Abstract
Evidence concerning the association between ambient gaseous air pollutant exposures and semen quality is sparse, and findings in previous studies remain largely inconsistent. We enrolled 1759 men with 2184 semen examinations at a large reproductive medical center in Wuhan, China between 2013 and 2015. Inverse distance weighting interpolation was performed to estimate individual exposures to SO2, NO2, CO and O3 during the entire period (lag 0–90 days) and key periods (lag 0-9, 10-14, 70-90 days) of sperm development. Linear mixed models were used to analyze exposure-response relationships. SO2 exposure with 0-90 days lag was significantly associated with monotonically decreased sperm concentration (β for each interquartile range increase of exposure: −0.14; 95% CI: −0.23, −0.05), sperm count (−0.21; −0.30, −0.12) and total motile sperm count (−0.16; −0.25, −0.08). Significant associations were observed for total and progressive motility only when SO2 exposure was at the highest quintile (all Ptrend < 0.05). Similar trends were observed for SO2 exposure with 70-90 days lag. NO2, CO, or O3 exposure was not significantly associated with semen quality. Our results suggest that ambient SO2 exposure adversely affects semen quality, and highlight the potential to improve semen quality by reducing ambient SO2 exposure during early stage of sperm development.
TOC image
INTRODUCTION
Air pollution continues to be a major public health concern worldwide.1 Sulfur dioxide (SO2), nitrogen dioxide (NO2), carbon monoxide (CO), and ozone (O3) are common gaseous air pollutants globally, especially in developing countries, and are being routinely monitored in many countries. A number of studies have linked these gaseous air pollutant exposures to various adverse health effects such as incidence of cardiopulmonary diseases, increased mortality, and reproductive disorders.2–6 Recent studies found a reduction in fertility rates with increasing traffic-related air pollution, including particulate matter (PM) and NO2, and suggested that all size fractions of PM exposure, as well as traffic-related air pollution were significantly associated with incidence of infertility.7,8 It should be noted that the prevalence of infertility is approximately 10% worldwide, and about 40% of infertility are due to male factors.9,10
Poor semen quality is well recognized as a condition that causes male infertility, and has drawn much attention recently.11–13 Several in vivo studies have provided preliminary evidence that exposure to SO2, NO2 or O3 might cause damage on the testes and adversely affect sperm count, motility, or morphology in mice or rats;14–17 however, only a limited number of epidemiological studies investigated the effects of gaseous air pollutant exposures on semen quality, and the results remain largely inconsistent.18 For example, both Sokol et al. and Hansen et al. conducted studies in the United States to examine the association between O3 exposure and semen quality, but only Sokol et al. found a positive link between O3 exposure and decreased sperm concentration.6,11 While a study in China and another study in Poland suggested that SO2 and NOx might adversely affect sperm morphology, rather than sperm concentration or motility, Farhat et al. did not observe any significant association for SO2 and NO2 exposures in Brazil.12,19,20
Taken together, the current evidence is limited and inconclusive on which gaseous air pollutant exposure may affect semen quality and which semen quality parameters could be affected. Due to limitations including inaccurate individual exposure assessment, selection bias, as well as small sample size, further studies are warranted.18 Therefore, we conducted this study among 1759 men to assess associations between SO2, NO2, CO, O3 exposures and semen quality in a megacity (Wuhan) in China. We employed the inverse distance weighting interpolation to estimate individual pollutant exposures during the entire period and key periods of sperm development, and conducted exposure-response analyses for semen quality parameters, including sperm concentration, count, and motility.
METHODS
Study population
The study design and population have been described elsewhere.21 In brief, we enrolled male partners of 2065 couples who visited the Reproductive Medical Center, Tongji Hospital in Wuhan, China seeking assisted reproductive technology (ART) procedures between Mar 27, 2013 and December 31, 2015. All these men lived in the central Wuhan for over 6 months, and underwent at least one semen examination during the study period. A subject typically underwent one semen examination. If the first ART procedure failed, and the subject seek another ART procedure again, the subject might undergo more semen examinations. To minimize potential confounding by poor semen quality unrelated to air pollution, we excluded 306 men with at least one of the following conditions: sexually transmitted diseases, mumps, urethral surgery, testicular surgery, epididymis surgery, vasectomy surgery, retrieve sperm difficulty, absent epididymis, absent vasectomy, azoospermia, varicocele, chromosomal abnormality, and too long (> 7 days) or too short (< 2 days) abstinence period before the date of semen examination. Therefore, we included 1759 men as the study subjects who underwent a total of 2184 semen examinations in the final analyses. This work has been approved by the Ethical Committee of Hubei Provincial Center for Disease Control and Prevention. The informed consent was waived by the committee because the data on study subjects were collected from previously routine clinical procedures and were anonymous to all research investigators.
Data collection
Gaseous air pollutant data between January 1, 2013 and December 31, 2015 were obtained from the Wuhan Environmental Protection Bureau (http://www.whepb.gov.cn/viewAirDarlyForestWaterInfo.jspx). We collected daily average individual air quality indices (IAQIs) of SO2, NO2, CO and O3, as well as PM2.5 (particulate matter < 2.5 μm in aerodynamic diameter), and then converted them to concentrations in μg/m3 according to the national standard operating procedures for air quality monitoring.22 The gaseous air pollutant concentrations were continuously monitored at each of 9 fixed air quality monitoring stations located in the central Wuhan (Figure 1). None of the 9 monitoring stations were close to industrial sources, traffic, buildings or residential sources of emissions from the burning of coal, waste or oil. The daily average temperature data were obtained from the Wuhan Regional Climate Center in Wuhan, China.
Figure 1.
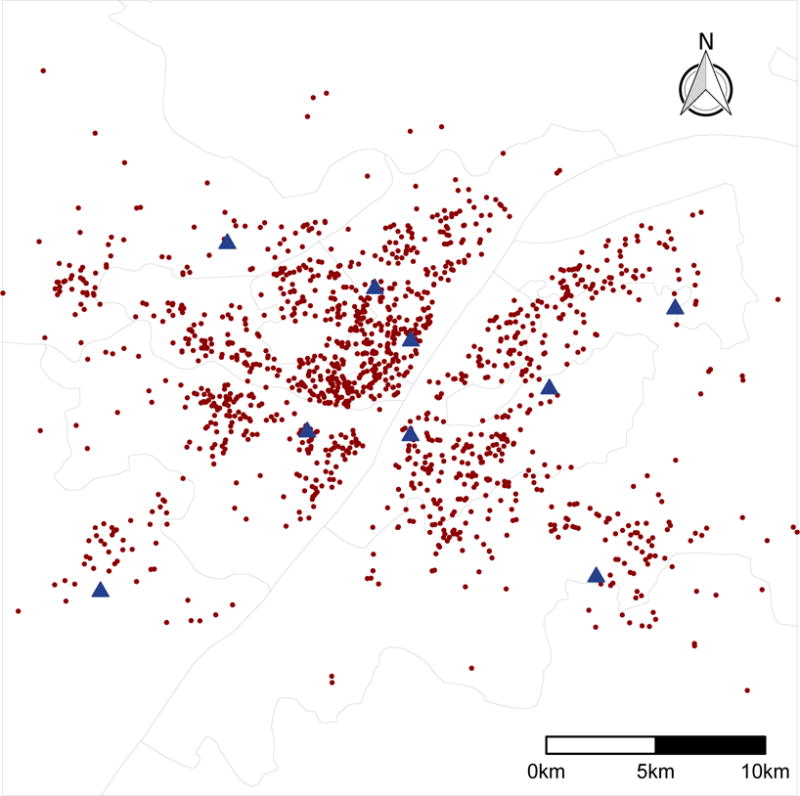
Spatial distribution of air quality monitoring stations and subjects’ residence addresses in Wuhan, China. The blue triangles indicate 9 air quality monitoring stations; the red period dots indicate subjects’ residence addresses. The grey lines represent administrative boundaries of the districts in central Wuhan (Data source: National Geomatics Center of China).
From the Reproductive Medical Center, we obtained demographic, lifestyle and clinical data, which included date of birth, race, education, residence address, cigarette smoking, alcohol consumption, height, weight, abstinence period, and semen quality. Race was categorized into Han and other. Self-reported education was categorized into college and higher, and less than college. Subjects who did not smoke during the past 6 months before enrollment was defined as non-smoking; subjects who smoked were divided into two categories including 1–10 and > 10 cigarettes/day. Body-mass index (BMI, kg/m2) was calculated as weight (kg) divided by the square of height (m). All subjects were asked for the number of abstinence days before semen examination. We categorized abstinence period as 2–3, 4–5, and 6–7 days.
All semen examinations were conducted by trained clinicians in the center according to the World Health Organization (WHO) laboratory manual for the examination and processing of human semen.23 Semen samples were collected from the subjects by masturbating into a sterile plastic specimen container in a semen collection room. The samples were then liquefied in a heating chamber and tested for semen quality parameters, including semen volume, sperm concentration, count and motility. The semen volume was tested using a serologic pipette; sperm concentration and motility (including total motility and progressive motility) were tested using a computer-aided semen analysis system (CASA, WLJY9000, Weili New Century Science & Tech Dev., Beijing, China). We calculated sperm count as semen volume multiplied by sperm concentration, and calculated total motile sperm count as sperm count multiplied by total motility.
Exposure assessment
Individual exposures of SO2, NO2, CO, O3 and PM2.5 for each subjects during 0–90, 0–9, 10–14, and 70–90 days before the date of semen examination were estimated using the inverse distance weighting (IDW) modelling method, which is commonly used in spatial interpolation to model air pollutant distribution based on data from fixed monitoring stations.21,24 We assessed exposures for the four time windows, because the development of human sperm takes approximately 90 days and includes three key periods, including epididymal storage, development of sperm motility, and spermatogenesis corresponding to 0-9, 10-14, and 70-90 days before semen ejaculation.6,11 Specifically, locations for both subjects’ residence and monitoring stations were geocoded to obtain the longitude and latitude coordinates. Using the monitoring air quality data, we then employed the IDW to predict daily pollutant concentrations at each of the 1759 subjects’ residence addresses between March 27, 2013 and December 31, 2015. The predicted daily concentration at each subject’s residence address was calculated as the average air pollutant concentration at all monitoring stations weighted by 1/d2, where d refers to distance between the residence address and each monitoring station. For each subject, we finally estimated his individual exposures to each gaseous air pollutant at different time windows by averaging the predicted daily pollutant concentrations during corresponding time windows.
Statistical analysis
The correlation between each gaseous air pollutant and temperature was examined using the Spearman’s rank correlation coefficient because these variables were not normally distributed. We examined exposure-response relationships between individual SO2, NO2, CO, O3 exposures (lag 0–90, 0–9, 10–14, and 70–90 days) and semen quality parameters, including sperm concentration, sperm count, total motility, progressive motility and total motile sperm count. All semen quality parameters were tested for normal distribution before analyses. For those with skewed distribution, we statistically transformed them to ensure that they were normally or approximately normally distributed. All semen quality parameters were standardized after data transformation as appropriate for better comparison and interpretation.
We analyzed exposure-response relationships using the linear mixed model with a subject-specific random intercept, which allows accounting for correlations between repeated semen examinations for the same subject.25 Multicollinearity was examined using the variance inflation factor (VIF). A general rule of thumb is that VIFs for exposure of interest exceeding 4 warrant further investigation, while VIFs for exposure of interest exceeding 10 are signs of serious multicollinearity requiring correction.26 For the base model, we adjusted for age, BMI, race, education, smoking amount, alcohol consumption, and abstinence period. We fitted models with further adjustment for temperature (average temperature during 0-90 days before semen examination), and temperature + season (at the date of semen examination) for comparison. Models with VIFs under 4 were considered as acceptable models, and models with adjustment for temperature and/or season were preferred if VIFs were under 4. We estimated regression coefficients and the 95% confidence intervals (CI) for each semen quality parameter associated with each interquartile (IQR) increase of each gaseous air pollutant exposure. We also grouped the gaseous air pollutant exposure into quintiles (Q1-Q5) based on its distribution among the study subjects, and estimated the regression coefficients for Q2-Q5 in comparison with Q1; linear trend across quintiles was tested by including the median of each quintile range in the model. To conduct exposure-response analyses between pollutant exposure during each key period of sperm development and semen quality, we included all exposures during three key periods in one single model simultaneously, and estimated their respective regression coefficients and 95% CIs.
We conducted sensitivity analyses to test the robustness of our results. In addition to the single-pollutant model that included only one pollutant exposure in each model, we also employed multi-pollutant models to account for the effects of other pollutants in assessing the association between a given pollutant and semen quality. Because there was possibility that poor semen quality of our study subjects might be related to risk factors unrelated to air pollution, we conducted further sensitivity analyses in a subgroup with exclusion of subjects with abnormal semen quality parameters according to the WHO standards (sperm concentration ≥ 15 × 106/mL; sperm count ≥ 39 × 106; total motility ≥ 40%; progressive motility ≥ 32%; total motile sperm count ≥ 15.6 × 106).23 All statistical analyses were performed with R version 3.3.2.27 All P values were 2-sided, and P < 0.05 was considered as statistical significant.
RESULTS
As shown in Table 1, we summarized characteristics of the 1759 study subjects. Over 60% of the subjects did not smoke in the past 6 months, and only approximately 15% smoked > 10 cigarettes per day. Over 98% of the subjects did not drink alcohol. The average abstinence period was 4.3 (SD: 1.4) days. Subjects with abnormal sperm concentration, sperm count, total motility, progressive motility, and total motile sperm count accounted for 9.3%, 14.7%, 35.2%, 33.2%, and 19.0% respectively. We also summarized the characteristics by quintiles of gaseous air pollutant exposures with 0-90 days lag. We did not find obvious different distribution for age, BMI, race, education, and abstinence period across quintiles of pollutant exposures. In Wuhan, the ambient temperature varies by season. Between 2013 and 2015, the average daily ambient temperature was 17.3, 27.5, 17.7, and 5.1 °C in spring, summer, autumn, and winter, respectively. During 0-90 days before semen examination, the average temperature decreased across SO2 exposure quintiles (Table 1; spearman’s rank correlation coefficient [r]: −0.78; P < 0.001). As also shown in Figure 2, we observed remarkable seasonality of SO2 exposure, which was relatively lower in warm season (summer, autumn) and higher in cool season (spring, winter). NO2 and CO exposures showed similar trends by temperature (data not shown) and season (Figure 2). Because O3 exposure was strongly and positively correlated with temperature (r: 0.95; P < 0.001), the temperature increased across O3 exposure quintiles (data not shown). Contrary to SO2, NO2 and CO, the O3 exposure was higher in warm season and lower in cool season (Figure 2).
Table 1.
Characteristics of study subjects by quintile of SO2 exposure during 0–90 days before the date of semen examination.
| Characteristic | All subjects | Quintile of SO2 exposure, μg/m3
|
||||
|---|---|---|---|---|---|---|
| 4.0–14.4 | 14.5–20.1 | 20.2–26.8 | 26.9–35.2 | 35.3–67.2 | ||
| No. subjects | 1759 | 327 | 343 | 355 | 364 | 370 |
| No. semen examinations | 2184 | 437 | 438 | 435 | 437 | 437 |
| Age, years | ||||||
| < 30 | 402 (18.4) | 76 (17.4) | 78 (17.8) | 75 (17.2) | 86 (19.7) | 87 (19.9) |
| 30–39 | 1475 (67.5) | 301 (68.9) | 287 (65.5) | 304 (69.9) | 280 (64.1) | 303 (69.3) |
| ≥ 40 | 307 (14.1) | 60 (13.7) | 73 (16.7) | 56 (12.9) | 71 (16.2) | 47 (10.8) |
| BMI, kg/m2 | ||||||
| < 18.5 | 43 (2.0) | 9 (2.1) | 7 (1.6) | 12 (2.8) | 5 (1.1) | 10 (2.3) |
| 18.5–23.9 | 1003 (45.9) | 207 (47.4) | 194 (44.3) | 201 (46.2) | 203 (46.5) | 198 (45.3) |
| 24.0–27.9 | 871 (39.9) | 171 (39.1) | 179 (40.9) | 178 (40.9) | 173 (39.6) | 170 (38.9) |
| ≥ 28.0 | 267 (12.2) | 50 (11.4) | 58 (13.2) | 44 (10.1) | 56 (12.8) | 59 (13.5) |
| Race, Han | 2131 (97.6) | 427 (97.7) | 421 (96.1) | 422 (97.0) | 431 (98.6) | 430 (98.4) |
| Education, college and higher | 1445 (66.2) | 298 (68.2) | 314 (71.7) | 295 (67.8) | 289 (66.1) | 249 (57.0) |
| Current smoking, cigarette/day | ||||||
| 0 (Non-smoking) | 1318 (60.3) | 261 (59.7) | 260 (59.4) | 262 (60.2) | 270 (61.8) | 265 (60.6) |
| 1–10 | 544 (24.9) | 122 (27.9) | 108 (24.7) | 110 (25.3) | 99 (22.7) | 105 (24.0) |
| > 10 | 322 (14.7) | 54 (12.4) | 70 (16.0) | 63 (14.5) | 68 (15.6) | 67 (15.3) |
| No alcohol consumption | 2152 (98.5) | 433 (99.1) | 436 (99.5) | 425 (97.7) | 429 (98.2) | 429 (98.2) |
| Abstinence period, days | ||||||
| 2–3 | 718 (32.9) | 147 (33.6) | 145 (33.1) | 136 (31.3) | 144 (33.0) | 146 (33.4) |
| 4–5 | 1056 (48.4) | 206 (47.1) | 222 (50.7) | 217 (49.9) | 201 (46.0) | 210 (48.1) |
| 6–7 | 410 (18.8) | 84 (19.2) | 71 (16.2) | 82 (18.9) | 92 (21.1) | 81 (18.5) |
| Temperature, °C | 16.7 (7.6) | 24.9 (2.2) | 21.3 (5.5) | 16.3 (6.3) | 12.5 (5.1) | 8.5 (3.6) |
| Season | ||||||
| Spring (Mar–May) | 841 (38.5) | 0 (0.0) | 96 (21.9) | 209 (48.0) | 310 (70.9) | 226 (51.7) |
| Summer (Jun–Aug) | 628 (28.8) | 255 (58.4) | 218 (49.8) | 125 (28.7) | 29 (6.6) | 1 (0.2) |
| Autumn (Sep–Nov) | 398 (18.2) | 181 (41.4) | 97 (22.1) | 66 (15.2) | 52 (11.9) | 2 (0.5) |
| Winter (Dec–Feb) | 317 (14.5) | 1 (0.2) | 27 (6.2) | 35 (8.0) | 46 (10.5) | 208 (47.6) |
| Semen quality parameter | ||||||
| Sperm concentration, 106/ml | 43.1 (41.9) | 43.7 (43.0) | 45.3 (47.3) | 43.8 (41.2) | 43.3 (42.6) | 39.4 (36.8) |
| Sperm count, 106 | 114.7 (129.5) | 117.8 (133.1) | 126.6 (135.6) | 115.0 (136.3) | 106.4 (134.0) | 108.4 (110.6) |
| Total motility, % | 46.3 (30.7) | 45.3 (32.0) | 45.2 (33.2) | 47.8 (32.8) | 46.0 (29.2) | 45.9 (30.3) |
| Progressive motility, % | 38.6 (26.8) | 36.9 (26.3) | 38.0 (29.3) | 40.5 (28.4) | 39.3 (25.2) | 38.9 (26.4) |
| Total motile sperm count, 106 | 51.4 (79.7) | 49.3 (75.8) | 57.6 (87.1) | 53.3 (83.8) | 49.1 (79.2) | 47.5 (70.3) |
Data are given as n (percent), except that temperature are given as mean (SD), and that semen quality parameters are given as median (IQR). Column percentages may not add up to 100 due to rounding.
Abbreviations: SO2, sulfur dioxide; BMI, body mass index; SD, standardized deviation; IQR, interquartile range.
Figure 2.
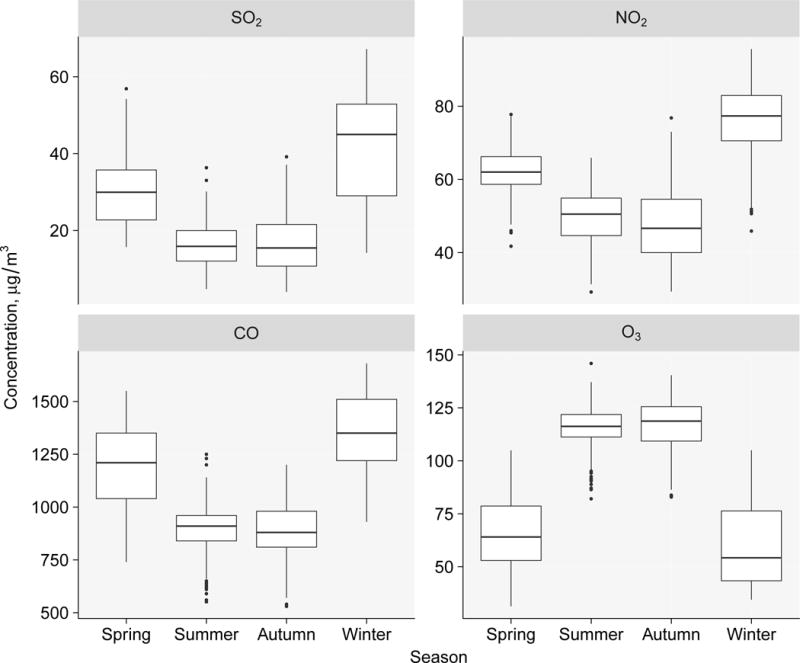
Box plot of SO2, NO2, CO and O3 exposures during 0-90 days before the date of semen examination by season.
Table S1 presents results of the exposure-response analyses for each gaseous air pollutant exposure (single-pollutant model) during 0–90 days lag and semen quality by including the exposure as a continuous variable. In the base model, SO2 exposure was only negatively associated with sperm concentration (β associated with each IQR increase of exposure: −0.07; P < 0.05). With further adjustment for temperature (base + temperature model), SO2 exposure was significantly associated with decreased sperm concentration (β: −0.12; P < 0.05), sperm count (β: −0.15; P < 0.05), total motility (β: −0.08; P < 0.05), and total motile sperm count (β: −0.12; P < 0.05) which still remained significant in models further adjusted for both temperature and season (base + temperature + season model), except for the association between SO2 exposure and total motility (β: −0.08; P > 0.05). We observed moderate or serious multicollinearity (VIF > 4) in the base + temperature and base + temperature + season models for NO2, CO and O3; for example, the VIF reached up to 25 for O3 in the base + temperature + season models. Nonetheless, all models did not yield any significant association between these gaseous air pollutant (NO2, CO, and O3) exposures and semen quality (all P > 0.05). We observed similar associations among subjects with normal sperm parameters (Table S2).
As shown in Figure 3, we further examined the shape of exposure-response associations between SO2 exposure during 0-90 days lag and semen quality by categorical analyses using the base + temperature + season model. Sperm concentration, sperm count, and total motile sperm count decreased monotonically across SO2 exposure quintiles, while total motility and progressive motility only significantly decreased at the highest quintile (Q5); nonetheless, we observed significant linear trends between SO2 exposure quintiles and all semen quality parameters (all P < 0.05). SO2 exposure at 0-9 days lag or 10-14 days lag were not significantly associated with any semen quality parameter (all P trend > 0.05); in contrast, we observed monotonic decreasing sperm concentration, sperm count, total motility, progressive motility and total motile sperm count with increasing SO2 exposure at 70-90 days lag, though the linear trend for progressive motility did not reach statistical significance (P = 0.06). The sensitivity analyses gave similar results, except that the association between SO2 exposure and motility became insignificant (all P > 0.05; Figure S1).
Figure 3.
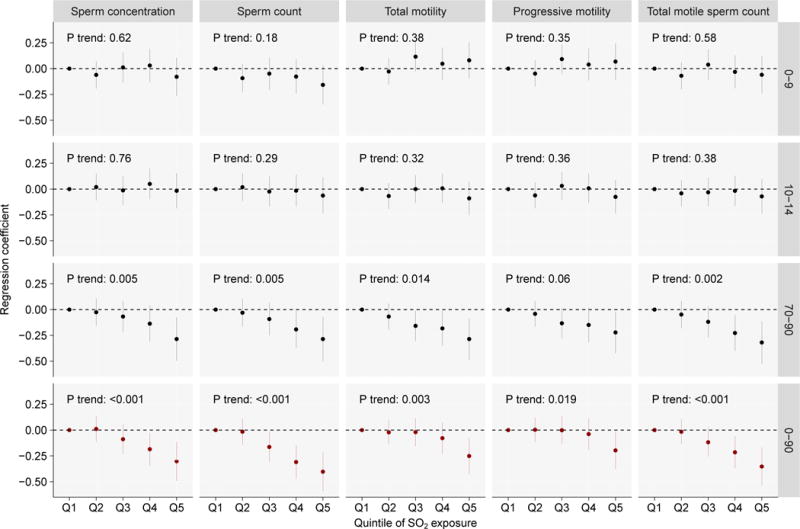
Regression coefficients (95% CIs) of semen quality associated with quintiles of SO2 exposure during 0-9, 10-14, 70-90, and 0-90 days before the date of semen examination estimated by single-pollutant models.
Table 2 presents results of the association between SO2 exposure (lag 0–90 days) and semen quality using multi-pollutant models. The SO2 + NO2, SO2 + CO, SO + O3, as well as SO2 + NO2 + CO + O3 models gave consistent results for all semen quality parameters, though the VIFs of SO2 exposures in these models reached up to 4.3, demonstrating some multicollinearity. We also tried SO2 + PM2.5 model, which demonstrated significant multicollinearity for both SO2 and PM2.5 due to high correlation between them (r: 0.90; P < 0.001); nonetheless, the model gave similar results for sperm count (β: −0.23; 95% CI: −0.37, −0.09) and total motile sperm count (β: −0.19; 95% CI: −0.32, −0.05).
Table 2.
Regression coefficients (95% CIs) of semen quality associated with quintiles of SO2 exposure during 0–90 days before the date of semen examination estimated by multi-pollutant models.
| Per IQR increase | VIF | Quintile of SO2 exposure, μg/m3
|
||||||
|---|---|---|---|---|---|---|---|---|
| 4.0–14.4 | 14.5–20.1 | 20.2–26.8 | 26.9–35.2 | 35.3–67.2 | P trend | |||
| SO2 + NO2 | ||||||||
| Sperm concentration | −0.16 (−0.26, −0.06) | 4.12 | 0 (ref) | 0.01 (−0.11, 0.13) | −0.09 (−0.24, 0.05) | −0.20 (−0.36, −0.04) | −0.33 (−0.54, −0.13) | < 0.001 |
| Sperm count | −0.24 (−0.34, −0.14) | 4.08 | 0 (ref) | −0.02 (−0.14, 0.11) | −0.17 (−0.32, −0.02) | −0.33 (−0.50, −0.16) | −0.44 (−0.65, −0.23) | < 0.001 |
| Total motility | −0.09 (−0.18, 0.01) | 4.08 | 0 (ref) | −0.02 (−0.14, 0.10) | −0.03 (−0.17, 0.11) | −0.10 (−0.26, 0.06) | −0.30 (−0.49, −0.10) | 0.002 |
| Progressive motility | −0.06 (−0.16, 0.03) | 4.07 | 0 (ref) | 0.00 (−0.12, 0.12) | −0.01 (−0.15, 0.13) | −0.06 (−0.22, 0.10) | −0.24 (−0.44, −0.05) | 0.011 |
| Total motile sperm count | −0.19 (−0.29, −0.09) | 4.10 | 0 (ref) | −0.02 (−0.14, 0.10) | −0.13 (−0.27, 0.01) | −0.24 (−0.40, −0.08) | −0.40 (−0.60, −0.20) | < 0.001 |
| SO2 + CO | ||||||||
| Sperm concentration | −0.17 (−0.26, −0.08) | 3.39 | 0 (ref) | 0.03 (−0.10, 0.15) | −0.07 (−0.21, 0.08) | −0.17 (−0.33, −0.01) | −0.35 (−0.54, −0.16) | < 0.001 |
| Sperm count | −0.23 (−0.33, −0.14) | 3.36 | 0 (ref) | −0.01 (−0.13, 0.12) | −0.15 (−0.30, −0.00) | −0.30 (−0.46, −0.14) | −0.43 (−0.63, −0.24) | < 0.001 |
| Total motility | −0.10 (−0.19, −0.01) | 3.35 | 0 (ref) | −0.00 (−0.12, 0.12) | 0.01 (−0.13, 0.15) | −0.06 (−0.21, 0.09) | −0.31 (−0.49, −0.13) | < 0.001 |
| Progressive motility | −0.07 (−0.16, 0.01) | 3.35 | 0 (ref) | 0.02 (−0.10, 0.14) | 0.02 (−0.11, 0.16) | −0.02 (−0.17, 0.13) | −0.25 (−0.43, −0.07) | 0.005 |
| Total motile sperm count | −0.19 (−0.28, −0.10) | 3.37 | 0 (ref) | −0.00 (−0.12, 0.12) | −0.09 (−0.24, 0.05) | −0.20 (−0.36, −0.04) | −0.40 (−0.59, −0.21) | < 0.001 |
| SO2 + O3 | ||||||||
| Sperm concentration | −0.15 (−0.24, −0.06) | 3.25 | 0 (ref) | 0.00 (−0.12, 0.13) | −0.11 (−0.25, 0.04) | −0.21 (−0.37, −0.05) | −0.32 (−0.51, −0.13) | < 0.001 |
| Sperm count | −0.22 (−0.31, −0.12) | 3.21 | 0 (ref) | −0.02 (−0.15, 0.11) | −0.18 (−0.33, −0.03) | −0.32 (−0.49, −0.16) | −0.41 (−0.61, −0.22) | < 0.001 |
| Total motility | −0.08 (−0.17, 0.00) | 3.21 | 0 (ref) | −0.03 (−0.15, 0.09) | −0.03 (−0.17, 0.10) | −0.10 (−0.25, 0.06) | −0.26 (−0.44, −0.08) | 0.002 |
| Progressive motility | −0.06 (−0.14, 0.03) | 3.21 | 0 (ref) | −0.00 (−0.12, 0.12) | −0.01 (−0.15, 0.13) | −0.05 (−0.21, 0.10) | −0.20 (−0.39, −0.02) | 0.014 |
| Total motile sperm count | −0.17 (−0.26, −0.08) | 3.23 | 0 (ref) | −0.02 (−0.14, 0.10) | −0.13 (−0.28, 0.01) | −0.23 (−0.39, −0.07) | −0.36 (−0.55, −0.18) | < 0.001 |
| SO2 + NO2 + CO + O3 | ||||||||
| Sperm concentration | −0.18 (−0.29, −0.08) | 4.34 | 0 (ref) | 0.02 (−0.11, 0.14) | −0.09 (−0.24, 0.06) | −0.20 (−0.37, −0.03) | −0.37 (−0.58, −0.17) | < 0.001 |
| Sperm count | −0.26 (−0.36, −0.15) | 4.29 | 0 (ref) | −0.01 (−0.14, 0.12) | −0.17 (−0.32, −0.01) | −0.33 (−0.50, −0.15) | −0.47 (−0.68, −0.25) | < 0.001 |
| Total motility | −0.11 (−0.21, −0.01) | 4.29 | 0 (ref) | −0.01 (−0.13, 0.11) | −0.01 (−0.15, 0.13) | −0.08 (−0.25, 0.08) | −0.34 (−0.53, −0.14) | < 0.001 |
| Progressive motility | −0.08 (−0.18, 0.02) | 4.28 | 0 (ref) | 0.02 (−0.10, 0.14) | 0.01 (−0.13, 0.15) | −0.04 (−0.21, 0.12) | −0.28 (−0.48, −0.08) | 0.004 |
| Total motile sperm count | −0.22 (−0.32, −0.12) | 4.31 | 0 (ref) | −0.01 (−0.13, 0.12) | −0.11 (−0.26, 0.03) | −0.23 (−0.40, −0.06) | −0.44 (−0.64, −0.23) | < 0.001 |
Abbreviations: CI, confidence interval; IQR, interquartile range; SO2, sulfur dioxide; NO2, nitrogen dioxide; CO, carbon monoxide; O3, ozone; VIF, variance inflation factor.
DISCUSSION
In this study, we investigated 1759 men who underwent 2184 semen examinations between 2013 and 2015 in a megacity in China, estimated their individual exposures to gaseous air pollutants during different periods of sperm development, and conducted quantitative exposure-response analyses for gaseous air pollutants and semen quality. Our results showed that SO2 exposures during both the entire period (lag 0-90 days) and the spermatogenesis stage (lag 70-90 days) of sperm development were significantly associated with decreased sperm concentration, sperm count, total motility, progressive motility, and total motile sperm count. We did not observe significant associations of NO2, CO, or O3 exposures with semen quality.
Although it is generally accepted that air pollution exposure may affect male reproduction, the impact of individual air pollutants on semen quality is still unclear. Only a few recent studies have examined the association between gaseous air pollutant exposure and semen quality, and the results remain largely inconsistent.18,28 Previous epidemiological studies suggested that SO2 exposure with 90 days lag was significantly associated with increased abnormalities in sperm morphology12,19, but not associated with sperm concentration, nor total motility.12,19,20 In comparison, Zhang et al. found that SO2 inhalation lowered sperm count and sperm motility in rats, though the decrease of sperm count did not reach statistical significance;16 another in vivo study reported that SO2 exposure significantly decreased sperm count, and significantly increased sperm shape abnormality percentage in mice.17 Our results were consistent with the in vivo studies that SO2 exposure was significantly associated with sperm count and motility, and inconsistent with results from the epidemiological studies. It should be noted that both epidemiological and in vivo studies suggested that SO2 exposure may affect sperm morphology;12,17,19 unfortunately, we were unable to provide further evidence due to lack of sperm morphology data.
This is the first study to report that SO2 exposure mainly adversely affects the spermatogenesis (lag 70-90 days) stage during sperm development. In studies that investigated air pollutant exposures during different key periods of sperm development and semen quality, they estimated the associations by including these exposures in separate models (e.g. included the lag 0-9 exposure in a single model to estimate its association with semen quality);6,11,21 however, this method did not take into account the effects caused by exposures during the other two key periods, and might lead to biased associations. In this study, we included all exposures during three key periods in one single model simultaneously, and estimated their respective effects.
The detailed mechanisms in which SO2 may adversely affect semen quality remain to be elucidated. One possible mechanism is oxidative stress, which is known to interfere with the fertilizing capacity of spermatozoa, damage sperm nuclear DNA, and affect the epigenetic profile of these cells.29 It has been reported that SO2 inhalation could cause oxidative damage to the testes in mice.30 SO2 exposure may lower semen quality via increasing expressions of cAMP-responsive element molecular (CREM) and activator of CREM (ACT) proteins in rats, which play a crucial role in the spermatogenesis and normal male reproduction.16 Changes in the BTB (blood-testis barrier)-associated junction proteins, which could cause dysfunction of BTB, may be another pathway that leads to low semen quality in SO2 exposed mice.17
For O3, our results were consistent with results reported by Hansen et al. that no statistically significant adverse effect on sperm concentration nor count were detected.11 Sokol et al. also reported similar results that O3 exposures during 0-9, 10-14, 70-90 days before semen examination were not associated with total motile sperm count;6 however, they did observe significant associations for sperm concentration, which was also reported by a recent study in China.6,31 An in vivo study found that O3 exposure could decrease sperm concentration in rats, but the sperm morphology and motility were not significantly affected.15
Previous studies suggested that exposure to NO2 (or NOx) and CO were associated with increased abnormalities in sperm morphology,12,19 but not associated with sperm concentration, count, motility, nor aneuploidy6,12,19,20,32, which was in line with our results. In contrast, an in vivo study concluded that NO2 inhalation might reduce the sperm production in rats;14 Boggia et al. found significantly lower sperm total motility, not sperm count, in occupational NO2 exposed workers;33 however, the NO2 exposures in these two studies tended to be much higher than that in the epidemiological studies for general population, which may partly explain the inconsistence.
Several reasons may account for the inconsistence of associations between gaseous air pollutant exposures and semen quality. First, most previous epidemiological studies investigating gaseous air pollutant exposures and semen quality employed exposure assessment approaches that have strong limitations and that are incapable of providing truly individual estimates of exposure.18 They typically considered only temporal variation of the exposure by averaging daily concentrations for a specific period of time (e.g. 90 days) before semen collection, while ignored spatial variation of the exposure by using an average concentration on a single day. Only two studies estimated individual exposure based on subjects’ residence addresses, one using 10 km × 10 km modelled grids,6 and the other using data from the closest monitoring station.12 The lack of spatial variation of exposure would induce exposure misclassifications which may further bias the exposure-response associations. In our study, we used the IDW interpolation for exposure assessment considering both spatial and temporal variations, which may better predict the individual exposures and thus reduce bias. Second, the association between gaseous air pollutants and semen quality may change with exposure levels (that is, nonlinearity), which varies in different region or countries. For example, our results showed that the SO2 exposure did not significantly affect sperm motility until the exposure increase to the highest quintile (Figure 3). The average SO2 concentration between 2013 and 2015 in our study was 23 μg/m3, which was lower than that in studies by Zhou et al. (69 μg/m3) and Radwan et al. (37 μg/m3).12,19 In contrast, the average O3 concentration in our study (134 μg/m3) was much higher in comparison with studies by Hansen et al. (~60 μg/m3) and Sokol et al. (~43 μg/m3).6,11 Third, the sample size in some studies might be insufficient to provide a sufficient statistical power, especially when a number of potential confounders were adjusted in the analyses.
Our results have important public health implications. SO2 is a very common ambient gaseous air pollutant worldwide, especially in developing countries. It has been recently reported that SO2 pollution in the eastern US and the eastern Europe decreased from 2005 to 2015.34 Though China has severe SO2 pollution, a decreasing trend has been observed since 2011, with about a 50% reduction in 2012-2015; in contrast, SO2 pollution in some developing countries such as India is growing at fast pace.34 In our study, the annual mean SO2 concentration in 2013, 2014 and 2015 in Wuhan were 33, 21, and 18 μg/m3 respectively, indicating a decreasing trend. Our results suggest that SO2 exposure at this level may have significant adverse effects on semen quality. Because it is difficult for the general population to prevent inhalation of ambient gaseous air pollutants using personal protective equipment (e.g. a N95 respirator against PM2.5), our results highlights the importance and needs to further reduce the ambient SO2 concentrations for reproductive health. The primary source of these air pollutants is the combustion of fossil fuels in power plants, various industrial processes, and motor vehicles and equipment, while the ground level O3 forms from the reaction of nitrogen oxides (NOx, NO2 and NO) and volatile organic compounds (VOCs) in the presence of sunlight. Our results provides clues for the government to adopt effective control measures against air pollution. Men who are trying to conceive may need to reduce ambient SO2 exposure as early as three months in advance, by using an air purifier and avoiding outdoor activity for example, especially when the air pollution is severe.
One unique strength of our study is that we employed the IDW interpolation to account for the spatial variation of air pollutant exposure in assessment for the individual exposure, which could help reduce exposure misclassifications in comparison with previous studies. With repeated measures, the sample size of this study is relatively large to provide sufficient statistical power in detecting positive associations. On the other hand, several limitations need to be discussed. First, as previous studies,6,8,11 we used ambient gaseous air pollutant exposure as a proxy for the individual exposure. We did not consider residence height, indoor exposures, as well as the time-activity patterns of the study subjects, which may lead to exposure misclassification and bias the exposure-response association. However, several studies have shown that ambient measurements of air pollutants are acceptable surrogate for individual level exposure, and the use of ambient exposures is helpful because regulation typically focuses on these levels.35,36 Second, decreased semen quality may be the result of a complex of inhaled multi-pollutants. Therefore, we modeled the associations with both single-pollutant and multi-pollutant approaches. However, the high correlation between SO2 and other air pollutants such as PM2.5 limited our ability to separate the independent effect for each pollutant. Further studies in other geographical locations with a different composition of air pollutants are warranted to confirm our findings. Third, we considered smoking information during the recent 6 months before semen examination, which may underestimate smoking exposure for those who did not smoke in the past 6 months (i.e. non-smoking) but had ever smoked before. Finally, we did not investigate the associations between gaseous air pollutant exposures and abnormalities in sperm morphology, because the sperm morphology data were unavailable.
In conclusion, the present study shows that ambient SO2 exposure may adversely affect sperm count and motility by mainly affecting the spermatogenesis (lag 70-90 days) stage of sperm development. We did not find a significant association of NO2, CO, or O3 exposure with semen quality. Given the widespread pollution by SO2 in China and other countries, our study highlights the importance and needs to take further control measures to reduce ambient SO2 concentrations.
Supplementary Material
Acknowledgments
We sincerely thank the Wuhan Regional Climate Center for providing us with the data on daily average temperature in Wuhan, China. This research was funded by the Hubei Province Health and Family Planning Scientific Research Project (WJ2015MA027). Dr. Wei Bao was supported by the University of Iowa Environmental Health Sciences Research Center (NIH P30 ES005605) and Center for Global and Regional Environmental Research.
Footnotes
Supporting Information Available
Table S1–S2 and Figure S1. This information is available free of charge via the Internet at http://pubs.acs.org.
Notes
The authors declare no competing financial interest.
References
- 1.Venkatesan P. WHO report: air pollution is a major threat to health. Lancet Respir Med. 2016;4:351. doi: 10.1016/S2213-2600(16)30014-5. [DOI] [PubMed] [Google Scholar]
- 2.Turner MC, Jerrett M, Pope CA, 3, Krewski D, Gapstur SM, Diver WR, Beckerman BS, Marshall JD, Su J, Crouse DL, et al. Long-Term Ozone Exposure and Mortality in a Large Prospective Study. Am J Respir Crit Care Med. 2016;193:1134–1142. doi: 10.1164/rccm.201508-1633OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moolgavkar SH, McClellan RO, Dewanji A, Turim J, Luebeck EG, Edwards M. Time-series analyses of air pollution and mortality in the United States: a subsampling approach. Environ Health Perspect. 2013;121:73–78. doi: 10.1289/ehp.1104507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin S, Liu X, Le LH, Hwang S-A. Chronic exposure to ambient ozone and asthma hospital admissions among children. Environ Health Perspect. 2008;116:1725–1730. doi: 10.1289/ehp.11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, Burnett R, Palmer JR, Rosenberg L. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012;125:767–772. doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokol RZ, Kraft P, Fowler IM, Mamet R, Kim E, Berhane KT. Exposure to environmental ozone alters semen quality. Environ Health Perspect. 2006;114:360–365. doi: 10.1289/ehp.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieuwenhuijsen MJ, Basagana X, Dadvand P, Martinez D, Cirach M, Beelen R, Jacquemin B. Air pollution and human fertility rates. Environ Int. 2014;70:9–14. doi: 10.1016/j.envint.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Mahalingaiah S, Hart JE, Laden F, Farland LV, Hewlett MM, Chavarro J, Aschengrau A, Missmer SA. Adult air pollution exposure and risk of infertility in the Nurses’ Health Study II. Hum Reprod. 2016;31:638–647. doi: 10.1093/humrep/dev330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Legare C, Droit A, Fournier F, Bourassa S, Force A, Cloutier F, Tremblay R, Sullivan R. Investigation of male infertility using quantitative comparative proteomics. J Proteome Res. 2014;13:5403–5414. doi: 10.1021/pr501031x. [DOI] [PubMed] [Google Scholar]
- 10.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen C, Luben TJ, Sacks JD, Olshan A, Jeffay S, Strader L, Perreault SD. The effect of ambient air pollution on sperm quality. Environ Health Persp. 2010;118:203–209. doi: 10.1289/ehp.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radwan M, Jurewicz J, Polanska K, Sobala W, Radwan P, Bochenek M, Hanke W. Exposure to ambient air pollution—does it affect semen quality and the level of reproductive hormones? Ann Hum Biol. 2016;43:50–56. doi: 10.3109/03014460.2015.1013986. [DOI] [PubMed] [Google Scholar]
- 13.Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum Reprod Update. 2017:1–14. doi: 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe N. Decreased number of sperms and Sertoli cells in mature rats exposed to diesel exhaust as fetuses. Toxicol Lett. 2005;155:51–58. doi: 10.1016/j.toxlet.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Jedlinska-Krakowska M, Gizejewski Z, Dietrich GJ, Jakubowski K, Glogowski J, Penkowski A. The effect of increased ozone concentrations in the air on selected aspects of rat reproduction. Pol J Vet Sci. 2006;9:11–16. [PubMed] [Google Scholar]
- 16.Zhang J, Zheng F, Liang C, Zhu Y, Shi Y, Han Y, Wang J. Sulfur dioxide inhalation lowers sperm quality and alters testicular histology via increasing expression of CREM and ACT proteins in rat testes. Environ Toxicol Phar. 2016;47:47–52. doi: 10.1016/j.etap.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Li Z, Qie M, Zheng R, Shetty J, Wang J. Sodium fluoride and sulfur dioxide affected male reproduction by disturbing blood-testis barrier in mice. Food Chem Toxicol. 2016;94:103–111. doi: 10.1016/j.fct.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Lafuente R, Garcia-Blaquez N, Jacquemin B, Checa MA. Outdoor air pollution and sperm quality. Fertil Steril. 2016;106:880–896. doi: 10.1016/j.fertnstert.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Zhou N, Cui Z, Yang S, Han X, Chen G, Zhou Z, Zhai C, Ma M, Li L, Cai M, et al. Air pollution and decreased semen quality: a comparative study of Chongqing urban and rural areas. Environ Pollut. 2014;187:145–152. doi: 10.1016/j.envpol.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 20.Farhat J, Farhat SCL, Braga ALF, Cocuzza M, Borba EF, Bonfa E, Silva CA. Ozone decreases sperm quality in systemic lupus erythematosus patients. Rev Bras Reumatol. 2016;56:212–219. doi: 10.1016/j.rbre.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Wu L, Jin L, Shi T, Zhang B, Zhou Y, Zhou T, Bao W, Xiang H, Zuo Y, Li G, et al. Association between ambient particulate matter exposure and semen quality in Wuhan, China. Environ Int. 2017;98:219–228. doi: 10.1016/j.envint.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Ministry of Environmental Protection of China. Technical regulation on ambient air quality index (On Trial) China Environmental Science Press; Beijing: 2012. [Google Scholar]
- 23.World Health Organization (WHO) WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th. World Health Organization; Geneva: 2010. [Google Scholar]
- 24.Kim E, Park H, Hong Y-C, Ha M, Kim Y, Kim B-N, Kim Y, Roh Y-M, Lee B-E, Ryu J-M, et al. Prenatal exposure to PM(1)(0) and NO(2) and children’s neurodevelopment from birth to 24 months of age: mothers and Children’s Environmental Health (MOCEH) study. Sci Total Environ. 2014;481:439–445. doi: 10.1016/j.scitotenv.2014.01.107. [DOI] [PubMed] [Google Scholar]
- 25.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. Springer-Verlag New York; New York: 2000. [Google Scholar]
- 26.Belsley DA, Kuh E, Welsch RE. Regression diagnostics: Identifying influential data and sources of collinearity. Wiley; New York: 1980. [Google Scholar]
- 27.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2017. Vienna, Austria. URL https://wwwR-projectorg/ [Google Scholar]
- 28.Deng Z, Chen F, Zhang M, Lan L, Qiao Z, Cui Y, An J, Wang N, Fan Z, Zhao X, et al. Association between air pollution and sperm quality: A systematic review and meta-analysis. Environ Pollut. 2016;208:663–669. doi: 10.1016/j.envpol.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 29.Aitken RJ, Smith TB, Jobling MS, Baker MA, de Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl. 2014;16:31–38. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng Z, Bai W. Oxidation damage of sulfur dioxide on testicles of mice. Environ Res. 2004;96:298–304. doi: 10.1016/j.envres.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Tian XJ, Wang XC, Ye B, Li CL, Zhang Y, Ma L. The effects of exposure to ozone on sperm quality in Wuhan. Zhonghua Yu Fang Yi Xue Za Zhi. 2017;51:197–202. doi: 10.3760/cma.j.issn.0253-9624.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Jurewicz J, Radwan M, Sobala W, Polanska K, Radwan P, Jakubowski L, Ulanska A, Hanke W. The relationship between exposure to air pollution and sperm disomy. Environ Mol Mutagen. 2015;56:50–59. doi: 10.1002/em.21883. [DOI] [PubMed] [Google Scholar]
- 33.Boggia B, Carbone U, Farinaro E, Zarrilli S, Lombardi G, Colao A, de Rosa N, de Rosa M. Effects of working posture and exposure to traffic pollutants on sperm quality. J Endocrinol Invest. 2009;32:430–434. doi: 10.1007/BF03346481. [DOI] [PubMed] [Google Scholar]
- 34.Krotkov NA, McLinden CA, Li C, Lamsal LN, Celarier EA, Marchenko SV, Swartz WH, Bucsela EJ, Joiner J, Duncan BN, et al. Aura OMI observations of regional SO2 and NO2 pollution changes from 2005 to 2015. Atmos Chem Phys. 2016;16:4605–4629. [Google Scholar]
- 35.Janssen NA, de Hartog JJ, Hoek G, Brunekreef B, Lanki T, Timonen KL, Pekkanen J. Personal exposure to fine particulate matter in elderly subjects: relation between personal, indoor, and outdoor concentrations. J Air Waste Manage Assoc. 2000;50:1133–1143. doi: 10.1080/10473289.2000.10464159. [DOI] [PubMed] [Google Scholar]
- 36.Sarnat JA, Schwartz J, Catalano PJ, Suh HH. Gaseous pollutants in particulate matter epidemiology: confounders or surrogates? Environ Health Perspect. 2001;109:1053–1061. doi: 10.1289/ehp.011091053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


