Abstract
Background
Chronic Kidney Disease (CKD) occurs in over one third of patients with Sickle Cell Disease (SCD) and can progress to end-stage renal disease. Unfortunately, current clinical assessments of kidney function are insensitive to early-stage CKD. Prior studies have shown that diffusion Magnetic Resonance Imaging (MRI) can sensitively detect regional renal microstructure changes associated with early-stage chronic kidney disease. However, previous MRI studies in sickle cell disease patients have been largely limited to detection of renal iron deposition assessed by T2* relaxometry. In this pilot imaging study, we compare MRI assessments of renal microstructure (diffusion) and iron deposition (T2*) in SCD patients and non-SCD control subjects.
Methods
Diffusion Tensor Imaging (DTI) and T2* relaxometry MRI data were obtained for pediatric (n=5) and adult (n=4) SCD patients as well as non-SCD control subjects (n=10) on a Siemens Espree 1.5T MRI scanner. A region-of-interest analysis was used to calculate mean medullary and cortical values for each MRI metric. MRI findings were also compared with clinical assessments of renal function and hemolysis.
Results
SCD patients showed a significant decrease in medullary Fractional Anisotropy (FA, p=0.0001) in comparison to non-SCD subjects indicative of microstructural alterations in the renal medulla of SCD patients. Cortical and medullary reductions in T2* (increased iron deposition, p = ≤ 0.0001) were also observed. Significant correlations were also observed between kidney T2* assessments and multiple measures of hemolysis.
Conclusion
This is the first DTI MRI study of SCD patients to demonstrate reductions in medullary FA despite no overt chronic kidney disease (eGFR > 100 ml/min/1.73m2). These medullary FA changes are consistent with prior studies in patients with chronic kidney disease and suggest that DTI MRI can provide a useful measure of kidney injury to complement MRI assessments of iron deposition.
Keywords: Magnetic Resonance Imaging, kidney, sickle cell disease, diffusion, relaxometry
Graphical Abstract
Chronic Kidney Disease (CKD) occurs in over one third of patients with Sickle Cell Disease (SCD), but is not easily detected at early stages. Prior studies have shown that Diffusion Tensor Imaging (DTI) can sensitively detect medullary microstructure changes associated with early-stage CKD, but have not been studied in SCD patients. In this pilot MRI study, we show that medullary Fractional Anisotropy as measured by DTI is significantly reduced in pediatric and adult SCD patients in comparison to control subjects.
INTRODUCTION
Sickle Cell Disease (SCD) is a life-threatening chronic illness that affects over 100,000 African-Americans in the United States. SCD is defined by an abnormal hemoglobin (sickle hemoglobin, HgbS) that causes abnormal polymerization when deoxygenated leading to red cell sickling, hemolysis, and vasculopathy.1,2 Multiple organs are affected by SCD, including the central nervous, cardiopulmonary, and reno-vascular systems.3–6 Within the kidneys, the renal medulla is particularly susceptible to red blood cell sickling and microvascular occlusion, given the lower oxygen tension, lower pH, and relatively low blood flow as compared to the renal cortex.7 The process of intravascular hemolysis likely contributes to the pathophysiology of kidney disease in SCD, as increased free hemoglobin can generate reactive oxygen species and scavenge nitric oxide, resulting in endothelial dysfunction.8 Additionally, free hemoglobin is filtered through the kidney where it is either excreted or reabsorbed in the proximal convoluted tubules to be stored as hemosiderin or ferritin where it is presumed to be toxic to the kidneys.9 Studies also suggest that hemosiderosis (iron overload) associates with proteinuria and that the renal iron load in SCD is primarily caused by hemolysis rather than by repeated red blood cell transfusions.10,11
Overt chronic kidney disease (CKD) occurs in over one third of patients with SCD, progresses over time, and is associated with considerable morbidity and mortality.6,12–14 Renal involvement in SCD initially manifests as “hyperfiltration” in infancy and childhood, with estimated glomerular filtration rates (eGFR) typically > 140 ml/min/1.73m2, and impaired urinary concentrating ability is also observed.15–18 While these abnormalities are present in almost all pediatric patients with SCD, only a subset of patients progress to overt CKD (decreased eGFR and/or proteinuria indicative of underlying glomerulosclerosis and tubulointerstitial scarring). Recent data from our group,19 examining cross-sectional data from multiple databases, as well as the longitudinal Jamaica Sickle Cell Study indicates that the mean decline in eGFR for adult sickle cell disease patients with homozygous sickle cell genes ranges between 1.78 and 3 ml/min/1.73m2/year,18 which is more than twice the rate of decline seen in the general population.20 However, analogous kidney disease progression data are not available in pediatric patients with SCD. Like almost all other forms of CKD, once overt renal impairment in SCD is established, it is not reversible, and end stage kidney disease occurs in some (but not all) patients.12,15,17,21,22
Notably, current methods for identifying CKD, including creatinine-based eGFR estimates or direct measurements of GFR (such as iothalamate clearance measurements), are insensitive to detecting early manifestations of CKD.23,24 This is particularly problematic in SCD, given the hyperfiltration / “supranormal” GFRs observed in many SCD patients. Albuminuria (microalbuminuria and/or overt proteinuria) is another marker for CKD progression that occurs commonly in adults with SCD.18 Microalbuminuria has been reported to occur in approximately 15% of children, especially those who demonstrate hyperfiltration. However, microalbuminuria has been shown to remit in a substantial portion of children and adults with other hyperfiltration disorders, notably diabetes mellitus.15,25,26 Therefore, the prognostic value of microalbuminuria in pediatric patients with SCD remains to be established. Newer serum and urinary biomarkers, such as soluble Fas/Fas-ligand ratio or serum FMS-like tyrosine kinase-1 (sFLT-1) are now being studied as surrogate measures of early renal involvement in SCD.27,28 However, as yet, none of these clinical markers have consistently and sensitively identified the complex processes underlying the development of CKD in patients with SCD. Given the limitations of current laboratory and clinical markers in detecting chronic kidney disease, it is imperative that new, sensitive methods of studying early functional and structural changes in the kidney be developed to better define kidney disease in this at-risk patient population.
Magnetic Resonance Imaging (MRI) is a safe, non-invasive imaging modality capable of providing multiple quantitative assessments of kidney structure and function without ionizing radiation or injectable contrast agents. In particular, our group and others have shown that quantitative MRI techniques can be used to detect changes in renal microstructure, perfusion, oxygenation, and iron deposition in patients with a variety of acute and chronic kidney diseases.29–38 Importantly, many of these MRI techniques detect early-stage, regional disease more sensitively than conventional clinical assessments of renal function (e.g., serum creatinine, albuminuria).37 Unfortunately, the use of these safe and sensitive MRI techniques to assess CKD in patients with SCD has been extremely limited. To date, only renal iron deposition (T2*) has been thoroughly studied.10,11,39,40
In this pilot imaging study, we have obtained Diffusion Tensor Imaging (DTI) MRI assessments to quantitatively assess kidney microstructure as well as iron deposition (T2*) in a cohort of pediatric and adult SCD patients in comparison to non-SCD volunteers. Importantly, prior studies have shown that DTI MRI is capable of detecting early-stage chronic kidney disease, but have not been previously studied in SCD patients. Furthermore, if DTI MRI provides detection of early alterations in kidney microstructure in SCD patients, the opportunity for more intensive interventional strategies as well as the development of new therapeutics aimed at preventing kidney disease progression in these patients may be possible.
MATERIALS AND METHODS
Study Population and Clinical Assessments
Pediatric (n=5, age 12–18 years) and adult (n=4, age 20–31 years) African American subjects homozygous for SCD with eGFR values >100 ml/min/1.73m2 were recruited from the Sickle Cell Clinics at Seidman Cancer Center / University Hospitals - Cleveland Medical Center and Rainbow Babies and Children’s Hospital. All patients were evaluated at clinical baseline (i.e. no SCD crises or acute complications within the prior 2 weeks). Adults (n=10, 20–43 years old) without SCD and without kidney disease by history were recruited as controls (4 African-American / 4 Caucasian / 2 Asian). The study was performed according to approved Institutional Review Board (IRB) protocols.
Clinical data for the SCD patients including age, gender, renal function assessments (serum creatinine, urine albumin excretion, blood pressure), and hematologic status (lactate dehydrogenase (LDH), white blood cell count, hemoglobin, absolute reticulocyte count (ARC), ferritin and aspartate transaminase (AST)) were obtained from each patient’s medical record. Most data were obtained at concurrent clinical visits or at a baseline visit within 12 months of the scan. For pediatric patients, eGFR was calculated using the modified (“bedside”) Schwartz formula (height (cm) / serum creatinine (mg/dL) X 0.413)24 whereas for adult patients, the Modification of Diet in Renal Disease (MDRD) method was used.41,42 Hypertension was defined using established age/gender/height percentile norms in children and the standard adult cutoff of ≥140/90 was used in the adult population.43
Quantitative MRI Acquisitions
All participants were consented and scanned in supine position with no sedation in a Siemens Espree 1.5T MRI scanner (Siemens Medical Systems, Erlangen, Germany). Image uniformity was maximized by way of spine array (posterior) and body array (anterior) coils which were positioned over each subject’s lower abdomen. Anatomic images of both the left and right kidneys were acquired using an initial localizer and proton density-weighted Half-fourier Acquisition Single shot Turbo spin Echo (HASTE) images (TR/TE = 1,000/25, slice thickness = 6 mm, 10 slices, FOV = 400 mm x 400 mm, 1 average). These images were used to position the slices for the DTI and T2* relaxometry MRI scans.
The DTI data were acquired using a respiratory-gated, echo planner imaging (EPI) MRI sequence (b=0, 400 s/mm2, 6 directions + null TR/TE = 2,000/66 ms, imaging slice thickness = 6mm, 8–10 imaging slices/subject). Six signal averages were acquired to obtain imaging data with a sufficiently high signal-to-noise ratio (SNR) to enable accurate quantification.37 Fat and slab suppression radiofrequency pulses were added to limit chemical shift and motion artifacts, respectively. Prospective and retrospective gating was implemented to limit respiratory motion artifacts.37 The DTI data were collected over 4–6 minutes depending on the participant’s respiratory rate. A multi-echo gradient recalled echo MRI technique was used to acquire the T2* (“T2-star”) relaxometry data for each subject (TR/TE = 56/2.4 ms, FOV = 300 mm x 300 mm, slice thickness = 5 mm, 12 echoes: 2.41 ms, 5.74 ms, 10.36 ms, 14.98 ms, 19.6 ms, 24.22 ms, 28.84 ms, 33.46 ms, 38.08 ms, 42.7 ms, 47.32 ms, and 51.94 ms). The T2* data were collected during a 12-second voluntary breath hold.
Image Processing and Analysis
All MRI images were exported from the clinical scanners and processed offline in Matlab (The Mathworks, Natick, MA). The DTI data were used to obtain renal Apparent Diffusion Coefficient (ADC) and Fractional Anisotropy (FA) maps, representing the magnitude and directionality of interstitial water diffusion, respectively.44 Renal T2* maps were generated from the relaxometric MRI data using a conventional least-squares regression to a mono-exponential decay model. A region of interest (ROI) analysis was performed on the FA maps and T2* weighted images of both the left and right kidneys in order to collect quantitative data on the mean cortical and medullary regions of the kidneys. A total of 16 kidney ROIs were drawn in each imaging slice to delineate the 4 medullary and 4 cortical regions of the left and right kidneys, respectively. A total of 3–4 imaging slices were analyzed for each subject. Subjects with smaller kidneys were limited to three imaging slices.
Statistical analysis
Mean values were calculated for the patients with SCD and control subjects for each MRI parameter: DTI (cortical and medullary ADC and FA) as well as cortical and medullary T2*. A Student’s t-test was performed to compare the imaging findings for the SCD patients with the control subjects. A Pearson’s correlation using the least squared error fit to a linear model was used to compare DTI and T2* assessments with the clinical assessments. Bonferroni corrections were applied as appropriate to the correlations with clinical assessments to accurately test for significance.
RESULTS
In this pilot study, we enrolled 5 pediatric and 4 adult patients with SCD as well as 10 non-SCD controls. SCD patient demographics and clinical features in addition to current clinical assessments of kidney function and hematologic status are shown in Table 1. The SCD patients had a range of eGFRs (100–180 ml/min/1.73 m2), with five patients exhibiting clinical hyperfiltration (eGFR > 140 ml/min/1.73m2). Out of the eight patients who had blood work taken within 12 months of the scan date, one SCD patient had elevated urine albumin measurements (urine albumin:creatinine >30mg/g) and one patient had overt hypertension. With respect to hematologic parameters, all of the patients with SCD had LDH and ARC levels consistent with elevated hemolysis rates. Two pediatric patients with SCD on chronic transfusions exhibited considerably higher ferritin levels (1,541 and 1,606 mcg/L) in comparison to the other pediatric patients (range = 93–310 mcg/L). Two adult patients with SCD exhibited higher ferritin levels (6,256 and 4,187 mcg/L) in comparison to the other adult SCD patients (range = 580–924 mcg/L). One of these SCD patients with elevated ferritin levels was currently prescribed with chronic transfusions.
TABLE 1.
Clinical features of the SCD patients
| Gender | Treatment | Age | BP Category |
Creatinine (mg/dL) |
eGFR (ml/min/ 1.73m2) |
Urine ACR (mg/g Creatinine) |
WBC (x109/L) |
Hgb (g/dL) |
ARC (x1012/L) |
AST (U/L) |
LDH (U/L) |
Ferritin (mcg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | HU | 14 | NL | 0.58 | 110 | 9.3 | 6.5 | 8.2 | 0.18 | 46 | 627 | 93 |
| Female | HU | 13 | NL | 0.49 | 126 | NA | 10.2 | 8.6 | 0.37 | 37 | 448 | 310 |
| Female | Transfusions | 12 | HTN | 0.45 | 143 | 11.1 | 8.3 | 7.4 | 0.47 | 44 | 346 | 1541 |
| Female | Transfusions | 18 | NL | 0.44 | 146 | 13 | 10.5 | 10 | 0.33 | 11 | 315 | 1606 |
| Female | HU | 14 | NL | 0.66 | 100 | 9.2 | 3 | 9.1 | 0.18 | 29 | 393 | 158 |
| Male | None | 20 | NL | 0.52 | 117 | 18 | 11.3 | 9.7 | 0.23 | 26 | 390 | 4187 |
| Male | Transfusions | 23 | NT | 0.48 | 180 | 10.5 | 16.4 | 8.2 | 1.20 | 120 | 1296 | 6256 |
| Male | None | 22 | NT | 0.89 | 146 | 687 | 6.7 | 10 | 0.37 | 44 | 461 | 924 |
| Male | Transfusions | 31 | NL | 0.5 | 166 | 7.6 | 11.6 | 9.7 | 0.91 | 94 | 548 | 580 |
HU, hydroxyurea; BP, blood pressure; NL, normal; HTN, hypertension; NT, normotensive; HBP, high blood pressure; eGFR, estimated glomerular filtration rate; ACR, albumin:creatinine ratio; WBC, white blood cell count; Hgb, hemoglobin; ARC, absolute reticulocyte count; AST, aspartate amniotransferase; LDH, lactate dehydrogenase; NA, not available (sample taken outside of one year window)
- ARC (0.022–0.118 x1012/L)
- AST (10–60 U/L)
- LDH (100–300 U/L)
- Ferritin (20–300 mcg/L)
- Hemoglobin levels for adults (8–10 g/dL)
- Hemoglobin levels for pediatrics (7–10 g/dL)
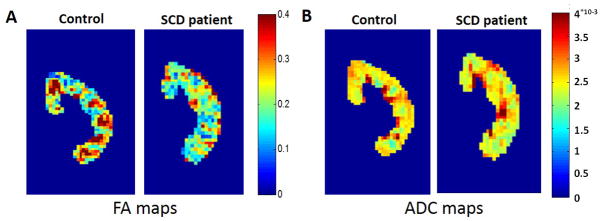
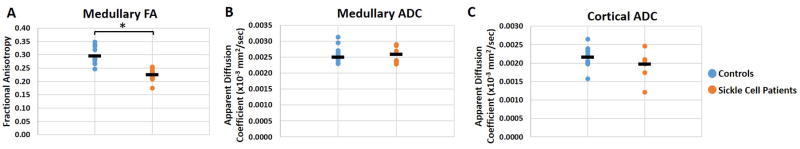
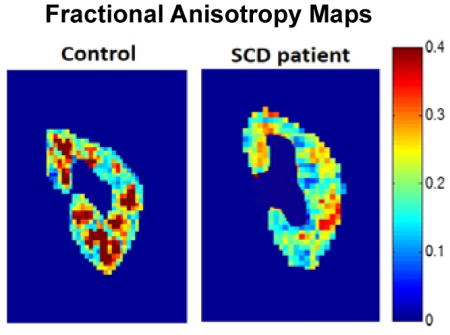
The DTI scans were processed to generate pixel-wise maps of Fractional Anisotropy (FA) and Apparent Diffusion Coefficient (ADC). Representative cortical and medullary ROIs used to analyze the DTI and T2* MRI data are shown as overlays on a T2*-weighted image (Figure 1A) and a FA map (Figure 1B), respectively. The FA map was used to calculate mean ADC and FA values for the cortical and medullary ROIs. Representative FA maps of the kidneys from a healthy control subject (age = 26) and an SCD patient (age = 14) are shown in Figure 2A. The FA map for the healthy control subject shows medullary regions with high FA values (red regions, FA > 0.3) representing normal renal pyramids with radially aligned tubules and vessels. By comparison, the medullary FA values for the SCD patients were visibly diminished. Representative ADC maps from a control subject (age = 26) and a patient with sickle cell (age = 14) are shown in Figure 2B. Mean medullary FA in patients with SCD was significantly reduced in comparison to control subjects, (0.23 ± 0.02 vs. 0.30 ± 0.03, p = 0.0001, Fig. 3A). In a sub-analysis of the adult and pediatric SCD patient cohorts, it was observed that both the adult and pediatric SCD patients had significant reductions in medullary FA as compared to controls (adult: 0.22 ± 0.04 vs. 0.30 ± 0.03, p=0.008 and pediatric: 0.22 ± 0.03 vs. 0.30 ± 0.03, p = 0.002). These results suggest that DTI MRI can provide early detection of microstructural changes in pediatric SCD patients. No significant difference was observed for the mean medullary and cortical ADC values (Fig. 3B and 3C).
Figure 1.
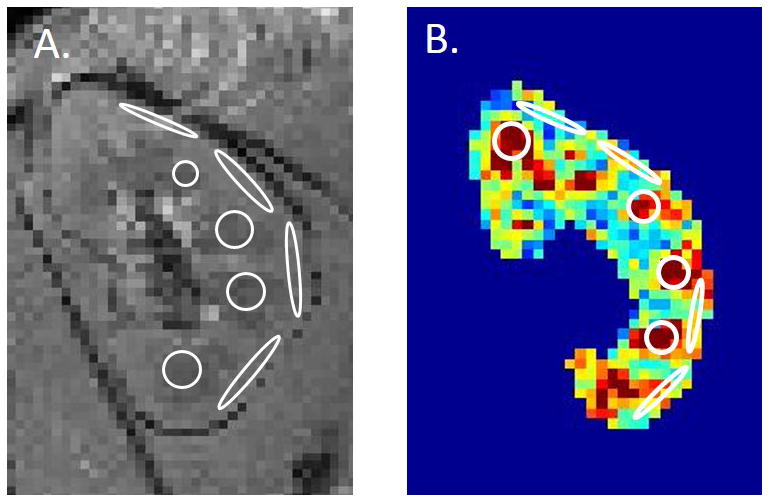
(A) Coronal T2*-weighted MRI image of a control kidney with representative cortical and medullary ROIs. (B) Cortical and medullary representative ROIs are overlaid onto a FA map. These ROIs were used to calculate mean cortical and medullary T2*, ADC, and FA for each subject.
Figure 2.
(A) Representative coronal kidney Fractional Anisotropy (FA) maps of a healthy control subject (age = 26) and a patient with SCD (age = 14). Note the distinctive medullary regions for the control subject (focal red regions, FA > 0.3) indicative of healthy radially-aligned tubules and vessels. These medullary FA values are visibly reduced for the patient with SCD (FA < 0.3). (B) Representative coronal kidney Apparent Diffusion Coefficient (ADC) maps of a healthy control subject (age = 26) and a patient with SCD (age = 14). Note the relative similarity of the ADC maps between the control and the SCD patient despite known iron loading in the SCD patient population.
Figure 3.
(A) Mean medullary FA values representative of the patients with SCD (n=9) and non-SCD healthy control subjects (n=10). The mean FA value for the patients with SCD is significantly lower than those of the control subjects (*p < 0.0001). Mean (B) medullary and (C) cortical ADC values for the same two cohorts. The mean medullary and cortical ADC values for the patients with SCD were not statistically different than those of the control subjects.
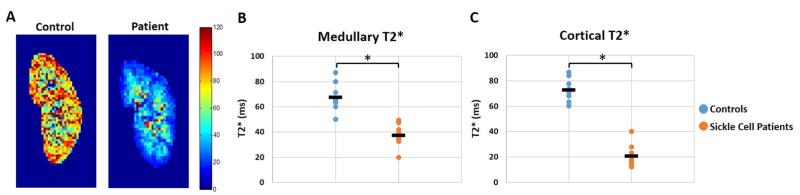
Relaxometric MRI assessments were used to generate T2* maps for the patients with SCD and controls as a relative measure of iron deposition. Representative T2* maps of a control subject (age = 20) and a patient with SCD (age = 13) are shown in Figure 4A. T2* reductions are clearly visible in the kidneys of patients with SCD, indicative of increased iron deposition (T2* < 60 ms). A comparison of the mean cortical and medullary T2* values showed significant T2* reductions (increased iron deposition) for the patients with SCD in comparison to the healthy control subjects (Fig. 4B, cortex: 20.7 ms ± 8.6 vs. 72.9 ms ± 7.9, p = ≤ 0.0001; Fig. 4C, medulla: 37.5 ms ± 8.7 vs. 67.4 ms ± 9.7, p = ≤ 0.0001). A slight difference was also observed between the cortical and medullary T2* values for the healthy control subjects (cortex: 72.9 ms ± 7.9 vs. medulla: 67.4 ms ± 9.7); however, this difference was not significant.
Figure 4.
(A) Representative kidney T2* maps of a healthy control subject (age = 20) and a patient with SCD (age = 13). The patients with SCD have visibly reduced cortical T2* (< 40 ms) and medullary T2* (< 80 ms) values compared to the healthy control subject. (B) Mean medullary T2* values for the patients with SCD were also significantly decreased (*p < 0.0001). (C) Mean cortical T2* values for the patients with SCD were significantly lower than those of the healthy control subjects (*p < 0.0001).
Mean medullary T2* values for each subject were plotted against corresponding conventional clinical assessments of SCD-associated hemolysis for each subject with SCD. Of the nine clinical assessments, the only significant correlation was between medullary T2* and AST (Fig. 5, R = 0.86, p = 0.003). Of note, ferritin did not correlate with T2* in this pilot study. In addition, cortical and medullary T2* did not result in a significant correlation with medullary FA suggesting that microstructural changes detected by DTI are complementary to renal iron loading in SCD. Note also, that medullary FA did not correlate with eGFR as shown previously in diabetic patients with CKD.37
Figure 5.
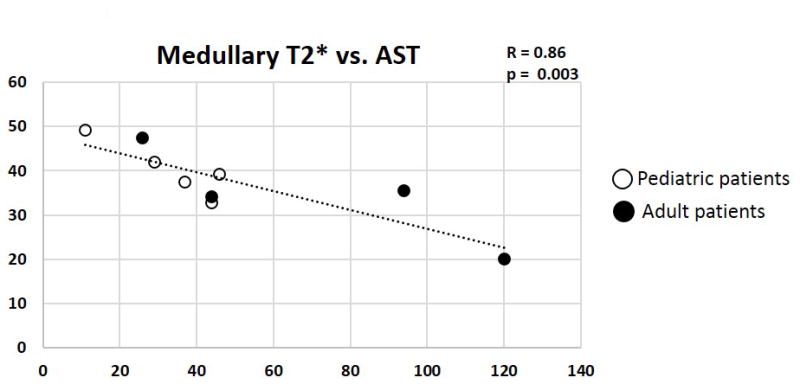
Scatterplot of medullary T2* values plotted against corresponding Aspartate Aminotransferase (AST) values for each patient with SCD (n=9). The pediatric patients are represented by the hollow circles and the adult patients as filled circles. Medullary T2* correlated significantly with AST (p = 0.003). The plot displays a linear regression line and Pearson Correlation coefficient (R).
DISCUSSION
In this cross-sectional pilot imaging study, we evaluated the sensitivity of DTI and T2* MRI assessments to non-invasively detect renal differences in SCD patients with intact renal function (eGFR > 100 ml/min/1.73m2) in comparison to young adult controls. DTI MRI has been used on multiple organs to quantify pathophysiology, including multiple studies in chronic kidney disease.37,45 This is one of the first studies, however, to utilize this clinically-established MRI technique in the kidneys of patients with SCD. Despite our relatively small cohort of patients (n=9), our results showed a significant reduction in medullary FA values (p = 0.0001) for SCD patients indicative of medullary microstructural alterations in the radially-aligned tubules and/or vessels of the renal medulla. Similar significant reductions in the directionality (FA) of water movement in the renal medulla have been previously observed in patients with diabetes mellitus.37 Overall, these MRI findings suggest that renal microstructure changes (assessed with DTI MRI) can provide a non-invasive, assessment of regional renal microstructural changes in both pediatric and adult SCD patients. Future studies may be needed to determine if these alterations in medullary FA are associated with renal iron loading assessed by cortical and medullary T2*.
While renal DTI studies have not been previously reported in patients with SCD, prior studies have shown that medullary FA is similarly reduced in diabetic nephropathy patients.37 It is important to note that, in this prior study, medullary FA changes: 1) were observed in diabetic patients with relatively early-stage CKD (eGFR > 60 ml/min/1.73m2) and 2) significantly correlated with declining eGFR (eGFR range = 27 – 100 ml/min/1.73m2).37 These clinical results in diabetic subjects have since been validated by other groups in animal models.45 Therefore, medullary FA measurements may be a sensitive imaging marker for early renal dysfunction in patients with CKD. In contrast to these previous studies, a significant correlation was not observed between medullary FA and eGFR for the SCD patients (data not shown). However, this lack of correlation may be due in part to the fact that the SCD patients scanned in this pilot study all had normal-to-supranormal eGFR values. In contrast, the diabetic subjects evaluated in the diabetic nephropathy study exhibited a larger range of kidney function with eGFR values ranging from 27–110 ml/min/1.73m2. Future DTI MRI studies will need to be conducted in SCD patients with declining eGFR values (e.g., eGFR < 90 ml/min/1.73m2) to more accurately determine if an association exists between medullary FA and eGFR in SCD patients. Regardless, these initial results suggest that renal microstructural alterations are present in patients with SCD despite no overt kidney dysfunction.
It is important to note that prior studies have shown that iron deposition can have a direct influence on both ADC and FA values obtained from DTI MRI acquisitions.46 The increased iron loading in the kidney of SCD patients can cause a reduced signal-to-noise ratio (SNR) in the DTI images. This reduction in SNR can then result in a relative decrease in ADC and a relative increase in FA values.46 Therefore, care must be taken to acquire the DTI MRI data with sufficient SNR to limit these effects. In this study, we observed that both cortical and medullary ADC values in the SCD patients were not significantly different from non-SCD controls despite significant cortical and medullary iron deposition (i.e., reduced cortical and medullary T2* values, Fig. 4). In addition, the medullary FA values were reduced in patients with SCD despite the expected increase in FA from iron loading / reduced SNR; Note that cortical FA results were not reported in this study as: 1) cortical FA values are typically much lower than medullary FA values, even in healthy subjects, due to non-uniform diffusion in the human renal cortex; and 2) high cortical iron loading may cause erroneous cortical FA assessments for the SCD subjects. Overall, these results suggest that reasonable medullary FA assessments can be obtained in both adult and pediatric SCD patients despite significant iron loading. It is also important to note that in this study we acquired the DTI MRI data with 6 averages. This high level of signal averaging may limit the impact of iron deposition, but does increase the acquisition time of the DTI MRI.
A major limitation to this pilot study was the small sample size. We expect that the limited number of significant correlations between DTI and T2* metrics against serologic and hemolytic markers was primarily due to the small cohort size. For example, while one of the serum markers of hemolysis (i.e., AST) resulted in significant correlations with renal iron loading as shown in Figure 5, significant correlations were not observed with other markers for hemolysis (e.g., ferritin). It could be expected that an expanded cohort of SCD patients would reveal additional significant associations with clinical markers of hemolysis and/or kidney function.
Another limitation for this pilot study is that the healthy control subjects were not specifically age-matched to the SCD patients, and only 4 out of the 10 control subjects were of African American ancestry. However, despite the limited number of SCD patients and mixed ancestry of the control cohort, the observed DTI alterations in CKD31,32,37 as well as the increased renal iron deposition (medullary and cortical T2*) are in alignment with a prior MRI study in SCD patients.11 It is important to note; however, that previous papers have shown a significantly lower T2* value in the renal medulla as compared to the cortex which may be due to larger cohorts in those prior studies. Additionally, the mean kidney DTI and T2* values for the healthy young adult controls in this study are consistent with multiple prior reports in healthy subjects.11,37,47,48 Therefore, the overall consistency between our results and multiple prior kidney MRI studies supports the significance of these DTI results in patients with SCD despite our small cohorts.
Given these limitations, future studies will need to be conducted to include larger cohorts of SCD patients, better matching of the healthy control subjects, and a wider range of eGFR values as described above. Additionally, in order to understand the development of these kidney microstructural variations in patients with SCD, it will be necessary to recruit a cohort of younger pediatric patients (< 12 yrs) to identify the age of onset for these observed medullary FA changes. Acquiring additional serum and urinary biomarkers to compare against these MRI assessments could provide additional information to better understand the onset and progression of chronic kidney disease. By understanding when the kidneys start to show medullary FA changes, we may be able to develop interventional and therapeutic strategies to prevent CKD progression. Along this same path, longitudinal MRI assessments would allow us to track these changes over time and gain a further understanding of disease progression.
In conclusion, this pilot kidney MRI study suggests that DTI can detect regional kidney changes in children and adults with SCD. Herein, renal microstructural alterations assessed by DTI MRI (i.e., medullary FA) may be indicative of early CKD in patients with SCD and were reliably detected despite renal iron loading. Taken together with prior work in diabetic patients, this work suggests that DTI MRI may provide a non-invasive assessment of early kidney structural / functional changes in SCD patients.
Acknowledgments
The authors would like to acknowledge the support of Case Comprehensive Cancer Center (NIH/NCI P30 CA43703) and the Clinical and Translation Science Collaborative of Cleveland (NIH/NCATS UL1 TR000439).
List of Abbreviations
- ADC
Apparent Diffusion Coefficient
- ARC
Absolute Reticulocyte Count
- AST
Aspartate Transaminase
- CKD
Chronic Kidney Disease
- DTI
Diffusion Tensor Imaging
- eGFR
Estimated Glomerular Filtration Rate
- EPI
Echo Planar Imaging
- FA
Fractional Anisotropy
- HASTE
Half Fourier Acquisition Single Shot Turbo Spin Echo
- HgbS
Sickle Hemoglobin
- IRB
Institutional Review Board
- LDH
Lactose Dehydrogenase
- MDRD
Modification of Diet in Renal Disease
- MRI
Magnetic Resonance Imaging
- ROI
Region of Interest
- SCD
Sickle Cell Disease
- SNR
Signal-to-Noise Ratio
References
- 1.Mills ML. Life-threatening complications of sickle cell disease in children. JAMA. 1985;254:1487–1491. [PubMed] [Google Scholar]
- 2.Schnog JB, et al. Sickle cell disease; a general overview. Neth J Med. 2004;62:364–374. [PubMed] [Google Scholar]
- 3.Kato GJ, Hebbel RP, Steinberg MH, Gladwin MT. Vasculopathy in sickle cell disease: Biology, pathophysiology, genetics, translational medicine, and new research directions. Am J Hematol. 2009;84:618–625. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsironi M, Aessopos A. The heart in sickle cell disease. Acta Cardiol. 2005;60:589–598. doi: 10.2143/AC.60.6.2004929. [DOI] [PubMed] [Google Scholar]
- 5.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359:2254–2265. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 6.Becker AM. Sickle cell nephropathy: challenging the conventional wisdom. Pediatr Nephrol. 2011;26:2099–2109. doi: 10.1007/s00467-010-1736-2. [DOI] [PubMed] [Google Scholar]
- 7.Piccone CaD, Katherine al., E.D.A.e, editor. Pediatric Nephrology. Springer; Berlin: 2016. Sickle Cell Nephropathy in Children; pp. 1523–1544. [Google Scholar]
- 8.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 2017;127:750–760. doi: 10.1172/JCI89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nath KA, Croatt AJ, Haggard JJ, Grande JP. Renal response to repetitive exposure to heme proteins: chronic injury induced by an acute insult. Kidney Int. 2000;57:2423–2433. doi: 10.1046/j.1523-1755.2000.00101.x. [DOI] [PubMed] [Google Scholar]
- 10.Vasavda N, et al. Renal iron load in sickle cell disease is influenced by severity of haemolysis. Br J Haematol. 2012;157:599–605. doi: 10.1111/j.1365-2141.2012.09093.x. [DOI] [PubMed] [Google Scholar]
- 11.Schein A, Enriquez C, Coates TD, Wood JC. Magnetic resonance detection of kidney iron deposition in sickle cell disease: a marker of chronic hemolysis. J Magn Reson Imaging. 2008;28:698–704. doi: 10.1002/jmri.21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ataga KI, Orringer EP. Renal abnormalities in sickle cell disease. Am J Hematol. 2000;63:205–211. doi: 10.1002/(sici)1096-8652(200004)63:4<205::aid-ajh8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Guasch A, Navarrete J, Nass K, Zayas CF. Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol. 2006;17:2228–2235. doi: 10.1681/ASN.2002010084. [DOI] [PubMed] [Google Scholar]
- 14.Nath KA, Katusic ZS. Vasculature and kidney complications in sickle cell disease. J Am Soc Nephrol. 2012;23:781–784. doi: 10.1681/ASN.2011101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aygun B, Mortier NA, Smeltzer MP, Hankins JS, Ware RE. Glomerular hyperfiltration and albuminuria in children with sickle cell anemia. Pediatr Nephrol. 2011;26:1285–1290. doi: 10.1007/s00467-011-1857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware RE, et al. Renal function in infants with sickle cell anemia: baseline data from the BABY HUG trial. J Pediatr. 2010;156:66–70 e61. doi: 10.1016/j.jpeds.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wigfall DR, Ware RE, Burchinal MR, Kinney TR, Foreman JW. Prevalence and clinical correlates of glomerulopathy in children with sickle cell disease. J Pediatr. 2000;136:749–753. [PubMed] [Google Scholar]
- 18.Drawz P, et al. Kidney Disease among Patients with Sickle Cell Disease, Hemoglobin SS and SC. Clin J Am Soc Nephrol. 2016;11:207–215. doi: 10.2215/CJN.03940415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodas P, Huang A, O’Riordan MA, Sedor JR, Dell KM. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease -a cross sectional review. BMC Nephrol. 2013;14:237. doi: 10.1186/1471-2369-14-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asnani M, Serjeant G, Royal-Thomas T, Reid M. Predictors of renal function progression in adults with homozygous sickle cell disease. Br J Haematol. 2016;173:461–468. doi: 10.1111/bjh.13967. [DOI] [PubMed] [Google Scholar]
- 21.Davenport A, Buscombe J. Sickle cell kidney. J Nephrol. 2008;21:253–255. [PubMed] [Google Scholar]
- 22.Kelly CJ, Singer I. Acute renal failure in sickle-cell disease. Am J Kidney Dis. 1986;8:146–150. doi: 10.1016/s0272-6386(86)80017-8. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz GJ, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son MK, et al. Regression and progression of microalbuminuria in adolescents with childhood onset diabetes mellitus. Ann Pediatr Endocrinol Metab. 2015;20:13–20. doi: 10.6065/apem.2015.20.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkins BA, et al. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 27.Adly AA, Ismail EA, Andrawes NG, Mahmoud MM, Eladawy R. Soluble Fas/FasL ratio as a marker of vasculopathy in children and adolescents with sickle cell disease. Cytokine. 2016;79:52–58. doi: 10.1016/j.cyto.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 28.Youssry I, et al. Novel marker for the detection of sickle cell nephropathy: soluble FMS-like tyrosine kinase-1 (sFLT-1) Pediatr Nephrol. 2015;30:2163–2168. doi: 10.1007/s00467-015-3172-9. [DOI] [PubMed] [Google Scholar]
- 29.Cutajar M, Clayden JD, Clark CA, Gordon I. Test-retest reliability and repeatability of renal diffusion tensor MRI in healthy subjects. Eur J Radiol. 2011;80:e263–268. doi: 10.1016/j.ejrad.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Gurses B, Kilickesmez O, Tasdelen N, Firat Z, Gurmen N. Diffusion tensor imaging of the kidney at 3 Tesla MRI: normative values and repeatability of measurements in healthy volunteers. Diagn Interv Radiol. 2011;17:317–322. doi: 10.4261/1305-3825.DIR.3892-10.1. [DOI] [PubMed] [Google Scholar]
- 31.Kataoka M, et al. Diffusion tensor imaging of kidneys with respiratory triggering: optimization of parameters to demonstrate anisotropic structures on fraction anisotropy maps. J Magn Reson Imaging. 2009;29:736–744. doi: 10.1002/jmri.21669. [DOI] [PubMed] [Google Scholar]
- 32.Kido A, et al. Diffusion tensor MRI of the kidney at 3.0 and 1.5 Tesla. Acta Radiol. 2010;51:1059–1063. doi: 10.3109/02841851.2010.504741. [DOI] [PubMed] [Google Scholar]
- 33.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 34.Fenchel M, et al. Perfusion MR imaging with FAIR true FISP spin labeling in patients with and without renal artery stenosis: initial experience. Radiology. 2006;238:1013–1021. doi: 10.1148/radiol.2382041623. [DOI] [PubMed] [Google Scholar]
- 35.Martirosian P, et al. Magnetic resonance perfusion imaging without contrast media. Eur J Nucl Med Mol Imaging. 2010;37(Suppl 1):S52–64. doi: 10.1007/s00259-010-1456-7. [DOI] [PubMed] [Google Scholar]
- 36.Martirosian P, Klose U, Mader I, Schick F. FAIR true-FISP perfusion imaging of the kidneys. Magn Reson Med. 2004;51:353–361. doi: 10.1002/mrm.10709. [DOI] [PubMed] [Google Scholar]
- 37.Lu L, et al. Use of diffusion tensor MRI to identify early changes in diabetic nephropathy. Am J Nephrol. 2011;34:476–482. doi: 10.1159/000333044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94:3271–3275. doi: 10.1161/01.cir.94.12.3271. [DOI] [PubMed] [Google Scholar]
- 39.Lande IM, et al. Sickle-cell nephropathy: MR imaging. Radiology. 1986;158:379–383. doi: 10.1148/radiology.158.2.3941863. [DOI] [PubMed] [Google Scholar]
- 40.Aubart M, et al. Longitudinal MRI and Ferritin Monitoring of Iron Overload in Chronically Transfused and Chelated Children With Sickle Cell Anemia and Thalassemia Major. J Pediatr Hematol Oncol. 2016;38:497–502. doi: 10.1097/MPH.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 41.Levey AS, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 42.Florkowski CM, Chew-Harris JS. Methods of Estimating GFR - Different Equations Including CKD-EPI. Clin Biochem Rev. 2011;32:75–79. [PMC free article] [PubMed] [Google Scholar]
- 43.National High Blood Pressure Education Program Working Group on High Blood Pressure in, C. & Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 44.Le Bihan D, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 45.Hueper K, et al. Magnetic resonance diffusion tensor imaging for evaluation of histopathological changes in a rat model of diabetic nephropathy. Invest Radiol. 2012;47:430–437. doi: 10.1097/RLI.0b013e31824f272d. [DOI] [PubMed] [Google Scholar]
- 46.Xu X, Wang Q, Zhong J, Zhang M. Iron deposition influences the measurement of water diffusion tensor in the human brain: a combined analysis of diffusion and iron-induced phase changes. Neuroradiology. 2015;57:1169–1178. doi: 10.1007/s00234-015-1579-4. [DOI] [PubMed] [Google Scholar]
- 47.Wang WJ, et al. MR diffusion tensor imaging of normal kidneys. J Magn Reson Imaging. 2014;40:1099–1102. doi: 10.1002/jmri.24450. [DOI] [PubMed] [Google Scholar]
- 48.Zhang JL, et al. New magnetic resonance imaging methods in nephrology. Kidney Int. 2014;85:768–778. doi: 10.1038/ki.2013.361. [DOI] [PMC free article] [PubMed] [Google Scholar]