated with LPS (1 μg/ml) for 3 h in vitro (n=4/group). *P<0.05 vs. saline-treated LPS, #P<0.05 vs. control groups.
Effects of L-4F and apoA-I on TNF-α-Induced IL-6 Production in Human Blood
It has been shown that major toxic effects of LPS are mediated through TNF-α signaling [33, 34]. Therefore, we performed the experiments to assess the effects of L-4F and apoA-I on TNF-α signaling. Whole human blood was stimulated with 10 ng/ml human TNF-α for 6 h (n=4). Both, L-4F and apoA-I had only minimal effects on TNF-α-mediated elevation of plasma IL-6 (Fig. 2). These results indicate that the effects of L-4F and apoA-I on inhibition of LPS-mediated effects are not via the TNF-α signaling as a major mechanism.
Fig. 2.
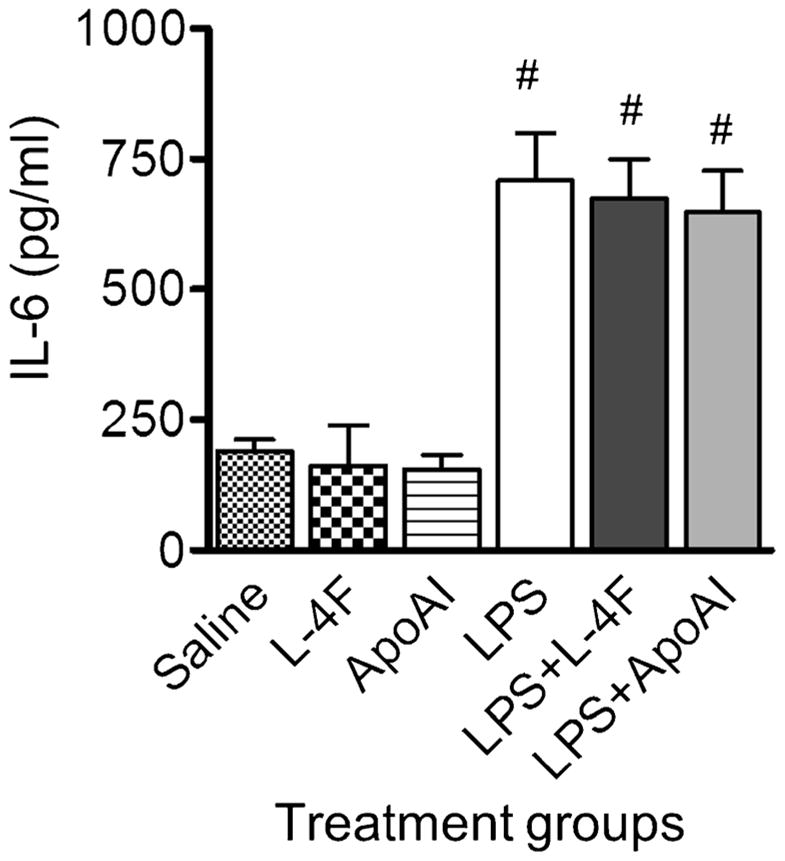
Effects of L-4F and apoA-I on TNF-α-mediated production of IL-6 in whole human blood. L-4F or apoA-I (40 μg/ml) does not reduce plasma levels of IL-6 in the healthy whole human blood incubated with T-NF-α (10 ng/ml) for 6 h in vitro (n=4/group). #P<0.05 vs. control groups.
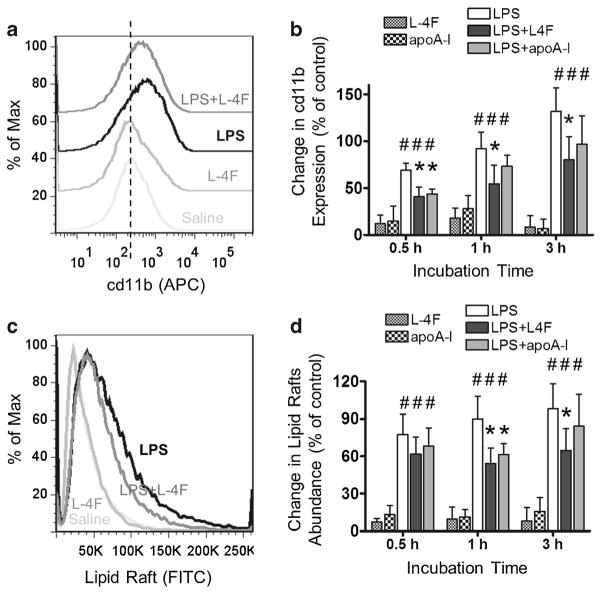
Effects of L-4F and apoA-I on CD11b Expression and Lipid Raft Abundance in LPS-Stimulated Human Neutrophils
Freshly isolated human neutrophils were incubated in 50 % donor plasma with LPS (1 μg/ml) in the presence and absence of L-4F (40 μg/ml) or apoA-I (40 μg/ml) for 30 min, 1 h, and 3 h. Neutrophils were then stained with anti-human CD11b (activation epitope) allophycocyanin (APC) and with cholera toxin B subunit FITC. Changes in CD11b expression and lipid raft abundance were measured by flow cytometry (Fig. 3). LPS significantly increases CD11b expression in neutrophils (P<0.05 vs. control at all time points). Peptide L-4F and apoA-I inhibits this effect (P<0.05 vs. LPS, for L-4F at all time points and for apoA-I at 30 min, Fig. 3A, B). LPS also significantly increases lipid raft abundance in neutrophils (P<0.05 vs. control at all time points), and L-4F or apoA-I inhibits this effect (P<0.05 vs. LPS, for L-4F at 1 and 3 h and for apoA-I at 1 h, Fig. 3C, D). Neither L-4F alone nor apoA-I alone affects CD11b expression and lipid raft abundance in neutrophils in our experiments (NS vs. saline control).
Fig. 3.
L-4F and apoA-I inhibit LPS-mediated increase in CD11b expression and lipid raft abundance in isolated human neutrophils. Isolated human neutrophils were incubated in donor plasma with saline, L-4F or apoA-I (40 μg/ml), LPS (1 μg/ml), and LPS plus L-4F or apoA-I for 30 min (n=5), 1 h (n=4), and 3 h (n=4). Following incubation, neutrophils were stained with anti-human CD11b (activation epitope) allophycocyanin (APC) and with cholera toxin B subunit FITC. Changes in CD11b expression and lipid raft abundance were measured by flow cytometry. A A representative histogram of relative changes of surface expression of CD11b for different treatments following 1 h incubation. Vertical dashed line represents median CD11b expression in control. B Statistical data presented as percent relative changes of surface expression of CD11b (mean fluorescent intensity, MFI) relative to the saline control. C A representative histogram of relative changes of lipid rafts abundance. D Statistical data presented as percent changes in lipid raft abundance (MFI) relative to the saline control. *P<0.05 vs. saline-treated LPS, #P<0.05 vs. control groups.
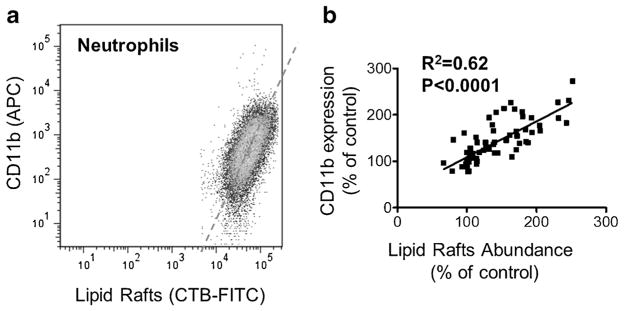
There is a correlation between CD11b expression and lipid raft abundance in human neutrophils. In Fig. 4A, CD11b expression is plotted against lipid raft abundance during flow cytometry analysis for all treatment groups, with distribution pattern of neutrophils specific for linear relation. A correlation analysis between CD11b expression (APC mean fluorescent intensity) and lipid raft abundance (FITC mean fluorescent intensity) in each treatment group at different time points reveals significant correlation with r=0.79 (P<0.0001, two-tailed Pearson correlation, Fig. 4B).
Fig. 4.
Correlation between CD11b expression and lipid raft abundance in isolated human neutrophils. A A representative plot of CD11b expression (APC) against lipid raft abundance (FITC) for a single-treatment sample. B Regression analysis of CD11b expression and lipid raft abundance for all treatment groups and time points.
In a separate experiment, we tested whether L-4F can inhibit neutrophil activation by neutrophil chemotactic factor IL-8, which stimulates neutrophils through its specific receptor and also induces cd11b activation [35]. We find that 40 μg/ml L-4F, a concentration that inhibits LPS-induced neutrophil activation, produces only minimal effect on IL-8-induced increase of membrane expression of CD11b and lipid rafts abundance (Fig. S3).
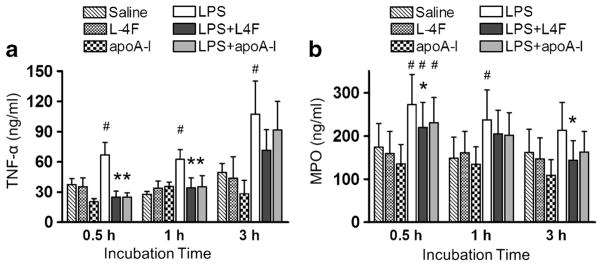
Effects of L-4F and apoA-I on LPS-Mediated TNF-α, IL-6, and MPO Production in Human Neutrophils
Following incubation of neutrophils with 1 μg/ml LPS in the presence and absence of L-4F (40 μg/ml) or apoA-I (40 μg/ml) for 30 min, 1 h, and 3 h, we measured plasma levels of TNF-α and MPO (Fig. 5). LPS increases TNF-α levels at all time points, whereas L-4F and apoA-I inhibit this effect (P<0.05 vs. LPS, for 30 min and 1 h, Fig. 5A). MPO levels are significantly elevated by LPS at 30 min and 1 h. Though both L-4F and apoA-I demonstrates inhibitory effect against LPS-mediated MPO elevation, only inhibitory effect of L-4F reaches statistical significance (P<0.05 vs. LPS, for 30 min and 3 h, Fig. 5B). LPS also increases levels of IL-6 in cell media by 3 h (Fig. 6). L-4F significantly inhibits LPS-mediated IL-6 increase (P<0.05 vs. LPS), whereas apoA-I has only modest effect (nonsignificant vs. LPS).
Fig. 5.
Effects of L-4F and apoA-I on LPS-mediated production of inflammatory biomarkers by isolated human neutrophils. Isolated human neutrophils were incubated in donor plasma with saline, L-4F or apoA-I (40 μg/ml), LPS (1 μg/ml), and LPS plus L-4F or apoA-I for 30 min (n=5), 1 h (n=5), and 3 h (n=4). A Plasma levels of TNF-α. B Myeloperoxidase (MPO) levels. *P<0.05 vs. saline-treated LPS, #P<0.05 vs. control groups.
Fig. 6.
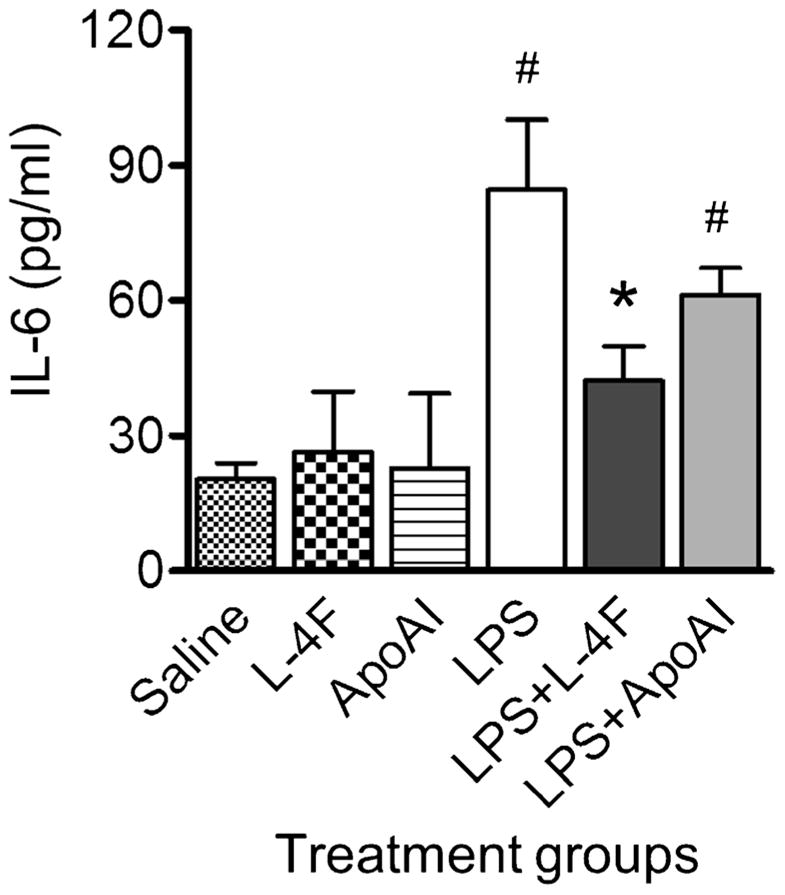
Effects of L-4F and apoA-I on LPS-mediated production of IL-6 by isolated human neutrophils. Isolated human neutrophils were incubated in donor plasma with saline, L-4F or apoA-I (40 μg/ml), LPS (1 μg/ml), and LPS plus L-4F or apoA-I for 3 h (n=3). IL-6 levels in cell media were measured by ELISA kit (BD) specific for human IL-6. *P<0.05 vs. saline-treated LPS, #P<0.05 vs. control groups.
L-4F Exhibits Enhanced Potency Compared to apoA-I in Inhibiting LAL Endotoxin Activity
We compared direct neutralizing effects of L-4F and apoA-I against endotoxin. L-4F or apoA-I at doses of 0, 1, 10, and 100 μg/ml were added to 1 μg/ml LPS in saline, and LAL endotoxin activity was immediately measured. L-4F and apoA-I neutralizes endotoxin activity in a dose-dependent manner (P<0.001, n=3, Fig. 7). L-4F also inhibits endotoxin activity more effectively than apoA-I (P<0.005, Fig. 7).
Fig. 7.
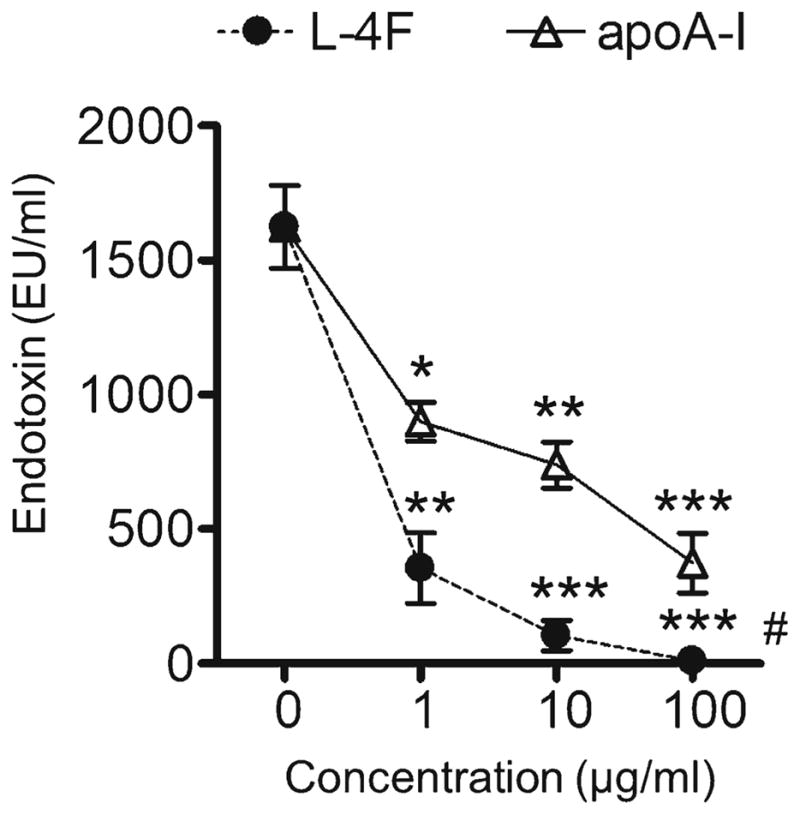
L-4F and apoA-I inhibit LAL endotoxin activity in aqueous solution. LPS from Escherichia coli from E. coli 026:B6 (1 μg/ml) was mixed with L-4F or apoA-I at indicated concentrations in saline and endotoxin activity was immediately measured with LAL assay. L-4F and apoA-I neutralized endotoxin activity in a dose-dependent manner (P<0.001, n=4), L-4F inhibited endotoxin activity more effectively than apoA-I. *P<0.05, **P<0.01, ***P<0.001 vs. LPS alone, #P<0.05 vs. apoA-I.
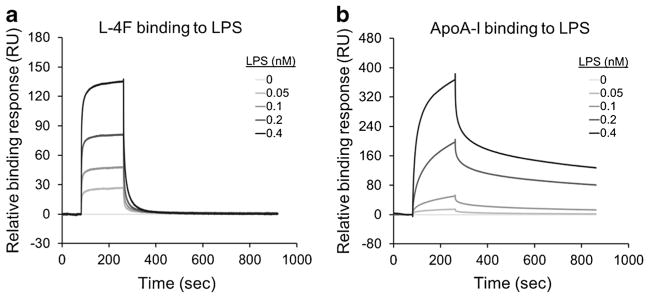
L-4F Binds LPS More Avidly Than apoA-I
To compare the kinetics of L-4F and apoA-I interaction with LPS, we utilized surface plasmon resonance (SPR) technique (Fig. 8). In the experiment, L-4F and apoA-I are immobilized on CM5 sensor chip surfaces, and 30 μl of LPS (0, 0.05, 0.1, 0.2, and 0.4 mg/ml, in duplicates) flowed over these surfaces. Compared to apoA-I, L-4F has a faster binding kinetics [ka=1.79E+5/Ms−1 and kd=0.067/s for L-4F vs. ka=531.5/Ms and kd=0.001/s for apoA-I) with higher steady-state affinity (Kd=3.727E-7 M for L-4F vs. Kd=2.073E-6 M for apoA-I).
Fig. 8.
L-4F and apoA-I bind LPS with different kinetics. Representative experimental sensograms of L-4F (A) and apoA-I (B) binding to LPS are obtained in surface plasmon resonance (SPR) studies. LPS (Escherichia coli 026:B6) binding was measured by observing the change in the SPR angle of the L-4F (A) or apoA-I (B) immobilized to the CM5 chip when LPS flowed over the chip. Compared to apoA-I, L-4F has a faster binding kinetics with a higher calculated steady-state affinity [Kd=3.727E-7 M for L-4F vs. Kd=2.073E-6 M for apoA-I].
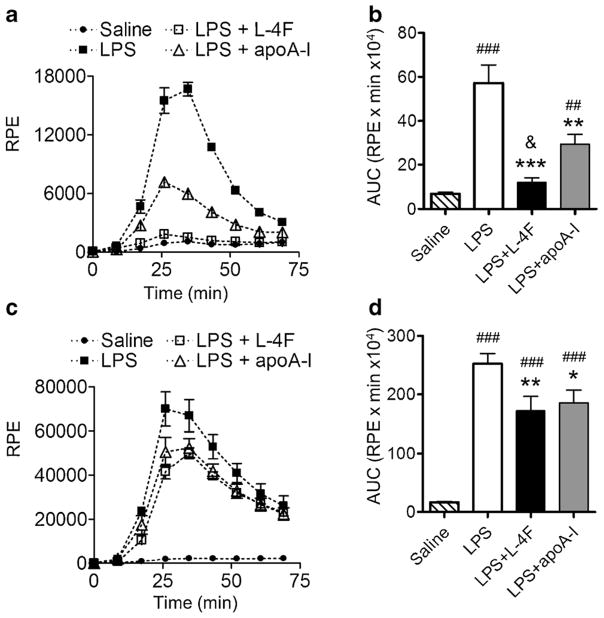
Effects of L-4F and apoA-I on LPS-Induced Oxidative Stress in Human Leukocytes
Freshly isolated human leukocytes were incubated in 5 or 50 % donor plasma with LPS (1 μg/ml) with and without L-4F (40 μg/ml) or apoA-I (40 μg/ml), and superoxide formation as a marker of oxidative stress was measured using lucigenin-amplified chemiluminescence (Fig. 9). LPS alone induces strong chemiluminescence in low and in high plasma levels (P<0.0001 vs. saline), whereas L-4F and apoA-I inhibits the rate as well as peak of LPS-mediated chemiluminescence (both P<0.05 vs. LPS, Fig. 9). However, L-4F more effectively inhibits oxidative stress than apoA-I in the presence of 5 % plasma (P<0.05 vs. apoA-I). In the presence of 50 % plasma, the inhibitory effects of L-4F and apoA-I are similar (NS vs. apoA-I).
Fig. 9.
Effect of plasma on relative efficiency of L-4F and human apoA-I in inhibiting of oxidative cascade in freshly isolated leukocytes. L-4F and apoA-I inhibit superoxide formation by isolated human leukocytes incubated with LPS (1 μg/ml) for 3 h. LPS-induced superoxide formation was measured with lucigenin-amplified chemiluminescence. Effects of L-4F are superior to apoA-I in media with low plasma levels. A, B A representative experiment (in triplicates) in 5 % plasma and statistical data on total superoxide formation (n=3). C, D A representative experiment (in triplicates) in 50 % plasma and statistical data (n=3). AUC area under curve, RPE relative photon emission. *P<0.05, **P<0.01, ***P<0.001 vs. LPS; #P<0.05, ##P<0.01, ###P<0.001 vs. saline; &P<0.05 vs. LPS and apoA-I.
DISCUSSION
In the present work, we find that L-4F peptide, analogous to apoA-I, effectively inhibits LPS-mediated activation of human neutrophils, which is associated with reduction in CD11b expression and lipid raft abundance. In the presence of L-4F, LPS-stimulated neutrophils release reduced levels of TNF-α and MPO. These L-4F inhibitory effects on neutrophils are likely due to reduced oxidative stress. In aqueous solutions, L-4F binds more avidly to LPS than apoA-I and neutralizes endotoxin activity more effectively than apoA-I, which might result in higher anti-endotoxin effects of L-4F, compared to apoA-I, in body fluids with limited plasma components.
In addition to enhanced binding ability of L-4F to LPS, there are multiple mechanisms that may be involved to explain L-4F actions described in the present work. It has been shown that HDL and apoA-I increase cholesterol efflux from neutrophils, which likely leads to disruption of lipid rafts and inhibition of CD11b expression [11]. It has been suggested that lipid rafts play an important role in LPS-mediated cell activation [36], while CD11b is the major mediator for LPS effects in neutrophils [6]. A strong correlation between lipid raft abundance and CD11b activation in neutrophils has been reported [11]. In our experiments, LPS increased lipid rafts abundance and CD11b expression in human neutrophils, which was associated with increased release of active MPO and TNF-α in plasma. L-4F reduced these effects of LPS, analogous to apoA-I. Ability of L-4F to stimulate cholesterol efflux and reduce lipid raft abundance has been previously demonstrated in primary human monocytes [28]. In human monocyte-derived macrophages, disruption of rafts by 4F are believed to alter the assembly of TLR–ligand complexes in cell membranes, thus attenuating the responsiveness of macrophages to LPS [29].
Oxidized lipids binding properties of L-4F and apoA-I may be another mechanism that can explain the results of the present work [37]. It is also important to note that L-4F binds pro-inflammatory oxidized lipids with higher affinity than human apoA-I [37]. LPS-mediated stimulation of neutrophils results in lipid oxidation [38, 39], which promotes inflammatory cascade [38, 40]. L-4F can potentiate anti-oxidant activity of HDL by increasing HDL-associated paraoxonase 1 activity [20, 41]. Paraoxonase-1 protects lipoproteins against oxidation by hydrolyzing lipid hydroperoxides [42] and is most active when anchored to functional HDL [43]. However, human HDL becomes dysfunctional in the presence of MPO, which selectively oxidizes apoA-I [44]. L-4F can substitute apoA-I as a reactive substrate for MPO-derived hypochlorous acid without losing its lipid binding and cholesterol effluxing capacities [45].
Recent data suggest that MPO released by neutrophils also exerts cytokine-like properties that are independent of MPO catalytic activity [46, 47]. MPO binds CD11b/CD18 integrins and induces neutrophil activation [46], which causes the delay in neutrophil apoptosis and prolongs inflammation [47]. As we have observed, L-4F reduces MPO release by LPS-stimulated neutrophils and thus should reduce abovementioned effects of MPO.
In the present work, we focused on another important mechanism of L-4F, which relates to the ability of L-4F to directly interact with LPS [21]. This has been supported by the experiments in which TNF-a and IL-8 were used for cell activation instead of LPS. SPR experiments suggest the binding of L-4F to LPS occurs much faster and with higher affinity than apoA-I to LPS. This is consistent with our previous in vitro and animal experiments where interaction of LPS and L-4F was strongly suggested due to co-localization of Bodipy-LPS and 14C-L-4F in FPLC fractions [21, 48]. Studies in vitro and in vivo suggest that HDL and apoA-I bind LPS, resulting in LPS neutralization [12, 15, 17]. Our results indicate that L-4F more efficiently neutralizes endotoxin LAL activity than human apoA-I. Direct binding to LPS can explain the ability of L-4F to compete with lipopolysaccharide-binding protein (LBP) for binding with LPS [21] and inhibit binding of LPS to leukocytes [24], which should inhibit cell activation by LPS. A higher LPS binding and neutralizing properties of L-4F over apoA-I in aqueous media could be responsible for stronger inhibitory effects of L-4F in leukocytes when incubated in only 5 % plasma as the media in the present work. In contrast, L-4F and apoA-I produced similar inhibition of LPS effects in human blood and in isolated cells when the media contained higher plasma levels. At least two factors likely contribute to this phenomenon: (1) Though untested, L-4F peptide might bind to different plasma components (e.g., albumins) in addition to LPS and HDL, thus reducing actual effective dose of peptide; (2) Plasma contains LBP, soluble CD14, PON1, and other components that could potentially affect the efficiency of anti-endotoxin activity of apoA-I/HDL [17, 42, 49, 50]. Many body fluids like interstitial and bronchoalviolar fluids contain very limited plasma components. Small size of the 18-aminoacid peptide L-4F and high water solubility should allow this compound to reach organ tissues more easily than apoA-I. This property of L-4F, along with higher LPS and oxidized lipids binding and neutralizing, might provide better anti-endotoxin and anti-oxidant protection than apoA-I. This might in part explain why L-4F exhibits high efficiently in animal models of severe sepsis at much lower than human apoA-I doses (10 mg/kg L-4F [22–24] vs. 80–100 mg/kg apoA-I [12, 13, 51]).
Neutrophils and LPS are actively involved in the processes of initiation and progression of inflammation [1, 2, 4]. Recent studies indicate that neutrophils and LPS may also be actively involved in the pathogenesis of atherosclerosis [5, 38, 52–54]. Inhibition of LPS-mediated activation of human neutrophils, as demonstrated in our work, may be an important mechanism of enhanced anti-atherogenic and anti-inflammatory activity of 4F peptide that has been shown previously in different animal models [19, 20, 22–25]. An acceptable safety profile of 4F peptide has been recently shown in selected groups of patients with high-risk cardiovascular disease [55, 56], which warrants clinical potential of this peptide.
Study Limitations
Our study has a number of limitations. First, in experiments in whole human blood and isolated neutrophils, we tested only one concentration of L-4F and apoA-I (40 μg/ml). We selected this dose after carefully incorporating the various considerations described in methodology section including use of different concentrations of L-4F for specific experiments (Fig. 7). Second, the tolerability of L-4F doses studied here has not been evaluated in human in vivo situations [55, 56]. However, based on our observations, rodents were able to tolerate L-4F in much higher doses (Fig. S4). Recent studies have also indicated that plasma concentrations of L-4F may not necessarily directly correlate to the biologic effects [56, 57]. Moreover, there may be intestinal targets for 4F effects [58, 59], which were not tested in the present study. Lastly, we used a high concentration of LPS to mimic severe inflammation, which may not encompass alternate mechanisms due to Gram-negative sepsis. We and others have previously demonstrated that L-4F has protective effects in cecal ligation and puncture model of polymicrobial sepsis [22].
Supplementary Material
Acknowledgments
SPR experiments and data analysis was performed in Multidisciplinary Molecular Interaction Core (MMIC) facility (NIH Grant 1S10RR026935). This work was supported by NIH grants NHLBI K08HL085282 (H.G.), R01 HL102371 (A.G.), 5R01GM 082952 (C.R.W.), NHLBI HL 34343 (G.M.A.).
Dr. G.M. Anantharamaiah, who is inventor of the peptide L-4F and co-investigator, is a principal in Bruin Pharma, a start-up biotech company.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10753-014-9864-7) contains supplementary material, which is available to authorized users.
References
- 1.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nature Reviews Immunology. 2011;11(8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 2.Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368(9530):157–169. doi: 10.1016/S0140-6736(06)69005-3. [DOI] [PubMed] [Google Scholar]
- 3.Davies MJ. Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention. Journal of Clinical Biochemistry and Nutrition. 2011;48(1):8–19. doi: 10.3164/jcbn.11-006FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munford RS. Severe sepsis and septic shock: the role of Gram-negative bacteremia. Annual Review of Pathology. 2006;1:467–496. doi: 10.1146/annurev.pathol.1.110304.100200. [DOI] [PubMed] [Google Scholar]
- 5.Pasterkamp G, Van Keulen JK, De Kleijn DP. Role of toll-like receptor 4 in the initiation and progression of atherosclerotic disease. European Journal of Clinical Investigation. 2004;34(5):328–334. doi: 10.1111/j.1365-2362.2004.01338.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhou X, Gao XP, Fan J, Liu Q, Anwar KN, Frey RS, Malik AB. LPS activation of toll-like receptor 4 signals CD11b/CD18 expression in neutrophils. American Journal of Physiology -Lung Cellular and Molecular Physiology. 2005;288(4):L655–662. doi: 10.1152/ajplung.00327.2004. [DOI] [PubMed] [Google Scholar]
- 7.Sorci-Thomas MG, Thomas MJ. High density lipoprotein biogenesis, cholesterol efflux, and immune cell function. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(11):2561–2565. doi: 10.1161/ATVBAHA.112.300135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circulation Research. 2004;95(8):764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 9.Blackburn WD, Jr, Dohlman JG, Venkatachalapathi YV, Pillion DJ, Koopman WJ, Segrest JP, Anantharamaiah GM. Apolipoprotein A-I decreases neutrophil degranulation and superoxide production. The Journal of Lipid Research. 1991;32(12):1911–1918. [PubMed] [Google Scholar]
- 10.Liao XL, Lou B, Ma J, Wu MP. Neutrophils activation can be diminished by apolipoprotein A-I. Life Sciences. 2005;77(3):325–335. doi: 10.1016/j.lfs.2004.10.066. [DOI] [PubMed] [Google Scholar]
- 11.Murphy AJ, Woollard KJ, Suhartoyo A, Stirzaker RA, Shaw J, Sviridov D, Chin-Dusting JP. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(6):1333–1341. doi: 10.1161/ATVBAHA.111.226258. [DOI] [PubMed] [Google Scholar]
- 12.Levine DM, Parker TS, Donnelly TM, Walsh A, Rubin AL. In vivo protection against endotoxin by plasma high density lipoprotein. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(24):12040–12044. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan YJ, Li Y, Lou B, Wu MP. Beneficial effects of ApoA-I on LPS-induced acute lung injury and endotoxemia in mice. Life Sciences. 2006;79(2):210–215. doi: 10.1016/j.lfs.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Shah PK, Nilsson J, Kaul S, Fishbein MC, Ageland H, Hamsten A, Johansson J, Karpe F, Cercek B. Effects of recombinant apolipoprotein A-I (Milano) on aortic atherosclerosis in apolipoprotein E-deficient mice. Circulation. 1998;97(8):780–785. doi: 10.1161/01.cir.97.8.780. [DOI] [PubMed] [Google Scholar]
- 15.Pajkrt D, Doran JE, Koster F, Lerch PG, Arnet B, van der Poll T, ten Cate JW, van Deventer SJ. Antiinflammatory effects of reconstituted high-density lipoprotein during human endotoxemia. The Journal of Experimental Medicine. 1996;184(5):1601–1608. doi: 10.1084/jem.184.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290(17):2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 17.Kitchens RL, Thompson PA, Viriyakosol S, O’Keefe GE, Munford RS. Plasma CD14 decreases monocyte responses to LPS by transferring cell-bound LPS to plasma lipoproteins. The Journal of Clinical Investigation. 2001;108(3):485–493. doi: 10.1172/JCI13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remaley AT, Amar M, Sviridov D. HDL-replacement therapy: mechanism of action, types of agents and potential clinical indications. Expert Review of Cardiovascular Therapy. 2008;6(9):1203–1215. doi: 10.1586/14779072.6.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garber DW, Datta G, Chaddha M, Palgunachari MN, Hama SY, Navab M, Fogelman AM, Segrest JP, Anantharamaiah GM. A new synthetic class A amphipathic peptide analogue protects mice from diet-induced atherosclerosis. The Journal of Lipid Research. 2001;42(4):545–552. [PubMed] [Google Scholar]
- 20.Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, Yu N, Ansell BJ, Datta G, Garber DW, Fogelman AM. Apolipoprotein A-I mimetic peptides. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(7):1325–1331. doi: 10.1161/01.ATV.0000165694.39518.95. [DOI] [PubMed] [Google Scholar]
- 21.Gupta H, Dai L, Datta G, Garber DW, Grenett H, Li Y, Mishra V, Palgunachari MN, Handattu S, Gianturco SH, Bradley WA, Anantharamaiah GM, White CR. Inhibition of lipopolysaccharide-induced inflammatory responses by an apolipoprotein AI mimetic peptide. Circulation Research. 2005;97(3):236–243. doi: 10.1161/01.RES.0000176530.66400.48. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Datta G, Zhang Y, Miller AP, Mochon P, Chen YF, Chatham J, Anantharamaiah GM, White CR. Apolipoprotein A-I mimetic peptide treatment inhibits inflammatory responses and improves survival in septic rats. American Journal of Physiology -Heart and Circulatory Physiology. 2009;297(2):H866–873. doi: 10.1152/ajpheart.01232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon WY, Suh GJ, Kim KS, Kwak YH, Kim K. 4F, apolipoprotein AI mimetic peptide, attenuates acute lung injury and improves survival in endotoxemic rats. Journal Trauma Acute Care Surgery. 2012;72(6):1576–1583. doi: 10.1097/TA.0b013e3182493ab4. [DOI] [PubMed] [Google Scholar]
- 24.Sharifov OF, Xu X, Gaggar A, Grizzle WE, Mishra VK, Honavar J, Litovsky SH, Palgunachari MN, White CR, Anantharamaiah GM, Gupta H. Anti-inflammatory mechanisms of apolipoprotein a-I mimetic peptide in acute respiratory distress syndrome secondary to sepsis. PLoS One. 2013;8(5):e64486. doi: 10.1371/journal.pone.0064486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madenspacher JH, Azzam KM, Gong W, Gowdy KM, Vitek MP, Laskowitz DT, Remaley AT, Wang JM, Fessler MB. Apolipoproteins and apolipoprotein mimetic peptides modulate phagocyte trafficking through chemotactic activity. The Journal of Biological Chemistry. 2012;287(52):43730–43740. doi: 10.1074/jbc.M112.377192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Datta G, Chaddha M, Hama S, Navab M, Fogelman AM, Garber DW, Mishra VK, Epand RM, Epand RF, Lund-Katz S, Phillips MC, Segrest JP, Anantharamaiah GM. Effects of increasing hydrophobicity on the physical–chemical and biological properties of a class A amphipathic helical peptide. Journal of Lipid Research. 2001;42(7):1096–1104. [PubMed] [Google Scholar]
- 27.Anantharamaiah GM, Garber DW. Chromatographic methods for quantitation of apolipoprotein A-I. Methods in Enzymology. 1996;263:267–282. doi: 10.1016/s0076-6879(96)63019-5. [DOI] [PubMed] [Google Scholar]
- 28.Smythies LE, White CR, Maheshwari A, Palgunachari MN, Anantharamaiah GM, Chaddha M, Kurundkar AR, Datta G. Apolipoprotein A-I mimetic 4F alters the function of human monocyte-derived macrophages. American Journal of Physiology -Cellular Physiology. 2010;298(6):C1538–1548. doi: 10.1152/ajpcell.00467.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White CR, Smythies LE, Crossman DK, Palgunachari MN, Anantharamaiah GM, Datta G. Regulation of pattern recognition receptors by the apolipoprotein A-I mimetic peptide 4F. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(11):2631–2639. doi: 10.1161/ATVBAHA.112.300167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Jackson PL, Tanner S, Hardison MT, Abdul Roda M, Blalock JE, Gaggar A. A self-propagating matrix metalloprotease-9 (MMP-9) dependent cycle of chronic neutrophilic inflammation. PLoS One. 2011;6(1):e15781. doi: 10.1371/journal.pone.0015781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iovine NM, Elsbach P, Weiss J. An opsonic function of the neutrophil bactericidal/permeability-increasing protein depends on both its N- and C-terminal domains. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(20):10973–10978. doi: 10.1073/pnas.94.20.10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharifov OF, Nayyar G, Ternovoy VV, Mishra VK, Litovsky SH, Palgunachari MN, Garber DW, Anantharamaiah GM, Gupta H. Cationic peptide mR18L with lipid lowering properties inhibits LPS-induced systemic and liver inflammation in rats. Biochemical and Biophysical Research Communications. 2013;436(4):705–710. doi: 10.1016/j.bbrc.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehmann V, Freudenberg MA, Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. The Journal of Experimental Medicine. 1987;165(3):657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowak M, Gaines GC, Rosenberg J, Minter R, Bahjat FR, Rectenwald J, MacKay SL, Edwards CK, 3rd, Moldawer LL. LPS-induced liver injury in D-galactosamine-sensitized mice requires secreted TNF-alpha and the TNF-p55 receptor. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2000;278(5):R1202–1209. doi: 10.1152/ajpregu.2000.278.5.R1202. [DOI] [PubMed] [Google Scholar]
- 35.Lundahl J, Jacobson SH, Paulsson JM. IL-8 from local subcutaneous wounds regulates CD11b activation. Scandinavian Journal of Immunology. 2012;75(4):419–425. doi: 10.1111/j.1365-3083.2012.02679.x. [DOI] [PubMed] [Google Scholar]
- 36.Olsson S, Sundler R. The role of lipid rafts in LPS-induced signaling in a macrophage cell line. Molecular Immunology. 2006;43(6):607–612. doi: 10.1016/j.molimm.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Van Lenten BJ, Wagner AC, Jung CL, Ruchala P, Waring AJ, Lehrer RI, Watson AD, Hama S, Navab M, Anantharamaiah GM, Fogelman AM. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. The Journal of Lipid Research. 2008;49(11):2302–2311. doi: 10.1194/jlr.M800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malle E, Marsche G, Arnhold J, Davies MJ. Modification of low-density lipoprotein by myeloperoxidase-derived oxidants and reagent hypochlorous acid. Biochimica et Biophysica Acta. 2006;1761(4):392–415. doi: 10.1016/j.bbalip.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 39.Carr AC, Frei B. Human neutrophils oxidize low-density lipoprotein by a hypochlorous acid-dependent mechanism: the role of vitamin C. Biological Chemistry. 2002;383(3–4):627–636. doi: 10.1515/BC.2002.065. [DOI] [PubMed] [Google Scholar]
- 40.Memon RA, Staprans I, Noor M, Holleran WM, Uchida Y, Moser AH, Feingold KR, Grunfeld C. Infection and inflammation induce LDL oxidation in vivo. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(6):1536–1542. doi: 10.1161/01.atv.20.6.1536. [DOI] [PubMed] [Google Scholar]
- 41.Nayyar G, V, Mishra K, Handattu SP, Palgunachari MN, Shin R, McPherson DT, Deivanayagam CC, Garber DW, Segrest JP, Anantharamaiah GM. Sidedness of interfacial arginine residues and anti-atherogenicity of apolipoprotein A-I mimetic peptides. The Journal of Lipid Research. 2012;53(5):849–858. doi: 10.1194/jlr.M019844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. Journal of Clinic Investment. 1998;101(8):1581–1590. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moren X, Deakin S, Liu ML, Taskinen MR, James RW. HDL subfraction distribution of paraoxonase-1 and its relevance to enzyme activity and resistance to oxidative stress. The Journal of Lipid Research. 2008;49(6):1246–1253. doi: 10.1194/jlr.M700439-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Undurti A, Huang Y, Lupica JA, Smith JD, DiDonato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. The Journal of Biological Chemistry. 2009;284(45):30825–30835. doi: 10.1074/jbc.M109.047605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White CR, Datta G, Buck AK, Chaddha M, Reddy G, Wilson L, Palgunachari MN, Abbasi M, Anantharamaiah GM. Preservation of biological function despite oxidative modification of the apolipoprotein A-I mimetic peptide 4F. The Journal of Lipid Research. 2012;53(8):1576–1587. doi: 10.1194/jlr.M026278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, Brummer J, Rudolph V, Munzel T, Heitzer T, Meinertz T, Baldus S. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(2):431–436. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El Kebir D, Jozsef L, Pan W, Filep JG. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circulation Research. 2008;103(4):352–359. doi: 10.1161/01.RES.0000326772.76822.7a. [DOI] [PubMed] [Google Scholar]
- 48.Dai L, Datta G, Zhang Z, Gupta H, Patel R, Honavar J, Modi S, Wyss JM, Palgunachari M, Anantharamaiah GM, White CR. The apolipoprotein A-I mimetic peptide 4F prevents defects in vascular function in endotoxemic rats. The Journal of Lipid Research. 2010;51(9):2695–2705. doi: 10.1194/jlr.M008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu B, Wright SD. Catalytic properties of lipopolysaccharide (LPS) binding protein. Transfer of LPS to soluble CD14. The Journal of Biological Chemistry. 1996;271(8):4100–4105. doi: 10.1074/jbc.271.8.4100. [DOI] [PubMed] [Google Scholar]
- 50.Wurfel MM, Kunitake ST, Lichenstein H, Kane JP, Wright SD. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. The Journal of Experimental Medicine. 1994;180(3):1025–1035. doi: 10.1084/jem.180.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McDonald MC, Dhadly P, Cockerill GW, Cuzzocrea S, Mota-Filipe H, Hinds CJ, Miller NE, Thiemermann C. Reconstituted high-density lipoprotein attenuates organ injury and adhesion molecule expression in a rodent model of endotoxic shock. Shock. 2003;20(6):551–557. doi: 10.1097/01.shk.0000097249.97298.a3. [DOI] [PubMed] [Google Scholar]
- 52.Baetta R, Corsini A. Role of polymorphonuclear neutrophils in atherosclerosis: current state and future perspectives. Atherosclerosis. 2010;210(1):1–13. doi: 10.1016/j.atherosclerosis.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 53.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circulation Research. 2012;110(6):875–888. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- 54.Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck study. Journal of the American College of Cardiology. 1999;34(7):1975–1981. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 55.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. The Journal of Lipid Research. 2008;49(6):1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watson CE, Weissbach N, Kjems L, Ayalasomayajula S, Zhang Y, Chang I, Navab M, Hama S, Hough G, Reddy ST, Soffer D, Rader DJ, Fogelman AM, Schecter A. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. The Journal of Lipid Research. 2011;52(2):361–373. doi: 10.1194/jlr.M011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Navab M, Reddy ST, Anantharamaiah GM, Imaizumi S, Hough G, Hama S, Fogelman AM. Intestine may be a major site of action for the apoA-I mimetic peptide 4F whether administered subcutaneously or orally. The Journal of Lipid Research. 2011;52(6):1200–1210. doi: 10.1194/jlr.M013144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meriwether D, Imaizumi S, Grijalva V, Hough G, Vakili L, Anantharamaiah GM, Farias-Eisner R, Navab M, Fogelman AM, Reddy ST, Shechter I. Enhancement by LDL of transfer of L-4F and oxidized lipids to HDL in C57BL/6 J mice and human plasma. The Journal of Lipid Research. 2011;52(10):1795–1809. doi: 10.1194/jlr.M016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Navab M, Reddy ST, Anantharamaiah GM, Hough G, Buga GM, Danciger J, Fogelman AM. D-4F-mediated reduction in metabolites of arachidonic and linoleic acids in the small intestine is associated with decreased inflammation in low-density lipoprotein receptor-null mice. The Journal of Lipid Research. 2012;53(3):437–445. doi: 10.1194/jlr.M023523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.