Abstract
Extant research documents impaired language among children with prenatal cocaine exposure (PCE) relative to nondrug exposed (NDE) children, suggesting that cocaine alters development of neurobiological systems that support language. The current study examines behavioral and neural (electrophysiological) indices of language function in older adolescents. Specifically, we compare performance of PCE (N = 59) and NDE (N = 51) adolescents on a battery of cognitive and linguistic assessments that tap word reading, reading comprehension, semantic and grammatical processing, and IQ. In addition, we examine event related potential (ERP) responses in in a subset of these children across three experimental tasks that examine word level phonological processing (rhyme priming), word level semantic processing (semantic priming), and sentence level semantic processing (semantic anomaly). Findings reveal deficits across a number of reading and language assessments, after controlling for socioeconomic status and exposure to other substances. Additionally, ERP data reveal atypical orthography to phonology mapping (reduced N1/P2 response) and atypical rhyme and semantic processing (N400 response). These findings suggest that PCE continues to impact language and reading skills into the late teenage years.
Introduction
Human and animal research indicates that prenatal cocaine exposure modifies the development of neural systems, particularly the monoaminergic systems (dopamine, serotonin and norepinephrine) involved in cortical development. Specifically, cocaine may act directly at the presynaptic level, where it can block the reuptake of monoaminergic neurotransmitters and disrupt neural circuitry development (Bhide, 2009; Mayes, 1999; Mayes & Bornstein, 1995). Additional mechanisms by which cocaine may impact fetal development include: (a) vasoconstriction, during which redistributed blood results in elevated plasma catecholamine levels and subsequent growth restriction (Basset & Hanson, 1998; Jones & Robinson, 1983), and (b) disruption of intrauterine homeostasis, which can damage the fetal-placental neuroendocrine environment (Lester & Padbury, 2009). These additional mechanisms in the broader prenatal environment also have negative implications for the developing neural systems that support cognition (Welberg & Seckl, 2001). Together, these alterations to the developing fetal brain and to the broader prenatal environment can produce lasting consequences on brain structure and function (Lidow, 1995; Morrow, Elsworth, & Roth, 2002).
Indeed, a significant body of developmental work now documents impaired cognitive performance among children prenatally exposed to cocaine (PCE) relative to nondrug exposed (NDE) children across multiple domains in childhood and adolescence (cf. Bandstra et al., 2011; Lewis et al., 2013). These deficits span a range of cognitive domains, including executive function, attentional processing, inhibitory control, spatial learning, and processing speed (Alessandri, Sullivan, Imaizumi, & Lewis, 1993; Heffelinger, Craft, & Shyken, 1997; Savage, Brodsky, Malmud, Giannetta, & Hurt, 2005; Schroder, Snyder, Sielski, & Mayes, 2004). One of the more consistent findings is that youth with PCE show impairment across a variety of language tasks, suggesting that cocaine alters development of neurobiological systems that support language processing. Specifically, linguistic deficits identified in PCE children encompass the domains of speech processing, expressive language, semantic processing, phonological processing, receptive language, and language delay (e.g., Bandstra et al., 2011, 2002; Bandstra, Vogel, Morrow, Xue, & Anthony, 2004; Cone-Wesson, 2005; Delaney–Black et al., 2000; Landi, Crowley, Wu, Bailey, & Mayes, 2012; Lewis et al., 2013; Malakoff, Mayes, Schottenfeld, & Howell, 1999; Singer, Arendt, Minnes, Farka, & Salvator, 2001). Furthermore, findings of impaired language function are present in both younger and older children, suggesting that these effects are not transient. For example, Bandstra and colleagues (2011) examined performance on the Clinical Evaluation of Language Fundamentals-3 (CELF-3) in children with PCE and noncocaine exposed controls at ages 3, 5, and 12 years of age, and found that PCE was associated with poorer total language and expressive language scores at all three time points, even after controlling for exposure to other drugs and socio-economic status (SES). Furthermore, these authors found a dose-dependent association, such that increased amounts of exposure were associated with more substantial language impairments. Another study by Lewis and colleagues (2013) found that 12-year-old children with PCE had poorer phonological processing and syntactic processing skills relative to nonexposed controls. Moreover, phonological processing skills in that study were related to word reading, reading fluency, and reading comprehension in PCE children, consistent with well-established links between reading skill and phonological processing (Liberman & Shankweiler, 1985). Notably, this finding highlights the possible cascading effects of PCE from general language function to later literacy, a progression that is less well documented in PCE, in part because few studies of PCE follow children through adolescence.
Despite these positive associations, other studies have failed to find effects of impaired language function in PCE (e.g., Betancourt et al., 2011; Espy, Kaufmann, & Glisky, 1999; Hawley, Halle, Drasin, & Thomas, 1995; Hurt, Brodsky, Roth, Malmud, & Giannetta, 1997; Kilbride, Castor, & Fuger, 2006). Further, across studies with positive findings there is variation in the skills examined, making it difficult to identify which effects are consistently impaired (e.g., receptive vs. expressive language). Two meta-analyses of the effects of PCE on language and cognitive function suggest that variable results are due to differences in sample size, amount of cocaine exposure,1 SES differences between exposed and control groups, exposure to other substances, and the age of the children tested (J. P. Ackerman, Riggins, & Black, 2010; Frank, Augustyn, Knight, Pell, & Zuckerman, 2001). Furthermore, as J. P. Ackerman et al. note, only a few studies on language processing in PCE include older children and adolescents. This is an important point, given that the effects of exposure to teratogenic substances may not necessarily be observed all at once, or even early in development (Sayal et al., 2009), and that language-based learning disabilities can be late emerging (cf. Torppa, Eklund, Van Bergen, & Lyytinen, 2015). Thus, more studies of PCE effects on language function in older children and adolescents are needed.
Using magnetic resonance imaging (MRI) and elelctroencephalography (EEG), researchers have now begun to examine the impact of PCE on post-natal brain function and structure in children and adolescents. These studies find that children with PCE have reduced volume in the caudate nucleus, a region implicated in attentional processing, learning, and language (Avants et al., 2007), and that PCE is associated with higher isotropic diffusion (DAV) in frontal white matter, which, unlike increased anisotropic diffusion (FA), may be associated with reduced integrity or slower maturation of this region (Warner et al., 2006). Other imaging work finds reduced gray matter volume in occipital and parietal lobes and reduced white matter in the corpus callosum in those with PCE (Singer et al., 2006). Moreover, studies utilizing event related potentials (ERPs) suggest slower and more distributed cognitive processing, implicating reduced regional specialization (e.g., Mayes, Molfese, Key, & Hunter, 2005). With respect to language related biomarkers, studies find that children with PCE show some early differences in basic auditory processing, a necessary precursor for spoken language acquisition. For example, infants with PCE show slower habituation to auditory stimuli compared to nonexposed infants, a process thought to be central to early language development (cf. Potter, Zelazo, Stack, & Papageorgio, 2000). Furthermore, PCE infants show larger auditory startle response and atypical auditory evoked potentials, suggesting impaired sensorineural processing early in development (Anday, Cohen, Kelley, & Lietner, 1988; Cone-Wesson, 2005; Singer et al, 2001; Tan-Laxa, Sison-Switala, Rintelman, & Ostrea, 2004). Moreover, Landi and colleagues (2012) report language-specific ERP effects, including reduced P600s and reduced P1/N2 complex to the onset of spoken nonwords in an old-new “learning” paradigm, suggesting poor phonological processing and reduced discrimination of old versus new speech tokens.
The current paper extends upon previous work (Landi et al., 2012), which examined ERPs to spoken nonwords in 13-year-old PCE and NDE adolescents and retrospective behavioral indices of language function from the Clinical Evaluation of Language Fundamentals III (CELF III; Semel, Wiig, & Secord, 1995). That work identified both an early phonological processing component (N1/P2 complex) and a later memory associated component to repeated tokens (P600), which showed differential response in PCE and NDE children, indicating aberrant neural response to speech in PCE. However, in that study, the retrospective behavioral findings were less clear: PCE children presented with some language dysfunction at age 9, but were no different from NDE children at age 11. Limitations of that work for studying neural and behavioral aspects of language function in PCE include: (a) lack of behavioral assessments of language ability from all children who completed the ERP study; (b) retrospective but not concurrent behavioral measures of language ability; (c) no ERP measures of higher level language (lexical or supra lexical processing); and (d) limited behavioral measures of language ability. The current study extends upon this prior work to include both behavioral and ERP measures of reading and language skills in older adolescents (age 14-20) with PCE and NDE controls. This study is first to examine the effects of PCE on the behavioral and neural bases of language and reading function in late adolescents.
In order to explore neurolinguistic function at multiple levels of processing, we included behavioral assessments of reading and language skill and three ERP paradigms designed to tap lexical-level phonological processing, lexical-level semantic processing, and sentence-level semantic processing. To explore lexical-level phonological processing, we used a rhyme priming task (RPT) that has been shown to be sensitive to phonological processing skill and reading skill (e.g., P. T. Ackerman, Dykman, & Oglesby, 1994; Grainger, Kiyonaga, & Holcomb, 2006; Landi & Perfetti, 2007; Rüsseler, Becker, Johannes, & Münte, 2007). In this task, participants were asked to judge whether two consecutively presented visual words rhymed or not. For this task, we were interested in both early components associated with orthography to phonology mapping (N1/P2) and later components associated with rhyme awareness and comparison (N200/N400). To explore lexical level semantic processing, we used a semantic priming task (SPT) that has previously been associated with reading comprehension skill (cf. Landi & Perfetti, 2007). In this task, participants were asked to judge whether two consecutively presented visual words were related in meaning. For this task, we were interested primarily in components associated with lexical-semantic processing and integration (e.g., the N400). Finally, to explore sentence level integration of meaning, we used a semantic anomaly task (SAT), in which a final word is either semantically congruent with the sentence context or not (cf. Kutas & Van Petten, 1988). In this task, which is sensitive to language impairment in aphasia and dyslexia (e.g., Schulz et al, 2008; Swaab, Brown, & Hagoort, 1998), participants were asked to decide whether visually presented sentences “made sense.” For this task, we focus on N400 response to the final word, which was either semantically congruent or anomalous (see detailed task descriptions in the following sections).
Given prior work implicating impaired reading (phonological recoding) for children with PCE (e.g., Lewis et al., 2013), we expect to see differences in word processing at both the behavioral and neurophysiological levels. Specifically, we expect to see poorer performance behaviorally among PCE individuals on measures of word reading and reading comprehension. Physiologically, we predict that PCE individuals would have smaller responses in early ERP components such as the N1 and P2 components, which are sensitive to individual differences in decoding and phonological processing (Dujardin et al., 2011; Meyler & Breznitz, 2005), and have been found to distinguish PCE and NDE young adolescents in spoken word processing tasks (e.g., Landi et al., 2012).
With respect to higher-level language function, such as access to and integration of meaning, studies that have examined vocabulary and comprehension in PCE children and adolescents have identified impaired performance (e.g., Singer et al., 2001). However, to our knowledge there have been no electrophysiological or neuroimaging studies that have examined semantic processing or word to text integration in PCE. Extant neurophysiological work utilizing ERPs suggests that the N400 response is highly sensitive (in both single word and sentence processing studies) to individual differences in comprehension skill and vocabulary knowledge (e.g., Landi & Perfetti, 2007; Perfetti, Wlotko, & Hart, 2005; Stafura, Rickles, & Perfetti, 2015). As such, we predict that we will see N400 differences between our PCE and NDE adolescents if we also see differences on our behavioral measures of reading comprehension and semantic knowledge. Specifically, we expect reduced N400 response in PCE, which would indicate reduced sensitivity to semantic relatedness/congruency.
Methods
Participants
Adolescent participants in the current study were recruited from a longitudinal cohort of 523 children, including both exposed children and nonexposed controls who were recruited at birth, initially enrolled at birth to examine the effects of cocaine exposure on physical, social, cognitive and emotional development (Landi et al., 2012; Mayes, Bornstein, Chawarska, & Granger, 1995; Mayes et al., 2005; Mayes, Snyder, Langlois, & Hunter, 2007).2 From the originally recruited longitudinal cohort we have maintained contact with 78% with no selective attrition between the cocaine-exposed (21.4% lost) and nondrug-exposed (24.4% lost).
Cocaine use and exposure to the fetus were determined using combinations of three factors: (a) self-report by the birth mother of cocaine addiction; (b) positive maternal urine toxicology screen upon entry into the study; and (c) positive meconium toxicology screen after the baby was born. Other drug use during the 30 days prior to delivery was assessed by birth mother self-report (see Table 1 for mean group use of other drugs during this period). Maternal use of cocaine was often associated with the use of other substances (tobacco, alcohol, and marijuana). However, cocaine was reported by the birth mother as the primary drug of addiction3 during the prenatal period; amount of other drug use during the 30 days prior to birth for all participants is reported in Table 1. For the NDE cohort, mothers reported no use of cocaine or other drugs; they had negative urine samples upon entry into the study, and their babies' meconium toxicology screens were negative for cocaine and other substances. Families were initially recruited when they sought prenatal care at a northeastern U.S. hospital or when they were admitted to the postpartum ward in the case of no prenatal care.
Table 1.
Effects, means and standard deviations for covariates included in the MANCOVA for full sample and ERP subsamples.
| Full Sample (N = 110) | PCE M (SD) | NDE M (SD) | F | p | partial η2 |
|---|---|---|---|---|---|
| KBIT: Nonverbal IQ | 85.500 (15.250) | 97.800 (14.650) | 8.030 | <.001 | .37 |
| SES | 4.020 (0.805) | 4.630 (1.170) | 1.445 | .190 | .09 |
| Marijuana | 0.510 (0.770) | 0 | 0.680 | .680 | .04 |
| Alcohol | 2.730 (4.740) | 0.100 (0.300) | 1.550 | .160 | .10 |
| Cigarettes | 5.580 (10.850) | 0 | 1.827 | .090 | .12 |
| Age | 17.370 (1.880) | 16.800 (1.990) | 5.350 | <.001 | .28 |
| grade | 11.020 (1.610) | 10.920 (1.470) | 4.190 | <.001 | .23 |
|
| |||||
| RPT & SPT (N = 70) | |||||
| KBIT: Nonverbal IQ | 89.570 (15.800) | 96.570 (13.580) | 5.68 | <.001 | .42 |
| SES | 3.940 (0.830) | 4.710 (1.100) | 1.58 | .160 | .17 |
| Marijuana | 0.571 (0.500) | 0 | 1.41 | .220 | .15 |
| Alcohol | 3.500 (5.260) | 0.057 (0.235) | 2.68 | .020 | .25 |
| Cigarettes | 8.310 (12.530) | 0 | 2.65 | .020 | .25 |
| Age | 17.370 (2.050) | 16.600 (1.900) | 3.45 | <.010 | .35 |
| grade | 11.060 (1.300) | 10.680 (1.600) | 2.58 | .020 | .25 |
|
| |||||
| SAT (N = 60) | |||||
| KBIT: Nonverbal IQ | 85.260 (15.300) | 95.500 (15.400) | 5.68 | <.001 | .47 |
| SES | 4.030 (0.850) | 4.500 (1.100) | 2.10 | .060 | .25 |
| Marijuana | 0.500 (0.510) | 0 | 0.94 | .480 | .13 |
| Alcohol | 2.100 (4.160) | 0.130 (0.340) | 1.80 | .110 | .22 |
| Cigarettes | 7.430 (12.170) | 0 | 2.54 | .020 | .28 |
| Age | 17.020 (1.850) | 16.770 (1.900) | 2.38 | .030 | .27 |
| grade | 10.800 (1.096) | 10.930 (1.510) | 1.77 | .110 | .22 |
Note. Substance use is reported as mean number of days in 30 days prior to birth. SES is reported as maternal education, which is a mean of the following: 1 = less than 7th grade; 2 = junior high; 3 = some high school; 4 = high school or GED; 5 = some college; 6 = undergrad degree; 7 = graduate degree.
Full sample
One hundred and ten adolescents (61 Males), between the ages of 14 and 20, M age = 17.11 (SD = 1.95) completed the behavioral portion of the study. All participants were monolingual English speakers and were neurotypical.4 Of the 110 adolescent participants, 59 (34 Males) were exposed to cocaine and comprised the PCE group, and 51 (26 Males) were not exposed to cocaine or other drugs and comprised the NDE group. The gender distribution did not differ between PCE and NDE groups X2 = .777, p = .38. Age at time of test did not differ between groups, t(108) = 1.35, p = .129, mean PCE age = 17.37 (SD = 1.99); NDE age = 16.80 (SD = 1.86), nor did grade in school t(108) = -.640, p = .615 mean PCE grade = 11.10 (SD = 1.13), mean NDE grade = 10.92 (SD = 1.55). Socioeconomic status as assessed by maternal education did differ between groups, with mothers of PCE adolescents on average having received less education t(108) = 3.12, p = .002. Specifically, more mothers of NDE children completed high school and had some college. The racial background of the current cohort is N = 54 African American; 2 Caucasian; 3 other/mixed race for the exposed group, and N = 23 African American; 23 Caucasian; 5 other/mixed race for the nonexposed group. Most participants (80% in NDE group, and 74% in PCE group) were right-handed based on self-report. All participants were compensated financially at an hourly rate of $20/hour.
ERP subsamples
The following section describes the subsamples for each EEG task of the total 110 participants that completed behavioral testing in the current study. Although all participants were invited to complete the EEG experiments, some participants were unwilling to wear or unable to tolerate the EEG sensor net. Ninety-eight participants (45 NDE and 53 PCE) were willing to attempt the EEG experiments. the remaining 12 participants (11% of the full sample) were unwilling to participate. Of those 98, data loss or incomplete data reduced our semantic and rhyme priming samples to 70 adolescents and our sentence anomaly sample to 60 adolescents (detailed demographics for each group for each sample, and data exclusion procedures are provided in the following section).
Semantic Prime Task (SPT) & Rhyme Prime Task (RPT) Participants: Participants included in our analyses were 35 NDE (17 males) and 35 PCE (24 males) adolescents who all had at least 50% artifact free trials per condition (artifact rejection and trial exclusion procedures are discussed below). The gender distribution did not differ between PCE and NDE groups X2 = 2.89, p = .09. Age at time of test did not differ between groups, t(68) = -1.62, p = .11, mean PCE age = 17.3 (SD = 2.05); mean NDE age = 16.6 (SD = 1.92), nor did grade in school, t(68) = -1.04, p = .30 mean PCE grade = 11.06 (SD = 1.34), mean NDE grade = 10.68 (SD = 1.64). Socio-economic status (SES), assessed by maternal education did differ between groups, with exposed mothers on average having received less education t(68) = 3.30, p = .002. Specifically, more mothers of NDE children completed high-school and had some college. The racial background of the PCE group was N = 33 African American; N = 1 Caucasian; N = 1 other/mixed race, and for the NDE group N = 16 African American; N = 15 Caucasian; N = 4 other/mixed race.
Semantic Anomaly Task (SAT) Participants: Participants included in our analyses were 30 NDE (15 males) and 30 PCE (20 males) adolescents who all had at least 50% artifact free trials per condition (artifact rejection and trial exclusion procedures are discussed in the further section). The gender distribution did not differ between PCE and NDE groups X2 = 1.714, p = .19. Age at time of test did not differ between groups t(58) = -.606, p = .55, mean PCE age = 17.07 (SD = 1.85); mean NDE age = 16.77 (SD = 1.97), nor did grade in school t(58) = .382, p = .70, mean PCE grade = 10.80 (SD = 1.09); mean NDE grade = 10.93 (SD = 1.51). Socio-economic status (SES), assessed by maternal education did not differ between groups, t(58) = 1.8, p = .072, but exposed mothers of PCE children had marginally less education than mothers of NDE children. The racial background of the PCE group was N = 28 African American; N = 1 Caucasian; N = 1 other/mixed race. The racial background of for the NDE group was N = 12 African American; N = 14 Caucasian; N = 4 other/mixed race.
Behavioral data collection and analysis procedures
Behavioral data on language and general cognitive functioning were collected using standardized assessments including (a) the Kaufman Brief Intelligence Test, second edition, KBIT II (Kaufman & Kaufman, 2004a), which was used to measure verbal and nonverbal IQ; (b) The Clinical Evaluation of Language Fundamentals, third edition, CELF III (Semel et al., 1995), which was used to measure semantic and syntactic competence; from this test we administered three subtests that we have found to discriminate among clinical groups with language deficits (e.g., Hoffmann, Turcios, Cook, Landi, & Irwin, under revision) including semantic relations, word associations, and recalling sentences; and (c) the Kaufman Test of Educational Achievement KTEA (Kaufman & Kaufman, 2004b), which was used to measure word reading ability, reading comprehension ability, and listening comprehension ability.5 The behavioral data collection procedure took 1.5–2 hours to complete. Each of these behavioral assessments has been shown to have medium-high to high reliability (correlation coefficients > .80) and validity (correlation coefficients > .54) across multiple independent studies (Bain & Jaspers, 2010; Semel et al., 1995; Vladescu, 2007). Behavioral assessment data were analyzed using a MANCOVA, with each of the behavioral assessment measures as a dependent variable, group (PCE, NDE) as a fixed factor, and age, grade, SES, and other drug use (based on self-reported tobacco, marijuana, and alcohol use during the 30 days prior to birth) included as covariates.
ERP tasks
Rhyme prime task (RPT)
The RPT consisted of 100 visually presented prime-target pairs, half of which rhymed when pronounced (plaid – glad), and half of which did not (life – shelf). Rhyme prime-target pairs were chosen to be as visually dissimilar as possible (e.g., homophone pairs such as meat – meet were not included). Rhyme targets and nonrhyme targets were matched on mean length and mean frequency using the Kucera & Francis (1967) norms obtained from the MRC database http://websites.psychology.uwa.edu.au/school/MRCDatabase/uwa_mrc.htm. All words selected for this experiment were relatively high frequency to ensure that vocabulary knowledge did not limit ability to perform the task. Additionally, the majority of primes and targets had regular spellings (Lint rather than Pint; see Table 2 for a summary of prime and target characteristics). Words were presented in lower case white Courier New size 18 font on a black background on a 14-inch square Dell LCD display that was measured to be 1 yard from the participant's head. Each trial started with a fixation cross (1000 ms), followed by the prime word (1500 ms), followed by a fixation cross (1000 ms), followed by the target word (1500 ms), followed by a question mark (4000 ms). Participants indicated whether the target rhymed with the prime via button press while the question mark was on the screen; 400 milliseconds after the question mark disappeared, the next trial began (note that null responses factored into the accuracy but were excluded from reaction time analyses). The full experiment lasted approximately 15 minutes.
Table 2.
Length and frequency for primes and targets.
| Experiment | Related Prime | Unrelated Prime | Related Target | Unrelated Target | |
|---|---|---|---|---|---|
| RPT | Length | 4.74 (0.83) | 4.42 (0.88) | 4.54 (0.73) | 4.66 (0.75) |
| Frequency | 136.00 (249.00) | 136.00 (252.00) | 87.00 (149.00) | 87.00 (163.00) | |
| SPT | Length | 4.32 (0.94) | 4.50 (0.84) | 4.66 (0.96) | 4.50 (0.81) |
| Frequency | 102.50 (161) | 102.50 (238.50) | 63.20 (86.20) | 63.20 (80.20) | |
|
| |||||
| SAT | Length | Congruent 4.90 Target (1.26) | Anomalous 4.78 Target (1.12) | ||
| Frequency | 142.16 (114.85) | 141.25 (190.38) | |||
Semantic prime task (SPT)
The SPT had identical structure to the RPT, with the sole difference being the relationship between related prime target pairs, which were either semantically related (west-east) or unrelated (church-scale). Again, targets for semantically related and semantically unrelated pairs were matched on number of letters and frequency (see Table 2 for prime and target stimulus characteristics). Participants indicated whether the target was semantically related to the prime via button press while the question mark was on the screen (note that null responses factored into the accuracy but were excluded from RT analyses). The full experiment lasted approximately 15 minutes.
Semantic anomaly task (SAT)
This task was designed to examine semantic processing/integration at the sentence level. To explore this level of processing we used a semantic anomaly detection task (SAT), which produces reliable ERP response to semantically anomalous relative to semantically congruent words embedded in sentences. The 80 sentence stimuli in this experiment were based on sentences used in Atchley et al. (2006). These sentences were chosen because they have been well normed for children and produce consistent, easily interpretable semantic anomaly-associated results. In particular, target sentences (½ of the sentences) contained a sentence final semantic violation “where does a chair like to play?” as compared to control semantically congruous sentences “where does a boy like to play?” ERP analyses were time locked to the onset of the final word. Target words were matched for length and frequency (Table 2). Individual words for each sentence were presented in white Courier New size 18 font on a black background. Each trial started with a fixation cross (1000 ms), followed by each word in the sentence (1500 ms each), followed by a question mark (4000 ms). After each sentence participants were asked to judge whether the sentence made sense and press one of two buttons (1 for yes, 4 for no) while the question mark remained on the screen. Again, null responses were included in the accuracy data, but not in the RT data. The total duration of this experiment was 22 minutes.
ERP data collection & analysis procedures
ERP data collection
ERP data was collected with an Electrical Geodesics Inc. (EGI) netamps 200 system, using 128 channel geodesic sponge electrode caps. EEG was continuously recorded while participants completed experimental tasks presented using E-Prime (Psychological Software Tools). The EEG sensor nets were soaked for up to ten minutes prior to use in a warm potassium chloride solution (2 teaspoons of potassium chloride, 1 liter of purified bottled water, and 3 ccs of Johnson & Johnson baby shampoo to remove oils from the scalp). The high density geodesic sensor nets and associated high impedance amplifiers have been designed to accept impedance values ranging as high as 100 kΩ, which permits the sensor nets to be applied in under ten minutes and without scalp abrasion, recording paste, or gel (e.g., Ferree, Luu, Russell, & Tucker, 2001; Pizzagalli, 2007). Impedance for all electrodes was kept below 40 kΩ throughout the experimental run (impedances were re-checked between blocks).
ERP preprocessing procedures
All initial processing was conducted using Netstation v.4.5.7 EEG data were band pass filtered at .3 to 30 hz and segmented by condition, 100 milliseconds pre-stimulus to 900 milliseconds post-stimulus. Artifacts were automatically detected and manually verified for exclusion from additional analysis. Eye blinks and vertical eye movements were evaluated using electrodes placed below and above the eyes (channels 8, 126, 25, 127). Horizontal eye movements were measured using channels 125 and 128, located at positions to the left and right of the eyes. Artifacts were automatically detected and manually verified for exclusion from additional analysis (bad channel >200 microvolts, eye blinks >140 microvolts and eye movement >55 microvolts). For every channel, 50% or greater bad segments was used as the criteria for marking the channel bad; for every segment, greater than 20 bad channels was used as a criterion for marking a segment bad.
Participants with less than 50% usable trials per condition in an experiment were excluded from our analysis for each experiment. Bad channels were spline interpolated from nearby electrodes. Data were baseline corrected using a 100-millisecond window prior to onset of all stimuli. Data were re-referenced from vertex recording to an average reference of all 128 channels. All processed, artifact-free segments were averaged by condition, producing a single event related potential for each condition for all participants and exported for plotting and statistical analysis in R.
ERP analysis procedures
Electrode montages for each experiment were chosen based on a combination of visual inspection and previous literature for maximum N400 effects (Coch, Hart, & Mitra, 2008; Deacon, Breton, Ritter, & Vaughan, 1991; Grossi, Coch, Coffey-Corina, Holcomb, & Neville, 2001). For the rhyme prime and semantic prime experiments, a central anterior montage of 12 electrodes (5, 6, 7, 12, 13, 31, 32, 55, 81, 106, 107, 113) was selected (Figure 1); for the anomaly experiment, a more posterior montage of 14 central electrodes (32, 53, 54, 55, 61, 62, 67, 68, 73, 78, 79, 80, 81, 87) was selected. The same montages were used for analysis of the early N1-P2 and P2-N2 complexes in the priming and sentence anomaly experiments respectively. See Figure 1 for montage display.
Figure 1.
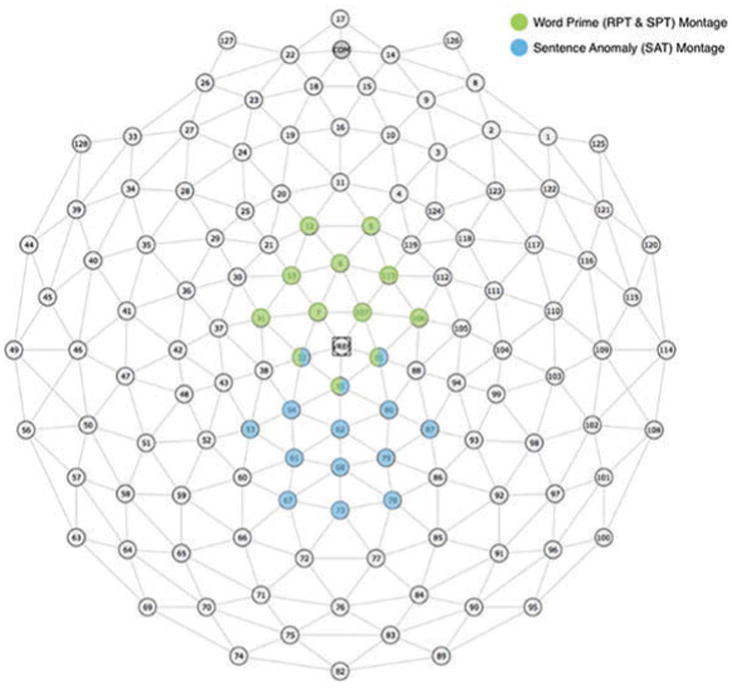
Electrode montages used for analysis of ERP components for the word priming experiments (green) and sentence anomaly experiment (blue).
Accuracy and reaction time for each experiment were analyzed to compare overall group performance for each experiment using two-tailed independent samples t tests. ERP data for each component of interest (N1-P2; N2-P2; N400) was analyzed using a mixed model repeated measures ANOVA with experimental condition (primed vs. unprimed; anomalous vs. congruent) as the within subjects variable and group (PCE, NDE) as the between subjects variable.
Results
Behavioral assessments
We report three sets of behavioral data: one for the entire sample, and one for each of our ERP task subsamples.
All participants
A MANCOVA that included participant age, grade, maternal education (SES), and exposure to other substances (alcohol, tobacco, marijuana), and nonverbal IQ as covariates revealed a main effect of group on language and reading measures F(1,7) = 3.61, p = .001, see Table 1 for pairwise group comparisons on the covariates included in the model. Bonferroni corrected tests of between subjects effects revealed that PCE adolescents performed more poorly than NDE adolescents on most of our reading and language subtests after controlling for age, grade, SES, exposure to other substances, and nonverbal IQ (the same covariates were included in the subsample analyses reported in the following section). Specifically, PCE adolescents performed more poorly than NDE adolescents on the recalling sentences subtest from the CELF F(1,7) = 7.7, p = .007, partial η2 = .07; on the word and letter reading subtest from the KTEA F(1,7) = 9.24, p = .003, partial η2 = .08; and on the reading comprehension subtest from the KTEA F(1,7) = 8.82, p = .004, partial η2 = .08. PCE adolescents performed marginally worse than NDE adolescents on the listening comprehension subtest from the KTEA F(1,7) = 3.49, p = .065 partial η2 = .033 and on the word associations subtest from the CELF F(1,7) = 2.97, p = .088, partial η2 = .029. PCE and NDE groups did not differ on verbal IQ F(1,7) = 1.3, p = .256 partial η2 = .013; or on the semantic relations subtest from the CELF F(1,7) = 2.23, p = .138, partial η2 = .022. Means and standard errors for the groups on these assessments are displayed in Table 3.
Table 3.
Group effects, means, and standard deviations for our assessments of reading and language.
| Full Sample (N=110) | PCE M (SD) | NDE M (SD) | F | p | partial η2 |
| KBIT: Verbal IQ | 84.66 (11.21) | 93.54 (12.18) | 1.30 | .260 | .01 |
| CELF: Word Associations | 7.92 (3.69) | 9.98 (3.11) | 2.97 | .080 | .03 |
| CELF: Recalling Sentences | 8.31 (3.36) | 10.10 (2.19) | 7.70 | <.010 | .07 |
| CELF: Semantic Relations | 6.07 (2.80) | 7.80 (2.74) | 2.23 | .140 | .02 |
| KTEA: Letter Word | 85.80 (11.15) | 95.61 (10.12) | 9.25 | <.010 | .08 |
| KTEA: Reading Comprehension | 55.64 (16.31) | 69.40 (11.98) | 8.88 | <.010 | .08 |
| KTEA: Listening Comprehension | 83.52 (14.28) | 93.14 (12.81) | 3.49 | .070 | .03 |
|
| |||||
| RPT & SPT Sample (N = 70) | |||||
| KBIT: Verbal IQ | 84.82 (12.44) | 94.33 (12.73) | 3.12 | .080 | .05 |
| CELF: Word Associations | 8.17 (3.77) | 9.91 (3.16) | 2.37 | .130 | .04 |
| CELF: Recalling Sentences | 8.41 (3.57) | 10.00 (1.85) | 17.2 | <.001 | .22 |
| CELF: Semantic Relations | 6.32 (3.05) | 7.37 (2.02) | 6.35 | .010 | .10 |
| KTEA: Letter Word | 86.31 (12.83) | 96.26 (10.36) | 6.99 | .010 | .10 |
| KTEA: Reading Comprehension | 56.39 (17.47) | 69.97 (12.13) | 11.8 | .001 | .16 |
| KTEA: Listening Comprehension | 85.47 (14.76) | 92.92 (12.44) | 6.581 | .010 | .09 |
|
| |||||
| SAT Sample (N = 60) | |||||
| KBIT: Verbal IQ | 83.66 (11.26) | 92.30 (10.21) | 3.48 | .070 | .064 |
| CELF: Word Associations | 7.10 (3.59) | 9.63 (2.97) | 3.72 | .060 | .068 |
| CELF: Recalling Sentences | 7.48 (3.14) | 10.00 (2.39) | 9.45 | <.010 | .056 |
| CELF: Semantic Relations | 5.70 (2.49) | 8.00 (3.11) | 4.42 | .040 | .080 |
| KTEA: Letter Word | 83.17 (11.79) | 95.83 (9.77) | 10.78 | <.010 | .175 |
| KTEA: Reading Comprehension | 52.45 (15.69) | 69.53 (12.48) | 11.59 | <.010 | .185 |
| KTEA: Listening Comprehension | 83.88 (13.16) | 94.17 (15.48) | 1.34 | .260 | .025 |
RPT & SPT participants
Overall, in this subsample, PCE adolescents performed more poorly than NDE adolescents on measures of language and reading. Our MANCOVA revealed a main effect of group F(1,7) = 3.05, p = .009, partial η2 = .280, and Bonferroni corrected tests of between subjects effects revealed that PCE adolescents performed more poorly on the recalling sentences subtest of the CELF F(1,7) = 17.21, p < .001 partial η2ss = .220; on the semantic relations subtest of the CELF F(1,7) = 6.35, p = .014, partial η2 = .094; on the word and letter reading subtest of the KTEA F(1,7) = 6.99, p = .01 partial η2 = .103; on the reading comprehension subtest of the KTEA F(1,7) = 11.88, p = .001, partial η2 = .185; on the listening comprehension subtest of the KTEA F(1,7) = 6.58, p = .013, partial η2 = .097. In addition, PCE adolescents also performed marginally more poorly on verbal IQ F(1,7) = 3.12, p = .082, partial η2 = .049. PCE and NDE adolescents in this sample did not differ on the word associations subtest of the CELF F(1,7) = 2.37, p = .129, partial η2 = .037. Means and standard errors for the groups on these assessments are displayed in Table 3.
SAT participants
Overall, in this subsample, PCE adolescents performed more poorly than NDE adolescents on measures of language and reading. Our MANCOVA revealed a main effect of group F(1,7) = 2.42, p = .034, partial η2 = .270, and Bonferroni corrected tests of between subjects effects revealed that PCE adolescents performed more poorly on the recalling sentences subtest of the CELF F(1,7) = 9.45, p = .003, partial η2 = .156; on the semantic relations subtest of the CELF F(1,7) = 4.42, p = .04, partial η2 = .080; on the word and letter reading subtest of the KTEA F(1,7) = 10.78, p = .002, partial η2 = .175; on the reading comprehension subtest of the KTEA F(1,7) = 11.59, p = .001, partial η2 = .185. In addition, PCE adolescents also performed marginally more poorly on verbal IQ F(1,7) = 3.478, p = .07, partial η2 = .064 and on the word associations subtest of the CELF F(1,7) = 3.71, p = .06, partial η2 = .059. PCE and NDE adolescents in this sample did not differ on the listening comprehension subtest of the KTEA F(1,7) = 1.3 p = .259, partial η2 = .025. Means and standard errors for the groups on these assessments are displayed in Table 3.
ERP findings
Rhyme prime task (RPT)
Accuracy and Reaction Time: On average, participants were highly accurate mean accuracy = 90.34%; SD = 8.85. An independent samples t test revealed that PCE adolescents were significantly less accurate than NDE adolescents t(68) = 2.74, p = 0.008; PCE mean accuracy = 87.59 (SD = 10.64); NDE mean accuracy = 93.09 (SD = 5.49). With respect to reaction time, an independent samples t test revealed that PCE and NDE adolescents did not differ significantly in RT for correct trails t(68) = -0.53, p = 0.597 (mean RT = 637.32 ms; SD = 179.63).
N1- P2: To calculate the N1-P2 complex amplitude we subtracted the adaptive mean amplitude of the first negative peak (between 100–150 ms) from the second positive peak (between 150–250 ms) for each condition of interest (as in Landi et al., 2012). A mixed model repeated measures ANOVA conducted on this difference revealed a main effect of Group F(1,68) = 9.06, p = 0.004, η2 = 0.118, but no significant effects of condition (p > .1) and no group by condition interaction (p > .1), such that NDE participants had larger peak to peak differences, regardless of condition (See Fig. 2).
Figure 2.
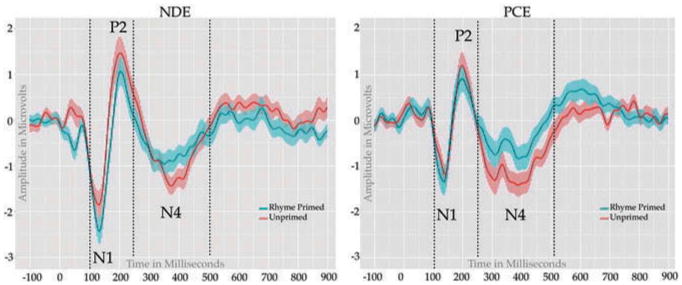
Rhyme Prime Task: ERP waveforms elicited by the rhyme primed (blue) and unprimed (red) targets.
N400: The N400 was calculated using the adaptive mean (± 25 ms on either side of the peak) amplitude of the most negative peak between 250–500 ms. A mixed model repeated measures ANOVA conducted revealed a main effect of condition with unrelated targets producing a more negative deflection than nonrhyming targets F(1,68) = 4.89, p = 0.03, η2 = 0.067. We also observed a main effect of group F(1,68) = 4.25, p = 0.043, η2 = 0.059, with PCE adolescents showing overall more negative amplitudes during this time window for both primed and unprimed targets, however there was no interaction between condition and group (p > .1).
Semantic priming task (SPT)
Accuracy and Reaction Time: On average, participants were accurate, mean accuracy = 85.16%, SD = 12.54. An independent samples t test revealed no difference in accuracy between PCE and NDE adolescents t(68) = 1.77, p = 0.081; PCE mean accuracy = 82.52 (SD = 15.18), NDE mean accuracy = 87.79 (SD = 8.62). With respect to reaction time, an independent samples t test revealed that PCE and NDE adolescents did not differ significantly in RT for correct trials t(68) = -0.192, p = 0.848 (mean RT = 705.78ms, SD = 237.62).
N1-P2: The N1-P2 was calculated in the same manner as in the RPT. A mixed model repeated measures ANOVA conducted on the amplitude difference revealed a main effect of condition with semantically related targets producing larger peak to peak differences than unrelated targets F(1,68) = 4.89, p = 0.030, η2 = 0.067. In addition, there was a marginal effect of group F(1,68) = 3.06, p = 0.085, η2 = 0.043, with NDE adolescents showing a trend for larger peak to peak amplitudes (see Figure 3).
Figure 3.
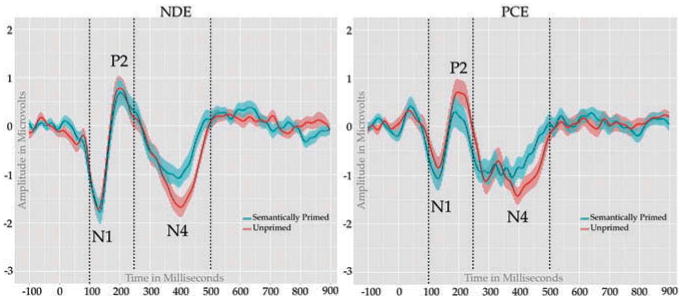
Semantic Priming Task: ERP waveforms elicited by semantically primed (blue) and unprimed (red) targets.
N400. The N400 was calculated in the same manner as in the RPT. A mixed model repeated measures ANOVA conducted revealed a main effect of condition, with semantically related targets producing a more negative deflection than unrelated targets F(1,68) = 21.68, p < 0.001, η2 = 0.241 (Figure 3). (all ps > .1).
Semantic anomaly task (SAT)
Accuracy and Reaction Time: On average participants were accurate, mean accuracy = 86.72% SD = 13.09. An independent samples t test revealed that PCE adolescents were significantly less accurate than NDE adolescents t(68) = 5.22, p < .001; PCE mean accuracy = 79.38 (SD = 13.89), NDE mean accuracy = 94.06 (SD = 6.66). With respect to reaction time, an independent samples t test revealed that PCE adolescents were also slower to respond than NDE adolescents on correct trials t(68) = -2.93, p = 0.005; PCE mean RT = 847.27 (SD = 304.37), NDE mean RT = 640.38 (SD = 239.35)
P2-N2: For this condition, the early complex was protracted relative to the word priming conditions, and thus measured using a subtraction of the adaptive mean amplitude of the first positive peak (between 100–150 ms) from the second negative peak (between 150–300 ms) for each condition of interest. For this complex, a mixed model repeated measures ANOVA conducted on the amplitude difference revealed main effect of group F(1,58) = 5.95, p = 0.018, η2 = 0.093, such that NDE adolescents had larger peak to peak amplitudes relative to PCE adolescents. Further, we observed an interaction between condition and group F(1,58) = 5.51, p = 0.022, η2 = 0.086; observation of Figure 4 reveals a reversal in the N2 effect that such NDE adolescents had a larger N2 for semantically congruent targets relative to semantically anomalous targets, whereas PCE adolescents had a larger N2 for anomalous targets. No other effects were observed.
Figure 4.
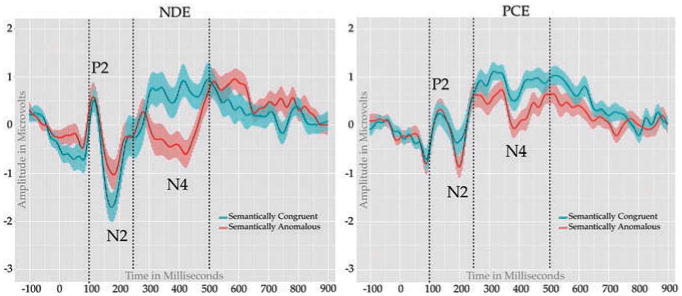
Semantic Anomaly Task: ERP waveforms elicited by semantically congruent (blue) and semantically anomalous (red) targets.
N400: The N400 was calculated in the same manner as in the two priming experiments. A mixed model repeated measures ANOVA conducted revealed a main effect of condition with semantically anomalous targets producing a more negative deflection than unrelated targets F(1,58) = 12.74, p = 0.0007, η2 = 0.079. We also observed a main effect of group, F(1,58) = 5.83, p = 0.0190, η2 = 0.062, with generally more negative amplitude for NDE relative to PCE adolescents. The interaction of group by condition (N400 effect) approached significance F(1,58) = 3.12, p = 0.0827, η2 = 0.018, with NDE adolescents showing a numerically larger (NDE mean = -2.097 (SD = 2.026); PCE mean = -1.271 (SD = 0.907; mean difference = -0.850) and visually larger N400 effect (see Figure 4).
Discussion
The behavioral and neural effects of PCE on reading and language function have not been well-examined in older adolescents. In terms of behavioral performance, overall, we observed that PCE adolescents performed more poorly than those who were not exposed to any drugs or alcohol (NDE) on all behavioral assessments of reading and some measures of general language function. Specifically, when the entire sample was analyzed, performance deficits were observed in single word reading and reading comprehension, and on the recalling sentences measure from the CELF. These findings held after controlling for age, grade, nonverbal IQ, maternal SES, and exposure to other drugs or alcohol (for the exposed group). When comparing behavioral performance for the subsets of participants who completed our ERP tasks, PCE adolescents performed more poorly than NDE controls on all reading measures, on the recalling sentences subtest from the CELF, and on at least one of the other language assessments from the CELF (semantic relations, word associations, listening comprehension, verbal IQ). These findings suggest that exposure to cocaine and other drugs in utero continues to exert downstream effects on language processing and reading skill into the late teenage years. Furthermore, when compared to our previous findings from this cohort, our findings suggest that behavioral differences between PCE and NDE children on measures of language and reading may increase over time. However, this difference is observational only, as the two samples have overlapping but not identical sets of participants, and we have included additional assessments in the current study. Our study is the longest prospective investigation of the behavioral and neural aspects of language and reading in PCE.
With respect to our ERP tasks, on average, PCE adolescents were less accurate than NDE adolescents in making linguistic judgments about the stimuli. These differences were significant when participants had to make judgments about whether two words rhymed (RPT) and whether sentences made sense (SAT); however, this group difference failed to reach significance when participants were asked to judge if two words were related in meaning (SPT). Although the ERP tasks contained relatively high frequency words and sentence constructions, group differences suggest that tasks that require metalinguistic knowledge (explicit awareness of rhyme or semantic coherence) were sensitive to overall language ability in our sample.
With respect to neural response, we found significant differences between adolescents who had been exposed to cocaine (PCE) and those who were not exposed (NDE) across all experiments in the early N1-P2 or P2-N2 complex (though the main effect of group in the SAT failed to reach statistical significance). These early effects likely reflect differences between the groups in the ability to rapidly identify letters and words and access their phonological representations. Consistent with this interpretation, these early effects were observed as main effects of group, and not group by condition interactions for the two priming experiments. This suggests that the relationship between prime and target (be it rhyme or semantic relatedness) did not impact the overall group difference in processing at this early stage. However, in the sentence anomaly experiment, we observed a condition (anomalous vs. congruent) by group interaction, suggesting that PCE and NDE adolescents differed in their ability to comprehend the text preceding the target such that the PCE adolescents were differentially impacted by the coherence of the target word. This may reflect differences in the degree to which PCE and NDE participants generate expectations about upcoming words in a text, or differences in the ability to integrate words into text. Substantial research documents that individuals with poorer reading comprehension skill are less likely to generate predictions about upcoming text when they read (e.g., Bowyer-Crane & Snowling, 2005; Denton et al., 2015; Yeari, Elentok, & Schiff, 2017), and are poorer at integrating words into text (e.g., Long & Chong, 2001; Stafura et al., 2015).
With respect to later processing, we did not find significant N400 group effects for either of the two priming tasks. This finding was somewhat unexpected given differences between the groups on our standardized assessments of decoding and lexical semantic processing. In the RPT, we did find more negative amplitudes for PCE participants in the N400 window overall, but these were not qualified by condition, and thus do not represent a larger N400 effect, per se. Though numerical differences between the two groups suggest a larger N400 effect for PCE adolescents, and visual inspection reveals a distinct morphology for the PCE group with two negative deflections and a prolonged separation in ERP response to unprimed versus unprimed words. At first consideration, this is a surprising result, as larger N400 effects would be expected for the NDE group given their superior reading and language skill, which typically reflects faster access to phonological codes and more intact phonological representations. However, when considering the larger N1/P2 amplitudes for NDE adolescents, this finding may suggest a delay in phonological recoding for PCE adolescents, which led to larger later emerging condition differences and a distinct N400 morphology.
In contrast to the RPT, we did not observe any overall difference in amplitude during the N400 window as a function of group in the SPT, nor did we observe any trends in the data, numerically or visually, that would suggest differential processing between PCE and NDE participants during this time window. Moreover, the N400 effect that we observed for both groups (main effect of condition) was small. This lack of a later ERP effect is consistent with the lack of any accuracy difference between the groups in this task. Thus, it is possible that the task demands at the metalinguistic level (here a semantic judgment) were less challenging (“decide whether the words are related in meaning”), or more ambiguous, and had the effect of yielding comparable neural responses for both groups. It's also possible that group differences in lower-level aspects of word processing (which we observed during the N1-P2 window) are simply swamping out later, higher-level semantic effects. This interpretation is consistent with the generally poor reading performance of PCE participants, and with the pattern of effects observed for the RPT. Given that that those with PCE are significantly worse on behavioral assessments of single word reading and tasks that tap processing of semantic and syntactic information (reading comprehension, recalling sentences), further exploration of these skills at the behavioral and neural levels is needed to identify which aspects of linguistic processing are most impacted in those with PCE.
As previously noted, the only task that was associated with a significant group difference in the N400 effect (a group by condition interaction) in the expected direction was the SAT. For this task, we observed a reduced N400 effect for PCE relative to NDE participants in response to sentence final target words. This finding likely reflects the poorer reading comprehension ability of PCE participants, such that reading of the target word was slower and more difficult to integrate into text, resulting in slower and more error prone detection of anomaly. Again, this neural finding is consistent with both the poorer reading comprehension performance of PCE adolescents on the KTEA and with poorer performance of PCE adolescents in the anomaly judgment task.
Taken together, our findings suggest that early disruptions to the monoaminergic systems of the developing fetus, resulting from PCE and other drugs, can have lasting impacts through adolescence on the cognitive and neural systems that support language and reading development. These negative impacts were observed at multiple levels, from lower-level phonological decoding skills to processing and integration of meaning during text comprehension. These findings are consistent with several other reports of impaired expressive language, syntactic processing, and phonological processing in older children and adolescents with PCE (Bandstra et al, 2011; Landi et al., 2012; Singer et al, 2013).
With respect to the neural systems implicated, our findings of impaired word and text processing and at the behavioral and neural levels are consistent with our prior ERP work that implicated impaired systems for phonological processing in adolescents with PCE. In addition, these findings are consistent with other imaging work that finds reduced gray matter volume in individuals with PCE in the caudate nucleus (Avants et al, 2007), a region that is important for language learning and reading (Chan, Ryan, & Bever, 2013; Pugh et al., 2013) as well as findings of atypical white matter integrity in tracts that connect frontal and temporal regions that are important for phonological and semantic access (Binder, Desai, Graves, & Conant, 2009; Burton, 2001; Fiez, 1997). In terms of mechanism, we speculate that the monoaminergic disruptions caused by PCE during the prenatal period alter basic cortical and subcortical learning systems (Mayes, 1999; Stanwood, Washington, Shumsky, & Levitt, 2001) that are necessary for language and reading acquisition. However, specific examinations of procedural learning mechanisms in relation to later language and literacy outcomes in PCE are needed to test this hypothesis.
Strengths of the current study include concurrent behavioral and neurobiological examinations of language and reading in a well characterized sample of PCE and NDE children that have been followed prospectively since birth into late adolescence. In addition, the inclusion of important environmental and cognitive covariates in our behavioral analyses, including SES, exposure to other substances, and nonverbal IQ is a distinct strength. However, several limitations should be noted. Although we included age and grade in our behavioral analysis, the age range of our participants was rather large (ages 14–20). In addition, given the reduced power in our ERP analyses, we were not able to include all the covariates from our behavioral analysis in our ERP analyses. Therefore, factors such as SES (e.g., maternal education), which differed between the two groups, and exposure to substances other than cocaine could be contributing to our observed ERP effects. Finally, because we do not have reliable measures of the amount of cocaine used by the mothers of our participants during the prenatal period, we could not examine dosage effects in this study. Although difficult to obtain, large samples of older adolescents prenatally exposed to cocaine and other substances of abuse with precise information on dosage and other environmental, economic, and social factors should be the target of future studies to tease apart the relative contributions of these variables to cognitive and language outcomes.
In sum, our behavioral findings suggest relatively broad effects of prenatal cocaine exposure on language and reading function in adolescents across ages 14–20. Differences between PCE and NDE adolescents were observed in expressive and receptive measures of language and in word reading, reading comprehension, and listening comprehension. We also observed differences in accuracy between the PCE and NDE adolescents during our ERP tasks when they were asked to make metalinguistic judgements about rhyme and semantic congruence. At the neural level, we observed differences between PCE and NDE adolescents in early ERP components associated with phonological decoding of words across all ERP tasks, and in later processing, in the RPT and SAT tasks, reflecting differences in metalinguistic aspects of rhyme detection and semantic anomaly detection. These neurobiological findings are generally consistent with our behavioral findings and with neurobiological findings from our group and others, suggesting that PCE is associated with anomaly in neural systems that support language and reading. Taken together, this set of findings suggests that PCE is associated with language and reading impairments, observed at both the behavioral and neural levels, into late adolescence.
Acknowledgments
This research was supported in part by funding from the National Institute on Drug Abuse (R21 DA030665).
Footnotes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/HDVN.
It should be noted that dose-dependent findings can be difficult to interpret because they are often based on self-reported drug use.
For all analyses age, grade, SES and other drug use were entered as covariates.
It is rare to find a sample of pregnant women who use only cocaine during pregnancy. In this sample, the drug of addiction was cocaine; however, some of these women used some alcohol, smoked some cigarettes and some smoked marijuana, or a combination of these drugs during the prenatal period, see Table 1.
No parents or children self-reported a neurological disorder or developmental disability including dyslexia, specific language impairment, or specific reading comprehension deficit (S-RCD). We did not screen for ADHD, however, we excluded any participants on psychoactive medications.
All RAs who completed assessments at any time were blind to exposure status.
References
- Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school aged children. Pediatrics. 2010;125(3):554–565. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman PT, Dykman RA, Oglesby DM. Visual event-related potentials of dyslexic children to rhyming and nonrhyming stimuli. Journal of Clinical and Experimental Neuropsychology. 1994;16(1):138–154. doi: 10.1080/01688639408402624. [DOI] [PubMed] [Google Scholar]
- Alessandri S, Sullivan M, Imaizumi S, Lewis M. Learning and emotional responsivity in cocaine exposed infants. Developmental Psychology. 1993;29:989–997. doi: 10.1037/0012-1649.29.6.989. [DOI] [Google Scholar]
- Anday EK, Cohen ME, Kelley NE, Leitner DS. Effect of in utero cocaine exposure on startle and its modification. Developmental Pharmacology and Therapeutics. 1988;12(3):137–145. [PubMed] [Google Scholar]
- Atchley RA, Rice ML, Betz SK, Kwasny KM, Sereno JA, Jongman A. A comparison of semantic and syntactic event related potentials generated by children and adults. Brain and Language. 2006;99(3):236–246. doi: 10.1016/j.bandl.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Avants B, Hurt H, Giannetta JM, Epstein BA, Shera D, Rao H, et al. Gee J. Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatric Neurology. 2007;37:275–279. doi: 10.1016/j.pediatrneurol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Bain SK, Jaspers KE. Journal of Psychoeducational Assessment. 2. Vol. 28. Bloomington, MN: Pearson, Inc; 2010. Test review: Review of Kaufman Brief Intelligence Test: Kaufman, AS, & Kaufman, NL (2004). Kaufman Brief Intelligence Test; pp. 167–174. [DOI] [Google Scholar]
- Bandstra ES, Morrow CE, Accornero VH, Mansoor E, Xue L, Anthony JC. Estimated effects of in utero cocaine exposure on language development through adolescence. Neurotoxicology and Teratology. 2011;33:25–35. doi: 10.1016/j.ntt.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Vogel AL, Fifer RC, Ofir A, Dausta AT, Anthony JC. Longitudinal influence of prenatal cocaine exposure on child language functioning. Neuroxicology and Teratology. 2002;24:297–308. doi: 10.1016/S0892-0362(02)00192-7. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Vogel AL, Morrow CE, Xue L, Anthony JC. Severity of prenatalcocaine exposure and child language functioning through age seven years: A longitudinal latent growth curve analysis. Substance Use and Misuse. 2004;39:25–59. doi: 10.1081/JA-120027765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JM, Hanson C. Catecholamines inhibit growth in fetal sheep in the absence of hypoxemia. American Journal of Physiology. 1998;274(part 2):R1536–R1545. doi: 10.1152/ajpregu.1998.274.6.R1536. [DOI] [PubMed] [Google Scholar]
- Betancourt LM, Yang W, Brodsky NL, Gallagher PR, Malmud EK, Giannetta JM, et al. Hurt H. Adolescents with and without gestational cocaine exposure: Longitudinal analysis of inhibitory control, memory and receptive language. Neurotoxicology and Teratology. 2011;33(1):36–46. doi: 10.1016/j.ntt.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhide PG. Dopamine, cocaine and the development of cerebral cortical cytoarchitecture: A review of current concepts. Seminars in Cell & Developmental Biology. 2009;20(4):395–402. doi: 10.1016/j.semcdb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer-Crane C, Snowling MJ. Assessing children's inference generation: What do tests of reading comprehension measure? British Journal of Educational Psychology. 2005;75(2):189–201. doi: 10.1348/000709904X22674. [DOI] [PubMed] [Google Scholar]
- Burton MW. The role of inferior frontal cortex in phonological processing. Cognitive Science. 2001;25(5):695–709. doi: 10.1207/s15516709cog2505_4. [DOI] [Google Scholar]
- Chan SH, Ryan L, Bever TG. Role of the striatum in language: Syntactic and conceptual sequencing. Brain and Language. 2013;125(3):283–294. doi: 10.1016/j.bandl.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Coch D, Hart T, Mitra P. Three kinds of rhymes: An ERP study. Brain and Language. 2008;104(3):230–243. doi: 10.1016/j.bandl.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Cone-Wesson B. Prenatal alcohol and cocaine exposure: Influences on cognition, speech, language and hearing. Journal of Communication Disorders. 2005;38:279–302. doi: 10.1016/j.jcomdis.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Deacon D, Breton F, Ritter W, Vaughan HG. The relationship between N2 and N400: Scalp distribution, stimulus probability, and task relevance. Psychophysiology. 1991;28(2):185–200. doi: 10.1111/psyp.1991.28.issue-2. [DOI] [PubMed] [Google Scholar]
- Delaney–Black V, Covington C, Templin T, Kershaw T, Nordstrom-Klee B, Ager J, Sokol R. Expressive language development of children exposed to cocaine prenatally: Literature review and report of a prospective cohort study. Journal of Communication Disorders. 2000;33:463–481. doi: 10.1016/S0021-9924(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Denton CA, Enos M, York MJ, Francis DJ, Barnes MA, Kulesz PA, et al. Carter S. Text-Processing differences in adolescent adequate and poor comprehenders reading accessible and challenging narrative and informational text. Reading Research Quarterly. 2015;50(4):393–416. doi: 10.1002/rrq.105. [DOI] [Google Scholar]
- Dujardin T, Etienne Y, Contentin C, Bernard C, Largy P, Mellier D, et al. Rebaï M. Behavioral performances in participants with phonological dyslexia and different patterns on the N170 component. Brain and Cognition. 2011;75(2):91–100. doi: 10.1016/j.bandc.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Espy KA, Kaufmann PM, Glisky ML. Neuropsychologic function in toddlers exposed to cocaine in utero: A preliminary study. Developmental Neuropsychology. 1999;15(3):447–460. doi: 10.1080/87565649909540761. [DOI] [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clinical Neurophysiology. 2001;112(3):536–544. doi: 10.1016/S1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Human Brain Mapping. 1997;5(2):79–83. doi: 10.1002/(SICI)1097-0193(1997)5:2<>1.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Frank DA, Augustyn M, Knight WG, Pell T, Zuckerman B. Growth Development, and behavior in early childhood following prenatal cocaine exposure, A systematic review. Journal of the American Medical Association. 2001;285:1613–1625. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger J, Kiyonaga K, Holcomb PJ. The time course of orthographic and phonological code activation. Psychological Science. 2006;17(12):1021–1026. doi: 10.1111/j.1467-9280.2006.01821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi G, Coch D, Coffey-Corina S, Holcomb PJ, Neville HJ. Phonological processing in visual rhyming: A developmental ERP study. Journal of Cognitive Neuroscience. 2001;13(5):610–625. doi: 10.1162/089892901750363190. [DOI] [PubMed] [Google Scholar]
- Hawley TL, Halle TG, Drasin RE, Thomas NG. Children of addicted mothers: Effects of the “crack epidemic” on the caregiving environment and the development of preschoolers. American Journal of Orthopsychiatry. 1995;65(3):364–379. doi: 10.1037/h0079693. [DOI] [PubMed] [Google Scholar]
- Heffelinger A, Craft S, Shyken J. Visual attention in children with prenatal cocaine exposure. Journal of the International Neurophysological Society. 1997;3:237–245. [PubMed] [Google Scholar]
- Hoffmann W, Turcios J, Cook B, Landi N, Irwin J. A comparison of social measures by parent report reveal developmental differences. Focus on Autism and Other Developmental Disabilities Under review. [Google Scholar]
- Hurt H, Malmud E, Betancourt L, Braitman LE, Brodsky NL, Giannetta J. Children with in utero cocaine exposure do not differ from control subjects on intelligence testing. Archives of pediatrics & adolescent medicine. 1997;151(12):1237–1241. doi: 10.1001/archpedi.1997.02170490063011. [DOI] [PubMed] [Google Scholar]
- Jones CT, Robinson JS. Studies on experimental growth retardation in sheep: Plasma catecholamines in fetuses with small placenta. Journal of Developmental Physiology. 1983;5:77–87. [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test, second edition (KBIT II) Bloomington, MN: Pearson; 2004a. [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Test of Educational Achievement-Second Edition (KTEA-II) Circle Pines, MN: American Guidance Service; 2004b. [Google Scholar]
- Kilbride HW, Castor CA, Fuger KL. School-age outcome of children with prenatal cocaine exposure following early case management. Developmental and Behavioral Pediatrics. 2006;27:181–187. doi: 10.1097/00004703-200606000-00001. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present-day American English. Dartmouth Publishing Group; 1967. [Google Scholar]
- Kutas M, Van Petten C. Event-related brain potential studies of language. Advances in Psychophysiology. 1988;3:139–187. [Google Scholar]
- Landi N, Crowley MJ, Wu J, Bailey C, Mayes LC. Deviant ERP response to spoken non-words among adolescents exposed to cocaine in utero. Brain and Language. 2012;120:209–216. doi: 10.1016/j.bandl.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi N, Perfetti CA. An electrophysiological investigation of semantic and phonological processing in skilled and less skilled comprehenders. Brain and Language. 2007;102:30–45. doi: 10.1016/j.bandl.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Lester B, Padbury J. Third pathophysiology of prenatal cocaine exposure. Developmental Neuroscience. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- Lewis BA, Minnes S, Short EJ, Min MO, Wu M, Lang A, et al. Singer LT. Language outcomes at 12 years for children exposed prenatally to cocaine. Journal of Speech Language and Hearing Research. 2013;56(5):1662–1676. doi: 10.1044/1092-4388(2013/12-0119). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman IY, Shankweiler D. Phonology and the problems of learning to read and write. Remedial and Special Education. 1985;6(6):8–17. doi: 10.1177/074193258500600604. [DOI] [Google Scholar]
- Lidow MS. Prenatal cocaine exposure adversely affects development of the primate cerebral cortex. Synapse. 1995;21:332–341. doi: 10.1002/(ISSN)1098-2396. [DOI] [PubMed] [Google Scholar]
- Long DL, Chong JL. Comprehension skill and global coherence: A paradoxical picture of poor comprehenders' abilities. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:1424. doi: 10.1037//0278-7393.27.6.1424. [DOI] [PubMed] [Google Scholar]
- Malakoff ME, Mayes LC, Schottenfeld R, Howell S. Language production in 24-month-old inner-city children of cocaine-and-other-drug-using mothers. Journal of Applied Developmental Psychology. 1999;20:159–180. doi: 10.1016/S0193-3973(99)80009-4. [DOI] [Google Scholar]
- Mayes LC. Developing brain and in utero cocaine exposure: Effects on neural ontogeny. Development and Psychopathology. 1999;11:685–714. doi: 10.1017/S0954579499002278. [DOI] [PubMed] [Google Scholar]
- Mayes LG, Bornstein MH. Developmental dilemmas for cocaine abusing parents and their children. In: Lewis M, Bendersky M, editors. Mothers, babies, and cocaine: The role of toxins in development. Hillsdale, NJ: Erlbaum; 1995. pp. 251–272. [Google Scholar]
- Mayes LC, Bornstein MH, Chawarska K, Granger RH. Information processing and developmental assessments in 3-month-old infants exposed prenatally to cocaine. Pediatrics. 1995;95(4):539–545. [PubMed] [Google Scholar]
- Mayes LC, Molfese DL, Key AP, Hunter NC. Event-related potentials in cocaine-exposed children during a Stroop task. Neurotoxicoogy and Teratology. 2005;27(6):797–813. doi: 10.1016/j.ntt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Snyder PJ, Langlois E, Hunter N. Visuospatial working memory in school-aged children exposed in Utero to cocaine. Child Neuropsychology. 2007;13:205–218. doi: 10.1080/09297040600888753. [DOI] [PubMed] [Google Scholar]
- Meyler A, Breznitz Z. Impaired phonological and orthographic word representations among adult dyslexic readers: Evidence from event-related potentials. The Journal of Genetic Psychology. 2005;166(2):215–240. doi: 10.3200/GNTP.166.2.215-240. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Roth RH. Male rats exposed to cocaine in utero demonstrate elevated expression of Fos in the prefrontal cortex in response to environment. Neuropsychopharmacology. 2002;26:275–285. doi: 10.1016/S0893-133X(01)00359-1. [DOI] [PubMed] [Google Scholar]
- Perfetti CA, Wlotko EW, Hart LA. Word learning and individual differences in word learning reflected in event-related potentials. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31(6):1281. doi: 10.1037/0278-7393.31.6.1281. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Electroencephalography and high-density electrophysiological source localization. Handbook of Psychophysiology. 2007;3:56–84. [Google Scholar]
- Potter SM, Zelazo PR, Stack DM, Papageorgiou AN. Adverse effects of fetal cocaine exposure on neonatal auditory information processing. Pediatrics. 2000;105(3):e40–e40. doi: 10.1542/peds.105.3.e40. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Landi N, Preston JL, Mencl WE, Austin A, Sibley D, et al. Frost SJ. The relationship between phonological and auditory processing and brain organization in beginning readers. Brain and Language. 2013;25:173–183. doi: 10.1016/j.bandl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüsseler J, Becker P, Johannes S, Münte TF. Semantic, syntactic, and phonological processing of written words in adult developmental dyslexic readers: An event-related brain potential study. BMC Neuroscience. 2007;8(1):52. doi: 10.1186/1471-2202-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage J, Brodsky E, Malmud E, Giannetta JM, Hurt H. Attentional functioning and impulse control in cocaine exposed and control children at age 10 years. Journal of Developmental and Behavioral Performance. 2005;26:42–47. [PubMed] [Google Scholar]
- Sayal K, Heron J, Golding J, Alati R, Smith GD, Gray R, Emond A. Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: Longitudinal population-based study. Pediatrics. 2009;123:e289–e296. doi: 10.1542/peds.2008-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder MD, Snyder PJ, Sieleski I, Mayes L. Impaired performance of children exposed in utero to cocaine on a novel test of visuospatial working memory. Brain and Cognition. 2004;55:409–412. doi: 10.1016/j.bandc.2004.02.062. [DOI] [PubMed] [Google Scholar]
- Schulz E, Maurer U, Van Der Mark S, Bucher K, Brem S, Martin E, Brandeis D. Impaired semantic processing during sentence reading in children with dyslexia: Combined fMRI and ERP evidence. Neuroimage. 2008;41(1):153–168. doi: 10.1016/j.neuroimage.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Semel EM, Wiig EH, Secord W. Psychological Corporation, Harcourt Brace Jovanovich. The Psychological Corporation; San Antonio, TX: 1995. CELF 3: Clinical evaluation of language fundamentals–examiner's manual. [Google Scholar]
- Singer LT, Arendt R, Minnes S, Salvator A, Seigel CA, Lewis BA. Developing language skills of cocaine-exposed infants. Pediatrics. 2001:1057–1064. doi: 10.1542/peds.107.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Lewin J, Minnes S, Weishampel P, Drake K, Satayathum S, et al. Evans A. Neuroimaging of 7–8 year-old children exposed prenatally to cocaine. Neurotoxicology and Teratology. 2006;28(3):386–402. [Google Scholar]
- Stafura JZ, Rickles B, Perfetti CA. ERP evidence for memory and predictive mechanisms in word- to-text integration. Language, Cognition and Neuroscience. 2015;30(10):1273–1290. doi: 10.1080/23273798.2015.1062119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Washington RA, Shumsky JS, Levitt P. Prenatal cocaine exposure produces consistent developmental alterations in dopamine-rich regions of the cerebral cortex. Neuroscience. 2001;106(1):5–14. doi: 10.1016/S0306-4522(01)00256-1. [DOI] [PubMed] [Google Scholar]
- Swaab TY, Brown C, Hagoort P. Understanding ambiguous words in sentence contexts: Electrophysiological evidence for delayed contextual selection in Broca's aphasia. Neuropsychologia. 1998;36:737–761. doi: 10.1016/S0028-3932(97)00174-7. [DOI] [PubMed] [Google Scholar]
- Tan-Laxa MA, Sison-Switala C, Rintelman W, Ostrea EM. Abnormal auditory brainstem response among infants with prenatal cocaine exposure. Pediatrics. 2004;113(2):357–360. doi: 10.1542/peds.113.2.357. [DOI] [PubMed] [Google Scholar]
- Torppa M, Eklund K, Van Bergen E, Lyytinen H. Late-emerging and resolving dyslexia: A Follow-up study from age 3 to 14. Journal of Abnormal Child Psychology. 2015;43(7):1389–1401. doi: 10.1007/s10802-015-0003-1. [DOI] [PubMed] [Google Scholar]
- Vladescu JC. Test review: Kaufman Test of Educational Achievement-(KTEA-II) Journal of Psychoeducational Assessment. 2007;25(1):92–100. doi: 10.1177/0734282906294708. [DOI] [Google Scholar]
- Warner TD, Behnke M, Eyler FD, Padgett K, Leonard C, Hou W, et al. Blackband S. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine exposed children. Pediatrics. 2006;118:2014–2024. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. Journal of Neuroendocrinology. 2001;13:113–128. doi: 10.1111/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Yeari M, Elentok S, Schiff R. Online and offline inferential and textual processing of poor comprehenders: Evidence from a probing method. Journal of Experimental Child Psychology. 2017;155:12–31. doi: 10.1016/j.jecp.2016.10.011. [DOI] [PubMed] [Google Scholar]


