Abstract
Maternal immune activation (MIA) can affect fetal brain development and thus behavior of young and adult offspring. Reports have shown that increased Interleukin-6 (IL-6) in the maternal serum plays a key role in altering fetal brain development, and may impair social behaviors in the offspring. Interestingly, these effects could be attenuated by blocking IL-6. The current study investigated the effects of luteolin, a citrus bioflavonoid, and its structural analog, diosmin, on IL-6 induced JAK2/STAT3 (Janus tyrosine kinase-2/signal transducer and activator of transcription-3) phosphorylation and signaling as well as behavioral phenotypes of MIA offspring. Luteolin and diosmin inhibited neuronal JAK2/STAT3 phosphorylation both in vitro and in vivo following IL-6 challenge as well as significantly diminishing behavioral deficits in social interaction. Importantly, our results showed that diosmin (10 mg/kg day) was able to block the STAT3 signal pathway; significantly opposing MIA-induced abnormal behavior and neuropathological abnormalities in MIA/adult offspring. Diosmin’s molecular inhibition of JAK2/STAT3 pathway may underlie the attenuation of abnormal social interaction in IL-6/MIA adult offspring.
Keywords: Flavonoids, Autism, JAK2/STAT3 signaling pathway, IL-6
1. Introduction
Although the root cause(s) of autism are far from being proven, in recent years, it has been hypothesized that maternal immune activation (MIA) may contribute to the development of autism through chronic induction of pro-inflammatory pathways which starts in utero. MIA is used to describe an increase in circulating maternal cytokines in response to an infection during pregnancy (Smith et al., 2007). The ensuing cytokine response and the highly susceptible developmental period in which it occurs may precipitate the neuropathological and behavioral deficits observed in autism and related disorders.
MIA-induced cytokines confer both direct and indirect effects on the fetus. Two methods have been used to study these effects: injecting or up-regulating cytokines during pregnancy in the absence of MIA, or blocking endogenous cytokines or preventing their induction during MIA. A study of the role of TNF-α in LPS-induced fetal loss and growth restriction indicated injection of anti-TNF-α antibodies or an inhibitor of TNF-α synthesis [pentoxifylline] can reduce these effects of LPS. Conversely, injection of TNF-α alone can induce fetal loss (Silver et al., 1994; Xu et al., 2006) which is significantly worse in IL-18 knockout mice, but not in IL-1α/β knockout mice (Wang et al., 2006).
Much of the previous investigations of cytokine mediation of MIA effects on neuropathology and behavior in the offspring have focused on IL-6. This cytokine is involved in the regulation of physiological processes including inflammation and neurodevelopment; making it a particularly appealing candidate molecule for MIA-induced neuropathology. Indeed, during neurodevelopment, the signal transducer and activator of transcription-3 (STAT3) pathway, activated by IL-6, maintains homeostasis between neuro- and gliogenesis (He et al., 2005; Murphy et al., 2000).
In support, Samuelsson et al. (2006) injected IL-6 i.p. in pregnant rats for 3 days resulting in severe effects on the offspring. An important finding was that IL-6 mRNA levels remain elevated in the hippocampi of the offspring at 4 and 24 weeks of age; indicative of the ongoing state of immune dysregulation in adult autistic brains. Spatial memory in the water maze, a hippocampal-dependent behavior, was observed in that study. Importantly, the IL-6-treated offspring displayed increased latency to escape and time spent near the pool wall. Therefore, prolonged exposure to elevated IL-6 in utero causes a deficit in working memory (for reviews see Patterson, 2008).
Blocking endogenous IL-6 in MIA also supports the central role of this cytokine (Smith et al., 2007). Co-injection of anti-IL-6 antibody with maternal poly(I:C) blocks the effects of MIA on the behavior of the offspring. Further, maternal injection of poly(I:C) in an IL-6 knockout mouse results in normal behaving offspring. In addition, the anti-IL-6 antibody also blocks the changes in brain transcription induced by maternal poly(I:C) (Patterson, 2008). Maternal injection of poly(I:C) induces expression of IL-6 mRNA in fetal brain and placenta, and this is also dependent on the IL-6 induced by maternal poly(I:C) (Patterson, 2008) (E. Hsiao and P.H. Patterson, unpublished). Taken together these previous works by other groups indicate both direct and indirect (positive feedback loop) mechanisms for IL-6 mediated MIA in the context of aberrant fetal brain development which could lead to an autism-like phenotype (Patterson, 2008).
The effect of flavonoids on JAK/STAT activation has also been previously described. For example, Park et al. (2008) found that EGCG inhibits STAT3 activation as an integral part of inhibition of keloid formation (Park et al., 2008). In an earlier study it was found that silibinin, a flavonoid, inhibits constitutive activation of Stat3, and causes caspase activation and apoptotic death of human prostate carcinoma cells (Agarwal et al., 2007). Prior to this, it was demonstrated that in vivo treatment of SJL/J mice with quercetin, a flavonoid, (i.p. 50 or 100 μg every other day) ameliorates experimental autoimmune encephalitis (EAE) by inhibiting IL-12 production and neural antigen-specific Th1 differentiation. In vitro treatment of activated T cells with this same flavonoid quartering blocks IL-12-induced tyrosine phosphorylation of JAK2, TYK2, STAT3, and STAT4, yielding a reduced IL-12-induced T cell proliferation and Th1 differentiation (Muthian and Bright, 2004). Our studies indicated that IL-6 activates the JAK2/STAT3 pathway, as N2a neuronal cells and brain homogenates from newborn IL-6-induced MIA (IL-6/MIA) offspring showed increased neuronal JAK2/STAT3 phosphorylation. In adulthood, these mice showed deficits in social interaction, suggesting that not only does IL-6 activate the JAK2/STAT3 pathway, but that it is also involved in the abnormal behavioral pathologies observed in MIA offspring and potentially autism and related disorders. Next we investigated if inhibition of JAK2/STAT3 signaling could attenuate MIA-induced pathologies. Previous research by our laboratory has shown that bioflavonoids such as epi-gallocatechin gallate (EGCG) or luteolin, inhibit IFN-γ induced STAT1 activation and attenuate production of pro-inflammatory cytokines in cultured and primary microglial cells (Giunta et al., 2006; Jagtap et al., 2009; Rezai-Zadeh et al., 2008).
The goal of the current study was to investigate possible prophylactic effects of two flavonoids which possess potentially better bioavailability and safety than these previously tested compounds. These two flavonoids are, luteolin, and its structural analog, diosmin. We hypothesized that JAK2/STAT3 phosphorylation and signaling as well as behavioral abnormalities in of IL-6 induced MIA offspring could be ameliorated with these naturally occurring compounds. Our results showed that diosmin (10 mg/kg day) was able to block the STAT3 signal pathway; significantly opposing IL-6-induced abnormal behavior and neuropathological abnormalities in MIA/adult offspring. Using guidelines put forth by the Food and Drug Administration (Reagan-Shaw et al., 2008), this 10 mg/kg day dose in mice is equivalent to 0.81 mg/kg/day in humans which translates into 48.6 mg/day for a 60 kg person.
2. Methods
2.1. Reagents
Luteolin (>95% purity by HPLC) was purchased from Sigma (St Louis, MO, USA). Diosmin (>90% purity by HPLC) was purchased from Axxora (San Diego, CA, USA). Antibodies against JAK2, phospho-JAK2, STAT3 and phospho-STAT3 were obtained from Cell Signaling Technology (Danvers, MA, USA). ELISA kits for tumor necrosis factor-α (TNF-α) and Interleukin-1β (IL-1β) were obtained from R&D Systems (Minneapolis, MN, USA). BCA protein assay kit was purchased from Pierce Biotechnology (Rockford, IL, USA). Murine recombinant IL-6 was purchased from eBioscience (San Diego, CA, USA).
2.2. Primary cell culture
Cerebral cortices were isolated from C57BL/6 mouse embryos, between 15 and 17 days in utero. After 15 min of incubation in trypsin (0.25%) at 37 °C, individual cortices were mechanically dissociated. Cells were collected after centrifugation at 1200 rpm, resuspended in DMEM supplemented with 10% fetal calf serum, 10% horse serum, uridine (33.6 g/mL; Sigma) and fluorodeoxyuridine (13.6 g/mL; Sigma), and plated in 24 well collagen coated culture plates at a density of 2.5×105 cells per well.
N2a (murine neuroblastoma) cells, purchased from the American Type Culture Collection (ATCC, Manassas, VA) were grown in complete EMEM supplemented with 10% fetal calf serum. Cells were plated in 24 well collagen coating culture plates at a density of 1×105 cells per well. After overnight incubation, N2a cells were incubated in neurobasal media supplemented with 3 mM dibutyryl cAMP in preparation for treatment.
Cells were treated with 50 ng/mL murine recombinant IL-6 for a range of time points (0, 15, 30, 45, 60 or 75min) in the presence or absence of various concentrations of luteolin (0, 1.25, 2.5, 5, 10, 20 μM) for 30 min.
2.3. Mice
Pregnant C57BL/6 mice, embryonic day 2 (E2) were obtained from Jackson Laboratory (Bar Harbor, MA) and individually housed and maintained in an animal facility of the University of South Florida (USF). All subsequent experiments were performed in compliance with protocols approved by the USF Institutional Animal Care and Use Committee. At E12.5, mice were intraperitoneally (i.p.) challenged (one time only) with murine recombinant IL-6 (5 μg dissolved in 200 μL of PBS/mouse) in the presence or absence of the STAT3 inhibitor S31-201 (4 μg dissolved in 200 μL of PBS/mouse) and/or treated with diosmin, administered orally (10 mg/kg/day, 0.005% in NIH31 chow). Intraperitoneal PBS injection (200 μL) was used as the control for IL-6 administration.
2.4. Western blot
Cultured cells were lysed in ice-cold lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% v/v Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM Na3VO4, 1 μg/mL leupeptin, 1 mM PMSF) as described previously (Tan et al., 2002). Mouse brains were isolated under sterile conditions on ice and placed in ice-cold lysis buffer. Brains were then sonicated on ice for approximately 3 min, allowed to stand for 15 min at 4 °C, then centrifuged at 15,000 rpm for 15 min at 4 °C. Total protein content was estimated using the BCA protein assay (Pierce Biotechnology) and aliquots corresponding to 100 μg of total protein were electrophoretically separated using 10% Tris gels. Electrophoresed proteins were then transferred to nitrocellulose membranes (Bio-Rad, Richmond, CA), washed in Tris buffered saline with 0.1% Tween-20 (TBS/T), and blocked for 1 h at ambient temperature in TBS/T containing 5% (w/v) non-fat dry milk. After blocking, membranes were hybridized overnight at 4 °C with various primary antibodies. Membranes were then washed 3× for 5 min each in TBS/T and incubated for 1 h at ambient temperature with the appropriate HRP-conjugated secondary antibody (1:1000, Pierce Biotechnology). Primary antibodies were diluted in TBS/T containing 5% BSA and secondary antibodies in TBS containing 5% (w/v) of non-fat dry milk. Blots were developed using the luminol reagent (Pierce Biotechnology). Densitometric analysis was conducted using a FluorS Multiimager with Quantity One™ software (Bio-Rad, Hercules, CA). For phospho-STAT3 detection, membranes were probed with a phospho-Ser727 STAT3 antibody (1:1000) and stripped with stripping solution and then re-probed with antibody that recognizes total STAT3 (1:1000). Similarly for phospho-JAK2 detection, membranes were probed with phospho-JAK2 (1:1000) and stripped and re-probed for total JAK2 (1:1000).
2.5. ELISA cytokine analysis
Mouse brain homogenates were prepared as described above and used at a dilution of 1:10 in PBS for these assays. Brain tissue-solubilized cytokines were quantified using commercially available ELISAs that allow for detection of IL-1β, and TNF-α. Cytokine detection was carried out according to the manufacturer’s instruction. Total protein content was determined as described above and data represented as pg of cytokine/mg total cellular protein for each cytokine.
2.6. Behavioral testing
Open field (OF)
The OF behavioral analysis is a test of both locomotor activity and emotionality in rodents (Radyushkin et al., 2009). Mice were placed in a 50×50 cm white Plexiglas box brightly lit by fluorescent room lighting and six 60 W incandescent bulbs approximately 1.5 m above the box. Activity was recorded by a ceiling-mounted video camera and analyzed from digital video files either by the automated tracking capabilities of Ethovision or counted using the behavior tracker (version 1.5, www.behaviortracker.com), a software-based event-recorder. The total distance moved and numbers of entries into the center of the arena (central 17 cm square) were determined in a 10minute session.
Social interaction (SI)
The testing apparatus consisted of a 60×40 cm Plexiglas box divided into three chambers. Mice were able to move between chambers through a small opening (6×6 cm) in the dividers. Plastic cylinders in each of the two side chambers contained the probe mice, and numerous 1 cm holes in the cylinders enabled test and probe mice to contact each other. Test mice were placed in the center chamber, with an overhead camera recording their movements. Mice were allowed 5 min of exploration time in the box, after which an unfamiliar, same-sex probe mouse from the same experimental group was placed in one of two restraining cylinders (Radyushkin et al., 2009). The Ethovision software (Noldus, Leesburg, VA) program measured time spent in each of the three chambers, and social preference was defined as follows: (% time spent in the social chamber)−(% time spent in the opposite chamber).
2.7. Statistical analysis
All data were normally distributed; therefore, in instances of single mean comparisons, Levene’s test for equality of variances followed by t-test for independent samples was used to assess significance. In instances of multiple mean comparisons, analysis of variance (ANOVA) was used, followed by post hoc comparison using Bonferroni’s method. Alpha levels were set at 0.05 for all analyses. The STATistical package for the social sciences release 10.0.5 (SPSS Inc., Chicago, IL, USA) was used for all data analysis.
3. Results
3.1. Luteolin inhibits IL-6 induced neuronal JAK2/STAT3 phosphorylation
To confirm the role of IL-6 in regulating the JAK2/STAT3 pathway, we treated murine neuron-like (N2a) cells and primary cultured neuronal cells with 50 ng/mL of murine recombinant IL-6 in a time-dependent manner. Western blot analysis of cell lysates showed that IL-6 treatment leads to a time-dependent increase in JAK2 (Fig. 1A) and STAT3 (Fig. 1C) phosphorylation. Densitometric analysis indicated significant and steady increases in JAK2 phosphorylation (**P<0.005) beginning at 30 min and continuing until 75 min when the timed analysis was concluded (Fig. 1A). Densitometric analysis of STAT3 phosphorylation (**P<0.005) showed a significant and maximal increase at 30 min, with no further significant increases at subsequent time points.
Fig. 1.
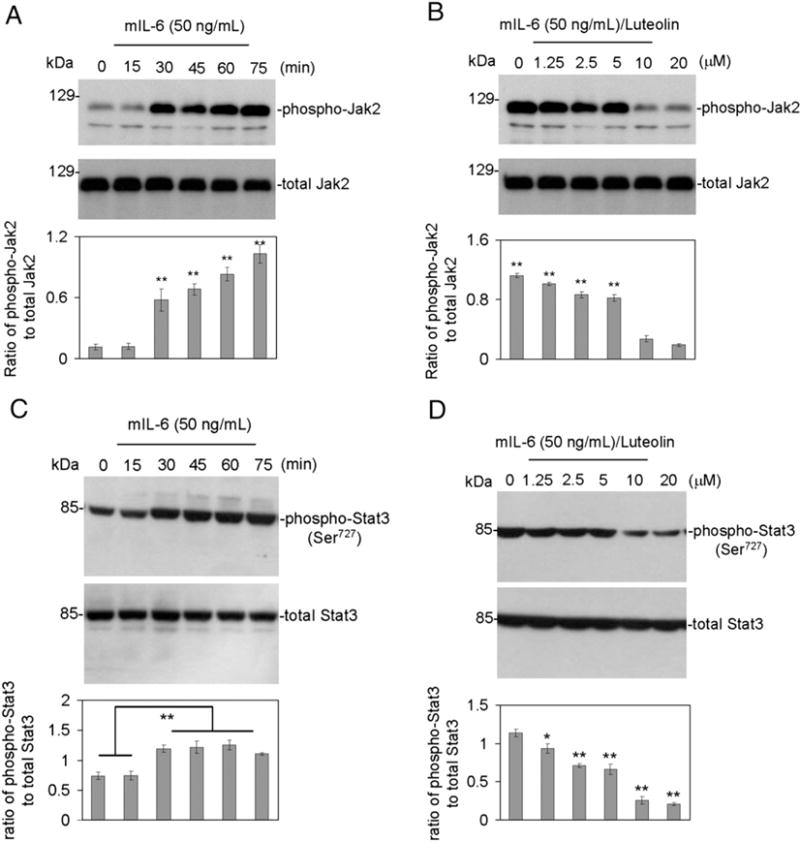
Luteolin inhibits JAK2/STAT3 phosphorylation induced by IL-6 in cultured neuronal cells. (A and C, top), analysis of results shows that IL-6 notably induced JAK2/STAT3 phosphorylation. Densitometry analysis shows the ratio of phospho-JAK2/STAT3 to total JAK2/STAT3 as indicated below the figures. One-way ANOVA showed that IL-6 significantly activates JAK2/STAT3 in a time-dependent manner (**P<0.005). Furthermore, (B and D, top), Western blot and densitometry analyses reveal the ratio of phospho-JAK2/STAT3 to total JAK2/STAT3 as indicated below the figures. Most notably, the presence of luteolin significantly inhibits IL-6-induced JAK2/STAT3 phosphorylation (*P<0.01; **P<0.001). These data shown are representative of three independent experiments. Similar results were obtained in murine primary cultured neuronal cells using antibody specifically against phospho-JAK2/STAT3 (Ser727) and in N2a cells using antibody specifically against phospho-STAT3 (Ser705).
We next examined the effects of luteolin on IL-6 induced JAK2/STAT3 phosphorylation. Murine N2a cells and primary cultured neuronal cells were again challenged with 50 ng/mL murine recombinant IL-6 and co-treated with increasing concentrations of luteolin (0–20 μM) for 30 min. Following Western blot analysis of cell lysates, we found that luteolin reduces IL-6 induced JAK2 phosphorylation (Fig. 1B) and STAT3 phosphorylation (Ser727) (Fig. 1D) in both murine N2a and primary neurons in a dose dependent manner with significant reductions beginning at 10 μM. Densitometric analysis showed that luteolin inhibited JAK2 and STAT3 (Ser727) phosphorylation by almost 50% (*P<0.01 and **P<0.001, respectively). It is important to note that luteolin did not affect apoprotein levels of JAK2 or STAT3.
3.2. STAT3 inhibitor, S31-201, and diosmin reduce JAK2/STAT3 phosphorylation and pro-inflammatory cytokine production in the brain tissues of IL-6/MIA newborn mice
In this study, we sought to extend the in vitro results to an animal model of MIA-induced autism by examining the effects of STAT3 inhibitor (S31-201) and diosmin a (flavonoid structural analog of luteolin), on JAK2/STAT3 phosphorylation and signaling. When either agent was co-administered to pregnant mice intraperitoneally with IL-6, JAK2/STAT3 phosphorylation and pro-inflammatory cytokine levels were both significantly reduced in the brain homogenates of newborn mice. Western blot analysis of brain homogenates shows that both S31-201 and diosmin significantly reduce IL-6 induced JAK2 (Fig. 2A) and STAT3 (Fig. 2B) phosphorylation (*P<0.005).
Fig. 2.
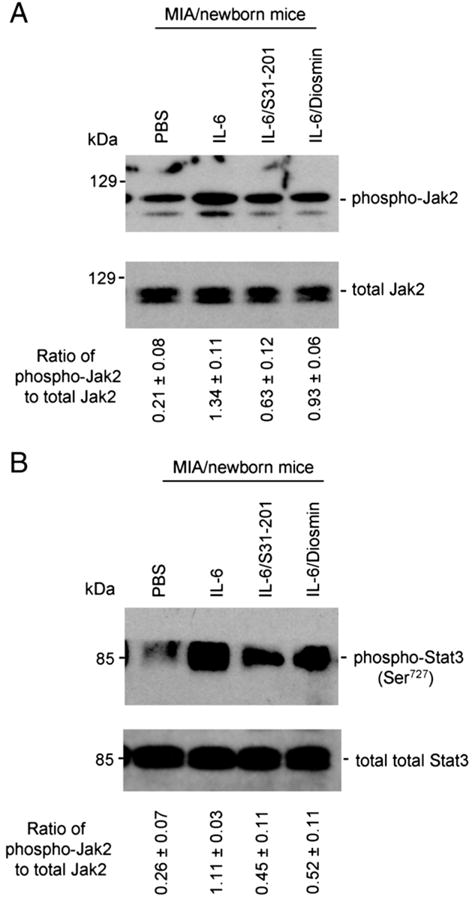
Both STAT3 inhibitor (S31-201) and diosmin (a glycoside of a structurally similar flavonoid to luteolin) reduce JAK2/STAT3 phosphorylation. Brain homogenates were prepared from newborn mice from mothers injected with IL-6, IL-6/S31-201, IL-6/diosmin or PBS (control) (n=6, 3 female/3 male) and subjected to Western blot analysis and cytokine ELISA. Most notably, the treatment of S31-201 or diosmin significantly inhibits IL-6-induced JAK2/STAT3 phosphorylation in brain tissues from newborn mice. Densitometry analysis shows the ratio of phospho-JAK2/STAT3 to total JAK2/STAT3 as indicated below the figures. One-way ANOVA showed that both significantly inhibit JAK2/STAT3 signaling (P<0.005).
Pro-inflammatory cytokine ELISA showed significant increases in TNF-α and IL-1β levels in the brain homogenates of new born mice from IL-6 treated dams compared to those of control dams (Fig. 3). These increases were significantly reduced by almost 50% in the presence of S31-201 or diosmin (**P<0.01) (Fig. 3).
Fig. 3.
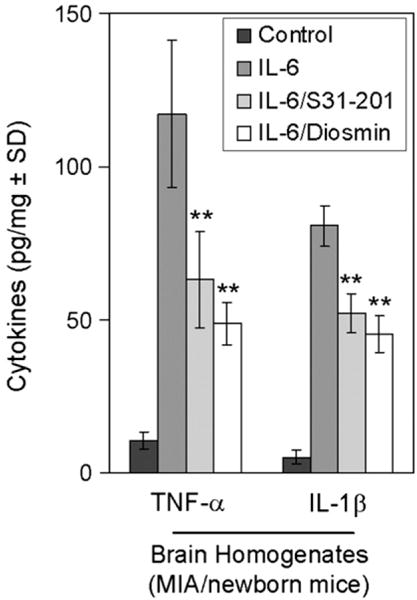
STAT3 inhibitor (S31-201) and diosmin reduce pro-inflammatory cytokines in the brain tissues of IL-6/MIA/newborn mice. Pro-inflammatory cytokine analysis by ELISA was conducted on these newborn mouse brain homogenates. Data are represented as mean±SD of each cytokine in brain homogenates (pg/mg total protein) from these newborn mice. Analysis of results revealed a significant reduction of TNF-α and IL-1β cytokines in brain homogenates from IL-6/S31-201 and IL-6/Diosmin/newborn mice when compared to IL-6 only (MIA model) newborn mice (**P<0.01).
3.3. Maternally blocking the STAT3 signal pathway with the STAT3 inhibitor, S31-201 or diosmin opposes IL-6-induced abnormal behavior in MIA/adult offspring
This experiment was aimed at determining whether diosmin would attenuate behavioral abnormalities observed in the adult offspring of IL-6 treated dams. Pregnant mice were treated one time with 5 μg/mL IL-6 in the presence or absence of S31-201 (4 μg/mL) or diosmin (10 mg/kg/day diosmin) administered orally in chow. We also treated control mice (non-IL-6 treated) with the STAT3 inhibitor, S31.
The adult offspring of these mice were examined for behavioral outcomes using the open field and social interaction tests to examine anxiety and social interaction, respectively. Our results demonstrate that S31-201 or diosmin co-treatment significantly attenuates the behavioral deficits seen in the adult offspring of IL-6 treated animals. In the open-field test, offspring of mice treated with either S31-201 or diosmin showed behaviors comparable to that of control mice, entering the center more often than IL-6 treated animals (**P<0.01) (Fig. 4A). In addition, an ANOVA on time spent in the inner section showed a significant main effect of group (P<0.05) and LSD post hoc tests showed that the IL-6/MIA mice spend less time in the inner section compared to S31-201 or diosmin treated mice (P<0.05). As these could be due to simply increased locomotion in one group, ANOVA on distance traveled in the inner section was performed and did not indicate a main effect (P=0.069). Analyses of distance traveled in the outer section did not reveal a significant difference or a statistical trend towards a significant difference (P>0.15) among groups. We did not find a significance between PBS-treated mice and S31-treated control (non-IL-6 injected) mice (P>0.05 with n=5).
Fig. 4.
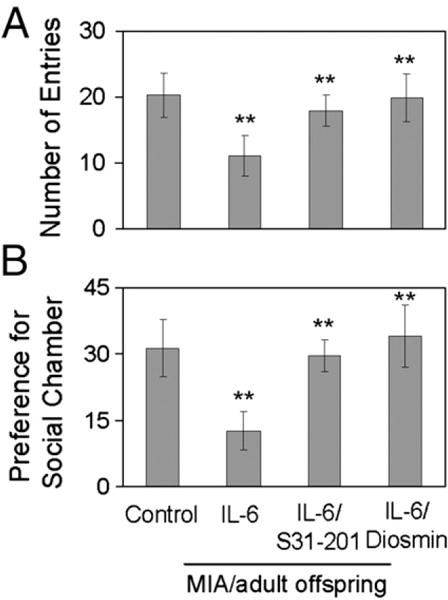
Maternally blocking STAT3 signal pathway with diosmin opposes IL-6-induced abnormal behavior in MIA/adult offspring. Offspring of mice (n=8, 4 female/4 male) intraperitoneally (i.p.) treated with IL-6 (5 μg/mouse) in the absence or presence of STAT3 inhibitor (S31-201; 4 μg/mouse; i.p.) (Siddiquee et al., 2007) or with diosmin [oral administration; (10 mg/kg/day)]. (A) In the open-field test, offspring of mice treated with either S31-201 or diosmin enter the center more often than IL-6 (**P<0.01) and are nearly similar to control mice. (B) In the social interaction test, as previously reported, the social chamber was defined as (percentage of time in social chamber)−(percentage of time in opposite chamber). Most notably, control mice reveal a strong preference for the social chamber. Interestingly, the social impairment of offspring is significantly improved by maternal administration of STAT3 inhibitor, S31-201 or diosmin (**P<0.005).
In the social interaction test, the adult offspring of control mice show a strong preference for the social chamber almost double that of the adult offspring of IL-6 treated mice. To quantify social interaction, exploration demonstrated as sniffing time was analyzed via ANOVA. There was a main effect of group (P<0.05); the S31-201 or diosmin mice exhibited an increase in sniffing compared to IL-6/MIA mice (P<0.05). We did not observe any social grooming, chasing, dominant mounts, pinning, boxing, or biting. There were no significant differences in total move time (P>0.07) and total distance traveled (P>0.05).
This social impairment observed in IL-6 adult offspring was significantly attenuated by maternal co-treatment with S31-201 or diosmin as these mice show a preference for the social chamber comparable to that of control adult offspring (**P<0.005) (Fig. 4B). Considering the above data, it can be seen that IL-6 induced JAK2/STAT3 phosphorylation plays an essential role in precipitating behavioral abnormalities seen in the adult offspring of IL-6 treated dams and regulation of this pathway by diosmin can attenuate these behavioral abnormalities.
3.4. Diosmin reduces pro-inflammatory cytokines and STAT3 phosphorylation in IL-/MIA adult offspring
After behavioral testing, adult offspring were scarified to confirm that inhibition of STAT3 phosphorylation by diosmin attenuates IL-6/JAK2/STAT3 induced behavioral abnormalities. At sacrifice, brain homogenates were prepared from offspring of control mice and mice treated with IL-6, IL-6/S31-201, and IL-6/Diosmin. Pro-inflammatory cytokine ELISA showed significant increase in TNF-α and IL-1β levels in the homogenates of IL-6 adult offspring. Maternal co-treatment with S31-201 or diosmin significantly reduces TNF-α and IL-1β cytokine levels significantly, with diosmin showing slightly more significant reductions as shown in Fig. 5 (**P<0.05).
Fig. 5.
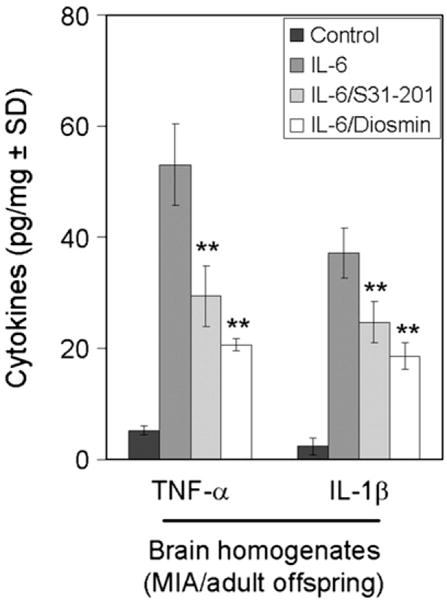
Diosmin reduces pro-inflammatory cytokines in IL-6/MIA adult offspring. At sacrifice, brain homogenates were prepared from offspring of mice treated with IL-6, IL-6/S31-201, IL-6/Diosmin or PBS (control) (n=8, 4 female/4 male). Pro-inflammatory cytokine analysis by ELISA was conducted on these mouse brain homogenates. Data are represented as mean±SD of each cytokine in brain homogenates (pg/mg total protein) from these mice. Analysis of results revealed a significant reduction of TNF-α and IL-1β cytokines in brain homogenates from IL-6/31-201 and IL-6/Diosmin adult offspring when compared to offspring of MIA only mothers (IL-6 treatment only) (**P<0.05).
Western blot analysis of phospho- and total STAT3 shows that IL-6 treatment of dams increases STAT3 phosphorylation in the brain homogenates of adult offspring while co-treatment with either S31-201 or diosmin leads to a significant reduction of STAT3 phosphorylation (**P<0.005) (Fig. 6).
Fig. 6.
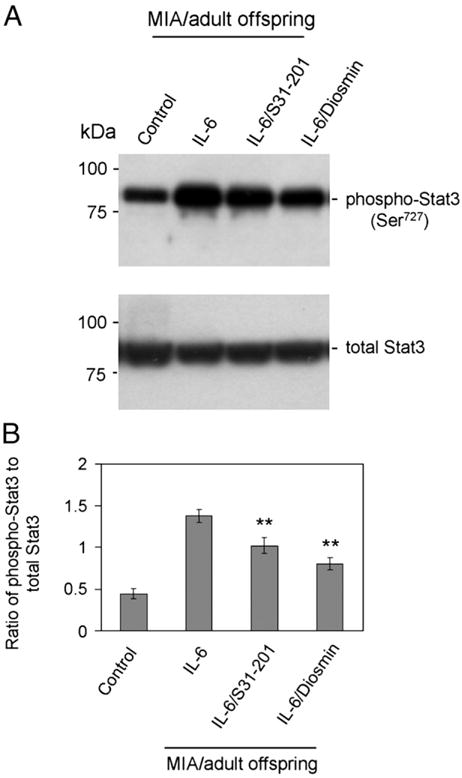
Diosmin reduces STAT3 phosphorylation in IL-6/MIA adult offspring. (A) Western blot analysis with antibodies specifically against phospho-STAT3 (Ser727) and total STAT3 shows a notable reduction of STAT3 phosphorylation in brain homogenates from IL-6/S31-201 and IL-6/Diosmin/adult offspring when compared to offspring of MIA only mothers (IL-6 treatment only). (B) Furthermore, densitometry analysis reveals the ratio of phospho-STAT3 to total STAT3 as indicated below the figure. In support, analysis of results showed a significant reduction of STAT3 phosphorylation from IL-6/S31-201 and IL-6/Diosmin/adult offspring when compared to offspring of MIA only mothers (IL-6 treatment only) (**P<0.005).
4. Discussion
Elucidating the mechanisms and pathways involved in neurodevelopmental disorders such as autism is important in not only understanding the etiology of these disorders but also to discover early diagnostic markers, and prophylactic therapies, in addition to therapeutic strategies to attenuate the associated symptoms. Previous research by Smith and colleagues has supported the role of exogenous IL-6 in precipitating the behavioral deficits and increases in pro-inflammatory cytokine release seen in MIA offspring, and potentially autistic individuals, in addition to demonstrating that its inhibition can attenuate these pathologies (Smith et al., 2007). In this study, we further examined the role of IL-6 in MIA by characterizing the role of JAK2/STAT3 phosphorylation in precipitating behavioral and pathological outcomes. We also sought to determine if inhibition of this phosphorylation had the ability to attenuate these behavioral deficits and/or pathologies observed in the adult offspring of IL-6/MIA mice. Previous research by our laboratory has shown that bioflavonoids regulate STAT1 phosphorylation, as we showed that luteolin (Rezai-Zadeh et al., 2008) or EGCG (Giunta et al., 2006) inhibited IFN-γ induced STAT1 phosphorylation. We therefore sought to examine if diosmin, a luteolin analog with better bioavailability, had similar effects, inhibiting IL-6 induced STAT3 phosphorylation.
We first aimed to confirm the role of IL-6 induced JAK2/STAT3 phosphorylation in precipitating pathological and behavioral deficits seen previously in IL-6/MIA animal models. The results of our study demonstrate that IL-6 induces JAK2/STAT3 phosphorylation resulting in the release of pro-inflammatory cytokines both in vitro and in vivo. Western blot analyses of cell lysates from murine N2a cells and primary cultured neuronal cells showed time-dependent increases in JAK2/STAT3 phosphorylation following IL-6 treatment. Analysis of brain homogenates of newborn mice from IL-6 treated dams similarly showed increases in JAK2/STAT3 phosphorylation, in addition to increases in released pro-inflammatory cytokines TNF-α and IL-1β.
We measured IL-6 levels in brain homogenates from the offspring using ELISA. The level of IL-6 was undetectable which is in contrast to the results previously reported by Samuelsson et al. (2006). As previously mentioned, this group found that IL-6 mRNA levels remain elevated in the hippocampi of the offspring at 4 and 24 weeks of age; indicative of the ongoing state of immune dysregulation in adult autistic brains. We thus suggest IL-6 itself may not be a contributory factor for the in vivo chronic inflammatory pathogenic affects, but rather for the in utero effects. Therefore measuring IL-6 in the offspring may not be representative of its exposure in utero. Rather, the indirect effects of IL-6 pro-inflammatory activation in utero of the JAK2/STAT3 pathway, as evidenced by increased TNF-α and IL-1β production, may be at play in an autism mouse phenotype. However, future studies will be required to clearly define the role of IL-6 in ongoing autism. Thus, with evidence that JAK2/STAT3 phosphorylation induced pathological outcomes in the offspring of IL-6 treated animals, we next sought to examine whether or not this phosphorylation contributed to the behavioral deficits observed in the offspring of IL-6 treated dams.
There is much epidemiologic evidence indicating that environmental contributions, including prenatal infections which can lead to MIA, may lead to the genesis of autism (Arndt et al., 2005; Libbey et al., 2005). Previous animal models, developed by Fatemi and Folsom (2009) and others, have shown that immune challenges during pregnancy lead to abnormal brain structure and function in the exposed offspring that replicate abnormalities observed in brains of subjects with autism (Fatemi et al., 2005, 2008; Meyer et al., 2006, 2007; Shi et al., 2005). Abnormal CNS changes in the offspring following infection at E9, which corresponds to infection at the middle of the first trimester (Fatemi et al., 2005; Shi et al., 2003), and E18, which corresponds to infection in late second trimester (Fatemi et al., 2008) have been previously shown. Importantly E16 immediately follows the period of neurogenesis of hippocampal pyramidal cells (E11–E15.5) (Rodier, 1980). Thus we hypothesized that middle second trimester infection (E12.5, E15 and E17) in mice would alter brain cytokine expression of the offspring since Fatemi and Folsom (2009) found that infection at E16 leads to altered expression of many brain genes in the hippocampi of the exposed mouse offspring.
Our results showed that the adult offspring of IL-6 treated animals displayed behavioral deficits in social interaction and regulation of anxiety that are reminiscent of autism. We also found neuropathology previously described (Turner, 1999) including increase pro-inflammatory cytokine release and increased STAT3 phosphorylation. In many previous works by other groups the pre-pulse inhibition (PPI) test is particularly informative for animal models of autism, because there are existing models for understanding the neural circuitry of startle gating; a process known to involve inhibitory cortico-striatal neural circuits (Braff et al., 2001). Furthermore, sensorimotor gating deficits have been reported in a family of neurodevelopmental disorders and are not specific to autism. Whereas Ornitz et al. (1993) reported equivocal PPI results in autistic children, others reported PPI deficits in adults with Asperger syndrome (McAlonan et al., 2002), children with Tourette syndrome (Swerdlow et al., 2001), and men with fragile X syndrome (Frankland et al., 2004). To date, only one published study on adults with autism (14 adult men diagnosed with autism and 16 typically developing normal comparison (NC) participants) has been conducted on the subject and it concluded that PPI deficits may only be indirectly linked to one of the hallmark features of autism. With this in mind we did not measure PPI in this study because if it were normal, it would have no scientific impact on the validity of the model, and it if were abnormal, it in itself would not be a measure of true neuropsychiatric impairment, rather the functional behavioral tests we performed would.
As a conformation of our results, we examined the effects of luteolin and diosmin in regulating IL-6 induced JAK2/STAT3 phosphorylation in vitro and if this could attenuate the pathologies and behavioral deficits previously described. When murine N2a cells and primary cultured neuronal cells were co-treated with IL-6 and luteolin, we observe a concentration dependent decrease in JAK2/STAT3 phosphorylation, as evidenced by Western blot analysis of cell lysates. We next examined the in vivo effects of diosmin, with the STAT3 inhibitor S31-201 as a positive control. When pregnant mice were co-treated with IL-6 and either diosmin, or S31-201, we observed an attenuation of the behavioral deficits in the adult offspring of diosmin and S31-201 co-treated animals as they showed social behaviors comparable to that of control mice. Furthermore, when brain homogenates of these adult offspring were examined, we saw decreased STAT3 phosphorylation with decreased pro-inflammatory cytokine secretion.
Taken together, our results show that IL-6 induced JAK2/STAT3 phosphorylation plays an integral role in the execution of IL-6/MIA mediated pathological effects. Indeed, inhibition of this phosphorylation was able to attenuate both behavioral deficits and pathological outcomes such as increased inflammation. In the future, we will further examine whether the JAK2/STAT3 signal pathway activation is specifically involved in brain histological abnormalities in IL-6-induced MIA (presumably resulting in development of abnormal behavior in adult offspring that mimics features of autism). In addition, we intend to fully characterize the mechanisms by which diosmin regulates JAK2/STAT3 phosphorylation in addition to qualifying its potential effect on improving abnormal, autistic like social behaviors in IL-6/MIA offspring in adulthood via its attenuation of the JAK2/STAT3 signal pathway. These studies could lay the foundation for autism clinical trials with diosmin diet supplementation in the future.
This may be not only effective, but safe. Indeed diosmin is a natural flavonoid isolated from various plant sources or derived from the flavonoid hesperidin. First used as a therapy in 1969, diosmin is currently considered a vascular-protecting agent and has been used for treatment of chronic venous insufficiency/varicose veins (Jantet, 2002), hemorrhoids (Jantet, 2000), lymphedema (Pecking et al., 1997), and diabetes (Lacombe et al., 1989). The compound also exhibits anti-inflammatory, antioxidant, and antimutagenic properties (Kuntz et al., 1999). Furthermore, marketed formulations 90% diosmin, 10% hesperiden pose little to no side effects. Taken together, there is convincing evidence from preliminary studies regarding efficacy, as well as published studies regarding safety in humans, that diosmin is a safe and potentially efficacious treatment for autism. Furthermore, the dose found to be effective in this animal model (10 mg/kg/day) translates into a human dose of approximately 48.6 mg/day for a 60 kg person. While not reasonably achievable through consumption of foods containing diosmin, this concentration may be provided through a daily oral supplement. Therefore the data from this study will be used to help determine the shape of the dose response, to begin to achieve the optimal dosing schedule for future human clinical trials.
Acknowledgments
This work was supported by the Silver Foundation. J.T. holds the Sliver Chair in Developmental Neurobiology. We thank Drs. Yuyan Zhu, Huayan Hou and Kavon Rezai-Zadeh for helpful advice.
Abbreviations
- MIA
maternal immune activation
- JAK2
Janus tyrosine kinase-2
- STAT3
signal transducer and activator of transcription-3
- IL-6
Interleukin-6
References
- Agarwal C, Tyagi A, Kaur M, Agarwal R. Silibinin inhibits constitutive activation of Stat3, and causes caspase activation and apoptotic death of human prostate carcinoma DU145 cells. Carcinogenesis. 2007;28:1463–1470. doi: 10.1093/carcin/bgm042. [DOI] [PubMed] [Google Scholar]
- Arndt TL, Stodgell CJ, Rodier PM. The teratology of autism. Int J Dev Neurosci. 2005;23:189–199. doi: 10.1016/j.ijdevneu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Pearce DA, Brooks AI, Sidwell RW. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: a potential animal model for schizophrenia and autism. Synapse. 2005;57:91–99. doi: 10.1002/syn.20162. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, Smee DF, Pearce DA, Winter C, Sohr R, Juckel G. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr Res. 2008;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, Ornitz EM, Silva AJ. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9:417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- Giunta B, Obregon D, Hou H, Zeng J, Sun N, Nikolic V, Ehrhart J, Shytle D, Fernandez F, Tan J. EGCG mitigates neurotoxicity mediated by HIV-1 proteins gp120 and Tat in the presence of IFN-gamma: role of JAK/STAT1 signaling and implications for HIV-associated dementia. Brain Res. 2006;1123:216–225. doi: 10.1016/j.brainres.2006.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Ge W, Martinowich K, Becker-Catania S, Coskun V, Zhu W, Wu H, Castro D, Guillemot F, Fan G, de Vellis J, Sun YE. A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat Neurosci. 2005;8:616–625. doi: 10.1038/nn1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagtap S, Meganathan K, Wagh V, Winkler J, Hescheler J, Sachinidis A. Chemoprotective mechanism of the natural compounds, epigallocatechin-3-o-gallate, quercetin and curcumin against cancer and cardiovascular diseases. Curr Med Chem. 2009;16:1451–1462. doi: 10.2174/092986709787909578. [DOI] [PubMed] [Google Scholar]
- Jantet G. RELIEF study: first consolidated European data. Reflux assEssment and quaLity of lIfe improvement with micronized Flavonoids. Angiology. 2000;51:31–37. doi: 10.1177/000331970005100107. [DOI] [PubMed] [Google Scholar]
- Jantet G. Chronic venous insufficiency: worldwide results of the RELIEF study. Reflux assEssment and quaLity of lIfe improvEment with micronized Flavonoids. Angiology. 2002;53:245–256. doi: 10.1177/000331970205300301. [DOI] [PubMed] [Google Scholar]
- Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr. 1999;38:133–142. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- Lacombe C, Lelievre JC, Bucherer C, Grimaldi A. Activity of Daflon 500 mg on the hemorheological disorders in diabetes. Int Angiol. 1989;8:45–48. [PubMed] [Google Scholar]
- Libbey JE, Sweeten TL, McMahon WM, Fujinami RS. Autistic disorder and viral infections. J Neurovirology. 2005;11:1–10. doi: 10.1080/13550280590900553. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, Simmons A, Sigmundsson T, Greenwood K, Russell A, Schmitz N, Happe F, Howlin P, Murphy DG. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;125:1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13:241–256. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- Murphy PG, Borthwick LA, Altares M, Gauldie J, Kaplan D, Richardson PM. Reciprocal actions of interleukin-6 and brain-derived neurotrophic factor on rat and mouse primary sensory neurons. Eur J Neurosci. 2000;12:1891–1899. doi: 10.1046/j.1460-9568.2000.00074.x. [DOI] [PubMed] [Google Scholar]
- Muthian G, Bright JJ. Quercetin, a flavonoid phytoestrogen, ameliorates experimental allergic encephalomyelitis by blocking IL-12 signaling through JAK-STAT pathway in T lymphocyte. J Clin Immunol. 2004;24:542–552. doi: 10.1023/B:JOCI.0000040925.55682.a5. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Lane SJ, Sugiyama T, de Traversay J. Startle modulation studies in autism. J Autism Dev Disord. 1993;23:619–637. doi: 10.1007/BF01046105. [DOI] [PubMed] [Google Scholar]
- Park G, Yoon BS, Moon JH, Kim B, Jun EK, Oh S, Kim H, Song HJ, Noh JY, Oh C, You S. Green tea polyphenol epigallocatechin-3-gallate suppresses collagen production and proliferation in keloid fibroblasts via inhibition of the STAT3-signaling pathway. J Invest Dermatol. 2008;128:2429–2441. doi: 10.1038/jid.2008.103. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2008;204(2):313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Pecking AP, Fevrier B, Wargon C, Pillion G. Efficacy of Daflon 500 mg in the treatment of lymphedema (secondary to conventional therapy of breast cancer) Angiology. 1997;48:93–98. doi: 10.1177/000331979704800115. [DOI] [PubMed] [Google Scholar]
- Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A, Winter D, Frahm J, Fischer J, Brose N, Ehrenreich H. Neuroligin-3 deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8(4):416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Ehrhart J, Bai Y, Sanberg PR, Bickford P, Tan J, Shytle RD. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J Neuroinflammation. 2008;5:41. doi: 10.1186/1742-2094-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM. Chronology of neuron development: animal studies and their clinical implications. Dev Med Child Neurol. 1980;22:525–545. doi: 10.1111/j.1469-8749.1980.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol, Regul Integr Comp Physiol. 2006;290:R1345–1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int J Dev Neurosci. 2005;23:299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, Sebti SM, Turkson J. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci U S A. 2007;104:7391–7396. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RM, Lohner WS, Daynes RA, Mitchell MD, Branch DW. Lipopolysaccharide-induced fetal death: the role of tumor-necrosis factor alpha. Biol Reprod. 1994;50:1108–1112. doi: 10.1095/biolreprod50.5.1108. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Karban B, Ploum Y, Sharp R, Geyer MA, Eastvold A. Tactile prepuff inhibition of startle in children with Tourette’s syndrome: in search of an “fMRI-friendly” startle paradigm. Biol Psychiatry. 2001;50:578–585. doi: 10.1016/s0006-3223(01)01164-7. [DOI] [PubMed] [Google Scholar]
- Tan J, Town T, Mori T, Obregon D, Wu Y, DelleDonne A, Rojiani A, Crawford F, Flavell RA, Mullan M. CD40 is expressed and functional on neuronal cells. EMBO J. 2002;21:643–652. doi: 10.1093/emboj/21.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. Annotation: repetitive behaviour in autism: a review of psychological research. J Child Psychol Psychiatry. 1999;40:839–849. [PubMed] [Google Scholar]
- Wang X, Hagberg H, Mallard C, Zhu C, Hedtjarn M, Tiger CF, Eriksson K, Rosen A, Jacobsson B. Disruption of interleukin-18, but not interleukin-1, increases vulnerability to preterm delivery and fetal mortality after intrauterine inflammation. Am J Pathol. 2006;169:967–976. doi: 10.2353/ajpath.2006.050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DX, Chen YH, Wang H, Zhao L, Wang JP, Wei W. Tumor necrosis factor alpha partially contributes to lipopolysaccharide-induced intra-uterine fetal growth restriction and skeletal development retardation in mice. Toxicol Lett. 2006;163:20–29. doi: 10.1016/j.toxlet.2005.09.009. [DOI] [PubMed] [Google Scholar]


