Abstract
Polyarteritis nodosa (PAN) is an inflammatory vasculitis that creates regions of stenosis and aneurysm formation. The authors describe a 66-year-old female with hepatic artery rupture as the first presentation of undiagnosed PAN, presenting with abdominal pain followed by hemorrhagic shock. This aneurysm was suture ligated with a successful outcome. A mesenteric arteriogram demonstrated lesions consistent with PAN including aneurysms of the left gastric branches, right and left hepatic arteries, and beaded appearance of the iliac artery. However, she developed massive pulmonary embolism from which she did not recover after discharge. Postmortem examination confirmed left hepatic artery aneurysm rupture and changes consistent with PAN on gross anatomical examination and histology. This report provides a unique overview of the disease process through imaging, gross anatomic specimen and pathology. Life-threatening hepatic artery aneurysm rupture is an uncommon presentation of PAN which may benefit readers in creating a more robust differential diagnosis.
INTRODUCTION
Polyarteritis nodosa (PAN) is a systemic inflammatory vasculitis that causes intimal proliferation and elastic lamina disruption. This multifocal disruption of the vessel results in aneurysm formation alternating with stenosis creating a characteristic ‘rosary sign’ on imaging. Spontaneous aneurysm rupture is rare and can cause life-threatening hemorrhage. Prompt diagnosis and appropriate intervention is paramount for survival in PAN aneurysm ruptures.
CASE REPORT
A 66-year old female with hypertension, cervical dysplasia and stage II breast cancer presented with sudden right upper quadrant abdominal pain associated with nausea, vomiting and anorexia. Initial laboratory tests showed a hematocrit of 33.9% (nl 37–47%), alanine aminotransferase of 518 U/L (nl 13–61 U/L), aspartate aminotransferase of 208 U/L (nl 15–37 U/L) and total bilirubin of 2.3 mg/dL (nl 0.2–1.0 mg/dL). Performance of an abdominal ultrasound was attempted but not completed as the patient became hypotensive, tachycardic and diaphoretic.
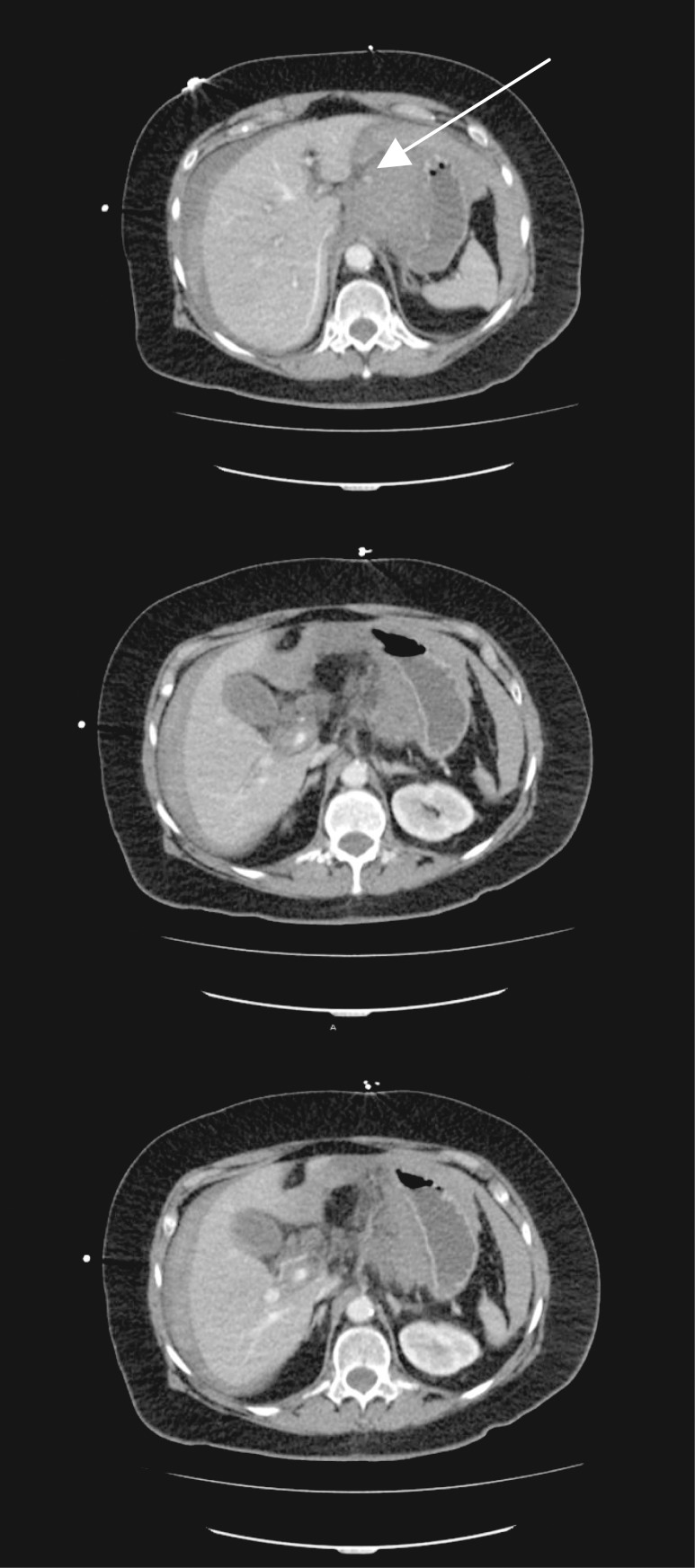
After fluid resuscitation and hemodynamic stabilization, IV contrast-enhanced CT revealed a large fluid collection consistent with hemoperitoneum (Fig. 1a). A bright area of contrast near the left hepatic artery was noted abutting the lesser curvature of the stomach with no active extravasation (Fig. 1b). She again became vitally unstable with a significant drop in hematocrit to 19% (nl 37–47%). Massive transfusion protocol was initiated and the patient was taken to the operating room for exploratory laparotomy rather than interventional radiology due to hemodynamic instability and a clinically acute surgical abdomen.
Figure 1:
(a) A contrast-enhanced CT of the abdomen shows a large fluid collection in the region of the left upper quadrant, abutting the lesser curvature of the stomach. Questionable active extravasation of contrast medium (a, white arrow) in the region of the left hepatic artery with shoddy celiac axis noted (c). The CT numbers range from 130 to 200 Hounsfield units in the area, consistent with possible contrast material.
Upon entering the abdomen, 2500 mL of blood was evacuated from the peritoneal space with an additional 400 mL hematoma abutting the lesser curvature of the stomach. Upon exploration of the hematoma, irregular vascular anatomy was noted, bleeding from the left hepatic artery was identified, suture ligation with distal and proximal control performed, and a drain placed.
IR mesenteric angiography was obtained and revealed a tortuous and aneurysmal takeoff of the celiac trunk (Fig. 2a), aberrant anatomy with a replaced right hepatic artery, splenic artery arising from the SMA and multiple mesenteric aneurysms including the intrahepatic right hepatic artery, left gastric branches and left hepatic artery (Fig. 2b) raising suspicion for PAN. Contrast examination of the right external iliac and common femoral arteries demonstrated an abnormal beaded appearance of the iliac artery (Fig. 2c). There was no active extravasation identified.
Figure 2:
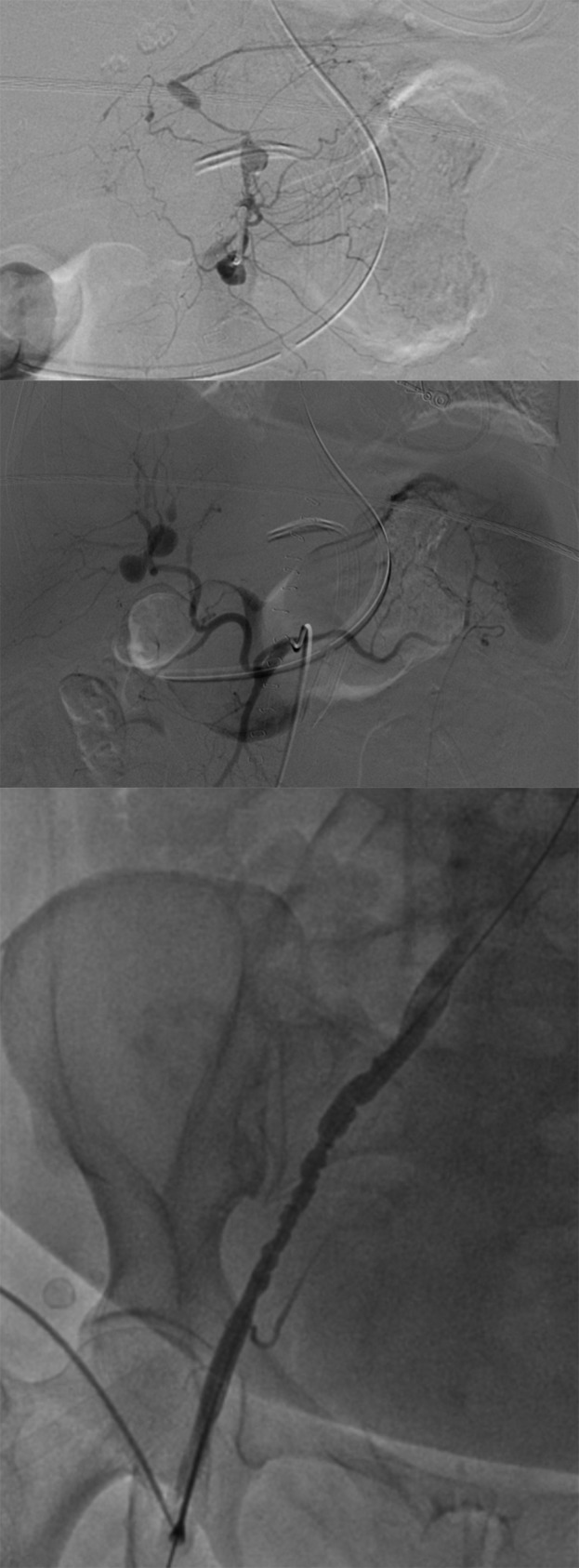
(a and b) Above: Mesenteric angiography showing aberrant anatomy as well as numerous visceral aneurysms including the right and left hepatic, and left gastric branches, without active contrast extravasation. (c) A contrasted angiogram examination of the right external iliac and femoral arteries showing a beaded appearance of the iliac and normal femoral artery.
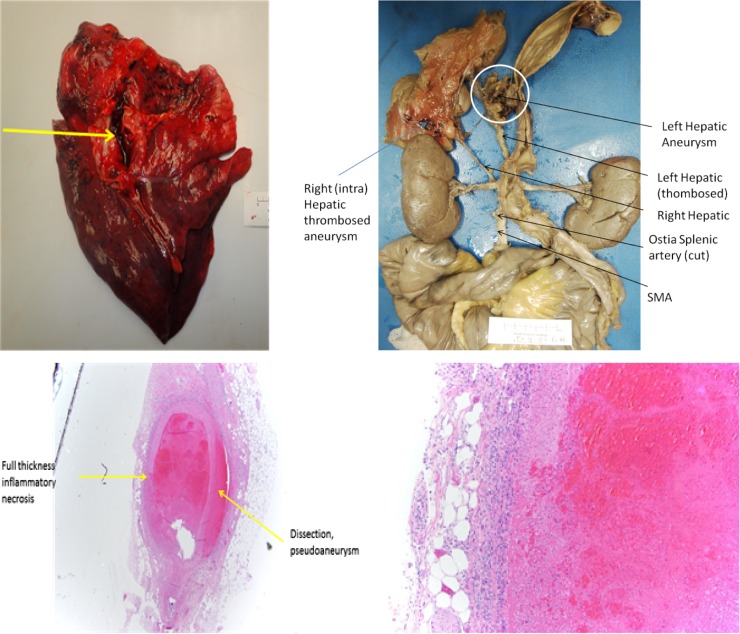
Prophylactic subcutaneous heparin was initiated on postoperative Day 3 since the acute bleed was directly visualized and suture ligated in the operating room with IR angiography and CT imaging demonstrating no extravasation. She remained stable on vital signs and serial hematocrits and was discharged on Day 10. Plans were made for outpatient autoimmune and vasculitis workup as an outpatient following discharge. The patient returned to the emergency department on postoperative Day 12 after a syncopal episode associated with chest pain. The patient lost vitals, underwent advanced cardiovascular life support, and subsequently expired. Postmortem autopsy revealed a massive pulmonary embolism and anomalous abdominal visceral vasculature (Fig. 3b). On microscopic examination, transmural inflammation with associated necrosis and dissected pseudoaneurysm was found in the left hepatic artery which was sutured and thrombosed (Fig. 3c and d). These findings were consistent with a diagnosis of PAN.
Figure 3:
(a–d) Above: Near saddle pulmonary embolism (a, yellow arrow). Aberrant arterial anatomy with hepatic artery originating from the SMA. Left hepatic artery with suture ligation and thrombosis (b). Left hepatic artery aneurysm (b, white circle). Full thickness inflammatory necrosis with a pseudoaneurysm that had dissected (c). Full thickness inflammatory necrosis of the left hepatic artery consistent with polyarteritis nodosa (d).
DISCUSSION
PAN is a systemic transmural inflammatory vasculitis that affects medium-sized arteries [1, 2]. Inflammation of the vessel wall and intimal proliferation creates luminal narrowing which can lead to stenosis and insufficiency. The same inflammatory process causes disruption of the elastic lamina leading to aneurysm formation and possible spontaneous rupture with life-threatening bleeding. Multifocal segments of stenosis and aneurysm formation are characteristically identified as a ‘rosary sign’ or ‘beads on a string’ as seen in this patient in Fig. 2c. Unlike other vasculitides, PAN does not involve small arteries or veins, and is not associated with anti-neutrophil cytoplasmic antibodies [3].
Inclusion of three of the following criteria has been found to have a sensitivity and specificity of 82 and 87%, respectively for the classification of PAN: unexplained weight loss, livedo reticularis, testicular pain, myalgias, neuropathy, new-onset diastolic blood pressure elevation, elevated blood urea nitrogen, hepatitis B infection, ateriographic abnormalities and biopsy of arteries containing polymorphonuclear cells [4]. Our patient endorsed a 25 pound weight loss, myalgia of bilateral lower extremities, neuropathy of the left proximal thigh, and was found to have arteriographic abnormalities as demonstrated (Fig. 2a–c). Autopsy yielded transmural inflammatory changes of medium arteries and regions of stenosis and aneurysm formation consistent with PAN (Fig. 3b–d).
Medical management of PAN is stratified by severity of disease with the goal of active disease remission and control of arterial disease and organ damage progression. Patients with mild disease manifest constitutional symptoms with no significant end-organ damage or life-threatening conditions. Initial monotherapy with oral glucocorticoids is followed by dose tapering once clinical improvement is noted. Moderate to severe disease is defined by serious manifestations such as any degree of renal insufficiency, hypertension secondary to vasculitis, symptomatic arterial stenosis, aneurysms or ischemic disease. This may be treated with high-dose glucocorticoids (intravenous in life-threatening disease) and a second immunosuppressive drug, such as cyclophosphamide. Once in remission, cyclophosphamide may be transitioned to azathioprine or methotrexate for maintenance due to its toxicity profile.
Arterial perforation in the setting of PAN is emergent and almost certainly fatal. Travers et al. described 17 patients with a clinical diagnosis of PAN in which 35% developed mesenteric perforation and hemorrhage. Despite interventional efforts, there was 100% mortality [5]. These results were mirrored in a similar study where 30% of PAN patients developed an acute abdomen with up to 100% mortality [6]. With acutely ruptured aneurysms, prompt diagnosis, resuscitation and hemostasis through transarterial embolization or surgery is paramount for patient survival. Patients with known PAN should be placed on a regimen of glucocorticoids in addition to an immunosuppressant depending on the severity of disease for disease remission and should receive routine evaluation to monitor disease progression. Aneurysms identified on imaging may benefit from prophylactic stenting to avoid rupture. Aneurysmal rupture and acute bleed should be considered in a robust differential diagnosis in patients with PAN presenting with hemodynamic instability and abdominal pain to aid in expedited treatment.
ACKNOWLEDGEMENTS
The researchers would like to thank the patient described for her willingness to participate in this research endeavor prior to her passing.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
No sources of funding.
ETHICAL APPROVAL
Aside from informed patient consent, ethical approval was not required for this case report.
CONSENT
Written informed consent was obtained directly from the patient for publication of this case report prior to her passing.
GUARANTOR
Shinban Liu is the guarantor for this case report.
REFERENCES
- 1. Balow JE. Renal vasculitis. Kidney Int 1985;27:954. [DOI] [PubMed] [Google Scholar]
- 2. Sato O, Cohn DL. Polyarteritis and microscopic polyangiitis In: Klippel JH, Dieppe PA, eds.. Rheumatology. St Louis: Mosby, 2003, 1290–99. [Google Scholar]
- 3. Kallenberg CG, Brouwer E, Weening JJ, Tervaert JW. Anti-neutrophil cytoplasmic antibodies: current diagnostic and pathophysiological potential. Kidney Int 1994;46:1. [DOI] [PubMed] [Google Scholar]
- 4. Lightfoot RW Jr, Michel BA, Bloch DA, Hunder GG, Zvaifler NJ, McShane DJ, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritisnodosa. Arthritis Rheum 1990;33:1088. [DOI] [PubMed] [Google Scholar]
- 5. Travers RL, Allison DJ, Brettie RP, Hughes GR. Polyarteritisnodosa: a clinical and angiographic analysis of 17 cases. Semin Arthritis Rheum 1979;8:184–99. [DOI] [PubMed] [Google Scholar]
- 6. Levine SM, Hellmann DB, Stone JH. Gastrointestinal involvement in polyarteritisnodosa (1986–2000): presentation and outcomes in 24 patients. Am J Med 2002;112:386. [DOI] [PubMed] [Google Scholar]