Abstract
Zinc is an essential micronutrient for the development of the fetal renal, cardiovascular, and metabolic systems; however, there is limited evidence of its effects on the postnatal cardiometabolic function. In this study, we evaluated the effect of maternal zinc supplementation during pregnancy on the cardiometabolic profile of the offspring in childhood. A total of 242 pregnant women were randomly assigned to receive a daily supplement containing iron + folic acid with or without zinc. A follow-up study was conducted when children of participating mothers were 4.5 years of age to evaluate their cardiometabolic profile, including anthropometric measures of body size and composition, blood pressure, lipid profile, and insulin resistance. No difference in measures of child cardiometabolic risk depending on whether mothers received supplemental zinc during pregnancy. Our results do not support the hypothesis that maternal zinc supplementation reduces the risk of offspring cardiometabolic disease.
Keywords: zinc, cardiovascular, metabolic
Introduction
The burden of non-communicable diseases (NCDs) is a major public health problem, and is particularly devastating in the developing world. According to the World Health Organization, in 2012, 68% of all deaths were from NCDs, and around three quarters occurred in low- and middle-income countries (1). Nutritional insufficiency in early life is also common in developing countries and is one additional risk factor for cardiometabolic diseases in adulthood (2, 3).
It has been proposed that deficiencies of key micronutrients involved in epigenetic processes affect fetal growth, organogenesis, and differentiation, leading to differences in cardiometabolic functional outcomes throughout life (4). Of particular interest is zinc. Studies in animals have demonstrated that prenatal zinc restriction is associated with decreased number and size of nephrons, glomerular filtration rate, lean body mass, and altered insulin response to glucose, and increased systolic blood pressure and body fat postnatally (5, 6). In humans, the prenatal effect of zinc deficiency on the risk of cardiometabolic diseases later in life is less well studied although the use of prenatal supplements containing zinc has been reported to result in a reduction in the risk of microalbuminuria and peripheral adiposity at 8 years of age (7, 8).
Zinc deficiency is common, particularly in periods critical for development. Around 82% of women worldwide do not consume the recommended intake of zinc during pregnancy, and in most low- or middle-income countries, the average zinc intake of pregnant women is below the Estimated Average Requirements (EAR) (9, 10). Based on earlier studies in the same community where this study was conducted, we have reported that an estimated 80–88% of pregnant women consume inadequate amounts of zinc and that serum zinc concentrations decline steeply throughout pregnancy at a faster rate than has been reported in other populations (11, 12).
We conducted a randomized clinical trial to evaluate the effect of prenatal zinc supplementation (25 mg/d) during pregnancy on health and nutritional outcomes in their offspring. Although no differences related to zinc supplementation were observed in maternal zinc indicators during pregnancy – except for a higher red blood cell zinc concentration at the end of pregnancy – improvements in growth and heart rate (HR) parameters were observed in fetuses of zinc-supplemented mothers (13–15). The HR differences persisted at 4.5 years of age (16).To further understand the role of zinc in the development of cardiometabolic diseases, in this report we examine the effects of prenatal zinc supplementation on the risk of metabolic syndrome and its individual components in early childhood (4.5 y). These are known to increase the risk of cardiovascular disease and diabetes in adults and track from childhood to adulthood (17).
Methods
Study design and participants
Between 1998 and 2000, a double-blind, randomized clinical trial was conducted in 242 pregnant women in a periurban area of Lima, Peru, to assess the effect of prenatal zinc supplementation on fetal neural development and growth. When the children were approximately 4.5 years, they participated in a follow-up study to evaluate the long-term effect of supplementation on health, nutritional, and developmental outcomes. Results for these primary outcomes have been previously reported (14-16, 18). The focus of this report is the effect of prenatal zinc supplementation on the cardiometabolic profile of these children at 4.5 years.
Details of recruitment, enrollment, and supplementation are provided in earlier reports (14–16). Briefly, women who were receiving antenatal care at a local hospital in periurban Lima, classified with a low-risk pregnancy, carrying a singleton fetus, and who had lived on the Coast of Peru (sea level) for at least 6 months before pregnancy were eligible for the study. Participants were enrolled between 10–16 weeks of gestation, and randomized within parity (primipara/multipara) and week of gestation (10–13/14–16 weeks) strata to receiving 60 mg iron (ferrous sulfate) and 250 µg folic acid, with or without 25 mg of zinc (zinc sulfate) daily throughout pregnancy. The dose of 25 mg of zinc was increased from the 15 mg used in our previous studies conducted in this same community, because although it was sufficient to improve the distribution of maternal indicators of zinc status ~0.21–0.23 SD, they were still below those reported for women with adequate zinc intakes.
Follow-up assessments
In 2003, children whose mothers participated in the prenatal supplementation trial were invited to take part in follow-up assessments. The evaluation was completed by trained study health professionals over two visits. The protocol included interviews with the caregiver, review of clinical records to collect information on socioeconomic conditions of the family, and the health, nutritional, and developmental history of the child, as well as a health exam, a nutritional evaluation, and behavioral and developmental testing. Anthropometric measures were conducted by a trained anthropometrist following standard procedures (19). Standing height of the child was measured to the nearest 0.1 cm using a stadiometer, and weight was recorded on a digital scale with 0.1 kg precision (Seca, Hamburg, Germany). Body Mass Index was calculated as BMI = [weight (kg)/height (m) 2]. BMI-for-age Z-scores were calculated using the World Health Organization (WHO) 2006 growth standards (20). Circumferences (waist, chest, mid-upper arm, calf) were measured to nearest 0.1 cm with a measuring tape (Seca, Hamburg, Germany). Fat mass (FM) was estimated from total body water (FM = TBW + Fat free mass) from an equation developed in Peruvian children (TBW = 0.276*weight (kg) + 0.105*height (cm) + 0.051* chest circumference (cm) – 0.319 (sex, female = 1) – 6.134) and validated using 18O dilution (21). Fat mass index was calculated as FMI = [FM (kg)/height (m) 2]. Blood pressure was measured by the study physician with the child in a seated position in triplicate at 1-min intervals using a pediatric sphygmomanometer (Reister, Jungingen, Germany). For the analyses we used the mean value of the three measures. Mean arterial blood pressure was calculated as MAP = [diastolic pressure + (systolic pressure – diastolic pressure)/3]. Blood pressure percentiles were calculated using the U.S. reference population according to sex, age, and height-for-age percentile of the child (25). Height-for-age percentiles were calculated using the CDC 2000 growth reference, which were required to estimate age- and sex-appropriate blood pressure percentiles using the U.S. blood pressure reference curves (22).
Children were asked to fast overnight and on the following morning they were picked up from their home and brought to the study clinic where the phlebotomist collected venous blood samples. Blood samples were drawn into tubes containing heparin, and within 30 minutes the samples were centrifuged at 600 g for 10 min for separation of plasma. Plasma samples were frozen at −20° C until micronutrient analyses were conducted. In 2011, the remaining cryopreserved plasma samples were thawed to conduct the analyses presented here. A Cholestech LDX analyzer (Cholestech Corporation, Hayward, California) was used to measure total and HDL-cholesterol, triglycerides, and glucose concentrations. LDL-cholesterol concentration was calculated using the Friedewald equation (23). Plasma insulin concentration was measured using an ultrasensitive immunoassay (Alpco Diagnostics, Salem, New Hampshire). Insulin resistance was estimated by using the homeostasis model assessment, HOMA-IR = fasting plasma insulin concentration (mU/L) X fasting plasma glucose (mmol/L)/22.5 (24).
Data analyses
Data analyses were conducted using Stata 12.1 (Stata Corporation, College Station, Texas). Selected maternal and child characteristics were compared between the treatment groups using t-tests or chi-square tests, as appropriate. We compared differences in cardiometabolic risk factors according to prenatal supplement type, in continuous and dichotomous scales. Anthropometric and blood pressure measures were normally distributed, therefore we used the t-test to compare differences by treatment group; insulin resistance measures followed a log-normal distribution hence we used the Wilcoxon ranksum test. A total of 17 children had triglycerides, total cholesterol, or HDL-cholesterol concentrations outside the detectable limit of the assay (triglycerides <0.51 mmol/L or >7.34 mmol/L, total cholesterol <2.59 mmol/L or >12.9 mmol/L, HDL-cholesterol <0.39 mmol/L or >2.59 mmol/L); we used tobit regression models for censored data to compare differences by supplement type (25). Because measures of lipid profile do not follow a normal distribution, they were transformed to normal using the ladder of powers developed by Tukey to meet normality assumptions for tobit regression (25, 26).
Because there is no accepted definition of metabolic syndrome for children under 10 y, we used modified criteria of the National Cholesterol Education Program (NCEP) ATP III guidelines for adults to identify children who are at risk of metabolic syndrome as described by Stewart et al. (7, 27). Children were considered to be at risk of metabolic syndrome if they met 3 or more of the following criteria: 1) abdominal obesity, defined as WC ≥ 90th percentile of the reference population (third National Health and Nutrition Examination Survey, NHANES III: 58.3 cm for girls and 57.6 cm for boys) (28); 2) high triglycerides, defined as TG ≥ 95th percentile of the reference population (American Academy of Pediatrics: 120 mg/dL [1.37 mmol/L] for girls and 85 mg/dL [0.97 mmol/L] for boys) (29); 3) low HDL-cholesterol, defined as HDL-cholesterol < 5th percentile of the reference population (American Academy of Pediatrics: 36 mg/dL [2.0 mmol/L] for girls and 38 mg/dL [2.1 mmol/L) for boys] (29); 4) high blood pressure, defined as SBP or DBP ≥ 90th percentile of the reference population (age-, sex-, and height-specific) (30); and 5) high glucose, defined as fasting plasma glucose ≥ 5.6 mmol/L (American Diabetes Association) (31).
To compare differences in the proportion of children at risk of metabolic syndrome or any of its individual components according to treatment type, we developed logistic regression models for each of the outcomes of interest (abdominal obesity, high triglycerides, low HDL-cholesterol, high blood pressure, insulin resistance, and at risk of metabolic syndrome). All models were adjusted for age and sex of the child. Interaction terms between treatment type and age or sex were tested, found not significant (p >0.10), and therefore were excluded from the final models. With our final sample size (n = 159), and assuming 80% power and a 2-tail significance level of 0.05, the smallest difference we could detect between mean values of continuous outcomes was 0.4 SD; a difference of 0.41 is considered the recommended minimum effect size. Under the same assumptions, we could detect a reduction of 90% (OR = 0.1) in the odds of metabolic syndrome or any of its individual components associated with zinc supplementation; this difference is considered to correspond to a very large effect size (32).
Results
Figure 1 shows the enrollment and analytical sample of the study, by supplement type. Of the 242 women enrolled in the supplementation trial, 20 were lost to follow-up, and 27 were excluded from the original analyses due to significant obstetrical or medical complications. Therefore, 195 were included in the analysis related to fetal outcomes (94 in the zinc group and 101 in the in the control group). For the follow-up at 4.5 years, we aimed to evaluate the 195 participants included in the analysis for fetal outcomes and 10 participants who were excluded from the analyses but whose mothers completed the prenatal protocol and were born free of congenital malformations. Of the 205 eligible children, a total of 184 were located and evaluated at follow-up. Of these, 25 had no frozen plasma samples to conduct biochemical measurements and/or had missing information on blood pressure. For some cases, plasma samples were missing due to refusal of the blood draw at follow-up or due to insufficient volume to conduct these biochemical analyses. The present report is therefore restricted to 159 children with complete biochemical and blood pressure data, 73 from the treatment group and 86 from the control group. Missing data between treatment groups were non-differential, as well as maternal and child characteristics at birth among participants and nonparticipants of the follow-up study, and those with complete or missing data on cardiometabolic risk factors at follow-up (p >0.05). Table 1 shows selected characteristics of study participants at enrollment. Participants were similar in maternal and child characteristics.
Figure 1.
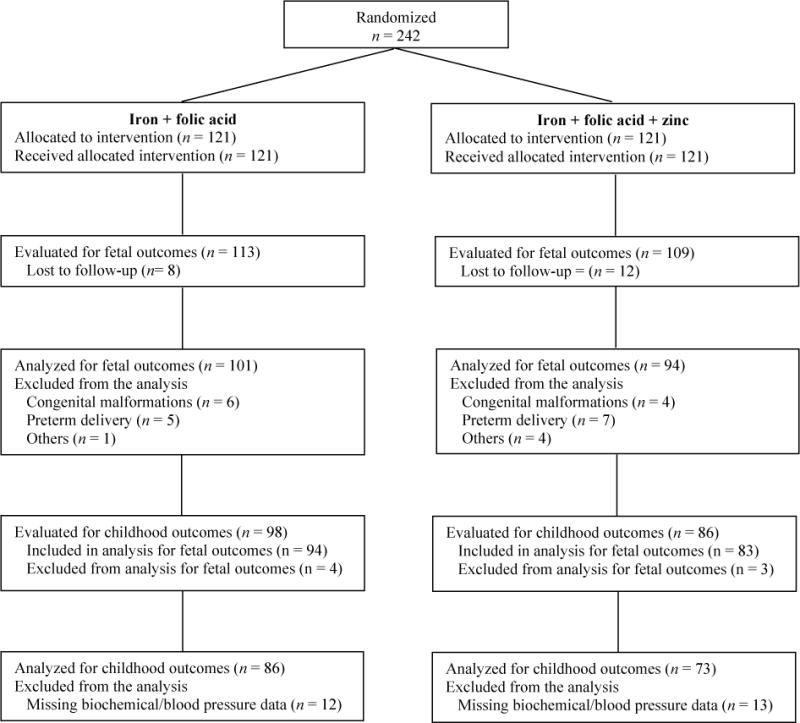
Enrollment and analytical sample in the study by prenatal supplement type
Table 1.
Selected maternal and child characteristics in 159 Peruvian children, by prenatal supplement type
| Maternal supplement type
|
||
|---|---|---|
| Characteristic | Iron + folate | Iron + folate + zinc |
| 86 | 73 | |
| Maternal† | ||
| Age, y | 23.4±4.9 | 23.5±4.9 |
| Height, cm | 152.4±5.1 | 152.2±5.5 |
| BMI, kg/m2 | 23.6±3.4 | 23.2±3.2 |
| Primiparity, % | 57.0 | 56.2 |
| Maternal education, % | ||
| Primary or less | 4.9 | 11.3 |
| Secondary incomplete | 28.0 | 25.3 |
| Secondary complete | 50.0 | 46.5 |
| Beyond secondary | 17.1 | 16.9 |
| Birth | ||
| Gestational age at birth, wk | 39.3±1.2 | 39.2±1.4 |
| Weight at birth, g | 3277±386 | 3309±440 |
| Length at birth, cm | 49.7±1.9 | 50.1±2.2 |
Values are mean ± SD or percent;
evaluated at a mean age of 13 weeks gestation.
No statistically significant differences by supplement type (p >0.05); p-values were calculated using t-test or chi-square test.
Shown in table 2 are the measures of central tendency and dispersion of the cardiometabolic risk factors according to treatment group. There were no statistically significant differences in variables related to anthropometry and body composition, lipid profile, or insulin resistance according to treatment group (p > 0.05).
Table 2.
Cardiometabolic risk factors in 159 Peruvian children at 4.5 years by prenatal supplement type
| Maternal supplement type
|
|||
|---|---|---|---|
| Characteristic | Iron + Folic acid | Iron + Folic acid + Zinc | p-value |
| n | 86 | 73 | |
| Anthropometry and body composition | |||
| Weight, kg | 17.4±2.4 | 17.2±2.2 | 0.58 |
| Height, cm | 102.4±3.4 | 102.9±4.0 | 0.72 |
| BMI, kg/m2 | 16.6±1.64 | 16.3±1.3 | 0.18 |
| BMI-for-age, Z-score | 0.82±1.0 | 0.65±0.9 | 0.24 |
| Waist circumference, cm | 54.7±4.2 | 55.1±4.2 | 0.60 |
| Fat mass, kg | 6.4±1.7 | 6.2±1.3 | 0.32 |
| Fat mass index, kg/m2 | 6.1±1.4 | 5.8±1.0 | 0.17 |
| Lipid profile | |||
| Plasma triglycerides, mmol/L | 0.88 (0.71, 1.10) | 0.86 (0.65, 1.06) | 0.59 |
| Plasma total cholesterol, mmol/L | 3.43 (3.08, 3.79) | 3.54 (3.28, 4.01) | 0.32 |
| Plasma HDL-cholesterol, mmol/L | 0.75 (0.54, 0.96) | 0.74 (0.59, 0.98) | 0.92 |
| Plasma LDL-cholesterol, mmol/L | 2.28 (1.84, 2.66) | 2.48 (1.87, 2.86) | 0.23 |
| Blood pressure | |||
| SBP, mmHg | 84.2±7.8 | 85.1±10.2 | 0.53 |
| DBP, mmHg | 59.9±8.1 | 57.7±9.6 | 0.12 |
| MAP, mmHg | 68.0±7.3 | 66.8±8.6 | 0.34 |
| Insulin resistance | |||
| Plasma glucose, mmol/L | 4.44 (4.11, 4.94) | 4.44 (4.11, 4.92) | 0.97 |
| Plasma insulin, µIU/ml | 9.00 (5.09, 16.00) | 10.02 (5.30, 15.01) | 0.96 |
| HOMA-IR | 1.73 (0.91, 3.00) | 1.98 (0.95, 2.97) | 0.92 |
Values are mean ± SD or median (IQR).
Data were missing for LDL-cholesterol (n=30).
p-values were calculated using t-test or Wilcoxon ranksum test. For left censored variables [triglycerides (n=11), total cholesterol (n=10), and HDL-cholesterol (n=6)], p-values were calculated using tobit regression.
BMI = Body Mass Index, SBP = systolic blood pressure, DBP = diastolic blood pressure, MAP = mean arterial pressure
The proportion of selected cardiometabolic risk factors and risk of metabolic syndrome and the corresponding ORs (and 95%CI) in study children by prenatal supplement type are shown in table 3. There were no statistically significant differences in the risk of developing metabolic syndrome or any of its individual components according to prenatal supplement type. A total of 20 (12.6%) children had no cardiometabolic risk factors present, 71 (44.7%) had 1, 52 (32.7%) had 2, 14 (8.8%) had 3, 2 (1.3%) had 4, and no children had all cardiometabolic risk factors present. Of the 38 children who were classified as having high blood pressure, 35 (92%) had high DBP. Of the 16 children who met the definition of being at risk for metabolic syndrome, 15 (94%) had low HDL-cholesterol, 12 (75%) had high blood pressure, 10 (63%) had high triglycerides, 7 (44%) had high waist circumference, and 6 (38%) had high glucose concentration.
Table 3.
Prevalence of cardiometabolic factors and being at risk of metabolic syndrome in 159 Peruvian children 4.5 years by prenatal supplement type
| Characteristic | Iron + Folic acid | Iron + Folic acid + Zinc | OR (95% CI) |
|---|---|---|---|
| n | 86 | 73 | |
| Abdominal obesity (WC ≥ 90th percentile) n (%) | 16 (18.6) | 13 (17.8) | 0.95 (0.42, 2.13) |
| High triglycerides (≥ 95th percentile) n (%) | 16 (18.6) | 15 (20.6) | 1.13 (0.52, 2.48) |
| Low HDL cholesterol (< 5th percentile) n (%) | 65 (75.6) | 54 (74.0) | 0.92 (0.45, 1.88) |
| High blood pressure (SBP or DBP ≥ 90th percentile) n (%) | 20 (23.3) | 18 (24.7) | 1.08 (0.52, 2.24) |
| High glucose (≥ 5.6 mmol/L) n (%) | 5 (5.8) | 3 (4.1) | 0.69 (0.16, 3.01) |
| At risk for metabolic syndrome (child met 3 of the above criteria) n (%) | 10 (11.6) | 6 (8.2) | 0.68 (0.23, 1.97) |
Cutoffs for cardiometabolic risk factors were as follows: WC ≥ 90th percentile (NHANES III: 58.3 cm for girls and 57.6 cm for boys) (28), triglycerides ≥ 95th percentile (ADA: 1.37 mmol/L for girls and 0.97 mmol/L for boys) (29), HDL-cholesterol < 5th percentile (ADA: 2.0 mmol/L for girls and 2.1 mmol/L for boys) (29).
p-values calculated using Fisher’s exact test; all p-values >0.05.
Discussion
We examined the effect of zinc supplementation among Peruvian pregnant women starting at 10-16 weeks of gestation on the cardiometabolic profile of the child at 4.5 y of age. In this study, we found no evidence of prenatal zinc supplementation affecting measures of anthropometry, lipid profile, blood pressure, or insulin resistance of participants up to this age of follow-up. No differences according to supplement type were observed when analyzing the measures as continuous variables, or using a definition of ‘at risk of’ metabolic syndrome or any of its individual components.
Zinc is considered to be critical for the development of fetal organs, and the deficiency of this micronutrient during the prenatal period has been associated with teratogenic consequences and long-term functional impact of the cardiovascular and metabolic function (4). Although studies in animal support the biological role of zinc on the development of the cardiometabolic function, evidence from human studies is lacking. We know of only one other study which has evaluated the long-term effects of prenatal zinc supplementation on cardiometabolic outcomes in childhood. Stewart et al. examined the effect of prenatal micronutrient supplementation on the risk of metabolic syndrome in children 6–8 y of age in rural Nepal (7). Specifically, they examined the effect of prenatal supplementation with 1) vitamin A (control), 2) folic acid (400 µg), 3) folic acid with iron (60 mg), 4) folic acid with iron and zinc (30 mg), and 5) a multiple micronutrient supplement containing folic acid, iron, zinc, and additional minerals and vitamins. In agreement with our study, prenatal supplementation with folic acid, iron, and zinc did not affect measures of blood pressure, lipid profile, or insulin resistance in children as compared to a control group. However, there was a significant reduction in microalbuminuria – a marker of kidney dysfunction and risk factor for cardiovascular disease – in the groups receiving folic acid (OR; 95% CI: 0.56; 0.33, 0.93), or folic acid, iron, and zinc (OR; 95% CI: 0.53; 0.32, 0.89), as compared to the control group. Even if the reduction in microalbuminuria was somewhat greater in the group receiving supplemental zinc, the effect observed cannot be attributed to zinc directly because the authors did not explicitly contrast the group receiving iron + folic acid with that receiving iron + folic acid + zinc, which is the contrast in our study.
There are two other studies examining the effect of prenatal multiple micronutrient supplementation (containing 15 mg of zinc) on individual cardiometabolic outcomes, particularly blood pressure, with contradictory results (33, 34). A study conducted in rural Bangladesh found that prenatal supplementation with multiple micronutrients was associated with higher diastolic blood pressure at 4.5 y (0.87 mmHg; 95% CI: 0.18, 1.56) when compared to those receiving folic acid (400 µg) and iron (30 mg or 60 mg) (33). No differences were found, however, between the prenatal treatment groups in systolic blood pressure, kidney volume, or glomerular filtration rate. A second study, also conducted in Nepal, found that prenatal supplementation with multiple micronutrients was associated with lower systolic blood pressure at 2.5 y (2.5 mmHg; 95% CI: 0.5, 4.6), when compared to those receiving folic acid (400 µg) with iron (60 mg) (34). In both studies, the dose of zinc used was lower than the one in our study and because zinc was one of the multiple micronutrients included in the supplement, the changes in blood pressure cannot be attributed solely to zinc.
The role of zinc status in the development of cardiometabolic diseases has been studied more frequently for the postnatal period but the evidence is not conclusive. Results from observational and intervention studies suggest that zinc deficiency may play a role in the development of insulin resistance, in abnormalities in lipid metabolism, and cardiovascular risk. Observational studies in adults from developing countries have shown that lower serum zinc concentrations and lower consumption of dietary zinc are associated with an increased prevalence of hypertension, hypertriglyceridemia, coronary artery disease, and diabetes (35, 36). Large, prospective, longitudinal studies in mid-aged females from developed countries have shown an association between higher dietary zinc intakes and lower incidence of type 2 diabetes (37, 38). Results from interventional studies also suggest a beneficial effect of zinc supplementation in glucose control and lipid metabolism, but mainly among adults with conditions know to influence zinc metabolism (39–41). We are aware of three different meta-analyses of randomized controlled trials examining the effect of supplemental zinc on cardiometabolic outcomes among adults. One found a modest but significant overall reduction in fasting glucose concentrations after zinc supplementation, with a greater effect among patients with type 2 diabetes, metabolic syndrome, and obesity, as compared to the effect observed in healthy individuals (39). A second meta-analysis conducted among patients with type 2 diabetes found that zinc supplementation was associated with a reduction in fasting blood glucose, 2 hr post-prandial blood glucose, HbA1c, total cholesterol, and LDL-cholesterol (40). A third meta-analysis found no overall effect of zinc supplementation on markers of lipid metabolism (41). However, when stratifying the analysis according to health status, a decrease in HDL-cholesterol among healthy subjects but an increase in HDL-cholesterol among subjects with type 2 diabetes or those undergoing hemodialysis was observed among zinc-supplemented individuals as compared to those receiving placebo. All these studies provide evidence of the role of zinc in the development of cardiometabolic disease, and the potential use of zinc in prevention and treatment of these disorders.
There are several strengths of our study that should be mentioned. First, the sample was drawn from a randomized, double-blind, controlled trial. Second, this is a long-term follow-up study evaluating the effect of prenatal supplementation on children exposed to a poor nutritional environment, in which durable effects of prenatal zinc supplementation on autonomic function during childhood were demonstrated. Here we expanded our evaluation to consider whether differences in related parameters of cardiometabolic risk would be detectable. Third, we evaluated cardiometabolic outcomes combined (being at risk of metabolic syndrome) and each of its individual components, allowing us to test the effects of zinc on different cardiometabolic domains.
Our study does have limitations. The major limitation is the reduced sample size, particularly related to the number of missing values for the cardiometabolic outcomes due to the non-availability of frozen plasma samples to conduct biochemical analyses (n = 20). Even if the missingness was not differential by treatment group, it decreased our sample size and therefore our power to detect differences between the two groups. As mentioned earlier, with our final sample size (n = 159) we could detect a reduction in the risk of metabolic syndrome or any of its individual components of 90% or greater, which is a very large effect size. We also analyzed the outcomes of interest in a continuous scale which provided enough power to detect differences of 0.4 SD or greater; these differences are considered to correspond to moderate effect sizes. Potentially, relevant differences in cardiometabolic outcomes between the two groups would have been detected; however, it is possible that the lack of differences observed are a result of insufficient statistical power. Second, we conducted measurements of lipids, glucose, and insulin on plasma samples which had been frozen for several years, and concentrations can decrease progressively with time (42–44). However, because the concentrations of metabolites assessed are within normal values for children their age and sex, it is likely that only minimal degradation occurred (45). Moreover, there is no reason to think that degradation occurred differentially by treatment group. As a result of the method used to measure the concentration of lipids in plasma, we were unable to detect values below or above particular limits; however, because censored data is more informative than missing data, we were able to impute those values and still include them in the analysis. Finally, because there is no standard definition of metabolic syndrome in children under 10 years of age, we used modified criteria available for adults with modified cut-offs appropriate for children to identify those at risk of metabolic syndrome. This approach makes comparison with other studies challenging.
In this study, prenatal supplementation with zinc had no discernable effects on cardiometabolic parameters or risk of disease at 4.5 y of age. These children may be too young to observe any changes in the cardiometabolic measures used; additional follow-up at later ages may reveal detectable differences. By not using markers that can identify changes in endothelial dysfunction or insulin resistance at earlier stages, such as intima media thickness and distensibility, or glucose tolerance and HbA1c, it is possible that we missed early differences by supplement type (46, 47). It is also possible that other environmental factors need to be present for cardiometabolic risk factors to evolve. For example, it has been suggested that rapid weight gain in infancy needs to occur for prenatal nutritional insults to have an effect on the risk of components of metabolic syndrome later in life; fast weight gain in infancy is not seen in this population (48–51). It is also possible that a stronger clustering of cardiometabolic factors is needed for a long-term effect of prenatal zinc to be observed. The degree of clustering of cardiometabolic factors varies with age, with stronger clustering and associations occurring in adulthood as compared to childhood (52). Our findings are in line with this suggestion; 14 (8.8%) children had 3 cardiometabolic risk factors present, 2 (1.2%) had 4, and none had all 5 factors present. Central adiposity and insulin resistance are considered instrumental in the development of the metabolic syndrome (53). In our study, abdominal obesity and glucose intolerance was observed in few participants – out of the 16 children classified at risk of metabolic syndrome only 7 (44%) had abdominal obesity and 6 (38%) had high glucose – therefore it may be that these children are too young to present alterations in these components. In addition, in our study population, lipid metabolism components had a high contribution when classifying children as being at risk of metabolic syndrome –, 15 (94%) had low HDL-cholesterol and 10 (63%) had high triglycerides. HDL-cholesterol concentrations have also been reported to be low in children from developing countries, and are particularly low as compared to those in children from developing countries (54–56). These differences could be attributed to specific components of the local diet; however, ethnic differences cannot be ruled out and therefore the clinical relevance of these low HDL-cholesterol concentrations in these populations is unknown. It is also possible that the zinc effects are only observed when classifying individuals by “normal” or “at risk” status, and we may not have had adequate power to detect differences that are potentially important; we found no evidence of differences according to supplement type when comparing cardiometabolic risk factors in a continuous scale, yet we observed a tendency of a protective effect of zinc on abdominal obesity, low HDL, and high glucose when comparing these risk factors in a dichotomous scale which did not reach statistical significance.
In conclusion, our study found no difference in measures of cardiometabolic risk at 4.5 y depending on whether mothers received supplemental zinc during pregnancy. Data currently available do not support the hypothesis that maternal zinc supplementation reduces the risk of offspring cardiometabolic disease, other than our previously reported findings on autonomic function. Because of the uniqueness of the study design and the findings on autonomic function, it would be important to evaluate the effect of prenatal zinc supplementation in larger studies, at older ages, and include additional markers of cardiometabolic diseases that may identify earlier changes in dyslipidemia, endothelial dysfunction and insulin resistance.
Acknowledgments
We are thankful to study participants, study team, staff of Hospital San Jose, and the local Ministry of Health authorities in Peru.
Financial support
The prenatal study was supported by the Nestle Research Foundation, Lausanne, Switzerland, and Mario Merialdi was supported in part by the Consiglio Nazionale delle Ricerche, Italy. The follow-up study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD: HD042675). The conduct of biochemical analyses was supported by the Division of Health Sciences at Idaho State University through their Enhancement Fund Competition. Monica Mispireta was supported in part by a predoctoral fellowship from the American Heart Association (09PRE2390038). As intramural scientists, the participation of Marc H. Bornstein and Diane L. Putnick in the study was supported by NICHD, NIH.
Footnotes
Conflicts of interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation in the United States and Peru and with the Helsinki Declaration of 1975, as revised in 2008. The prenatal trial, the follow-up study, and the conduct of biochemical analysis in cryopreserved plasma samples were approved by the Institutional Review Boards of the Instituto de Investigación Nutricional, Lima, Peru, and The Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, and written informed consent was obtained for the prenatal trial and the follow-up study.
References
- 1.World Health Organization. Global Status Report on Noncommunicable diseases. 2014 [Google Scholar]
- 2.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341(8850):938–41. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ. The developmental origins of insulin resistance. Horm Res. 2005;64(Suppl 3):2–7. doi: 10.1159/000089311. [DOI] [PubMed] [Google Scholar]
- 4.Christian P, Stewart CP. Maternal micronutrient deficiency, fetal development, and the risk of chronic disease. J Nutr. 2010;140(3):437–45. doi: 10.3945/jn.109.116327. [DOI] [PubMed] [Google Scholar]
- 5.Tomat A, Inserra F, Veiras L, Vallone M, Balaszczuk A, Costa M, et al. Moderate zinc restriction during fetal and postnatal growth of rats: effects on adult arterial blood pressure and kidney. Am J Physiol Regul Comp Physiol. 2008;295:R543–9. doi: 10.1152/ajpregu.00050.2008. [DOI] [PubMed] [Google Scholar]
- 6.Padmavathi IJ, Kishore YD, Venu L, Ganeshan M, Harishankar N, Giridharan NV, et al. Prenatal and perinatal zinc restriction: effects on body composition, glucose tolerance and insulin response in rat offspring. Exp Physiol. 2009;94(6):761–9. doi: 10.1113/expphysiol.2008.045856. [DOI] [PubMed] [Google Scholar]
- 7.Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KP, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. J Nutr. 2009;139(8):1575–81. doi: 10.3945/jn.109.106666. [DOI] [PubMed] [Google Scholar]
- 8.Stewart CP, Christian P, LeClerq SC, West KP, Khatry SK. Antenatal supplementation with folic acid + iron + zinc improves linear growth and reduces peripheral adiposity in school-age children in rural Nepal. Am J Clin Nutr. 2009;90(1):132–40. doi: 10.3945/ajcn.2008.27368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caulfield LE, Zavaleta N, Shankar AH, Merialdi M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am J Clin Nutr. 1998;68(2 Suppl):499S–508S. doi: 10.1093/ajcn/68.2.499S. [DOI] [PubMed] [Google Scholar]
- 10.Lee SE, Talegawkar SA, Merialdi M, Caulfield LE. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr. 2013;16(8):1340–53. doi: 10.1017/S1368980012004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacco LM, Caulfield LE, Zavaleta N, Retamozo L. Dietary pattern and usual nutrient intakes of Peruvian women during pregnancy. Eur J Clin Nutr. 2003;57(11):1492–7. doi: 10.1038/sj.ejcn.1601716. [DOI] [PubMed] [Google Scholar]
- 12.Caulfield LE, Zavaleta N, Figueroa A. Adding zinc to prenatal iron and folate supplements improves maternal and neonatal zinc status in a Peruvian population. Am J Clin Nutr. 1999;69(6):1257–63. doi: 10.1093/ajcn/69.6.1257. [DOI] [PubMed] [Google Scholar]
- 13.Caulfield LE, Donangelo CM, Chen P, Junco J, Merialdi M, Zavaleta N. Red blood cell metallothionein as an indicator of zinc status during pregnancy. Nutrition. 2008;24(11–12):1081–7. doi: 10.1016/j.nut.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, Costigan KA, Dominici F, et al. Randomized controlled trial of prenatal zinc supplementation and fetal bone growth. Am J Clin Nutr. 2004;79(5):826–30. doi: 10.1093/ajcn/79.5.826. [DOI] [PubMed] [Google Scholar]
- 15.Merialdi M, Caulfield LE, Zavaleta N, Figueroa A, Dominici F, Dipietro JA. Randomized controlled trial of prenatal zinc supplementation and the development of fetal heart rate. Am J Obstet Gynecol. 2004;190(4):1106–12. doi: 10.1016/j.ajog.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 16.Caulfield LE, Zavaleta N, Chen P, Lazarte F, Albornoz C, Putnick DL, et al. Maternal zinc supplementation during pregnancy affects autonomic function of Peruvian children assessed at 54 months of age. J Nutr. 2011;141(2):327–32. doi: 10.3945/jn.110.128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camhi SM, Katzmarzyk PT. Tracking of cardiometabolic risk factor clustering from childhood to adulthood. Int J Pediatr Obes. 2010;5(2):122–9. doi: 10.3109/17477160903111763. [DOI] [PubMed] [Google Scholar]
- 18.Caulfield LE, Putnick DL, Zavaleta N, Lazarte F, Albornoz C, Chen P, et al. Maternal gestational zinc supplementation does not influence multiple aspects of child development at 54 mo of age in Peru. Am J Clin Nutr. 2010;92(1):130–6. doi: 10.3945/ajcn.2010.29407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohman T, Roche A, Martorell R. Anthropometric standardization reference manual. Human Kinetics Pub; 1988. p. 18. [Google Scholar]
- 20.World Health Organization. WHO child growth standards: methods and development: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. Geneva, Switzerland: WHO; 2006. [Google Scholar]
- 21.Boutton TW, Trowbridge FL, Nelson MM, Wills CA, Smith EO, Lopez de Romana G, et al. Body composition of Peruvian children with short stature and high weight-for-height. I. Total body-water measurements and their prediction from anthropometric values. Am J Clin Nutr. 1987;45(3):513–25. doi: 10.1093/ajcn/45.3.513. [DOI] [PubMed] [Google Scholar]
- 22.National Cholesterol Education Program (NCEP) highlights of the report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 1992;89(3):495–501. [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;26(1):24–36. [Google Scholar]
- 26.Tukey J. Exploratory Data Analysis. Reading, MA: Addison-Wesley; 1977. [Google Scholar]
- 27.Adults EPoDEaToHBCi. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III); JAMA. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 28.Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145(4):439–44. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 29.Daniels SR, Greer FR, Nutrition Co Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122(1):198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 30.Adolescents NHBPEPWGoHBPiCa. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl):555–76. 4th Report. [PubMed] [Google Scholar]
- 31.American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson C. An effect size primer: a guide for clinicians and researchers. Professional psychology: Research and Practice. 2009;40(5):532–8. [Google Scholar]
- 33.Hawkesworth S, Wagatsuma Y, Kahn AI, Hawlader MD, Fulford AJ, Arifeen SE, et al. Combined food and micronutrient supplements during pregnancy have limited impact on child blood pressure and kidney function in rural Bangladesh. J Nutr. 2013;143(5):728–34. doi: 10.3945/jn.112.168518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaidya A, Saville N, Shrestha BP, Costello AM, Manandhar DS, Osrin D. Effects of antenatal multiple micronutrient supplementation on children’s weight and size at 2 years of age in Nepal: follow-up of a double-blind randomised controlled trial. Lancet. 2008;371(9611):492–9. doi: 10.1016/S0140-6736(08)60172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh RB, Niaz MA, Rastogi SS, Bajaj S, Gaoli Z, Shoumin Z. Current zinc intake and risk of diabetes and coronary artery disease and factors associated with insulin resistance in rural and urban populations of North India. J Am Coll Nutr. 1998;17(6):564–70. doi: 10.1080/07315724.1998.10718804. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Guo H, Wu M, Liu M. Serum and dietary antioxidant status is associated with lower prevalence of the metabolic syndrome in a study in Shanghai, China. Asia Pac J Clin Nutr. 2013;22(1):60–8. doi: 10.6133/apjcn.2013.22.1.06. [DOI] [PubMed] [Google Scholar]
- 37.Sun Q, van Dam RM, Willett WC, Hu FB. Prospective study of zinc intake and risk of type 2 diabetes in women. Diabetes Care. 2009;32(4):629–34. doi: 10.2337/dc08-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vashum KP, McEvoy M, Shi Z, Milton AH, Islam MR, Sibbritt D, et al. Is dietary zinc protective for type 2 diabetes? Results from the Australian longitudinal study on women’s health. BMC Endocr Disord. 2013;13:40. doi: 10.1186/1472-6823-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capdor J, Foster M, Petocz P, Samman S. Zinc and glycemic control: a meta-analysis of randomised placebo controlled supplementation trials in humans. J Trace Elem Med Biol. 2013;27(2):137–42. doi: 10.1016/j.jtemb.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Jayawardena R, Ranasinghe P, Galappatthy P, Malkanthi R, Constantine G, Katulanda P. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. 2012;4(1):13. doi: 10.1186/1758-5996-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foster M, Petocz P, Samman S. Effects of zinc on plasma lipoprotein cholesterol concentrations in humans: a meta-analysis of randomised controlled trials. Atherosclerosis. 2010;210(2):344–52. doi: 10.1016/j.atherosclerosis.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 42.Kuchmak M, Taylor L, Olansky AS. Suitability of frozen and lyophilized reference sera for cholesterol and triglyceride determinations. Clin Chim Acta. 1982;120(2):261–71. doi: 10.1016/0009-8981(82)90163-2. [DOI] [PubMed] [Google Scholar]
- 43.Tiedink HG, Katan MB. Variability in lipoprotein concentrations in serum after prolonged storage at −20 degrees C. Clin Chim Acta. 1989;180(2):147–55. doi: 10.1016/0009-8981(89)90346-x. [DOI] [PubMed] [Google Scholar]
- 44.Meigs JB, Haffner SM, Nathan DM, D’Agostino RB, Wilson PW. Sample exchange to compare insulin measurements between the San Antonio Heart Study and the Framingham Offspring Study. J Clin Epidemiol. 2001;54(10):1031–6. doi: 10.1016/s0895-4356(01)00367-5. [DOI] [PubMed] [Google Scholar]
- 45.Haney EM, Huffman LH, Bougatsos C, et al. Screening for Lipid Disorders in Children and Adolescents. Rockville, MD: Agency for Healthcare Research and Quality (US); 2007. [PubMed] [Google Scholar]
- 46.Litwin M, Niemirska A. Intima-media thickness measurements in children with cardiovascular risk factors. Pediatr Nephrol. 2009;24(4):707–19. doi: 10.1007/s00467-008-0962-3. [DOI] [PubMed] [Google Scholar]
- 47.Buse JB, Kaufman FR, Linder B, Hirst K, El Ghormli L, Willi S, et al. Diabetes screening with hemoglobin A(1c) versus fasting plasma glucose in a multiethnic middle-school cohort. Diabetes Care. 2013;36(2):429–35. doi: 10.2337/dc12-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evelein AM, Visseren FL, van der Ent CK, Grobbee DE, Uiterwaal CS. Excess early postnatal weight gain leads to increased abdominal fat in young children. Int J Pediatr. 2012;2012:141656. doi: 10.1155/2012/141656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evelein AM, Visseren FL, van der Ent CK, Grobbee DE, Uiterwaal CS. Excess early postnatal weight gain leads to thicker and stiffer arteries in young children. J Clin Endocrinol Metab. 2013;98(2):794–801. doi: 10.1210/jc.2012-3208. [DOI] [PubMed] [Google Scholar]
- 50.Ong KK, Petry CJ, Emmett PM, Sandhu MS, Kiess W, Hales CN, et al. Insulin sensitivity and secretion in normal children related to size at birth, postnatal growth, and plasma insulin-like growth factor-I levels. Diabetologia. 2004;47(6):1064–70. doi: 10.1007/s00125-004-1405-8. [DOI] [PubMed] [Google Scholar]
- 51.Iannotti LL, Zavaleta N, León Z, Caulfield LE. Growth and body composition of Peruvian infants in a periurban setting. Food Nutr Bull. 2009;30(3):245–53. doi: 10.1177/156482650903000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen W, Bao W, Begum S, Elkasabany A, Srinivasan SR, Berenson GS. Age-related patterns of the clustering of cardiovascular risk variables of syndrome X from childhood to young adulthood in a population made up of black and white subjects: the Bogalusa Heart Study. Diabetes. 2000;49(6):1042–8. doi: 10.2337/diabetes.49.6.1042. [DOI] [PubMed] [Google Scholar]
- 53.Liese AD, Mayer-Davis EJ, Haffner SM. Development of the multiple metabolic syndrome: an epidemiologic perspective. Epidemiol Rev. 1998;20(2):157–72. doi: 10.1093/oxfordjournals.epirev.a017978. [DOI] [PubMed] [Google Scholar]
- 54.Corvalán C, Uauy R, Kain J, Martorell R. Obesity indicators and cardiometabolic status in 4-y-old children. Am J Clin Nutr. 2010;91(1):166–74. doi: 10.3945/ajcn.2009.27547. [DOI] [PubMed] [Google Scholar]
- 55.Cowin I, Emmett P. Cholesterol and triglyceride concentrations, birthweight and central obesity in pre-school children. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. Int J Obes Relat Metab Disord. 2000;24(3):330–9. doi: 10.1038/sj.ijo.0801133. [DOI] [PubMed] [Google Scholar]
- 56.Williams CL, Strobino B, Bollella M, Brotanek J. Body size and cardiovascular risk factors in a preschool population. Prev Cardiol. 2004;7(3):116–21. doi: 10.1111/j.1520-037x.2004.03224.x. [DOI] [PubMed] [Google Scholar]


