Abstract
BACKGROUND
Epidemiologic data suggest cholesterol-lowering drugs may prevent the progression of prostate cancer, but not the incidence of the disease. However, the association of combination therapy in cholesterol reduction on prostate or any cancer is unclear. In this study, we compared the effects of the cholesterol lowering drugs simvastatin and ezetimibe alone or in combination on the growth of LAPC-4 prostate cancer in vivo xenografts.
METHODS
Proliferation assays were conducted by MTS solution and assessed by Student’s t-test. 90 male nude mice were placed on a high-cholesterol Western-diet for 7 days then injected subcutaneously with 1 × 105 LAPC-4 cells. Two weeks post-injection, mice were randomized to control, 11 mg/kg/day simvastatin, 30 mg/kg ezetimibe, or the combination and sacrificed 42 days post-randomization. We used a generalized linear model with the predictor variables of treatment, time, and treatment by time (i.e., interaction term) with tumor volume as the outcome variable. Total serum and tumor cholesterol were measured. Tumoral RNA was extracted and cDNA synthesized from 1 ug of total RNA for quantitative real-time PCR.
RESULTS
Simvastatin directly reduced in vitro prostate cell proliferation in a dose-dependent, cell line-specific manner, but ezetimibe had no effect. In vivo, low continuous dosing of ezetimibe, delivered by food, or simvastatin, delivered via an osmotic pump had no effect on tumor growth compared to control mice. In contrast, dual treatment of simvastatin and ezetimibe accelerated tumor growth. Ezetimibe significantly lowered serum cholesterol by 15%, while simvastatin had no effect. Ezetimibe treatment resulted in higher tumor cholesterol. A sixfold induction of low density lipoprotein receptor mRNA was observed in ezetimibe and the combination with simvastatin versus control tumors.
CONCLUSIONS
Systemic cholesterol lowering by ezetimibe did not slow tumor growth, nor did the cholesterol independent effects of simvastatin and the combined treatment increased tumor growth. Despite lower serum cholesterol, tumors from ezetimibe treated mice had higher levels of cholesterol. This study suggests that induction of low density lipoprotein receptor is a possible mechanism of resistance that prostate tumors use to counteract the therapeutic effects of lowering serum cholesterol.
Keywords: prostate cancer, cholesterol, simvastatin, ezetimibe
INTRODUCTION
Hypercholesterolemia, or high cholesterol, is a problematic issue in the United States. It is estimated that 102.2 million adults suffer from high cholesterol, which is characterized by total serum cholesterol levels >200 mg/dL [1]. Typically, adults with high cholesterol are at greater risk for heart disease and stroke [2]. Several treatment options exist for patients with hypercholesterolemia including HMG-CoA reductase inhibitors (statins), cholesterol uptake inhibitors such as niacin, fenofibrates, or ezetimibe [3], as well as dietary intervention [4].
Aside from cardiovascular disorders, recent data suggest consuming factors that are associated with high cholesterol (i.e., a high-fat, high-cholesterol “Western” diet) may be a risk factor for many solid tumors, including breast, colorectal, pancreatic, liver, and prostate cancers [5–8]. In terms of cholesterol and prostate cancer (PCa) risk, although not all studies support an association between cholesterol levels and PCa, the preponderance of the evidence supports a role for HDL, LDL and, total cholesterol (TC) in overall PCa risk [9–12] and in PCa progression [13]. However, the strongest evidence to date supports selective association between hypercholesterolemia and increased incidence of lethal or advanced prostate cancer with no association with total PCa risk [14,15]. In line with this, several pre-clinical studies suggest that high cholesterol promotes PCa progression [16–18], including a study that demonstrated that the cholesterol-uptake inhibitor ezetimibe reduced PCa growth of an LNCaP xenograft model [19]. The aforementioned study revealed an association between lowering serum cholesterol with slower tumor growth and decreased angiogenesis in mice treated with ezetimibe, despite consuming a high-fat, high-cholesterol diet.
As cholesterol appears to be important in tumor progression, in part, by increasing lipid raft formation and subsequent cell survival pathways [16] and/or by contributing to androgen synthesis [17], it has been hypothesized that cholesterol-lowering drugs may have anti-cancer properties. Inhibitors of the enzyme HMGCoA reductase, statins block the rate-limiting step in the de novo cholesterol synthesis pathway which might explain their anti-PCa properties. However, statins also reduce signaling of survival pathways involved in inflammation, angiogenesis, cell proliferation, invasion, and migration independent of cholesterol lowering and thus may have non-cholesterol mediated effects [20–24].
In human studies, we previously showed that PCa-free men significantly reduce their PSA levels within one year of starting a statin [25]. Interestingly, the reduction in PSA was both proportional to the amount of LDL decline (i.e., cholesterol-mediated) as well as the statin dose even after controlling for the LDL decline in the analysis (i.e., non-cholesterol mediated). Moreover, in preclinical models, two studies looking at combinatorial therapy with atorvastatin and celecoxib in vitro as well as in PC-3 and LNCaP xenograft models showed a statistically significant inhibition of cellular growth upon treatment with both drugs, but only modest effects when either was used alone [26,27]. This suggests that targeting multiple pathways, both cholesterol-dependent and -independent, may be key to slow PCa. It is important to note that while statins can have effects on both of these pathways in humans, data suggest that statins do not lower circulating levels of cholesterol in mice [28,29]. The inability of statins to affect circulating cholesterol levels in mice provides an effective model to understand the mechanisms through which these drugs may work. Do the effects of statins originate from lowering cholesterol or from other effects exerted by these drugs? In this study, we sought to determine the ability of the cholesterol-lowering drugs simvastatin and ezetimibe to slow PCa growth in a xenograft model. We hypothesized that these agents will slow PCa growth via cholesterol-mediated and non-cholesterol-mediated pathways, and the combination of the two will be more effective than either drug alone.
MATERIALS AND METHODS
Cell Culture
DU145, LnCaP, PC-3, LNCaP, and CWR22rv1 human PCa cell lines were purchased from the American Type Culture Collection in 2010 (ATCC; Manassas, VA) and were authenticated by STR analysis. LAPC-4 human PCa cells were a generous gift from William J. Aronson, UCLA School of Medicine in 2009. LnCaP, PC-3, and CWR22rv1 were cultured in RPMI 1640 medium, while DU145 cells were cultured in Dulbecco’s Modified Eagle Medium containing 0.1 mM Non-Essential Amino Acids and 1 mM Sodium Pyruvate. LAPC-4 cells were maintained in Iscove’s Modified Medium supplemented with 1 nM of the synthetic androgen R1881. All cell culture media contained 10% Fetal Bovine Serum and 1% Penicillin/Streptomycin, and cells were grown in 5% CO2 at 37°C and harvested by trypsinization at ~80% confluence in log phase growth.
In Vitro Proliferation Assays
Cells were seeded into 96-well plates at a concentration of 5,000–7,500 cells/well. Twenty-four hours after plating, media was removed and replaced with complete medium containing varying concentrations of simvastatin (0–1,000 nM; Sigma–Aldrich, St. Louis, MO) or ezetimibe (0–100 μM; Schering-Plough, Kenilworth, NJ). The cells were then incubated 0–5 days post-treatment and analyzed for changes in cell proliferation using the Celltiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI). All experiments were performed in triplicate and executed three times in independent experiments.
Animal Studies
After approval from the Duke University Institutional Animal Care and Use Committee, 90 male nude (Hsd:Athymic Nude-Foxn1Nu) mice, aged 6 weeks, were purchased from Harlan Laboratories (Somerville, NJ). All diets were prepared by Research Diets (New Brunswick, NJ). Animals were fed an ad libitum high-cholesterol Western-diet (40% fat, 43% carbohydrate, 17% protein kcals; 1.25% cholesterol) for 7 days, after which they were injected subcutaneously with 1 × 105 LAPC-4 tumor cells in 0.1 ml of Matrigel (BD Biosciences, Franklin Lakes, NJ). The rationale to use LAPC4 is that it is an androgen sensitive cell line that has a wild-type androgen receptor. Moreover, we have previously shown this cell line is sensitive to dietary manipulations [30–32]. Thus we felt this was a good model of androgen-sensitive PCa wherein less toxic approaches (i.e., diet, lifestyle, cholesterol manipulation) would have the greatest clinical benefit. All mice were housed five per cage. Two weeks post-injection, all mice were randomized (day 0) to one of four treatment groups: Control (no intervention), 11 mg/kg/day simvastatin, 30 mg/kg ezetimibe, or combination (11 mg/kg/day simvastatin +30 mg/kg ezetimibe). The dose of ezetimibe was based upon prior publications in the field of PCa that used this dose with significant effects on serum cholesterol and tumor growth [19]. The highest clinical dose of simvastatin used in patients is 80 mg. Assuming a 90 kg man (overweight—typical PCa patient who would need a statin), adjusted for mouse dosing, the equivalent mouse dose would be 10.93 mg/kg/day [33]. This was then rounded to 11 mg/kg/day. While human dosing is typically oral, given the large first-pass effect on simvastatin levels, herein we used continuous subcutaneous dosing to try to increase serum levels to increase delivery to the tumor. All mice were subcutaneously implanted with Alzet osmotic pumps (DURECT Corporation, Cupertino, CA). These pumps, which contained 250 μl of solution, continuously administered either vehicle control (40% DMSO, 60% PBS; used for the control and ezetimibe alone groups) or simvastatin. Mice receiving ezetimibe did so through their diet. Mice were weighed and tumor dimensions measured twice a week with calipers once palpable. Tumor volumes were then calculated using the formula: width × height × length × 0.5236 [34].
Animals were euthanized using a lethal dose of Nembutol 42 days post-randomization or when the health of the animal appeared compromised per Duke institutional criteria (ruffled fur, hunched posture, lethargy, severe weight loss, etc.). A total of five mice were euthanized early from the study—3 for improper wound healing at the site of pump implantation (1 control, 2 combination), and two for meeting health criteria for sacrifice (1 control, 1 combination). For the 85 mice that remained on study, serum was obtained via cardiac puncture at harvest. Livers were removed and snap-frozen for analysis of fatty deposition. Tumor samples were snap-frozen for necrosis and cholesterol analysis. Tissue weights were recorded prior to snap-freezing and all samples were stored at −80°C for subsequent analysis.
Liver Function Analysis
Samples from the median 10 mice (by tumor size) of each experimental group were analyzed in all secondary analyses. Serum was assayed for bilirubin (both direct and total), alanine transaminase (ALT), and aspartate transaminase (AST) activities via ELISA (BioAssay Systems, Hayward, CA; ID Labs, London, ON).
Tumor Necrosis and Liver Fatty Deposition Analysis
Slides of frozen tumors and livers were stained with hematoxylin and eosin (H&E) for necrosis (tumors) and fatty deposition (livers). All slides were blinded and read by an independent board-certified pathologist (SVP) and graded as follows: 0 = less than 10% necrosis/fatty deposition, 1 = 10–25% necrosis/fatty depositions, 2 = 25–50% necrosis/fatty deposition, 3 = 50–75% necrosis/fatty deposition, and 4 = greater than 75% necrosis/fatty deposition.
Serum and Tumor Cholesterol Analysis
Measurements for total serum cholesterol were performed using a Beckman D × C600 autoanalyzer (Fullerton, CA). Tumor cholesterol was extracted as previously described [35] and measured using the Infinity Cholesterol Liquid Stable Reagent (Thermo Scientific, Middletown, VA).
RNA Extraction and qRT-PCR Analyses
After cryogrinding tumors, total RNA was extracted using RNeasy mini kit (Qiagen, Germantown, MD). cDNA was synthesized from 1 μg total RNA using the BioRad iScript cDNA Synthesis Kit. Quantitative RT-PCR (qRT-PCR) was performed with 2 μl 1:20 diluted cDNA, 0.2 μmol/L primers and the iQ SYBR Green supermix (Bio-Rad, Hercules, CA), the results calculated using the 2−ΔΔcT method and data normalized to a 36B4 internal control. Primer sequences were previously described [36].
Western Blot Analysis
LAPC4, LNCaP, and 22RV1 cells were plated in their respective base media supplemented with FBS. At 50% confluency, cells were rinsed with 2× PBS and treated with respective media supplemented with 10% lipodeficient FBS (Alfa Aesar, MA) and varying concentrations of water-soluble cholesterol (Sigma Aldrich, MO). After 72 hr, protein from the treated cells was harvested in 1×RIPA buffer supplemented with protease inhibitor cocktail (Thermo Fisher, MA). Protein supernatant was then collected from treated cells after a 10,000g spin at 4°C for 15 min, quantified using the DC protein assay kit (Bio-Rad) and 30 μg was loaded onto each lane on a 7.5% SDS page gel after reducing with β-mercaptoethanol (Bio-Rad) and heating at 95°C for 5 min. Separated proteins were transferred to nitrocellulose membranes, which were blocked for 1 hr in 7% Milk+ 1× TBST, followed by incubation with primary antibodies, LDLR (Abcam, Cambridge, MA) or Actin (Cell Signaling, Danvers, MA), followed by 1 hr incubation with anti-rabbit secondary antibody (Cell Signaling). Densitometry was used for quantification of protein bands using ImageJ gel analysis tool on scanned immunoblot images (National Institutes of Health, Bethesda, MD).
Statistical Analysis
In vitro proliferation rates were compared between control and each treatment group using the Student’s t-test. In the simvastatin studies, the IC50 was calculated at Day 3 using BioDataFit 1.02 (Chang Bioscience, http://www.changbioscience.com/stat/ec50.html).
For the animal studies, to test whether treatment affected tumor growth over the 42-day post randomization period, we used a generalized linear model with the predictor variables of treatment, time, and treatment by time (i.e., interaction term) with tumor volume as the outcome variable. Time was treated as categorical since growth patterns in each treatment group were non-linear.
Secondary analyses included group comparisons of body weight, liver weight, tumor necrosis level, liver fat deposition, cholesterol, bilirubin, AST, and ALT by linear regression modeling. For secondary outcome variables that were not normally transformed, we applied techniques such as logarithmic, cubic, x4, and square-root transformations to generate a normally distributed data. Statistical analyses were performed using both a main effect term for treatment arm and an interaction term. If the interaction term was not significant (P >0.05), then the interaction term was removed from the model and the analyses repeated using only variables for the primary treatment arms. All statistical analyses were performed using STATA 11.0 (Stata Corp., College Station, TX) with P ≤ 0.05 considered statistically significant.
RESULTS
In Vitro Cell Proliferation Assays
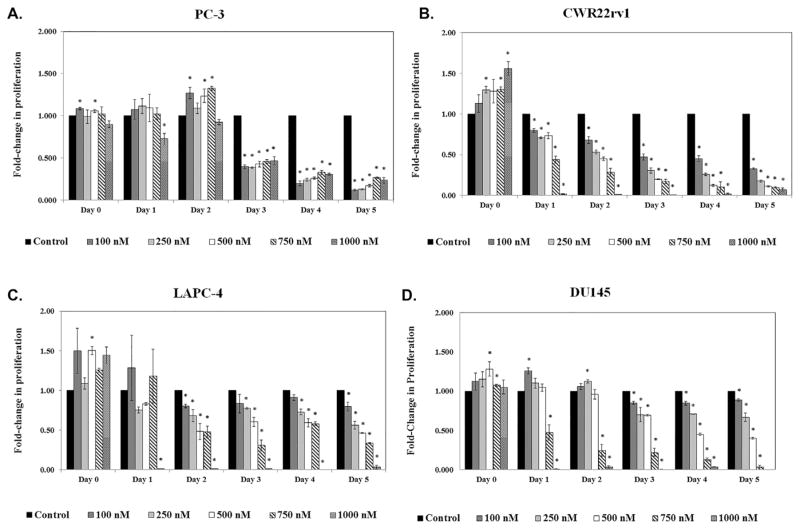
Treatment of four PCa cells lines with varying concentrations of simvastatin showed significant direct inhibition of cell growth in vitro (Fig. 1). We further calculated the IC50 for each cell line at Day 3, which was the median duration of treatment. The inhibitory response appeared to be cell-line specific, with the PC-3 cell line being the most sensitive (IC50 = 75 nM; Fig. 1A), followed by CWR22rv1 (IC50 = 175 nM; Fig. 1B), LAPC-4 (IC50 = 625 nM; Fig. 1C), and DU145 (IC50 = 628 nM; Fig. 1D).
Fig. 1.
Simvastatin inhibits cell proliferation in a cell line-specific manner in vitro. (A) PC-3, (B) CWR22rv1, (C) LAPC-4, and (D) DU145 cells were treated with 0–1,000 nM of simvastatin for 0–5 days. MTS assay was performed to determine the effect of treatment on cell proliferation. Data is presented as fold induction above control treated cells. Error presented as +/− SD of results from four tumors. *Denotes P ≤ 0.05.
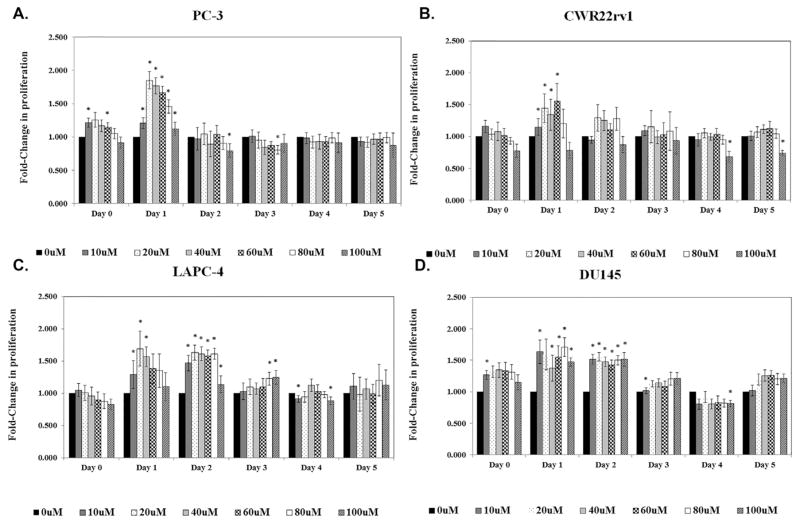
We also treated these same four cell lines with varying concentrations of ezetimibe to investigate whether ezetimibe could directly inhibit cell proliferation (Fig. 2). Interestingly, all cell lines showed a significant initial spike in cell proliferation on Day 1 at low and mid-range treatment concentrations (10–60 μM, respectively), but this spike did not occur with treatments ≥80 μM. Furthermore, all changes in cell proliferation disappeared by Day 2 for the PC-3 and CWR22rv1 cell lines (Fig. 2A and B) and Day 3 for LAPC-4 and DU145 (Fig. 2C and D). Among all cell lines, however, we saw no direct inhibitory effects of ezetimibe on cell proliferation at any dose or time point tested.
Fig. 2.
Ezetimibe has little effect on androgen-dependent or -independent cell lines in vitro. (A) PC-3, (B) CWR22rv1, (C) LAPC-4, and (D) DU145 cells were treated with 0–100 μMM of ezetimibe for 0–5 days. MTS assay was performed to determine the effect of treatment on cell proliferation. Data and error bars are presented as in Figure 1. *Denotes P ≤ 0.05.
Body Weights and Tumor Volumes
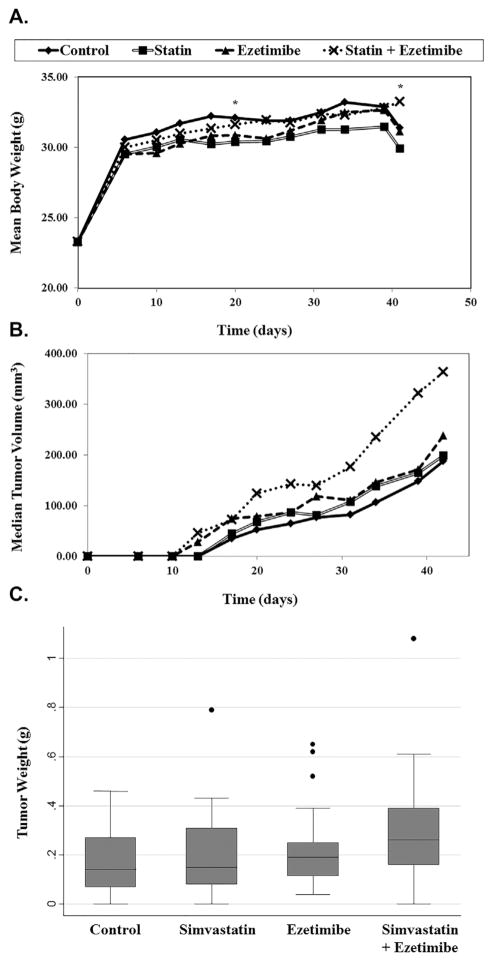
Throughout the course of this study, there were no significant differences in body weights across the groups except at Days 24 and 42 post-randomization, when there was a significant interaction between treatment and body weight. Specifically, mice treated with the combination of statin + ezetimibe were significantly heavier than predicted based upon the single individual arms of statins and ezetimibe (Fig. 3A).
Fig. 3.
The in vivo effects of simvastatin and ezetimibe alone or in combination. Nude mice were subcutaneously injected in the right flank with 1 × 105 LAPC-4 cells. Two weeks after tumor injection, all mice were randomized to receive control, simvastatin (11 mg/kg/day), ezetimibe (30 mg/kg), or the combination of both. Simvastatin was received via osmotic pump, and ezetimibe via the diet. Body weights and tumor volumes were measured twice weekly for 42 days. *Denotes a significant P-interaction < 0.05. Tumor weights were measured at sacrifice.
At every time point throughout the entire study, the control mice had the smallest tumors (Fig. 3B). Likewise, throughout the entire study, the combination group had the largest tumors, with median tumor volumes being 93% larger than control at day 42. Indeed, starting at day 31 and continuing through the remainder of the study, the combination group had significantly larger tumor volumes (P = 0.012 to <0.001) compared to controls. The same trend was observed with final tumor weights at sacrifice with the combination group having the largest tumor weights, however, no significant differences were observed overall in treatment groups compared to control group (P = 0.089) (Fig. 3C). Our results also show that neither statin nor ezetemibe treatment affected tumor growth compared to controls although there was a tendency for tumors that received ezetimibe to be larger at day 34 (P = 0.089) and at day 39 (P = 0.095) though this did not reach significance.
Tumor Necrosis and Fat Deposition in the Liver
Upon harvest, we observed no difference in the amount of necrosis present in the tumors of mice receiving simvastatin (P = 0.47) or ezetimibe (P = 0.83) compared to control (Table I; P-interaction not significant). Overall, mice receiving any of the treatments had significantly lighter livers compared to the control group (P <0.001). Consistent with these results, we found that livers from the control group contained greater fat infiltration than mice receiving simvastatin or ezetimibe (P = 0.01 and <0.001, respectively), but there was no synergistic effect when mice received both drugs (P-interaction not significant).
TABLE I.
Tissue and Serum Analyses
| Analysis | Control | Simvastatin | Ezetimibe | Simvastatin + Ezetimibe | Simvastatin | Ezetimibe (P-value) | Interaction |
|---|---|---|---|---|---|---|---|
| Tumor necrosis grad | 2.50 (1.88–3.50) | 2.25 (1.75–3.50) | 2.25 (1.13–3.13) | 3.00 (1.75–4.00) | 0.47 | 0.83 | ns |
| Liver weight | 1.71 (1.50–1.95) | 1.37 (1.25–1.68) | 1.29 (1.21–1.45) | 1.36 (1.26–1.48) | <0.001 | <0.001 | <0.001 |
| Liver fat deposition grade | 4.00 (3.75–4.00) | 3.50 (3.38–4.00) | 3.25 (1.00–4.00) | 2.00 (1.00–3.00) | 0.01 | <0.001 | ns |
| Total bilirubin (ng/dL) | 0.08 (0.07–0.11) | 0.07 | 0.08 | 0.09 | 0.07 | 0.26 | 0.03 |
| Direct bilirubin (ng/dL) | 0.006 (0.003–0.008) | 0.009 (0.004–0.011) | 0.009 (0.006–0.012) | 0.007 (0.004–0.011) | 0.25 | 0.57 | ns |
| AST (ng/dL) | 1.08 (0.54–2.28) | 0.54 (0.00–2.14) | 1.07 (0.54–1.88) | 0.54 (0.00–0.54) | 0.009 | 0.33 | ns |
| ALT (ng/dL) | 6.97 (2.81–19.97) | 6.16 (2.95–10.59) | 4.82 (2.41–9.38) | 8.58 (2.14–24.79) | 0.48 | 0.82 | ns |
| Serum cholesterol (ng/dL) | 150.00 (142.25–158.75) | 148.00 (127.25–169.00) | 128.50 (121.25–147.50) | 122.00 (109.50–131.00) | 0.25 | <0.001 | ns |
| Tumor cholesterol (mg/mg tumor) | 6.59 (2.64–8.70) | 3.71 (0.89–7.34) | 8.99 (6.52–16.10) | 8.09 (5.08–12.32) | 0.25 | 0.02 | ns |
Analysis of Liver Function
To assess whether simvastatin and/or ezetimibe treatment caused liver toxicities, we analyzed serum for bilirubin, ALT, and AST concentrations. From these analyses, we found no significant difference in total bilirubin of mice receiving simvastatin or ezetimibe (P = 0.07–0.26), but found the combination of the two increased these levels beyond what was predicted (P-interaction = 0.03; Table I). There was no difference in direct bilirubin or ALT levels (P = 0.25–0.82, p-interactions not significant). Interestingly, mice receiving simvastatin had lower AST levels compared to control (P = 0.009), but treatment with ezetimibe had no effect (P = 0.33). Furthermore, the combination had no further effect than single treatment (p-interaction not significant).
Serum and Tumor Cholesterol
Overall, we found that treatment with ezetimibe significantly lowered serum cholesterol levels by approximately 15% compared to control (P <0.001), but treatment with simvastatin had no effect (P = 0.25). Although the combination of simvastatin and ezetimibe lowered serum cholesterol by 19%, the synergistic effect of these two drugs did not reach statistical significance (P-interaction not significant). Despite lower serum cholesterol, mice in the ezetimibe-treated group had higher tumor concentrations of cholesterol compared to control (P = 0.02). There was no statistically significant difference between control and simvastatin-treated mice (P = 0.25), and there was no interaction between the two drugs (P-interaction not significant).
Tumor Levels of LDL Receptor (LDLR)
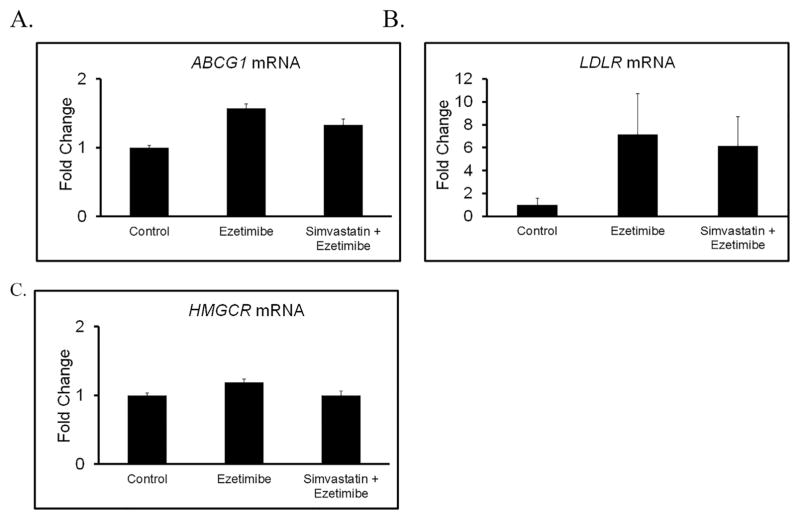
Given that mice that received ezetimibe had significantly higher tumor cholesterol than control tumors despite lower serum cholesterol, we hypothesized this may be explained in part by activation of cholesterol uptake, inhibition of cholesterol efflux, or greater cholesterol de novo production by the tumors. To test this, we compared tumor expression of LDLR between control and ezetimibe treated mice and found that treatment with ezetimibe (alone or in combination with simvastatin) was associated with approximately six fold induction in the transcript levels of LDLR (Fig. 4). Expression levels of other key genes that contribute to cholesterol homeostasis in the tumors such as ABCG1 (cholesterol efflux) and HMGCoA reductase (cholesterol synthesis) did not change as a result of ezetimibe treatment.
Fig. 4.
Mice receiving Ezetimibe treatment have higher expression levels of LDLR in their tumors. RNA was extracted from tumors of each of the following experimental groups (vehicle control, ezetimibe, simvastatin + ezetimibe) and reverse transcribed into cDNA. The expression of ABCG1, LDLR, and HMG-CoR was assessed using qPCR. Data is presented as fold induction above vehicle treated tumors. The data shown are representative of three independent experiments. Error presented as +/− SD of results from four tumors.
LDLR Protein Levels in Response Decreasing Cholesterol In Vitro
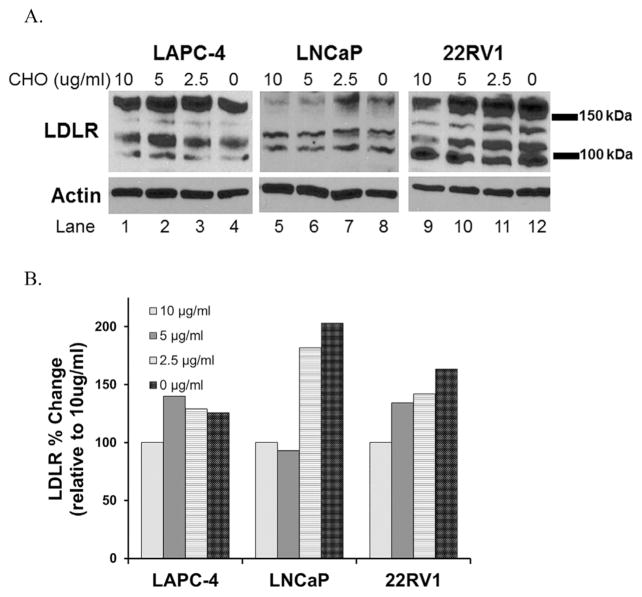
To examine this phenotype in vitro, we cultured PCa cells in media supplemented with lipodeficient serum and added back decreasing doses of cholesterol (10–0 μg/ml) [37] for 72 hr and found that cholesterol-lowering increased LDLR protein in a dose-dependent manner in LAPC4, 22RV1, and LNCaP cells (Fig. 5).
Fig. 5.
Cholesterol lowering induces LDLR in vitro. (A) Western blot analysis of LDLR in response to decreasing doses of cholesterol (CHO). Blot shows various LDLR bands corresponding to glycosylated LDLR, unglycosylated LDLR and LDLR monomers. B) Densitometry analysis (Image J) of summed LDLR bands normalized to Actin loading control, shown as percent induction relative to 10 ug/ml cholesterol control.
DISCUSSION
Hyperlipidemia is an epidemic in the United States, with nearly 50% of all adults categorized as having high cholesterol [1]. Currently there are several options for the treatment of high cholesterol, including statins, which block de novo cholesterol synthesis, and ezetimibe, which blocks cholesterol uptake in the gut. Along with lowering serum cholesterol, there is observational epidemiological data to suggest that cholesterol-lowering drugs, particularly statins, may prevent the progression of PCa, but not necessarily the incidence of the disease [38–41]. Of note, most pre-clinical studies tested whether statins prevent PCa, not whether they can be used as treatment for established tumors [26,27,42]. Furthermore, these studies used intraperitoneal injections as their mode of drug delivery, which yields a large bolus dose and not a timely release as occurs with oral intake in humans. Therefore, in our study, we wanted to test continuous low doses of simvastatin and ezetimibe as treatment for PCa both in vitro and in vivo. We hypothesized these cholesterol-lowering drugs would slow PCa growth via cholesterol-mediated and non-cholesterol-mediated pathways, and the combination of the two would be more effective than either drug alone.
In our study, we found that constant treatment with simvastatin (0–1000 nM) in vitro inhibited proliferation of four PCa cell lines with varying sensitivity. Our treatment concentrations are higher than physiologic conditions, with mean peak serum concentrations for a standard 20 mg simvastatin pill in humans being 3.2–8.7 nM [43,44]. However, our treatment concentrations are much lower than other studies that used simvastatin in micromolar ranges for shorter periods of time [26,27,42] and showed similar results. Only one study to date investigated simvastatin in vitro at physiologically relevant concentrations and found similar results as ours [45]. In contrast, we found no inhibitory effects when PCa cells were treated with varying concentrations of ezetimibe (0–100 μM). These concentrations are multiple log-fold higher than mean peak plasma concentrations of 3.4–5.5 nM in adults prescribed a standard 10 mg ezetimibe dose [46]. Being the first study to investigate the direct effects of ezetimibe on PCa cells in vitro, these data suggest that any anti-tumor activity of ezetimibe witnessed in vivo is due solely to systemic effects, not direct inhibition of tumor cells themselves. Given that expression of ezetimibe’s target, NPC1L1, is generally restricted to intestinal epithelial cells and hepatocytes, this result is consistent with expectations.
In vivo, we found no differences in body weights among the four treatment groups except at two times point: Days 24 and 42, wherein we found the combination-treated mice were significantly heavier than predicted. Upon examination of the livers, we found the control group to have heavier livers and more fatty deposition compared to the treatment arms. Although the mice consuming ezetimibe had the smallest livers by weight and the least amount of fatty deposition among the treatment arms, we did see an interaction between the two drugs in terms of liver weight. In terms of liver function enzymes, we found that simvastatin lowered AST levels, the combination led to slightly higher total bilirubin levels, but there were no effects on direct bilirubin or ALT levels. These data suggest our treatments caused little to no toxicities in the mice and support current studies showing statins and/or ezetimibe have either no effect or sometimes even improve liver function tests in mice [47] and humans [48–50].
The analysis of serum cholesterol showed that ezetimibe treatment resulted in approximately 15–20% lower concentrations than control mice while simvastatin had no effect on serum cholesterol. Of note, liver weights were significantly lower with statins suggesting that sufficient drug levels reached the liver to have an effect. Thus, insufficient statin dose is not the reason for the lack of lower cholesterol levels with statins and in fact prior studies using much higher statin dosing have shown similar lack of effect of statins on serum cholesterol in mice [51]. Most interestingly, this drop in serum cholesterol was associated with increased tumor cholesterol levels. This appears to be explained, in part, by enhanced cholesterol uptake through induction of LDLR, a mechanism that tumors may employ to sustain intracellular cholesterol and support tumor growth despite lowered serum cholesterol. In humans, simvastatin lowers serum cholesterol levels due to the high portion of LDL present. However, previous studies suggest that statins are unable to lower circulating cholesterol in mice [28,29]. This may explain in part why we were unable to detect a difference in circulating and tumor cholesterol levels in the simvastatin group compared to control.
In terms of tumor growth, by the end of the study, there were no significant differences between control, simvastatin, or ezetimibe-treated groups. This is contradictory to several studies indicating the tumor inhibitory effects of simvastatin or ezetimibe in multiple xenograft models [17,19]. However, none of these studies used the LAPC-4 cell line as their model. Moreover, the drug doses in these previous studies were much higher than in our study, wherein mice were implanted with 42-day osmotic pumps containing a concentrated simvastatin solution. Although our mice received continuous dosing, we were limited by the small total volume allowed within the pump reservoir, prohibiting us from dosing the mice any higher than 11 mg/kg/day. We did, however, find increased tumor growth in the combination-treated mice compared to the other three groups, suggesting combinatorial, simvastatin, and ezetimibe treatment may promote tumor growth in our model.
The role of the combination of simvastatin and ezetimibe and cancer is controversial. Recent secondary analyses from three independent human randomized clinical trials (SEAS, SHARP, and IMPROVE-IT) [52–54] provide interesting, but conflicting results. In the SEAS trial, there was a significant increase in all-cancer incidences and deaths in the simvastatin-ezetimibe treatment arm compared to placebo, while analysis of SHARP and IMPROVE-IT “combined” showed no association [55]. To date, our study is the first preclinical study to investigate the combination of simvastatin and ezetimibe as treatment for PCa. As such, the observation that tumor growth may be worse in the combination arm certainly requires validation in other studies, but if confirmed may have profound clinical implications.
Currently, we can only speculate on possible reasons for this effect. In mice treated with chronic low doses of simvastatin, circulating cholesterol levels remain unchanged due to the inability of statins to lower these levels as expected. This implies that any systemic changes that took place as a result of simvastatin treatment were non-cholesterol-mediated. This may possibly relate to the serum levels of simvastatin achieved with the dosage regimen applied in this study, which may not have been enough to directly inhibit tumor growth in vivo, despite evidence of direct inhibition in vitro, though as noted the simvastatin dosing was sufficient to lower liver weight. On the other hand, when mice consume ezetimibe, although this leads to lower circulating cholesterol, this change is not sufficient to modify tumor growth rate. While this may suggest altering serum cholesterol does not affect tumor growth, it is also possible that “slow-growing” tumors such as the LAPC-4 model have time to adapt to these decreases in serum cholesterol. In agreement with the above hypothesis we found that LDLR levels were higher in tumors of mice that received ezetimibe allowing tumors to maintain not just normal, but higher cholesterol levels. This would partially explain why our study contradicts the previous study using a “fast-growing” LNCaP cell line by Solomon et al. [[19]], wherein ezetimibe treatment did significantly slow tumor growth. As such, this suggests, at least in the LAPC-4 xenograft model, that LDLR upregulation to increase cholesterol uptake thereby maintaining cholesterol homeostasis may be a key resistance mechanism to the systemic effects of cholesterol lowering. Moreover, cholesterol-lowering in vitro in LAPC-4, LNCaP, and 22RV1 cells also increased LDLR protein in a dose-dependent manner suggesting that PCa cells can adapt to a reduced cholesterol environment and maintain higher intracellular cholesterol levels by increasing LDLR. However, why the combination of both simvastatin and ezetimibe increased tumor growth is unclear. The combination arm was the heaviest, albeit slightly, and obesity has been shown to promote tumor growth in LAPC4 cells. Whether this explains the findings is unknown. The greater concern is the possibility that inhibiting serum levels (ezetimibe) and potentially inhibiting cholesterol production within the tumor (simvastatin), resulted in upregulated cholesterol machinery above and beyond either drug alone that resulted in excess tumor growth. Though tumor cholesterol levels were similar among groups, it is possible cholesterol flux was increased or tumor androgen levels (cholesterol is a precursor for androgens) were higher thereby promoting tumor growth. As cholesterol flux and tumor androgens were not measured, we cannot assess this possibility. Future studies are necessary to decipher the resistance mechanisms by which cholesterol-lowering increases LDLR and other proteins involved in dysregulated cholesterol homeostasis. Given the number of men in the United States currently being treated for high cholesterol and/or PCa, it is important to better understand the mechanisms of these widely used compounds to provide more effective and personalized treatments for patients.
CONCLUSION
We tested the direct effects of ezetimibe and simvastatin on PC cells in vitro and found only simvastatin had a direct effect on PC cell growth in vitro. In mice, we found treatment with ezetimibe or simvastatin provided no benefit to slowing tumor growth compared to vehicle control, while the combination of simvastatin and ezetimibe promoted tumor growth. Ezetimibe effectively lowered serum cholesterol levels by approximately15–20%, yet tumors from ezetimibe treated groups had higher intratumoral cholesterol levels. LDLR levels were found elevated in ezetimibe treated tumors and after cholesterol-lowering in vitro, suggesting that LDLR upregulation is a potential resistance mechanism to lowering systemic cholesterol.
Acknowledgments
Grant sponsor: Department of Veterans Affairs; Division of Urology; Grant sponsor: Department of Surgery, Duke University; National Institutes of Health; Grant number: NIH R01 CA131235.
Footnotes
Conflict of interest: None
References
- 1.American Heart Association. Cholesterol Statistics. 2010 Available from: http://www.americanheart.org/presenter.jhtml?identifier=4506.
- 2.Almutairi F, Peterson TC, Molinari M, Walsh MJ, Alwayn I, Peltekian KM. Safety and effectiveness of ezetimibe in liver transplant recipients with hypercholesterolemia. Liver Transpl. 2009;15(5):504–508. doi: 10.1002/lt.21710. [DOI] [PubMed] [Google Scholar]
- 3.Foger B. Lipid lowering therapy in type 2 diabetes. Wien Med Wochenschr. 2011;161(11–12):289–296. doi: 10.1007/s10354-011-0908-4. [DOI] [PubMed] [Google Scholar]
- 4.Golan R, Tirosh A, Schwarzfuchs D, Harman-Boehm I, Thiery J, Fiedler GM, Bluher M, Stumvoll M, Shai I. Dietary intervention induces flow of changes within biomarkers of lipids, inflammation, liver enzymes, and glycemic control. Nutrition. 2011;28(2):131–137. doi: 10.1016/j.nut.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krejs GJ. Pancreatic cancer: Epidemiology and risk factors. Dig Dis. 2010;28(2):355–258. doi: 10.1159/000319414. [DOI] [PubMed] [Google Scholar]
- 6.Turner LB. A meta-analysis of fat intake, reproduction, and breast cancer risk: An evolutionary perspective. Am J Hum Biol. 2011;23(5):601–608. doi: 10.1002/ajhb.21176. [DOI] [PubMed] [Google Scholar]
- 7.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda K, Mutoh M, Teraoka N, Nakanishi H, Wakabayashi K, Taguchi R. Increase of oxidant-related triglycerides and phosphatidylcholines in serum and small intestinal mucosa during development of intestinal polyp formation in Min mice. Cancer Sci. 2011;102(1):79–87. doi: 10.1111/j.1349-7006.2010.01754.x. [DOI] [PubMed] [Google Scholar]
- 9.Van Hemelrijck M, Walldius G, Jungner I, Hammar N, Garmo H, Binda E, Hayday A, Lambe M, Holmberg L. Low levels of apolipoprotein A-I and HDL are associated with risk of prostate cancer in the Swedish AMORIS study. Cancer Causes and Control. 2011;22(7):1011–1019. doi: 10.1007/s10552-011-9774-z. [DOI] [PubMed] [Google Scholar]
- 10.Farwell WR, D’Avolio LW, Scranton RE, Lawler EV, Gaziano JM. Statins and prostate cancer diagnosis and grade in a veterans population. J Natl Cancer Inst. 2011;103(11):885–892. doi: 10.1093/jnci/djr108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mondul AM, Weinstein SJ, Virtamo J, Albanes D. Serum total and HDL cholesterol and risk of prostate cancer. Cancer Causes Control. 2011;22(11):1545–1552. doi: 10.1007/s10552-011-9831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kok DE, van Roermund JG, Aben KK, den Heijer M, Swinkels DW, Kampman E, Kiemeney LA. Blood lipid levels and prostate cancer risk; A cohort study. Prostate Cancer Prostatic Dis. 2011;14(4):340–345. doi: 10.1038/pcan.2011.30. [DOI] [PubMed] [Google Scholar]
- 13.Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK, Amling CL, Freedland SJ. Postoperative statin use and risk of biochemical recurrence following radical prostatectomy: Results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. BJU Int. 2014;114(5):661–666. doi: 10.1111/bju.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98(24):1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 15.Mucci LA, Stampfer MJ. Mounting evidence for prediagnostic use of statins in reducing risk of lethal prostate cancer. J Clin Oncol. 2014;32(1):1–2. doi: 10.1200/JCO.2013.53.2770. [DOI] [PubMed] [Google Scholar]
- 16.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115(4):959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mostaghel EA, Solomon KR, Pelton K, Freeman MR, Montgomery RB. Impact of circulating cholesterol levels on growth and intratumoral androgen concentration of prostate tumors. PLoS ONE. 2012;7(1):e30062. doi: 10.1371/journal.pone.0030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Adam RM, Solomon KR, Freeman MR. Involvement of cholesterol-rich lipid rafts in interleukin-6-induced neuroendocrine differentiation of LNCaP prostate cancer cells. Endocrinology. 2004;145(2):613–619. doi: 10.1210/en.2003-0772. [DOI] [PubMed] [Google Scholar]
- 19.Solomon KR, Pelton K, Boucher K, Joo J, Tully C, Zurakowski D, Schaffner CP, Kim J, Freeman MR. Ezetimibe is an inhibitor of tumor angiogenesis. Am J Pathol. 2009;174(3):1017–1026. doi: 10.2353/ajpath.2009.080551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goncalves I, Cherfan P, Soderberg I, Nordin Fredrikson G, Jonasson L. Effects of simvastatin on circulating autoantibodies to oxidized LDL antigens: Relation with immune stimulation markers. Autoimmunity. 2009;42(3):203–208. doi: 10.1080/08916930802668602. [DOI] [PubMed] [Google Scholar]
- 21.Kusama T, Mukai M, Iwasaki T, Tatsuta M, Matsumoto Y, Akedo H, Nakamura H. Inhibition of epidermal growth factor-induced RhoA translocation and invasion of human pancreatic cancer cells by 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors. Cancer Res. 2001;61(12):4885–4891. [PubMed] [Google Scholar]
- 22.Nubel T, Dippold W, Kleinert H, Kaina B, Fritz G. Lovastatin inhibits Rho-regulated expression of E-selectin by TNFalpha and attenuates tumor cell adhesion. FASEB J. 2004;18(1):140–142. doi: 10.1096/fj.03-0261fje. [DOI] [PubMed] [Google Scholar]
- 23.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105(6):739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, Wong WW, Khosravi F, Minden MD, Penn LZ. Blocking the Raf/MEK/ERK pathway sensitizes acute myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer Res. 2004;64(18):6461–6468. doi: 10.1158/0008-5472.CAN-04-0866. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton RJ, GK, Platz EA, Freedland SJ. The influence of statin medications on prostate-specific antigen levels. J Natl Cancer Inst. 2008;100:1511–1518. doi: 10.1093/jnci/djn362. [DOI] [PubMed] [Google Scholar]
- 26.Zheng X, Cui XX, Avila GE, Huang MT, Liu Y, Patel J, Kong ANT, Paulino R, Shih WJ, Lin Y, Rabson AB, Reddy BS, Conney AH. Atorvastatin and Celecoxib inhibit prostate PC-3 tumors in immunodeficient mice. Clin Cancer Res. 2007;13(18):5480–5487. doi: 10.1158/1078-0432.CCR-07-0242. [DOI] [PubMed] [Google Scholar]
- 27.Zheng X, Cui XX, Gao Z, Zhao Y, Lin Y, Shih WJ, Huang MT, Liu Y, Rabson A, Reddy B, Yang CS, Conney AH. Atorvastatin and celecoxib in combination inhibits the progression of androgen-dependent LNCaP xenograft prostate tumors to androgen independence. Cancer Prev Res. 2010;3(1):114–124. doi: 10.1158/1940-6207.CAPR-09-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Endo A. Chemistry, biochemistry, and pharmacology of HMG-CoA reductase inhibitors. Klin Wochenschr. 1988;66(10):421–427. doi: 10.1007/BF01745510. [DOI] [PubMed] [Google Scholar]
- 29.Tang W, Ma Y, Yu L. Plasma cholesterol is hyperresponsive to statin in ABCG5/ABCG8 transgenic mice. Hepatology. 2006;44(5):1259–1266. doi: 10.1002/hep.21380. [DOI] [PubMed] [Google Scholar]
- 30.Lloyd JC, Masko EM, Wu C, Keenan MM, Pilla DM, Aronson WJ, Chi JT, Freedland SJ. Fish oil slows prostate cancer xenograft growth relative to other dietary fats and is associated with decreased mitochondrial and insulin pathway gene expression. Prostate Cancer Prostatic Dis. 2013;16(4):285–291. doi: 10.1038/pcan.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masko EM, Thomas JA, 2nd, Antonelli JA, Lloyd JC, Phillips TE, Poulton SH, Dewhirst MW, Pizzo SV, Freedland SJ. Low-carbohydrate diets and prostate cancer: Ow low is “low enough“? Cancer Prev Res (Phila) 2010;3(9):1124–1131. doi: 10.1158/1940-6207.CAPR-10-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein KA, Reiter RE, Redula J, Moradi H, Zhu XL, Brothman AR, Lamb DJ, Marcelli M, Belldegrun A, Witte ON, Sawyers CL. SCID mice. Nat Med. 1997;3(4):402–408. doi: 10.1038/nm0497-402. [DOI] [PubMed] [Google Scholar]
- 33.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas JA, II, Antonelli JA, Lloyd JC, Masko EM, Poulton SH, Phillips TE, Pollak M, Freedland SJ. Effect of intermittent fasting on prostate cancer tumor growth in a mouse model. Prostate Cancer Prostatic Dis. 2010;13(4):350–355. doi: 10.1038/pcan.2010.24. [DOI] [PubMed] [Google Scholar]
- 35.Boucher K, Siegal R, Sharma P, Hauschka PV, Solomon KR. HMG-CoA reductase inhibitors induce apoptosis in pericytes. Microvasc Res. 2006;71:91–102. doi: 10.1016/j.mvr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Norris JD, Chang CY, Wittmann BM, Kunder RS, Cui H, Fan D, Joseph JD, McDonnell DP. The homeodomain protein HOXB13 regulates the cellular response to androgens. Mol Cell. 2009;36(3):405–416. doi: 10.1016/j.molcel.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Hughes-Fulford M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int J Cancer. 2001;91(1):41–45. doi: 10.1002/1097-0215(20010101)91:1<41::aid-ijc1009>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 38.Flick ED, Habel L, Chan KA, Van Den Eeden SK, Quinn VP, Haque R, Orav EJ, Seeger JD, Sadler MC, Quesenberry CP, Jr, Sternfeld B, Jacobsen SJ, Whitmer RA, Caan BJ. Statin use and risk of prostate cancer in the California Men’s Health Study Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2218–2225. doi: 10.1158/1055-9965.EPI-07-0197. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U. S. cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2213–2217. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 40.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: A population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2226–2232. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 41.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, WIllett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98(24):1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 42.Kochuparambil ST, Al-Husein B, Goc A, Soliman S, Somanath PR. Anticancer efficacy of simvastatin on prostate cancer cells and tumor xenografts is associated with inhibition of Akt and reduced prostate-specific antigen expression. J Pharmacol Exp Ther. 2011;336(2):496–505. doi: 10.1124/jpet.110.174870. [DOI] [PubMed] [Google Scholar]
- 43.Backman JT, Kyrklund C, Kivisto KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68(2):122–129. doi: 10.1067/mcp.2000.108507. [DOI] [PubMed] [Google Scholar]
- 44.Ziviani L, Da Ros L, Squassante L, Milleri S, Cugola M, Iavarone LE. The effects of lacidipine on the steady/state plasma concentrations of simvastatin in healthy subjects. Br J Clin Pharmacol. 2001;51(2):147–152. doi: 10.1111/j.1365-2125.2001.bcp119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murtola TJ, Pennanen P, Swala H, Blauer M, Ylikomi T, Tammela TL. Effects of simvastatin, acetylsalicylic acid, and rosiglitazone on proliferation of normal and cancerous prostate epithelial cells at therapeutic concentrations. Prostate. 2009;69(9):1017–1023. doi: 10.1002/pros.20951. [DOI] [PubMed] [Google Scholar]
- 46.PDR Network, L. 2011 [cited 2011 October 26]; Available from: http://www.pdr.net/drugpages/productlabeling.aspx?mpcode=52402965.
- 47.Dold S, Laschke MW, Lavasani S, Menger MD, Jeppsson B, Thorlacius H. Simvastatin protects against cholestasis-induced liver injury. Br J Pharmacol. 2009;156(3):466–474. doi: 10.1111/j.1476-5381.2008.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Athyros VG, Tziomalos K, Daskalopoulos GN, Karahiannis A, Mikhailidis DP. Statin-based treatment for cardiovascular risk and non-alcoholic fatty liver disease. Killing two birds with one stone? Ann Med. 2011;43(3):167–171. doi: 10.3109/07853890.2011.561363. [DOI] [PubMed] [Google Scholar]
- 49.Almutairi F, Peterson TC, Molinari M, Walsh MJ, Alwayn I, Peltekian KM. Safety and effectiveness of ezetimibe in liver transplant recipients with hypercholesterolemia. Liver Transpl. 2009;15(5):504–508. doi: 10.1002/lt.21710. [DOI] [PubMed] [Google Scholar]
- 50.Gazi IF, Daskalopoulou SS, Nair DR, Mikhailidis DP. Effect of ezetimibe in patients who cannot tolerate statins or cannot get to the low density lipoprotein cholesterol target desipite taking a statin. Curr Med Res Opin. 2007;23(9):2183–2192. doi: 10.1185/030079907X226267. [DOI] [PubMed] [Google Scholar]
- 51.Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP. 27-Hydroxycholesterol links hyper-cholesterolemia and breast cancer pathophysiology. Science. 2013;342(6162):1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baigent C, Landry M. Study of heart and renal protection (SHARP) Kidney Int Suppl. 2003;(84):S207–S210. doi: 10.1046/j.1523-1755.63.s84.4.x. [DOI] [PubMed] [Google Scholar]
- 53.Cannon CP, Giugliano RP, Blazing MA, Harrington RA, Peterson JL, Sisk CM, Strony J, Musliner TA, McCabe CH, Veltri E, Braunwald E, Califf RM, Investigators I-I. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): Comparison of ezetimbe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. Am Heart J. 2008;156(5):826–832. doi: 10.1016/j.ahj.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 54.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Eqstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Mienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R SEAS Investigators. Intensive lipid lowering with simvastatin and exetimibe in aortic stenosis. N Engl J Med. 2008;359(13):1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 55.Peto R, Emberson J, Landray M, Baigent C, Collins R, Clare R, Aliff R. Analyses of cancer data from three ezetimibe trials. N Engl J Med. 2008;259(13):1357–1366. doi: 10.1056/NEJMsa0806603. [DOI] [PubMed] [Google Scholar]