Abstract
Extracellular plaques of β-amyloid (Aβ) peptides are implicated in Alzheimer's Disease (AD) pathogenesis. Aβ formation is precluded by α-secretase, which cleaves within the Aβ domain of APP generating soluble APP-α(sAPP-α). Thus, α-secretase upregulation may be a target AD therapy. We previously showed green tea derived EGCG increased sAPP-α in AD mouse models. However, the comparable effective dose of EGCG in humans may exceed clinical convenience and/or safety. Epidemiological studies suggested fish oil consumption is associated with reduced dementia risk. Here we investigated whether oral co-treatment with fish oil (8 mg/kg/day) and EGCG (62.5 mg/kg/day, or 12.5 mg/kg/day) would reduce AD-like pathology in Tg2657 mice. In vitro co-treatment of N2a cells with fish oil and EGCG enhanced sAPP-α production compared to either compound alone (P<.001). Fish oil enhanced bioavailability of EGCG versus EGCG treatment alone (P<.001). Fish oil and EGCG had a synergetic effect on inhibition of cerebral Aβ deposits (P<.001) suggesting moderate supplementation with EGCG and fish oil have significant therapeutic potential for treatment of AD.
Keywords: Omega 3, fish oil, EGCG, Alzheimer's Disease, APP, A-beta
Introduction
Amyloid precursor protein (APP) “amyloidogenic” proteolysis by β and γ-secretases yields β-amyloid (Aβ) peptides implicated in Alzheimer's Disease (AD) [5, 14, 16]. In the “nonamyloidogenic” pathway, APP is cleaved at the α-secretase site, yielding soluble APP-α (sAPP-α); and precluding Aβ generation [2]. We previously found green tea derived (−)-epigallocatechin-3-gallate (EGCG) upregulates non-amyloidogenic processing of APP in mouse models of AD [13,15].
We recently found Tg2576 mice intraperitoneally (i.p) treated with EGCG show decreased memory deficits and Aβ plaques, and elevated sAPP-α, at an oral dose of 50 mg/kg/day [13,15]. Such a dose in humans would require oral delivery at least six times this concentration [14]. Epidemiologic studies suggest consumption of fish oils (ω-3 polyunsaturated fatty acids) confers reduced AD risk by ~40% [3, 4, 8, 10]. Fish oil is known to induce hippocampal transthyretin (TTR) expression in the aged-rat hippocampus [17]. Further, coexpression of Aβ-peptide and TTR in C. elegans lead to significant reductions of amyloid plaques [18].
We hypothesized, based on these previous studies; fish oil may synergize the effectiveness of EGCG in terms of amyloid reduction in Tg2576 mice and found it enhances α-secretase activity promoted by EGCG in APP-overexpressing N2a cells. We further demonstrate bioavailability of EGCG is enhanced by fish oil, as is its ability for promotion of nonamyloidogenic APP processing in Tg2576 mice.
Experimental procedures
Reagents
EGCG (> 95% purity), was purchased from Sigma (St Louis, MO). For in vivo works, fish oil was obtained from Trilogy International, Inc. (Palm City, Florida) and contains EPA (eicosapentaenonic acid), 1.8 g; DHA (docosahexaenoic acid), 0.9 g per 5 mL of the fish oil. It is also supplemented with Vitamin E (47 IU/5mL). For in vitro works, menhaden oil (salt form) was used (Sigma). BCA protein assay kit was purchased from Pierce Biotechnology (Rockford, IL). Anti-human amyloid-β antibody 4G8 was obtained from Signet (Dedham, MA). VectaStain Elite™ ABC kit was purchased from Vector Laboratories (Burlingame, CA). Aβ1–40, 42 ELISA kits were obtained from IBL-American (Minneapolis, MN).
Mice
Tg2576 mice (n = 12/group, female) were purchased from Taconic (Germantown, New York) [7] and fed 2018 Teklad Global 18% Protein Rodent Diet (http://www.harlan.com/research_models_and_services/laboratory_animal_diets/teklad_global_diets/global_rodent_diets/teklad_global_18_protein_rodent_diet_2018.hl). It is not enriched with DHA or EPA, but rather contains 6% crude oil (fat). Mice were in the following groups: (1) control (regular Teklad diet only), (2) fish oil only, (3) high dose EGCG, (4) low dose EGCG, and (5) low dose EGCG with fish oil.
To enrich food with EGCG, 20 mg (low dose) or 100mg (high dose) of >97% pure EGCG was mixed with 400 grams chow. The diet was prepared by first moistening the chow with DI water. For EGCG only diets, EGCG was dissolved in 1-2 ml of water, and mixed (via automatic mixer × 10 min) into the moistened chow to homogeneity. This translates into an EGCG dose of 62.5 mg/kg/day or a daily consumption of 1.875mg EGCG/day for the high dose. For the lower EGCG dose, this translates to 12.5 mg/kg/day or consumption of 0.375mg EGCG/day/mouse. From these homogenous chow mixes, we formed standard block size portions (~1”×1”×1”), and let them dry overnight in the dark .
Mice were also treated with fish oil only at 8 mg/kg/day which translates into approximately 240mg fish oil consumed/day/mouse. To generate this mixture, 1.6 mL fish oil was homogenized into 50g moistened chow as above.
For the EGCG plus fish oil diets , 12.8 mg of fish oil was added to 400 grams chow already moistened and premixed with either 20mg (low dose) or 100mg (high dose) of EGCG. This translated to a dose of 0.25 mg fish oil plus 1.875mg EGCG or 0.25mg fish oil plus 0.375 mg EGCG /mouse/day, respectively.
All animals were treated at 8 months of age for 6 months. Approximation of total EGCG or fish oil intake is based on average mouse food intake of 25% of body weight/day. Mouse weight range varied between 28 and 32g. Tg2576 mice (n=6 female) were also treated at the same age with water only. Age-matched non-transgenic mice (n = 6) also received water alone (data not shown). Transgene expression was confirmed by genotyping as previously described [13,15]. Mice were sacrificed at 14 months of age for analyses of Aβ levels and Aβ load in the brain according to previous methods [13,15]. Experiments were in compliance with protocols approved by the USF Animal Care /Use Committee.
Immunohistochemistry
Mice were anesthetized with isofluorane and transcardinally perfused with ice-cold physiological saline containing heparin (10 U/mL). Brains were isolated and quartered using a mouse brain slicer (Muromachi Kikai Co., Japan). The 1st and 2nd anterior quarters were homogenized for ELISA and Western blot analysis (as above), and the 3rd and 4th posterior quarters used for microtome or cryostat sectioning. Brains were fixed in 4% paraformaldehyde in PBS at 4 °C overnight and processed in paraffin in a core facility at the Department of Pathology (USF). Five, 5-μm thick coronal sections/brain were cut (150-μm intervals). Sections were deparaffinized and hydrated in a graded series of ethanols then pre-blocked for 30 min at ambient temperature with serum-free protein (Dakocytomation, Denmark). Aβ immunohistochemical staining was performed using anti-human amyloid-β antibody (clone 4G8, 1:100) with the VectaStain Elite™ ABC kit coupled with diaminobenzidine substrate. 4G8-positive Aβ deposits were examined under bright-field via Olympus BX-51 microscope. Quantitative image analysis (conventional “Aβ burden” analysis) was performed for 4G8 immunohistochemistry.
Image analysis
Quantitative image analysis for “Aβ burden” was performed for 4G8 immunohistochemistry for brains from all mice. Images were obtained using an Olympus BX-51 microscope and digitized via MagnaFire™ imaging system (Olympus, Tokyo). Briefly, images of five 5-μm sections (150 μm apart) through each region of interest were captured and threshold optical density was obtained, discriminating staining form background. Manual editing of fields was used to eliminate artifacts. Data are reported as percentage of immunolabeled area captured (positive pixels)/the full area captured (total pixels). Quantitative image analysis was performed by a single examiner (TM) blinded to sample identities.
Western blot
Brain homogenates were obtained as described above. 100 μg aliquots of total protein were electrophoretically separated using 10% Tris gels, transferred to PVDF membranes (Bio-Rad, Richmond, CA, USA), washed in dH2O, and blocked for 1 h at ambient temperature in Tris-buffered saline (TBS) containing 5% (w/v) non-fat dry milk. Next, membranes were hybridized for 1 h at ambient temperature with various primary antibodies. Membranes were then washed 3× for 5 min each in dH2O and incubated for 1 h at ambient temperature with the appropriate HRP-conjugated secondary antibody (1:1000, Pierce Biotechnology). All antibodies were diluted in TBS containing 5% (w/v) of non-fat dry milk. Blots were developed with luminol (Pierce Biotechnology). Densitometric analysis was done as previously described using a FluorS Multiimager with Quantity One™ software (Bio-Rad, Hercules, CA, USA). Antibodies used for Western blot included 22CL.
Plasma Collection and Brain Homogenate Preparation
At sacrifice, 1- 2 mL of blood was collected by cardiac puncture and centrifuged at 2,000 × g for 10 min to separate out plasma which was then mixed with 1/10th the volume of a preservative solution (20% ascorbic acid and 0.05% Na2EDTA in a 0.4 M sodium phosphate buffer, with pH of 7.2), in sterile heparinized tubes and frozen at −80°C. Brain homogenates were prepared as previously described [15]. To enhance sensitivity of HPLC, we used a vacuum centrifuge concentrator to maximize the amount of total EGCG present per volume of sample.
HPLC identification and analysis of EGCG
Concentrated plasma samples were thawed and centrifuged at 15,000 rpm ×15 min. Macromolecules were removed using 3 K Nanosep (Pall Life Sciences, Ann Arbor, MI) centrifugal filters at 14,000 × g. Filtered plasma samples were mixed with 10 μL of a mixture of β-glucuronidase and sulfatase, and incubated at 37°C ×45 min. HPLC was performed via BioLogic HPLC system (Bio-Rad, Hercules, CA,) equipped with a Duo Flow pump, BioFrac fraction collector, and a Quadtec UV/Vis detector (280 nm).
Samples were injected onto the reverse-phase column (Agilent, SB-C8 80A 5μ, length 150 mm, i.d. 4.6 mm) via injection valve with a 50 μL loop. Mobile phase was isocratic flow of water:acetonitrile:trifluoroacetic acid (87/13/0.1) at a flow rate of 1mL/min at 25°C for 40 min. Standards of >95% pure EGCG (Sigma) in water were run before and after the experimental samples for standard curve creation. Remote control of the HPLC system, data acquisition and peak calculation areas were performed via computer-based data system (Bio-Rad).
Cell Culture
N2a cells transfected with mutant “Swedish” APP gene were grown/maintained in 45% DMEM, 45% Opti-MEM, 10% FBS, containing 200 μg/ml G418 and 1% Penn/Strep at 37°C and seeded at 108 cells/well. Viability was determined with Trypan Blue dye exclusion. Cells were treated with concentrations of EGCG (5/20μM) with or without fish oil (10 μg/mL in salt form) overnight. Conditioned media were harvested, treated with protease inhibitor, centrifuged at 10,000g at 4°C for 10 min, and then supernatant protein-concentration determined (BCA, Pierce) [15]. 100μl aliquots containing equal amounts of protein were subjected to sAPP-α ELISA.
Aβ and sAPP-α ELISA
Mouse brains were isolated under sterile conditions on ice and placed in ice-cold lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% v/v Triton X-100, 2.5 mM sodium pyropgosphate, 1 mM β-glycerolphosphate, 1 mM Na3VO4, 1 μg/mL leupeptin, 1 mM PMSF). sAPP-α was detected in cultured cell media or brain homogenates prepared with lysis buffer by a 1:4 or 1:10 dilution, respectively. Brains were sonicated on ice for 3 min, allowed to stand for 15 min at 4 °C, and centrifuged at 15,000 rpm for 15 min. Aβ1–40, 42 species were detected by acid extraction of brain homogenates in 5 M guanidine buffer [13,15], followed by a 1:10 dilution in lysis buffer. Soluble Aβ1–40, 42 were detected in brain homogenates prepared with lysis buffer by a 1:10 dilution. Protein levels of homogenate samples were normalized by BCA protein assay prior to dilution. Aβ1–40, 42 was quantified in these samples using the Aβ1–40, 42 ELISA kits according to manufacturer's instructions, except standards included 0.5 M guanidine buffer in some cases.
The sAPP-α ELISA method was adapted from a previous protocol used [2, 12]. High binding 96-well plates were coated with monoclonal antibody 22C11 in 100 μL (1 μg/mL) of carbonate buffer (pH 9.6) and incubated overnight at 4°C. Plates were washed 5× with PBS- 0.05% Tween 20 and blocked with 300 μL of 1% BSA, 5% Horse Serum in PBS for 2 hrs at 37°C. Synthetic sAPP-α protein (Abgent, San Diego, CA) was used for positive control. Samples were analyzed in duplicate. Cell cultured media were diluted 1:1 and 1:2 respectively in reagent diluent (1% BSA in PBS) and added to each well. The plate was incubated for 2 hr at 37°C. After washing 5×, 100 μL of goat anti-human antibody 6E10 (Biosource; diluted 1:3,000 in reagent diluent) was added to each well. Following 2 hr incubation at 37°C and 5× washing, 100 μL of HRP conjugated anti-goat IgG (1:1500) was added to each well. The plate was incubated for 1 hr at 37°C. Following 5× washing, 100 μL of substrate solution (TMB) was added to each well and plate was incubated at room temperature. Twenty minutes later, 50 μL stop solution (2 N H2SO4) was added to each well. Optical density was determined immediately by microplate reader at 450 nm. Data reported as ng sAPP-α/mg total intracellular protein or total plasma protein.
Statistical analysis
All data were normally distributed. For single mean comparisons, Levene's equality of variances followed by t test for independent samples was used to assess significance. For multiple mean comparisons, ANOVA, followed by post-hoc Bonferonni's comparison were used. Alpha levels were 0.05 for all analyses. Statistical package for the social sciences 10.0.5 was used for data analysis.
Results
Fish oil synergizes the effects of EGCG on reducing cerebral Aβ/β-amyloid deposits in Tg2576 mice
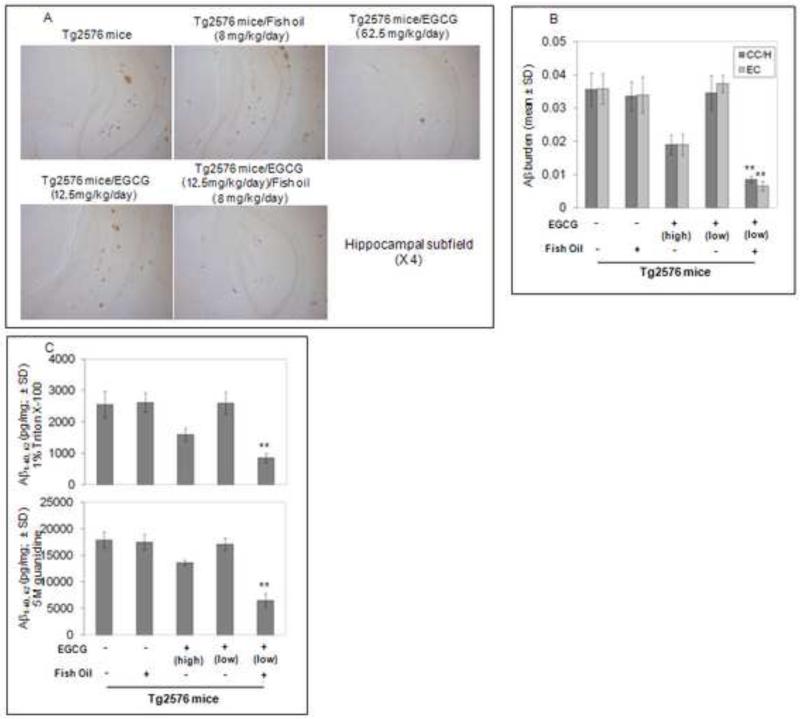
Tg2576 mice (n =12 female/group) were orally treated with EGCG at a low dose (12.5 mg/kg/day, in chow) with fish oil (8mg/kg/day, in chow) at 8 months of age for 6 months. This oral low dose EGCG alone does not result in reduction of Aβ deposits (Fig. 1A). However, we observed marked reductions of Aβ deposition in mice co-treated with fish oil (8mg/kg/day) and EGCG (12.5mg/kg/day). Micrographs from Aβ antibody stained sections reveal plaque burdens significantly reduced in brain regions examined from these mice (cingulate cortex [CC], hippocampus [H], and entorhinal cortex [EC]) (Fig 1B). These data suggest co-treatment of Tg2576 mice with fish oil and EGCG synergistically inhibits β-amyloid deposition. Furthermore we performed Aβ ELISA on anterior quarter brain homogenates. Again, fish oil and low dose EGCG markedly decreased soluble and insoluble forms of Aβ1-40, 42 (Fig. 1C).
Fig. 1. Reduction of cerebral Aβ/β-amyloid pathology in PSAPP mice that received chow containing EGCG or EGCG + Fish Oil for eight weeks.
(A) Coronal sections from frozen mouse brain were stained with rabbit polyclonal anti-human Aβ antibody (4G8). (B) Percentages of Aβ antibody-immunoreactive Aβ plaque (mean ± SD) were calculated by quantitative image analysis. (C) Aβ1-40, 42 brain levels were measured by ELISA. T- test for independent samples revealed significant differences (**P < 0.001) between groups for each brain region examined in EGCG alone (low dose) and EGCG at the same dose in conjunction with fish oil.
Fish oil enhances oral bioavailability of EGCG in Tg2576 mice
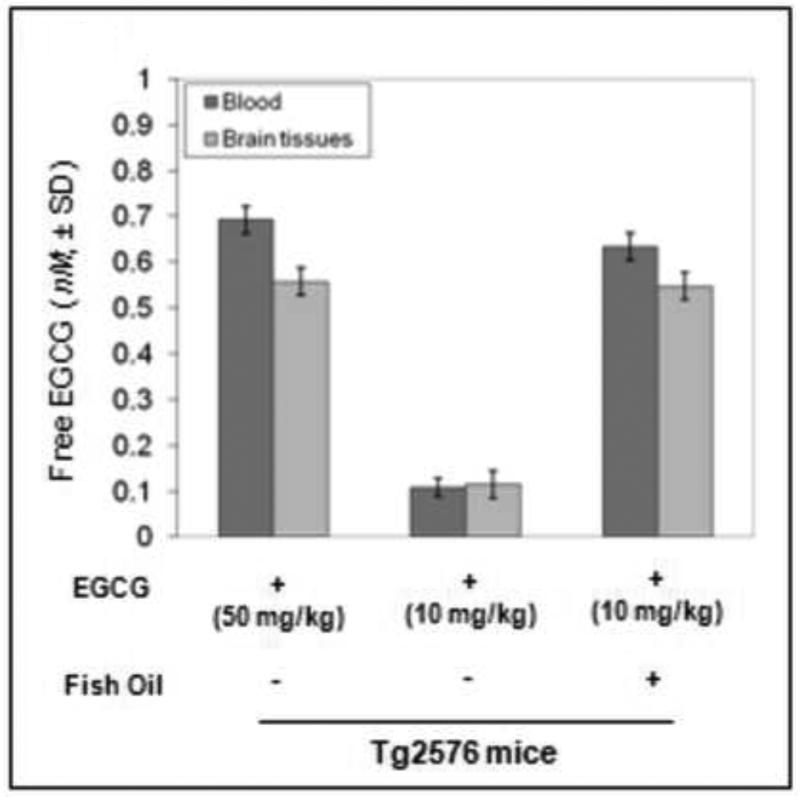
At sacrifice, blood and brains were collected from Tg2576 treated with EGCG alone (high and low doses) and low dose EGCG with fish oil for measurement of plasma free EGCG. We found significantly elevated plasma free EGCG in the mice co-treated with fish oil + EGCG versus EGCG treatment alone at the same dose (Fig. 2). Non-significance between blood and brain tissues was observed for this elevated plasma level of free EGCG (P > 0.05), suggesting plasma free EGCG could freely penetrate the blood-brain-barrier (BBB).
Fig. 2. Fish oil may enhance oral bioavailability of green tea-EGCG in Tg2576 mice.
Analysis of free EGCG in mice treated with EGCG alone (including the high and low doses), and also from Tg2576 mice treated with EGCG (low dose) and fish oil. Percent differences of blood and brain EGCG were quantified via HPLC. T- test for independent samples revealed a significant difference in plasma levels of free EGCG in the mice co-treated with fish oil compared to mice treated with EGCG alone (**P < 0.001). Most importantly, nonsignificance between blood and brain tissues was observed for this elevated plasma level of free EGCG in these mice (P > 0.05); suggesting plasma free EGCG could freely penetrate the blood-brain-barrier (BBB).
Fish oil enhances EGCG's promotion of α-secretase APP proteolysis
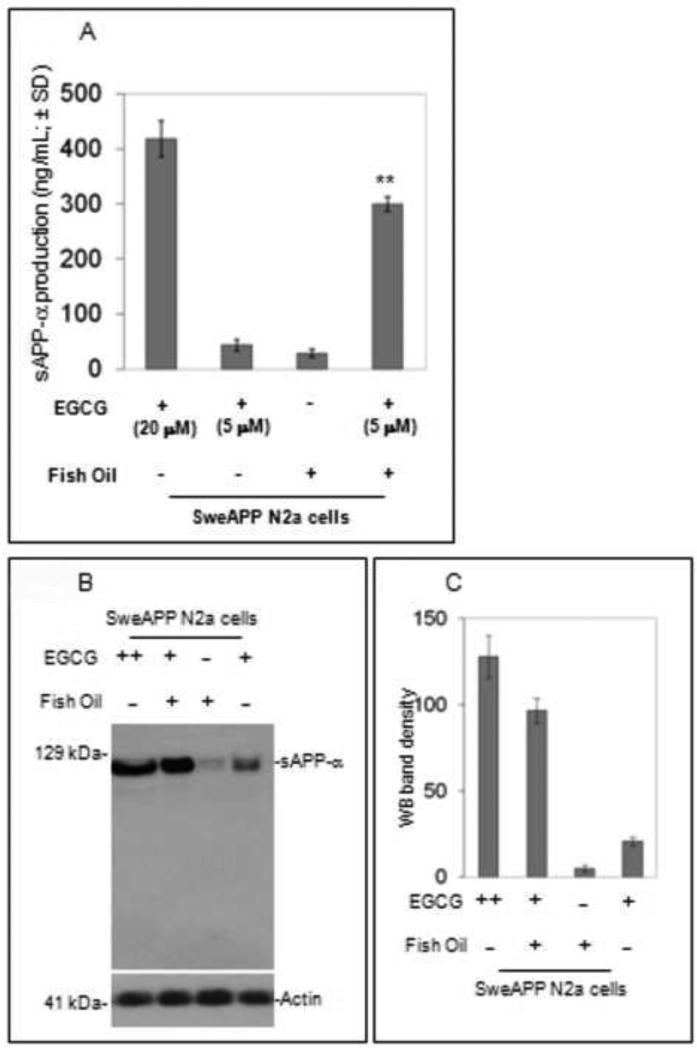
We co-treated SweAPP N2a cells with fish oil and EGCG overnight. Following, sAPP-α ELISA in cultured media was performed and indicated fish oil markedly enhances sAPP-α production induced by EGCG (5μM) versus EGCG or fish oil alone (Fig. 3A). Additionally Western blot (WB; Fig. 3B) on these samples showed increased sAPP-α in cultured media from co-treated condition versus EGCG at 5 μM or fish oil alone. Densitometry analysis shows significantly increased western blot band density in Fish oil + EGCG group (Fig3C; P < 0.001). Data presented as mean ± SD of WB band density. (representative of 3 independent experiments; Note: EGCG ++, 20 μM; EGCG +, 5 μM).
Fig. 3. Fish oil may enhance green tea-EGCG promoted non-amyloidogenic APP processing.
(A). sAPP-α levels were measured in cell-cultured media by ELISA. Data are presented as mean ± SEM of sAPP-α (ng/mg total plasma protein). (B) Western blot (WB) analysis analysis consistently shows increased sAPP-α levels in cultured media from co-treated condition versus EGCG at 5 μM or fish oil alone. (C)As quantified in comparison to total protein (normalization), densitometry analysis shows significantly increased WB band density as indicated in Figure 3C (P < 0.001). Data presented as mean ± SD of WB band density. Results are representative of three independent experiments. Note: EGCG ++, 20 μM; EGCG +, 5 μM; fish oil dose, 10 γg/mL in a salt form (Sigma).
Discussion
We previously found i.p. EGCG administration (20 mg/kg/day) significantly reduced Aβ deposits [15] . This translates into a human oral dose over six times this concentration in part due to first pass metabolism [18]. Here we found significantly elevated plasma and brain levels of free EGCG in mice cotreated with fish oil, suggesting a mechanism of increased bioavailability conferred by addition of fish oil to EGCG. Indeed we were able to lower the oral EGCG dose to 12.5mg/kg/day in chow in the presence of fish oil (8mg/kg/day in chow); markedly reducing both soluble and insoluble Aβ deposition (p<0.5).
Importantly, fish oil enhanced production of s-APPα, indicating increased non-amyloidenic processing. We hypothesize this is due to the increased bioavailibity of EGCG, as no previous reports indicated α-secretase enhancing ability for fish oil. Green and colleagues found dietary DHA supplementation in the 3×Tg-AD mouse model of AD reduced accumulation of both Aβ and tau due to a decrease in levels of presenilin 1, not altered processing of APP by α- secretase [6].
Our results are in accord with others showing activation of α-secretase reduces Aα-associated pathology in animal models of AD [13, 15]. Recent reports demonstrated sAPP-α is reduced in the CSF of AD patients [1]; indirectly suggesting α-secretase processing is impaired in AD. Since α-secretase cleaves within the Aβ peptide domain, its activation may even have the added advantage of, not only generating the putatively neuroprotective sAPP-α [2,11], but also precluding neurotoxic Aβ peptide formation. Thus, supplementation with minimal amounts of natural compounds promoting nonamyloidogenic α-secretase APP processing have significant potential for AD treatment.
Epidemiologic studies suggest consumption of fish oils reduces AD risk by some 40% [3, 4, 8, 10] and that fish oil is known to induce hippocampal transthyretin (TTR) in the aged-rat hippocampus [17]. Also increased TTR expression by fish oil was observed in APPSW transgenic mice which uncharacteristically lacked neurodegeneration [19]. Additionally, an inverse correlation between CSF TTR levels and severity of AD was established [20].
Here, we attribute the increased effectiveness of this combined fish oil/EGCG treatment to increased bioavailability of brain and plasma EGCG found in the mice that were co-treated with fish oil (Fig. 2). This is further substantiated by our in vitro treatment of SweAPP N2A cells with combinations of EGCG alone at various doses or with fish oil in terms of increased α-secretase activity (Fig. 3). Essentially, the low dose EGCG (12.5 mg/kg/day) translates into a daily consumption of approximately 0.375mg EGCG/day for these mice. This is compared to the higher EGCG dose of 62.5mg/kg/day of approximately 1.875mg EGCG/day/ mouse. This reduction in EGCG dosage was possible by the addition of 8 mg/kg/day fish oil in the chow which translates into approximately 0.25mg fish oil consumed/day/mouse.
Although increased TTR activity may be involved in our results, since the control group treated with fish oil only (Fig. 1b) did not manifest significant amyloid reduction, we deduce the effect of EGCG is enhanced by increased bioavailability conferred by fish oil (Fig.2); leading to improved efficacy of EGCG (as EGCG alone is efficacious, but at a dose > five times higher than in the presence of fish oil [Fig. 1B]). Nevertheless, it will be important to determine the relative importance of a possible fish oil- induced transthyretin (TTR) expression in future studies.
Taken together, supplementation with minimal amounts of natural compounds promoting nonamyloidogenic α-secretase APP processing have significant potential for AD treatment and safe combinations such as low doses of EGCG with fish oil present a potential anti-AD therapeutic option.
Acknowledgments
This work was supported by two NIH/NIMH grants: Clinical Scientist Career Development Award (1K08MH082642-01A1) (BG) and NIH/NIMH (1R43AT004871-01) (JT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Almkvist O, Basun H, Wagner SL, Rowe BA, Wahlund LO, Lannfelt L. Cerebrospinal fluid levels of alpha-secretase-cleaved soluble amyloid precursor protein mirror cognition in a Swedish family with Alzheimer disease and a gene mutation. Arch Neurol. 1997;54(5):641–644. doi: 10.1001/archneur.1997.00550170111022. [DOI] [PubMed] [Google Scholar]
- 2.Bailey AR, Giunta BN, Obregon D, Nikolic WV, Tian J, Sanberg CD, Sutton DT, Tan J. Peripheral biomarkers in Autism: secreted amyloid precursor protein- alpha as a probable key player in early diagnosis. Int J Clin Exp Med. 2008;1(4):338–344. [PMC free article] [PubMed] [Google Scholar]
- 3.Barberger-Gateau P, Raffaitin C, Letenneur L, Berr C, Tzourio C, Dartigues JF, Alperovitch A. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69(20):1921–1930. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 4.Colussi G, Catena C, Baroselli S, Nadalini E, Lapenna R, Chiuch A, Sechi LA. Omega-3 fatty acids: from biochemistry to their clinical use in the prevention of cardiovascular disease. Recent Patents Cardiovasc Drug Discov. 2007;2(1):13–21. doi: 10.2174/157489007779606158. [DOI] [PubMed] [Google Scholar]
- 5.Funamoto S, Morishima-Kawashima M, Tanimura Y, Hirotani N, Saido TC, Ihara Y. Truncated carboxyl-terminal fragments of beta-amyloid precursor protein are processed to amyloid beta-proteins 40 and 42. Biochemistry. 2004;43(42):13532–13540. doi: 10.1021/bi049399k. [DOI] [PubMed] [Google Scholar]
- 6.Green KN, Martinez-Coria H, Khashwji H, Hall EB, Yurko-Mauro KA, Ellis L, LaFerla FM. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci. 2007;27(16):4385–4395. doi: 10.1523/JNEUROSCI.0055-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsiao K, Chapman P, Nilsen S, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 8.Huang TL, Zandi PP, Tucker KL, Fitzpatrick AL, Kuller LH, Fried LP, Burke GL, Carlson MC. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology. 2005;65(9):1409–1414. doi: 10.1212/01.wnl.0000183148.34197.2e. [DOI] [PubMed] [Google Scholar]
- 9.Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Gordon M, Tan H, Games D, Lieberburg I, Schenk D, Seubert P, McConlogue L. Amyloid precursor protein processing and A beta42 deposition in a transgenic mouse model of Alzheimer disease. Proc Natl Acad Sci U S A. 1997;94(4):1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42(5):776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 11.Bell KF, Zheng L, Fahrenholz F, Cuello AC. ADAM-10 over-expression increases cortical synaptogenesis. Neurobiol Aging. 2008;29(4):554–65. doi: 10.1016/j.neurobiolaging.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Olsson A, Hoglund K, Sjogren M, Andreasen N, Minthon L, Lannfelt L, Buerger K, Hampel H, Blennow K. Measurment of alpha and beta secretase cleaved amyloid precursor protein in cerebrospinal fluid from Alzheimer patients. Exp. Neurol. 2003;183(1):74–80. doi: 10.1016/s0014-4886(03)00027-x. [DOI] [PubMed] [Google Scholar]
- 13.Obregon DF, Rezai-Zadeh K, Bai Y, Sun N, Hou H, Ehrhart J, Zeng J, Mori T, Arendash GW, Shytle D, Town T, Tan J. ADAM10 activation is required for green tea (−)-epigallocatechin-3-gallate-induced alpha-secretase cleavage of amyloid precursor protein. J Biol Chem. 2006;281(24):16419–16427. doi: 10.1074/jbc.M600617200. [DOI] [PubMed] [Google Scholar]
- 14.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 15.Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K, Zeng J, Morgan D, Hardy J, Town T, Tan J. Green tea epigallocatechin-3- gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25(38):8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambamurti K, Greig NH, Lahiri DK. Advances in the cellular and molecular biology of the beta-amyloid protein in Alzheimer's disease. Neuromolecular Med. 2002;1(1):1–31. doi: 10.1385/NMM:1:1:1. [DOI] [PubMed] [Google Scholar]
- 17.Puskas LG, Kitajka K, Nyakas C, Barcelo-Coblijn G, Farkas T. Short-term administration of omega 3 fatty acids from fish oil results in increased transthyretin transcription in old rat hippocampus. Proc Natl Acad Sci U S A. Feb 18. 2003;100(4):1580–5. doi: 10.1073/pnas.0337683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Link CD. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci U S A. September 26. 1995;92(20):9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein TD, Johnson JA. Lack of neurodegeneration in transgenic mice overexpressing mutant amyloid precursor protein associated with increased levels of transthyretin and the activation of cell survival pathways. J Neurosci. 22(17):7380–7388. doi: 10.1523/JNEUROSCI.22-17-07380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serot JM, Christmann D, Dubost T, Couturier M. Cerebrospinal fluid transthyretin: aging and late onset Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1997;63:506–508. doi: 10.1136/jnnp.63.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]