Since its discovery approximately fifteen years ago, cytoglobin (Cygb) has been studied extensively. Because it is found outside the red cell, Cygb is categorized as a non-erythroid globin, along with (in humans) proteins such as myoglobin (Mb), neuroglobin, androglobin, and hemoglobin α (Hb α). The putative functions of these non-erythroid globins are linked to tissue protection from conditions such as hypoxia, ischemia, and oxidative stress.1 Cygb not only fulfills these functions, but also has been related to other roles including tumor suppression and the regulation of fibrosis in cell and animal models.2–7 Like other heme globins, Cygb can reversibly bind oxygen and other small molecules. The ability of Cygb to store and sense oxygen, as well as its involvement in nitrite and nitric oxide (NO) metabolism, being able to both scavenge NO and produce NO from nitrite, are probably key to its function(s).8, 9 However, in spite of significant progress in understanding the structure, localization, and functional characteristics of Cygb, the central physiological roles of this protein have yet to be fully elucidated.10–12
In this issue of Arteriosclerosis, Thrombosis, and Vascular Biology, Jourd’heuil et al. examine the role of Cygb in controlling apoptosis and vascular remodeling after injury. Cygb appears to be the predominant globin in vessel walls of humans, rats, and mice with expression levels substantially higher than those of Mb. The protein is found in medial smooth muscle cells (SMCs), and dedifferentiation of SMCs by culture or vascular injury leads to a loss of Cygb expression, although this loss is only temporary after injury, with Cygb expression recovering after several days.
The authors use two different injury models: a rat model of unilateral carotid artery balloon angioplasty and a mouse model of unilateral carotid artery ligation. Following either vascular injury, animals that do not express Cygb show substantially impaired remodeling, specifically decreased neointima formation. Analyses of apoptotic and proliferative markers suggest higher levels of apoptosis and cell death with Cygb loss but unchanged levels of proliferation, indicating increased apoptosis as the primary cause of disrupted neointima formation.
In experiments with rat aortic SMCs, the authors observe increased Cygb expression under hypoxic conditions or after treatment with inflammatory cytokines. While hypoxia is known to increase Cygb expression, the induction of Cygb by cytokines has not been documented before and suggests Cygb may modulate inflammatory responses to non-ischemic tissue damage. Cygb silencing significantly increases rates of cell death, indicating a cytoprotective role. 1400W, a selective NOS2 inhibitor, largely reversed this increase in cell death, suggesting NO-dependent cytotoxicity that can be prevented by Cygb expression. Worth mentioning, Cygb-KO mice show exacerbated expression of NOS2 and inflammation markers, suggesting a link between Cygb function and immune response.10 The increase in cell death was also reversed with the use of the reducing agent N-acetyl cysteine or a pan-caspase inhibitor, implicating oxidative stress in promoting apoptosis. Specifically, Cygb loss appears to activate caspase-3, a finding that had previously been observed in animal models of brain ischemia-reperfusion injuries but is novel in the context of vascular injury.13
The implication that Cygb protects cells from NO-dependent toxicity is particularly compelling as numerous researchers have explored Cygb’s nitric oxide dioxygenase (NOD) activity, which was first proposed by Jourd’heuil et al.14 NO dioxygenation occurs when oxygen-bound Cygb reacts with NO, resulting in the production of nitrate and the oxidation of the heme iron from the ferrous the ferric state.15–17 This reaction is extremely rapid for globins (nearly diffusion-limited).18 NO dioxygenation is considered to significantly contribute to NO metabolism in vivo, with physiologic effects including cytoprotection and regulation of vascular tone.16, 19–23 For example, endothelial Hb α, localized to myoendothelial junctions, has been shown to consume NO generated in the endothelium, regulating vascular tone.21 Inhibition of this NO consumption results in significant decreases in blood pressure.22 Loss of Cygb in the SMCs appears to elicit similar effects, showing increased vasodilation and decreased blood pressure in Cygb knockout mice.23
Catalytic NO dioxygenase activity is limited by the reduction of the heme iron. A reducing system has been characterized for Hb α; inhibition of CYB5R3, a reducing enzyme present in endothelium, slows NO consumption by Hb α.21 Recent data from our group and others show a highly efficient reduction of Cygb by the NADH/CYB5/CYB5R3 reducing system; in fact, the reduction of Cygb is at least an order of magnitude faster than that of other heme globins.23, 24 Taken together, these results suggest the existence of a Cygb/CYB5/CYB5R3 metabolon in vascular SMCs, enabling rapid consumption of NO and thus modulating NO bioactivity and signaling. Interestingly, Mb in vascular smooth muscle has previously been shown to contribute to hypoxic vasodilation via nitrite reduction to NO.25 The responses of Mb and Cygb to oxygen and the relative efficiency of their reducing systems could underlie different roles for both proteins on vascular wall NO signaling.16, 24
This work by Jourd’heuil et al. showcases a new role for cytoglobin as an important regulator of apoptosis and vascular remodeling in SMCs after injury, acting independent of other globins. This role is supported by the novel observation that inflammatory cytokines trigger re-expression of Cygb in de-differentiated SMCs in vitro, preventing NOS2-dependent cytotoxicity and promoting proper remodeling of vascular tissue. Rapid NO consumption by Cygb in vascular SMCs, which has previously only been shown to influence vascular tone and blood pressure, may mediate this effect. This work provides compelling evidence that Cygb may be a key regulator of vascular function under both normal and pathologic conditions, indicating that Cygb and other non-erythroid globins may have value as therapeutic targets or agents for myriad disease states. The particular properties of the non-erythroid globins keep revealing new possibilities, from the potential of modulating NO signaling to their use as potential carbon monoxide scavengers and/or oxygen carriers.26, 27
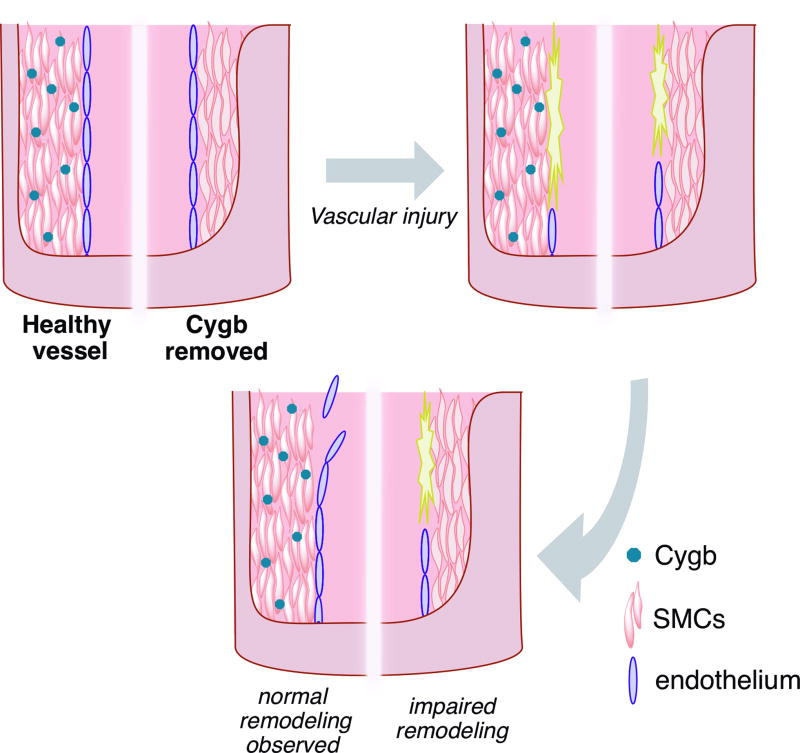
Figure 1.
Murine vascular injury is induced via arterial balloon angioplasty, leading to denuding of the endothelium. In a healthy vasculature (left half of vessel), such an injury results in remodeling (neointima formation) after several days. However, the loss of cytoglobin (Cygb) via either RNA silencing or genetic knockouts (right half of vessel) results in impaired remodeling over the same period.
Acknowledgments
Sources of Funding
M.B. Amdahl is funded by National Institutes of Health Grants T32 HL076124 and F30 DK112560. J. Tejero is funded by National Institutes of Health Grant R21 ES027390. M.T. Gladwin receives research support from National Institutes of Health grants R01HL098032, R01HL125886, P01HL103455, T32 HL110849, and T32 HL007563; and the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania.
Footnotes
Disclosures
None.
References
- 1.Ascenzi P, Gustincich S, Marino M. Mammalian Nerve Globins in Search of Functions. IUBMB Life. 2014;66:268–276. doi: 10.1002/iub.1267. [DOI] [PubMed] [Google Scholar]
- 2.Fordel E, Thijs L, Martinet W, Schrijvers D, Moens L, Dewilde S. Anoxia or Oxygen and Glucose Deprivation in Sh-Sy5y Cells: A Step Closer to the Unraveling of Neuroglobin and Cytoglobin Functions. Gene. 2007;398:114–122. doi: 10.1016/j.gene.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Hodges NJ, Innocent N, Dhanda S, Graham M. Cellular Protection from Oxidative DNA Damage by over-Expression of the Novel Globin Cytoglobin in Vitro. Mutagenesis. 2008;23:293–298. doi: 10.1093/mutage/gen013. [DOI] [PubMed] [Google Scholar]
- 4.Xu R, Harrison PM, Chen M, Li L, Tsui TY, Fung PC, Cheung PT, Wang G, Li H, Diao Y, Krissansen GW, Xu S, Farzaneh F. Cytoglobin Overexpression Protects against Damage-Induced Fibrosis. Mol Ther. 2006;13:1093–1100. doi: 10.1016/j.ymthe.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Nishi H, Inagi R, Kawada N, Yoshizato K, Mimura I, Fujita T, Nangaku M. Cytoglobin, a Novel Member of the Globin Family, Protects Kidney Fibroblasts against Oxidative Stress under Ischemic Conditions. Am J Pathol. 2011;178:128–139. doi: 10.1016/j.ajpath.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shivapurkar N, Stastny V, Okumura N, et al. Cytoglobin, the Newest Member of the Globin Family, Functions as a Tumor Suppressor Gene. Cancer Res. 2008;68:7448–7456. doi: 10.1158/0008-5472.CAN-08-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang J, Ma I, Allalunis-Turner J. Knockdown of Cytoglobin Expression Sensitizes Human Glioma Cells to Radiation and Oxidative Stress. Radiat Res. 2011;176:198–207. doi: 10.1667/rr2517.1. [DOI] [PubMed] [Google Scholar]
- 8.Vinogradov SN, Moens L. Diversity of Globin Function: Enzymatic, Transport, Storage, and Sensing. J Biol Chem. 2008;283:8773–8777. doi: 10.1074/jbc.R700029200. [DOI] [PubMed] [Google Scholar]
- 9.Tejero J, Gladwin MT. The Globin Superfamily: Functions in Nitric Oxide Formation and Decay. Biol Chem. 2014;395:631–639. doi: 10.1515/hsz-2013-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thuy le TT, Van Thuy TT, Matsumoto Y, Hai H, Ikura Y, Yoshizato K, Kawada N. Absence of Cytoglobin Promotes Multiple Organ Abnormalities in Aged Mice. Sci Rep. 2016;6:24990. doi: 10.1038/srep24990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hankeln T, Wystub S, Laufs T, Schmidt M, Gerlach F, Saaler-Reinhardt S, Reuss S, Burmester T. The Cellular and Subcellular Localization of Neuroglobin and Cytoglobin -- a Clue to Their Function? IUBMB Life. 2004;56:671–679. doi: 10.1080/15216540500037794. [DOI] [PubMed] [Google Scholar]
- 12.Burmester T, Hankeln T. Function and Evolution of Vertebrate Globins. Acta Physiol (Oxf) 2014;211:501–514. doi: 10.1111/apha.12312. [DOI] [PubMed] [Google Scholar]
- 13.Tian SF, Yang HH, Xiao DP, Huang YJ, He GY, Ma HR, Xia F, Shi XC. Mechanisms of Neuroprotection from Hypoxia-Ischemia (Hi) Brain Injury by up-Regulation of Cytoglobin (Cygb) in a Neonatal Rat Model. J Biol Chem. 2013;288:15988–16003. doi: 10.1074/jbc.M112.428789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halligan KE, Jourd'heuil FL, Jourd'heuil D. Cytoglobin Is Expressed in the Vasculature and Regulates Cell Respiration and Proliferation Via Nitric Oxide Dioxygenation. J Biol Chem. 2009;284:8539–8547. doi: 10.1074/jbc.M808231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner AM, Cook MR, Gardner PR. Nitric-Oxide Dioxygenase Function of Human Cytoglobin with Cellular Reductants and in Rat Hepatocytes. J Biol Chem. 2010;285:23850–23857. doi: 10.1074/jbc.M110.132340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Tong J, Zweier JR, Follmer D, Hemann C, Ismail RS, Zweier JL. Differences in Oxygen-Dependent Nitric Oxide Metabolism by Cytoglobin and Myoglobin Account for Their Differing Functional Roles. FEBS J. 2013;280:3621–3631. doi: 10.1111/febs.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gladwin MT, Ognibene FP, Pannell LK, Nichols JS, Pease-Fye ME, Shelhamer JH, Schechter AN. Relative Role of Heme Nitrosylation and Beta-Cysteine 93 Nitrosation in the Transport and Metabolism of Nitric Oxide by Hemoglobin in the Human Circulation. Proc Natl Acad Sci U S A. 2000;97:9943–9948. doi: 10.1073/pnas.180155397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD. Rate of Reaction with Nitric Oxide Determines the Hypertensive Effect of Cell-Free Hemoglobin. Nat Biotechnol. 1998;16:672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 19.Gardner PR. Nitric Oxide Dioxygenase Function and Mechanism of Flavohemoglobin, Hemoglobin, Myoglobin and Their Associated Reductases. J Inorg Biochem. 2005;99:247–266. doi: 10.1016/j.jinorgbio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Gardner PR, Gardner AM, Martin LA, Salzman AL. Nitric Oxide Dioxygenase: An Enzymic Function for Flavohemoglobin. Proc Natl Acad Sci U S A. 1998;95:10378–10383. doi: 10.1073/pnas.95.18.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE. Endothelial Cell Expression of Haemoglobin Alpha Regulates Nitric Oxide Signalling. Nature. 2012;491:473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Straub AC, Butcher JT, Billaud M, Mutchler SM, Artamonov MV, Nguyen AT, Johnson T, Best AK, Miller MP, Palmer LA, Columbus L, Somlyo AV, Le TH, Isakson BE. Hemoglobin Alpha/Enos Coupling at Myoendothelial Junctions Is Required for Nitric Oxide Scavenging During Vasoconstriction. Arterioscler Thromb Vasc Biol. 2014;34:2594–2600. doi: 10.1161/ATVBAHA.114.303974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, El-Mahdy MA, Boslett J, Varadharaj S, Hemann C, Abdelghany TM, Ismail RS, Little SC, Zhou D, Thuy LT, Kawada N, Zweier JL. Cytoglobin Regulates Blood Pressure and Vascular Tone through Nitric Oxide Metabolism in the Vascular Wall. Nat Commun. 2017;8:14807. doi: 10.1038/ncomms14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amdahl MB, Sparacino-Watkins CE, Corti P, Gladwin MT, Tejero J. Efficient Reduction of Vertebrate Cytoglobins by the Cytochrome B5/Cytochrome B5 Reductase/Nadh System. Biochemistry. 2017;56:3993–4004. doi: 10.1021/acs.biochem.7b00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Totzeck M, Hendgen-Cotta UB, Luedike P, et al. Nitrite Regulates Hypoxic Vasodilation Via Myoglobin-Dependent Nitric Oxide Generation. Circulation. 2012;126:325–334. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azarov I, Wang L, Rose JJ, et al. Five-Coordinate H64q Neuroglobin as a Ligand-Trap Antidote for Carbon Monoxide Poisoning. Sci Transl Med. 2016;8:368ra173. doi: 10.1126/scitranslmed.aah6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose JJ, Wang L, Xu Q, McTiernan CF, Shiva S, Tejero J, Gladwin MT. Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy. Am J Respir Crit Care Med. 2017;195:596–606. doi: 10.1164/rccm.201606-1275CI. [DOI] [PMC free article] [PubMed] [Google Scholar]